During the 2017–2018 fiscal year, the number of Canadians authorized to receive medical cannabis (ie, products derived from the cannabis plant, as opposed to the pharmaceutical cannabinoids nabilone and nabiximols) increased from 174 503 to 296 702, and that number continues to climb.1 In this commentary, we argue that the evidence for the therapeutic benefits of medical cannabis is very limited, and that this evidence is misrepresented by the medical cannabis industry. We further argue that the lack of regulation and oversight of medical cannabis clinics by Health Canada has allowed clinicians to prescribe unsafe doses. We comment on the influence that the medical cannabis industry has had on the public’s perception of cannabis and consider the effect that this influence has within the context of legalization. Finally, we outline strategies that family physicians can use to protect their patients from the harms of cannabis.
Evidence of benefit for medical cannabis
Advocates claim that cannabis is effective for a variety of medical and psychiatric conditions; however, the only conditions for which cannabis has credible evidence of benefit are neuropathic pain, spasticity from multiple sclerosis, palliative care, and chemotherapy-induced vomiting.2
In spite of a lack of supporting evidence, the medical cannabis industry commonly makes exaggerated claims about the therapeutic benefits of cannabis. A website belonging to a chain of cannabis clinics provides a list of “common conditions treated with cannabis” that includes several conditions that have little to no evidence and for which clinical guidelines do not recommend the use of cannabis. These conditions include back pain, headaches, anxiety, and posttraumatic stress disorder (PTSD).3 To take PTSD as an example, medical cannabis has been promoted as a viable treatment without a reasonable body of evidence behind its use. A prominent medical cannabis company states the following:
We’ve heard from a number of our patients that suffer from PTSD that medical cannabis is very effective at helping find relief …. More and larger studies need to be done on the effects of cannabis on treating PTSD, although recent studies are showing that there is much promise in this form of treatment.4
The company bases this conclusion on 2 studies of uncertain relevance to medical cannabis—one on nabilone5 and the other on pure oral tetrahydrocannabinol (THC)6—and on anecdotal evidence from studies that cannabis improves PTSD symptoms among veterans.4 However, these studies are not reflective of the wider body of literature. A recent systematic review7 of systematic reviews, clinical trials, and observational studies with control groups identified 2 systematic reviews and 3 primary studies on cannabis and PTSD. Both systematic reviews8,9 concluded that there was insufficient evidence to draw any conclusions about the efficacy of cannabis in PTSD treatment. One of the 3 primary studies, a retrospective cohort study of more than 2000 US veterans who were assessed before and after attending residential treatment for PTSD, found that those who started or continued using cannabis had worse PTSD symptoms, more violent behaviour, and greater alcohol use compared with those who never used or stopped using cannabis.10 In the other 2 studies, cannabis use was not associated with severity of PTSD symptoms.11,12 Another review of cannabis and PTSD concluded that
marijuana use has been linked to … depression, anxiety, psychosis, and substance misuse. Marijuana use is also associated with worse treatment outcomes in naturalistic studies .… Known risks of marijuana thus currently outweigh unknown benefits for PTSD.13
A broader literature review shows that the evidence for cannabis as a treatment for PTSD is far from conclusive, indicating that the cannabis company’s claim about recent studies showing promise4 is misleading and disingenuous.
Furthermore, when considering the evidence for medical cannabis, it is important to distinguish pharmaceutical cannabinoids from other preparations: cannabis advocates tend to equate the benefits and safety of medical cannabis with that of pharmaceutical cannabinoids, yet the 2 have different effectiveness profiles. Evidence for medical cannabis is far weaker than for the pharmaceutical cannabinoids nabiximols and nabilone, in part because of the difficulty of designing a trial of sufficient quality for inhaled cannabis. In a systematic review of cannabinoids for neuropathic pain, inclusion criteria were met by 10 randomized controlled trials (RCTs) on nabiximols, 3 RCTs on nabilone and dronabinol, and only 2 on medical cannabis.14 A systematic review of 11 high- and moderate-quality systematic reviews of RCTs and prospective long-term observational studies on the effectiveness of cannabinoids (both pharmaceutical cannabinoids and cannabis) in pain management15 found no evidence of benefit for medical cannabis (and limited evidence of benefit for nabiximols in the management of neuropathic pain).
