ABSTRACT
During the Brazilian slavery period, many African migrants were brought to the American continent. Historically, some of these migrants escaped from the Brazilian gold mines and farms to which they had been brought and settled in remote valleys and this was the main mode of resistance to the slavery system. These runaway-slave descendant communities are called quilombos, a group with distinct ethnic identity, specific behavioral habits, including geographic isolation and conservative practices. The objective of this study was to investigate the prevalence of rodent-borne viruses in two Afro-descendent communities from Mato Grosso do Sul State, Midwestern Brazil. A total of 319 individuals from rural and urban quilombola communities were enrolled. Twelve (3.76%) had anti-rodent-borne virus IgG antibodies. Seven (2.19%) were anti-mammarenavirus reactive and nine (2.82%) had anti-orthohantavirus antibodies. The literature includes limited data on the health status of quilombola communities, but all the studies emphasize the disparity of attention of local healthcare personnel to these communities compared to the general population. The findings of this study highlight the vulnerability and the precarious health conditions of quilombola groups, especially those living in rural areas and thus, point to the need of preventive measures to improve access to healthcare for this ethnic group.
Keywords: Afro-descendent communities, Arenavirus, Hantavirus, Rodent-borne diseases, Zoonosis, Quilombolas
Rodent-borne diseases have been a global health concern since there has been an increase in the number of different pathogens whose cycles depend on rodents in many ways 1 . Moreover, global climate change and transitions in human settlement patterns are considered the main factors responsible for the increase of problems with rodent-transmitted pathogens, especially in developing countries 1 , 2 .
Hantaviruses and arenaviruses are naturally occurring viruses of rodents, and human infections are classic examples of emerging diseases caused by the invasion of urban, agricultural, and livestock areas that are rodent habitats 1 .
These two distinct groups of negative-stranded RNA viruses are associated with different species of rodent hosts of the family Cricetidae 3 . The infected host sheds viruses into the environment through urine, feces and saliva; humans usually become infected by inhalation of aerosolized viral particles, but infections can also occur through rodent bites, and more rarely through person-to-person transmission (including solid-organ transplantation recipients) 2 , 3 .
Brazil has very heterogeneous rural populations, including settlers and campesinos, indigenous people, extractivists, family farmers and quilombolas (afro-descendent communities), representing about 36% of the entire country population 4 , 5 . During the Brazilian slavery period, many African migrants were brought to the American continent as slaves. Historically, some of them escaped from Brazilian gold mines and farms and settled in remote valleys and this was the main mode of resistance to the slavery system 5 . These runaway-slave descendents’ communities are called quilombos since their establishment in early times during the colonization of South American countries. Groups with distinct ethnic identities and specific behavioral characteristics, including geographic isolation and conservative practices, inhabit these territories 4 - 6 .
In Brazil, there are 2,474 communities (13.087 families) whose history and tradition allow them to be identified as remnants of quilombos, 77% of these individuals are located in rural areas, where the main activities are based on family farming and extractivism 5 , 6 . Thus, low access to health services, education, electrical light and sanitation are still issues faced by some quilombola communities 5 . The aim of the present study was to investigate the prevalence of rodent-borne viruses (hantavirus and arenavirus) IgG antibodies and its association with demographic variables and risk factors in two afro-descendent communities in Mato Grosso do Sul State, Midwestern Brazil.