Pharmaceutical cannabinoids also have a different safety profile than cannabis. Smoking is the most common route for cannabis ingestion among US adults,16 and cannabis smoke contains multiple toxins, some of which are carcinogenic and atherogenic. Smoking delivers very high concentrations of THC to the brain within seconds, which can cause acute cognitive impairment and increase the risk of motor vehicle accidents.17 Smoking 2 g of cannabis containing 20% THC, a concentration that is high but available for purchase from producers of medical cannabis, will deliver up to 400 mg of THC to the brain (although some THC will be lost through side smoke), whereas 12 sprays of nabiximols, the maximum daily dose, only delivers 33 mg of THC. Creating an equivalency between these 2 different products is misleading to consumers and contributes to a lack of public knowledge about the effects of medical cannabis.
Cannabis and opioids
Invoking a public health angle, advocates have asserted that cannabis can help prevent or treat opioid use disorder.18 This claim is based on a study that found that US states that had legalized cannabis had reductions in opioid overdose rates.19 However, this is an ecologic study that did not analyze individual-level data on cannabis use and overdose risk. Other factors not related to cannabis laws might have contributed to lower overdose rates, such as prescription monitoring systems or the crackdown on “pill mills.” A recent analysis of statewide long-term time trends in overdose deaths found that the states that had legalized cannabis had lower rates of prescription opioid overdose before legalization, and controlling for this eliminated the association.20
Contrary to advocates’ claims, observational studies have found a positive association between cannabis use and opioid use disorder. A review of observational studies concluded that opioid misuse is more common among cannabis users than among nonusers,8 and a large American epidemiologic survey found that cannabis use was associated with nonmedical prescription opioid use (odds ratio of 5.78, 95% CI 4.23 to 7.90) and with opioid use disorder (odds ratio of 7.76, 95% CI 4.95 to 12.16)21; other studies have had similar results.22
Medical cannabis users have self-reported that cannabis has helped them reduce their use of prescription opioids for pain,23 but there is no objective evidence that cannabis reduces use of opioid analgesics. In a 4-year Australian cohort study of 1500 patients taking opioids for chronic pain, frequent cannabis users had higher pain scores, higher pain interference scores, and lower pain self-efficacy scores; and they were not using lower opioid doses and did not have higher rates of opioid discontinuation.24
Regulation and oversight of the medical cannabis industry
There is little transparency about the clinical practices of medical cannabis clinics. To our knowledge, Canadian cannabis clinics have not published the indications, contraindications, or dosing protocols for the products they prescribe. The College of Family Physicians of Canada, in its 2014 guidance document,25 recommends restricting the prescribing of dried cannabis to patients with severe neuropathic pain unresponsive to all first-line medications. It also recommends a maximum dose of 700 mg of dried cannabis with 9% THC per day. However, in 2017, the average daily dose of prescribed medical cannabis in Canada was 2.3 g,1 well in excess of this recommended maximum. The average concentration of THC prescribed has not been reported, but many of the industry’s cannabis products contain concentrations of THC that are far above the 9% recommended by the guidance document, with some containing concentrations of 20% or more. To discourage inappropriate prescribing, some US states have imposed medical requirements on cannabis clinics, including mandatory training in cannabis prescribing, prescriptions lasting no more than 30 days, and a requirement to closely follow the patient to ensure the safety and effectiveness of the cannabis prescription.26
The provincial regulatory colleges have put out position statements on medical cannabis. For example, the College of Physicians and Surgeons of Ontario position statement27 advises physicians that they must do a comprehensive assessment before prescribing cannabis and identify patients at high risk of cannabis-related harms, such as psychosis or mood disorders. The college also recommends that prescribers use a low dose and monitor for complications such as cannabis use disorder (CUD).
However, evidence from the United States suggests that physicians frequently prescribe medical cannabis to patients who have conditions for which cannabis is contraindicated, such as CUD. In a US survey study, family doctors reported that 31% of their patients who were prescribed medical cannabis by another doctor had a medical condition that could be worsened by cannabis.28 Another large-scale epidemiologic study found that, out of a total of 3784 respondents with past-year cannabis use, 32% of medical cannabis users had past-year CUD, compared with 25% of recreational cannabis users.29 A US study of at-risk youth in Denver and San Francisco found that CUD was significantly (
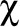 2 = 22.8, P < .001) associated with having a medical cannabis card.30 A review of US medical cannabis programs noted that
2 = 22.8, P < .001) associated with having a medical cannabis card.30 A review of US medical cannabis programs noted that
in many states people receive authorizations for medical marijuana from physicians whom they have seen for a single visit …. Initial studies have shown that the typical medical marijuana patient in these states is a young male with a nonspecific indication of chronic or severe pain and a history of recreational marijuana use.26
Health Canada is responsible for ensuring that pharmaceutical products are safe and effective. It approves products for sale after rigorous review of their safety and effectiveness, and it requires companies to develop a product monograph containing the indications, contraindications, and dosing for the product. Companies are not allowed to promote “off-label” uses of their product (ie, for nonindicated conditions). Physicians are expected to be consistent with the product monograph in their prescribing of the product and in their educational presentations on the product. However, Health Canada does not require cannabis companies to produce and abide by a product monograph, listing the indications, contraindications, and dosing of their products. As a result, the educational programs the industry sponsors have no restrictions on their claims about their product. Furthermore, Health Canada has allowed the companies to produce cannabis with THC concentrations of 20% or more. Industry involvement in medical cannabis marketing and education has a very dangerous precedent: Purdue’s marketing of OxyContin.
Health Canada failed in its obligation to protect the public in the OxyContin epidemic. Health Canada approved Purdue’s product monograph even though it contained misleading and inaccurate information. An affidavit, submitted to the court as part of the successful national class-action lawsuit against Purdue,31 concluded that Purdue’s 2009, 2010, and 2011 OxyContin monographs contained inaccurate statements that encouraged physicians to prescribe very high doses of OxyContin to patients at high risk of addiction, overdose, and other harms. The monographs suggested that OxyContin is indicated for all types of pain; they did not provide a therapeutic range or an upper dose limit; they did not warn physicians that high opioid doses are associated with an increased risk of overdose and addiction; and they did not warn physicians that certain patient groups were at high risk of these harms. This allowed Purdue, in its many publications, conferences, and workshops, to tell physicians that OxyContin could be “dosed to effect,” with no upper limit; that it was effective for all types of pain; and that addiction was rare in pain patients. Purdue’s campaign profoundly changed physicians’ prescribing practices and directly or indirectly caused the deaths of many thousands of people across North America. Health Canada also allowed Purdue to produce tablets containing up to 80 mg of oxycodone, 16 times the amount contained in an acetaminophen-oxycodone tablet, with the result that physicians began prescribing high doses of oxycodone even for benign pain conditions.
While medical cannabis will not lead to overdose deaths, it could potentially cause harm and disability for many. Health Canada has put strict limits on advertising, and has set the minimum legal age for cannabis purchase at 18, but much more needs to be done. Canadians were given access to medical cannabis because of a Supreme Court decision, not because of Health Canada regulatory approval. Nonetheless, it has the regulatory authority, and the public health obligation, to regulate medical cannabis just as it does with other pharmaceutical products. Health Canada should require the industry to produce a product monograph, stating the evidence-based indications, precautions, contraindications, and dosing protocols for medical cannabis. Health Canada should also prevent the industry from making products containing levels of THC higher than those used in trials (ie, 9%).
Legalization
Legalization of recreational cannabis has created uncertainty about the future of medical cannabis. The Canadian Medical Association has recommended that the medical cannabis program be scrapped.32 However, even if this happens, the marketing of medical cannabis has enhanced the public’s perception that cannabis is safe and beneficial, which in turn will increase the use and the harms of recreational cannabis. People who have been persuaded that cannabis will relieve their pain, anxiety, insomnia, or PTSD will purchase recreational cannabis if they cannot access medical cannabis. They will also be more resistant to concerns from family and friends about their cannabis use. There is evidence of an association between positive social attitudes about cannabis and population-level use of cannabis. Canada has a more positive attitude toward cannabis and a higher per capita use of cannabis than Sweden or Finland.33 Exposure to advertising of medical cannabis was associated with greater intention to use cannabis by students in grades 6 through 8 in California.34 While Canada does not permit direct advertising of cannabis, the industry and the clinics are able to market their products through media stories, websites, direct marketing to physicians, and “agents” who provide advice to consumers about how to access cannabis.