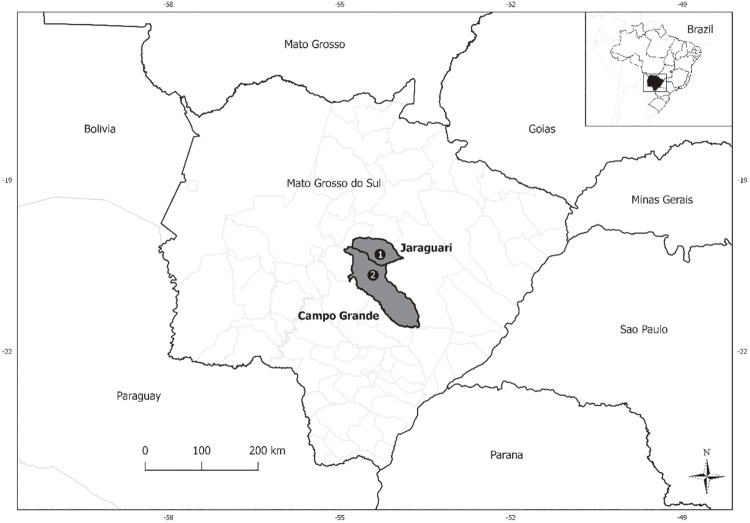
Mato Grosso do Sul State has 22 quilombola communities consisting of approximately 441 families. One of the studied quilombola communities was Furnas dos Dionisios, the largest rural quilombo in the State with 1,018.27 hectares in the Jaraguari municipality ( Figure 1 ) with approximately 450 residents 5 , 6 . Its economy is based on subsistence agriculture, vegetable production, flour and sugar cane derivates and livestock. The other studied population was Sao Benedito ( Figure 1 ), an urban quilombola community, located in the neighborhood of Campo Grande (capital of Mato Grosso do Sul State) with approximately 300 indiviuals 5 . Many inhabitants of this quilombo are employed in urban jobs but they have low access to sanitation. This study was approved by Fundacao Oswaldo Cruz/Instituto Oswaldo Cruz Ethical Committee, Nº CAAE 61629416.2.1001.5248 and by the Research and Ethics Committee of Universidade Federal do Mato Grosso do Sul, Nº CAAE 35103214.0.1001.0021.
Figure 1. Map of the studied quilombola communities regarding hantavirus and arenavirus IgG antibodies: 1) Furnas dos Dionisios; 2) Sao Benedito.
The Fisher exact test was used to evaluate factors associated with hantavirus and arenavirus infections (defined as IgG reactivity for one or both viruses). Statistical significance was set at 0.05 for all analyses. Odds ratios (OR) and 95% confidence intervals (IC 95%) were also estimated. Statistical evaluations were performed using the statistical package R for windows (version 3.4.3).
A total of 319 individuals consisting of 189 participants from Furnas dos Dionisios and 130 from Sao Benedito were voluntarily enrolled in the study between June and December 2015. Serum samples were screened for IgG antibodies against hantavirus and arenavirus using antigens described by Riera et al . 7 and Guzmán et al . 8 , which were tested by an enzyme-linked immunosorbent assay (ELISA) protocol in 96-round-bottom-well-microplates (Thermo Scientific TM ) coated with 100 µL of cell lysate diluted in phosphate-buffered saline (PBS) pH 7.4; one-half of the plate was coated with infected cell lysate (Junin mammarenavirus strain XJC13 or Maciel orthohantavirus strain #9) and the other half with uninfected cell lysate (Vero C76 - ATCC ® CRL-1587™) as the normal control antigens. The plates were kept at 4 o C overnight and then washed five times with 0.1% Tween 20 (Merck & Co., Inc., Kenilworth, NJ, USA) in PBS. The wells were then filled with 100 µL of diluted test serum samples starting at 1:100 dilution in PBS with 0.1% Tween 20 (Merck & Co., Inc., Kenilworth, NJ, USA) and 5.0% skimmed milk (BD Difco™). The plates were incubated for 1 h at 37 o C, washed as previously described, and 100 µL of goat anti-human IgG peroxidase-conjugated secondary antibody (Sigma-Aldrich ® , USA) at 1:2000 dilution was placed in each well and incubated for 1 h at 37 o C. Plates were washed five times, and 100 µL of ABTS TM substrate (Sigma-Aldrich ® , USA) was added to each well and left on the well for 30 min at 37 o C. Objective readings of ELISA results were performed by determination of absorbances at 405 and 450 nm. The cut-off was determined by the mean optical density (OD) of negative controls plus three standard deviations at 1:100 dilution, after subtracting the OD of the negative antigen from that of the positive one. A serum dilution was considered positive if its OD was > 0.2 7 , 8 .
The population ranged in age from 2 to 89 years old (average 34.44 years), and 57.05% (182/319) were females. Most of these individuals had low educational leveland 74.92% (239/319) had 1–9 years of formal education, 94.34% (300/319) received less than three minimum wages (US$330.00, Brazilian minimum wage in 2015) per family, per month, 71.47% (228/319) of the houses had no sewage system, and 52.35% (167/319) had no tap water service. Sociodemographic differences by community are shown in Table 1 . Afro-descendent populations along with Amerindians continue to be one of the most disadvantaged groups of people, presenting higher rates of segregation, poverty, unemployment, illiteracy and migration, associated with lower access to healthcare services and environmental sanitation 4 - 6 .
Table 1. Demographic variables, seroprevalence (%), Odds Ratio (with Confidence Interval) and the Fisher exact test p-value for Furnas do Dionisios and Sao Benedito Afro-descendent communities, Mato Grosso do Sul State, Brazil (2015).
| VARIABLES | FURNAS DO DIONISIOS | SAO BENEDITO | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Seropositivity (%) | Odds Ratio (95% CI) | Fisher exact test p-value | N (%) | Seropositivity (%) | Odds Ratio (95% CI) | Fisher exact test p-value | |
| Age | 0.07 | 0.31 | ||||||
| <18 | 58 (30.69) | 2 (3.45) | 23 (17.69) | 1 (4.35) | ||||
| 18-40 | 62 (32.8) | 1 (1.61) | 52 (40) | 0 | ||||
| 41-60 | 52 (27.51) | 3 (5.77) | 40 (30.77) | 2 (5.0) | ||||
| >60 | 17 (8.99) | 3 (17.65) | 15 (11.54) | 0 | ||||
| Gender | 0.73 | 0.56 | ||||||
| Women | 103 (54.5) | 4 (3.88) | 1.52 | 79 (60.77) | 1 (1.27) | 3.15 | ||
| Men | 86 (45.5) | 5 (5.81) | (0.39-5.87) | 51 (39.23) | 2 (3.92) | (0.16-189.95) | ||
| Scholarity | 0.55 | 0.67 | ||||||
| < 1 year | 15 (7.94) | 1 (6.67) | 8 (6.15) | 0 | ||||
| 1 – 9 years | 139 (73.54) | 8 (5.76) | 77 (59.23) | 3 (3.9) | ||||
| 10 – 12 years | 31 (16.4) | 0 | 40 (30.77) | 0 | ||||
| Higher education | 4 (2.12) | 0 | 5 (3.85) | 0 | ||||
| Monthly income | 0.07 | 0.22 | ||||||
| <1 minimum wage* | 84 (44.44) | 5 (5.95) | 9 (6.98) | 1 (11.11) | ||||
| 1-3 minimum wages | 102 (53.97) | 3 (2.94) | 105 (81.4) | 2 (1.9) | ||||
| >3 minimum wages | 3 (1.59) | 1 (33.33) | 15 (11.63) | 0 | ||||
| Occupation | 0.69 | 0.61 | ||||||
| Farmer | 80 (42.33) | 3 (3.75) | 6 (4.62) | 0 | ||||
| Housewife | 28 (14.81) | 2 (7.14) | 40 (30.77) | 1 (2.5) | ||||
| Retired/Unemployed | 8 (4.23) | 1 (12.5) | 13 (10) | 0 | ||||
| Student | 47 (24.87) | 2 (4.26) | 20 (15.38) | 1 (5.0) | ||||
| Work in school | 5 (2.65) | 0 | 10 (7.69) | 0 | ||||
| Child (non-school age) | 6 (3.17) | 0 | 3 (2.31) | 0 | ||||
| Construction | 2 (1.06) | 0 | 12 (9.23) | 1 (8.33) | ||||
| Others | 13 (6.88) | 1 (7.69) | 26 (20.0) | 0 | ||||
| Access to potable water | 0.55 | 1.00 | 1.00 | |||||
| No | 154 (81.91) | 8 (5.19) | (0.01-4.38) | 12 (9.23) | 0 | |||
| Yes | 34 (18.09) | 1 (2.94) | 118 (90.77) | 3 (2.54) | ||||
| Sanitation | 1.99 | 0.39 | 0.74 | 1.00 | ||||