Whether or not medical cannabis is still available, legalization will likely cause an overall increase in the public health harms of cannabis. In Colorado, where the legal age for cannabis purchase is 21, emergency department visits among adolescents for cannabis-related reasons rose from 1.8 per 1000 visits in 2009 to 4.9 per 1000 visits in 2015.35
Managing the risks of cannabis use
Family physicians can help protect their patients and the public from the harms of cannabis through the following steps.
Follow the College of Family Physicians of Canada guidance document25 when prescribing cannabis.
Use pharmaceutical preparations (nabilone or nabiximols) for patients with neuropathic pain who have not responded to a trial of adequate dose and duration of first-line medications (serotonin-norepinephrine reuptake inhibitors such as duloxetine, tricyclic antidepressants, and gabapentin or pregabalin), and if an adequate trial of nabilone or nabiximols is ineffective, consider a trial of vaporized dried cannabis slowly titrated to a maximum dose of 400 mg containing no more than 9% THC and at least 9% cannabidiol.25
For patients who request a cannabis prescription for a nonindicated or contraindicated condition, emphasize that cannabis lacks evidence of benefit for these conditions and has considerable evidence of harm, including motor vehicle accidents,36,37 psychosis,38,39 wor sening anxiety,40 long-term cognitive impairment,41 and CUD.
Explain that the risk of these harms is increased in young patients42–44 and with high THC doses.45
When patients are already using cannabis for symptom control, assess them for CUD.
Cannabis use disorder is characterized by frequent or daily use, spending a lot of time using, poor performance at work or school, deteriorating social relationships, anxiety, depression and fatigue, and inability to reduce or stop cannabis.46 Inform patients with CUD that their mood and daily function will markedly improve with treatment and reduction or cessation of cannabis use. As with other substance use disorders, CUD is often precipitated by a mental disorder, particularly PTSD and anxiety and mood disorders. Family physicians should identify these conditions in patients with suspected CUD, and make appropriate interventions and referrals.
Use a harm-reduction approach with cannabis users who do not have CUD.
Counsel them on the following safety measures outlined in the lower-risk cannabis use guidelines,47 which include the following:
Do not drive for at least 6 to 8 hours after using.
Use a vaporizer rather than smoking.
Do not mix with alcohol, opioids, or other sedating drugs.
Avoid high doses of potent THC, especially if you are younger than age 25, pregnant, or have an active psychiatric disorder.
Only refer patients to a cannabis clinic that adheres to a published, evidence-based prescribing guideline that has been endorsed by at least 1 unbiased medical organization.
The guideline should specify the indications, precautions, and contraindications for medical cannabis, as well as a dosing protocol.2,25
Conclusion
The evidence for medical cannabis is very limited, and its harms are substantial. Health Canada should require the industry to produce an evidence-based product monograph listing the indications, contraindications, and dosing of cannabis. Health Canada should also impose a limit on the THC concentration of cannabis products of no more than 9%. Family physicians should only prescribe cannabis to patients with neuropathic pain, at a maximum dose of 400 mg per day with 9% THC and an equal amount of cannabidiol. Patients who use cannabis regularly should be assessed for CUD and given advice on avoiding cannabis-related harms. It is not too late to impose evidence-based practice standards and guidelines that can help prevent the overprescribing of cannabis.
Footnotes
Competing interests
None declared
The opinions expressed in commentaries are those of the authors. Publication does not imply endorsement by the College of Family Physicians of Canada.
This article has been peer reviewed.
Cet article se trouve aussi en français à la page 873.
References
- 1.Government of Canada [website] Market data under the Access to Cannabis for Medical Purposes Regulations. Ottawa, ON: Government of Canada; 2018. Available from: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/licensed-producers/market-data.html. Accessed 2019 Oct 16. [Google Scholar]
- 2.Allan GM, Ramji J, Perry D, Ton J, Beahm NP, Crisp N, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician. 2018;64:111–20. (Eng), e64–75 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 3.Apollo Cannabis Clinics [website] Apollo’s medical cannabis guide. Toronto, ON: Apollo Cannabis Clinics; Available from: https://d2s2p4r6.stackpathcdn.com/wp-content/uploads/2019/07/medical_cannabis_guide_onepager2.pdf. Accessed 2019 Oct 16. [Google Scholar]
- 4.How cannabis can help PTSD [blog] CanniMed.ca. 2016. Jul 18, Available from: www.cannimed.ca/blogs/blog/cannabis-and-ptsd. Accessed 2019 Oct 16.
- 5.Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–8. doi: 10.1016/j.psyneuen.2014.11.002. Epub 2014 Nov 8. [DOI] [PubMed] [Google Scholar]
- 6.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34(8):587–91. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- 7.O’Neil ME, Nugent SM, Morasco BJ, Freeman M, Low A, Kondo K, et al. Benefits and harms of plant-based cannabis for posttraumatic stress disorder: a systematic review. Ann Intern Med. 2017;167(5):332–40. doi: 10.7326/M17-0477. Epub 2017 Aug 15. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050–64. doi: 10.4088/JCP.15r10036. [DOI] [PubMed] [Google Scholar]
- 9.Walsh Z, Gonzalez R, Crosby K, Thiessen MS, Carroll C, Bonn-Miller MO. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15–29. doi: 10.1016/j.cpr.2016.10.002. Epub 2016 Oct 12. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(9):1174–80. doi: 10.4088/JCP.14m09475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MJ, Pierce JD, Mavandadi S, Klaus J, Defelice D, Ingram E, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439–42. doi: 10.1016/j.jad.2015.10.048. Epub 2015 Oct 28. [DOI] [PubMed] [Google Scholar]
- 12.Ruglass LM, Shevorykin A, Radoncic V, Smith KM, Smith PH, Galatzer-Levy IR, et al. Impact of cannabis use on treatment outcomes among adults receiving cognitive-behavioral treatment for PTSD and substance use disorders. J Clin Med. 2017;6(2):E14. doi: 10.3390/jcm6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steenkamp MM, Blessing EM, Galatzer-Levy IR, Hollahan LC, Anderson WT. Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: a literature review. Depress Anxiety. 2017;34(3):207–16. doi: 10.1002/da.22596. Epub 2017 Feb 28. [DOI] [PubMed] [Google Scholar]
- 14.Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies [article in German] Schmerz. 2016;30(1):62–88. doi: 10.1007/s00482-015-0089-y. Erratum in: Schmerz 2017;31(6):619. [DOI] [PubMed] [Google Scholar]
- 15.Häuser W, Fitzcharles MA, Radbruch L, Petzke F. Cannabinoids in pain management and palliative medicine. An overview of systematic reviews and prospective observational studies. Dtsch Arztebl Int. 2017;114(38):627–34. doi: 10.3238/arztebl.2017.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steigerwald S, Wong PO, Cohen BE, Ishida JH, Vali M, Madden E, et al. Smoking, vaping, and use of edibles and other forms of marijuana among U.S. adults. Ann Intern Med. 2018;169(12):890–2. doi: 10.7326/M18-1681. Epub 2018 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas P. Rationale for cannabis-based interventions in the opioid overdose crisis. Harm Reduct J. 2017;14(1):58. doi: 10.1186/s12954-017-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174(10):1668–73. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohl RV. Time trends matter: the case of medical cannabis laws and opioid overdose mortality. SSRN. 2018. Jun 22, Available from: https://ssrn.com/abstract=3192703. Accessed 2019 Oct 16.