| No | 150 (79.37) | 6 (4.0) | (0.30-9.87) | 78 (60.0) | 2 (2.56) | (0.01-14.69) | ||
| Yes | 39 (20.63) | 3 (7.69) | 52 (40.0) | 1 (1.92) | ||||
From the total of 319 individuals, 12 (3.76%) were positive for rodent-borne viruses, seven (2.19%) were anti-mammarenavirus reactive and 9 (2.82%) had anti-orthohantavirus antibodies. Four samples were reactive for both viruses (1.25%). The prevalence for rodent-borne viruses by community was 2.40% (3/125) in Sao Benedito and 4.64% (9/194) in Furnas dos Dionisios, but no statistical differences were observed (p-value = 0.37; OR = 2.11; 95% CI = 0.56–7.97). Although expected, this difference is not always higher in rural population studies as we would suppose. Some studies have demonstrated that an urban population can be more exposed to rodent-borne diseases, resulting in a higher prevalence 9 . The prevalence of reactive men was 5.11% (7/137), while the prevalence in women was 3.29% (6/182). There was no job related activities shared between the seroreactive individuals, three were students (3/12), had activities related to housekeeping (3/12), agriculture (3/12), were retired (1/12) or had other non specified activities (2/12).
Arenaviruses are the causative agents of South American hemorrhagic fevers in Argentina, Bolivia, Venezuela and Brazil, producing hundreds of cases annually with a case-fatality ratio as high as 35% 3 , 10 . Only one case of Brazilian hemorrhagic fever in the Cotia municipality, Sao Paulo State was reported in 1990, and the reservoir of Sabia virus is still unknown 10 . Recent studies have detected the occurrence of Apore, Latino and Oliveros mammarenaviruses in Oligoryzomys mattogrossae (= fornesi ), Calomys callosus (67.8%) and Necromys lasiurus (14.2%), respectively, in three counties of Mato Grosso do Sul State 11 , 12 . Although there is no evidence of human diseases caused by these viruses, there is an increasing amount of serological evidence of arenavirus infection in different rural populations from the Midwest in which the prevalence ranges from 1.80% (2/108) to 2.12% (3/141) 13 , 14 .
Interestingly, Andersen et al . 15 demonstrated some evidence of a positive selection in the acetylglucosaminyltransferase-like protein (LARGE) and interleukin 21 (IL21), two genes implicated in arenavirus infectivity and immunity. Their results suggest that natural selection may have targeted variants giving rise to alternative splicing or differential gene expressions of LARGE and IL21 in West African populations. Overall, their results support the hypothesis that selective pressures imposed by the Lassa virus may have led to the emergence of particular alleles conferring resistance to the Lassa virus, and the Lassa fever an endemic arenavirus hemorrhagic disease in West Africa 15 , 16 . Perhaps some genetic recombination taking place in their ancestors may indicate some degree of Afro-descendent-associated resistance in quilombola communities to arenavirus infections, resulting in milder disease or even asymptomatic undetected cases.