- 21.Olfson M, Wall MM, Liu SM, Blanco C. Cannabis use and risk of prescription opioid use disorder in the United States. Am J Psychiatry. 2018;175(1):47–53. doi: 10.1176/appi.ajp.2017.17040413. Epub 2017 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiBenedetto DJ, Weed VF, Wawrzyniak KM, Finkelman M, Paolini J, Schatman ME, et al. The association between cannabis use and aberrant behaviors during chronic opioid therapy for chronic pain. Pain Med. 2018;19(10):1997–2008. doi: 10.1093/pm/pnx222. [DOI] [PubMed] [Google Scholar]
- 23.Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17(6):739–44. doi: 10.1016/j.jpain.2016.03.002. Epub 2016 Mar 19. [DOI] [PubMed] [Google Scholar]
- 24.Campbell G, Hall WD, Peacock A, Lintzeris N, Bruno R, Larance B, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3(7):e341–50. doi: 10.1016/S2468-2667(18)30110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.College of Family Physicians of Canada . Authorizing dried cannabis for chronic pain or anxiety. Preliminary guidance. Mississauga, ON: College of Family Physicians of Canada; 2014. [Google Scholar]
- 26.Williams AR, Olfson M, Kim JH, Martins SS, Kleber HD. Older, less regulated medical marijuana programs have much greater enrollment rates than newer ‘medicalized’ programs. Health Aff (Millwood) 2016;35(3):480–8. doi: 10.1377/hlthaff.2015.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.College of Physicians and Surgeons of Ontario . Marijuana for medical purposes. Position statement no. 8–16. Toronto, ON: College of Physicians and Surgeons of Ontario; 2016. [Google Scholar]
- 28.Kondrad EC, Reed AJ, Simpson MJ, Nease DE. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31(5):805–8. doi: 10.3122/jabfm.2018.05.170462. [DOI] [PubMed] [Google Scholar]
- 29.Choi NG, DiNitto DM, Marti CN. Nonmedical versus medical marijuana use among three age groups of adults: associations with mental and physical health status. Am J Addict. 2017;26(7):697–706. doi: 10.1111/ajad.12598. Epub 2017 Aug 18. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Coors ME, Young SE, Raymond KM, Hopfer CJ, Wall TL, et al. Cannabis use disorder and male sex predict medical cannabis card status in a sample of high risk adolescents. Drug Alcohol Depend. 2018;183:25–33. doi: 10.1016/j.drugalcdep.2017.11.007. Epub 2017 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay v Purdue Pharma Inc. 2017. ONSC No. 07-CV-343201CP.
- 32.Doctors’ group wants to scrap Canada’s medical cannabis program CBC Radio. 2018. Apr 27, Available from: www.cbc.ca/radio/quirks/scrap-medical-weed-women-in-space-and-more-1.4636793/doctors-group-wants-to-scrap-canada-s-medical-cannabis-program-1.4636810. Accessed 2019 Oct 23.
- 33.Cunningham JA, Blomqvist J, Koski-Jannes A, Raitasalo K. Societal images of cannabis use: comparing three countries. Harm Reduc J. 2012;9(1):21. doi: 10.1186/1477-7517-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Amico EJ, Miles JN, Tucker JS. Gateway to curiosity: medical marijuana ads and intention and use during middle school. Psychol Addict Behav. 2015;29(3):613–9. doi: 10.1037/adb0000094. Epub 2015 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GS, Davies SD, Halmo LS, Sass A, Mistry RD. Impact of marijuana legalization in Colorado on adolescent emergency and urgent care visits. J Adolesc Health. 2018;63(2):239–41. doi: 10.1016/j.jadohealth.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478–92. doi: 10.1373/clinchem.2012.194381. Epub 2012 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109–19. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Davis GP, Compton MT, Wang S, Levin FR, Blanco C. Association between cannabis use, psychosis, and schizotypal personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Schizophr Res. 2013;151(1–3):197–202. doi: 10.1016/j.schres.2013.10.018. Epub 2013 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195(6):488–91. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18(32):4966–79. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 41.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657–64. doi: 10.1073/pnas.1206820109. Epub 2012 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dragt S, Nieman DH, Becker HE, van de Fliert R, Dingemans PM, de Haan L, et al. Age of onset of cannabis use is associated with age of onset of high-risk symptoms for psychosis. Can J Psychiatry. 2010;55(3):165–71. doi: 10.1177/070674371005500308. [DOI] [PubMed] [Google Scholar]
- 43.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5(1):1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brook JS, Lee JY, Brown EN, Finch SJ, Brook DW. Developmental trajectories of marijuana use from adolescence to adulthood: personality and social role outcomes. Psychol Rep. 2011;108(2):339–57. doi: 10.2466/10.18.PR0.108.2.339-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–91. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 47.Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, et al. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. 2017;107(8):e1–12. doi: 10.2105/AJPH.2017.303818. [DOI] [PMC free article] [PubMed] [Google Scholar]