The hantavirus prevalence found in quilombolas is similar to those found in highly endemic areas for hantavirus cardiopulmonary syndrome (HCPS) in Santa Catarina (2.30%–3.50%) 17 and Sao Paulo State (1.23%–4.33%) 18 . These values are higher than the ones reported in rural populations from HCPS non-endemic areas in Amazonas State (0.80%–0.90%) 9 . Mato Grosso do Sul State has been a silent area for hantavirus circulation for a long time. Only in recent years (2012), have the first human cases been reported, and to date, only seven cases of HCPS have been detected. The hantavirus genotype responsible for HCPS cases in this State is still unknown. Nonetheless, the Juquitiba virus has been characterized in Oligoryzomys mattogrossae (1.45%), in Cassilandia municipality 19 . These rodents have peridomestic affinities and have been captured in and around human habitats and could contribute to human infections in those involved in housekeeping activities or others who spend most of the day indoors, such as children and elderly people 20 . Studies conducted in apparently healthy individuals from rural and urban slum communities in Chile have shown serological evidence for hantavirus infections associated with household-related occupations (homemaker, retiredor student) reinforcing the idea of the indoor environment as a possible site of infection 20 .
The literature includes limited data related to diseases affecting quilombola communities. Most studies are related to sexually-transmitted infections, blood-borne pathogens and intestinal parasites; nonetheless, all the studies emphasize the importance of carrying out researches involving this social group, given that there is still a disparity with respect to local healthcare attention concerning these individuals compared to the general population 4 - 6 .
The findings of this work reinforce the social vulnerability and the precarious conditions of quilombola groups, especially those living in rural areas. Lack of access to basic sanitation and degradation of the environment could contribute to the emergence of infectious diseases in this population. Thus, this study along with others point to the need of implementation of measures to improve health access via medical assistance, basic sanitation and the sustainable use of environment resources, which would faciliate to improve their health conditions.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Instituto Nacional de Enfermedades Virales Humanas Dr. Julio I. Maiztegui, Monteagudo, Pergamino, Argentina for the donation of antigens used in this work.
Footnotes
FINANCIAL SUPPORT
This research was supported by FIOCRUZ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES), Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul – FUNDECT-MS, Universidade Federal do Mato Grosso do Sul and Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq), grant Nº 404762/2016-6.
REFERENCES
- 1.Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- 2.Prist PR, D’Andrea PS, Metzger JP. Landscape, climate and hantavirus cardiopulmonary syndrome outbreaks. Ecohealth. 2017;14:614–629. doi: 10.1007/s10393-017-1255-8. [DOI] [PubMed] [Google Scholar]
- 3.Charrel RN, Coutard B, Baronti C, Canard B, Nougairede A, Frangeul A, et al. Arenaviruses and hantaviruses: from epidemiology and genomics to antivirals. Antiviral Res. 2011;90:102–114. doi: 10.1016/j.antiviral.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Conde BE, Ticktin T, Fonseca AS, Macedo AL, Orsi TO, Chedier LM, et al. Local ecological knowledge and its relationship with biodiversity conservation among two Quilombola groups living in the Atlantic Rainforest, Brazil. PLoS One. 2017;12:e0187599. doi: 10.1371/journal.pone.0187599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urquiza AH, Santos L. Regularização fundiária de comunidades quilombolas em Mato Grosso do Sul/Brasil. Rev Bras Polit Publicas. 2017;7:231–247. [Google Scholar]
- 6.Motta-Castro AR, Yoshida CF, Lemos ER, Oliveira JM, Cunha RV, Lewis-Ximenez LL, et al. Seroprevalence of Hepatitis B virus infection among an Afro-descendant community in Brazil. Mem Inst Oswaldo Cruz. 2003;98:13–17. doi: 10.1590/s0074-02762003000100002. [DOI] [PubMed] [Google Scholar]
- 7.Riera LM, Feuillade MR, Saavedra MC, Ambrosio AM. Evaluation of an enzyme immunosorbent assay for the diagnosis of Argentine haemorrhagic fever. Acta Virol. 1997;41:305–310. [PubMed] [Google Scholar]
- 8.Guzmán C, Mattar S, Levis S, Pini N, Figueiredo T, Mills J, et al. Prevalence of antibody to hantaviruses in humans and rodents in the Caribbean region of Colombia determined using Araraquara and Maciel virus antigens. Mem Inst Oswaldo Cruz. 2013;108:167–171. doi: 10.1590/0074-0276108022013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimaque JB, Bastos MS, Braga WS, Oliveira CM, Castilho MC, Figueiredo RM, et al. Serological evidence of hantavirus infection in rural and urban regions in the state of Amazonas, Brazil. Mem Inst Oswaldo Cruz. 2012;107:135–137. doi: 10.1590/s0074-02762012000100019. [DOI] [PubMed] [Google Scholar]
- 10.Coimbra TL, Nassar ES, Burattini MN, Souza LT, Ferreira IB, Rocco IM, et al. New arenavirus isolated in Brazil. Lancet. 1994;343:391–392. doi: 10.1016/s0140-6736(94)91226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes J, Oliveira RC, Guterres A, Carvalho Serra F, Bonvicino CR, D’Andrea PS, et al. Co-circulation of Clade C New World Arenaviruses: new geographic distribution and host species. Infect Genet Evol. 2015;33:242–245. doi: 10.1016/j.meegid.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes J, Guterres A, Oliveira RC, Jardim R, Dávila AM, Hewson R, et al. Aporé virus, a novel mammarenavirus (Bunyavirales: Arenaviridae) related to highly pathogenic virus from South America. Mem Inst Oswaldo Cruz. 2019;114:e180586. doi: 10.1590/0074-02760180586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes J, Oliveira RC, Coelho TA, Martins RM, Caetano KA, Horta MA, et al. Rodent-borne viruses survey in ruralsettlers from Central Brazil. Mem Inst Oswaldo Cruz. 2018;114:e180448. doi: 10.1590/0074-02760180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes J, Silva TA, Oliveira RC, Guterres A, Oliveira EC, Terças AC, et al. Silent Arenavirus infection in individuals living in Colniza, Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2018;51:881–882. doi: 10.1590/0037-8682-0071-2018. [DOI] [PubMed] [Google Scholar]
- 15.Andersen KG, Shylakhter I, Tabrizi S, Grossman SR, Happi CT, Sabeti PC. Genome-wide scans provide evidence for positive selection of genes implicated in Lassa fever. Philos Trans R Soc Lond B Biol Sci. 2012;367:868–877. doi: 10.1098/rstb.2011.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torriani G, Galan-Navarro C, Kunz S. Lassa virus cell entry reveals new aspects of virus-host cell interaction. J Virol. 2017;91:e01902-16. doi: 10.1128/JVI.01902-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira GW, Teixeira AM, Souza MS, Braga AD, Santos GS, Junior, Figueiredo GG, et al. Prevalence of serum antibodies to hantavirus in a rural population from the Southern State of Santa Catarina, Brazil. Rev Soc Bras Med Trop. 2012;45:117–119. doi: 10.1590/s0037-86822012000100022. [DOI] [PubMed] [Google Scholar]
- 18.Badra SJ, Maia FG, Figueiredo GG, Santos GS, Junior, Campos GM, Figueiredo LT, et al. A retrospective serologic survey of hantavirus infections in the county of Cássia dos Coqueiros, State of São Paulo, Brazil. Rev Soc Bras Med Trop. 2012;45:468–470. doi: 10.1590/s0037-86822012005000005. [DOI] [PubMed] [Google Scholar]
- 19.Guterres A, Oliveira RC, Fernandes J, Strecht L, Casado F, Gomes de Oliveira FC, et al. Characterization of Juquitiba virus in Oligoryzomys fornesi from Brazilian Cerrado. Viruses. 2014;6:1473–1482. doi: 10.3390/v6041473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Zanzi C, Saavedra F, Otth C, Domancich L, Hott M, Padula P. Serological evidence of hantavirus infection in apparently healthy people from rural and slum communities in southern Chile. Viruses. 2015;7:2006–2013. doi: 10.3390/v7042006. [DOI] [PMC free article] [PubMed] [Google Scholar]