Summary
Unlike juveniles, adult animals engage in suites of behaviors related to the search for and selection of potential mates and mating, including appropriate responses to sex pheromones. As in other species [1], male sex pheromones modulate several behaviors and physiological processes in C. elegans hermaphrodites [2-5]. In particular, one of these small-molecule signals, an ascaroside ascr#10, causes reduced exploration, more avid mating, and improved reproductive performance [Aprison & Ruvinsky, this issue]. Here we investigated the mechanism that restricts pheromone response to adult hermaphrodites. Unexpectedly, we found that attainment of developmental adulthood was not alone sufficient for the behavioral response to the pheromone. To modify exploratory behavior in response to male pheromone adult hermaphrodites also require functional germline and egg-laying apparatus. We show that this dependence of behavior on the reproductive system is due to feedback from the vulva muscles that reports ongoing reproduction to the nervous system. Our results reveal an activity-dependent conduit by which the reproductive system continuously licenses adult behaviors, including appropriate responses to the pheromones of the opposite sex. More broadly, our results suggest that signals from peripheral organs may serve as an important component of assuring age-appropriate functions of the nervous system.
eTOC Blurb:
Aprison and Ruvinsky demonstrate that a retrograde signal from the vulva is required to permit adult-specific responses of C. elegans hermaphrodites to a male pheromone. The underlying circuit continuously apprises the nervous system regarding egg-laying to match reproductive output with an appropriate behavioral repertoire.
Results and Discussion
Behavioral response to male pheromone is restricted to adult hermaphrodites with a functional reproductive system
An asacroside ascr#10 is a major component of the male sex pheromone blend in C. elegans [6]. Previous studies demonstrated that exposure to ascr#10 elicits several physiological responses in the germline of C. elegans hermaphrodites [2-4]. This molecule also alters hermaphrodite’s behavior – it substantially reduces exploratory movement and promotes behaviors that contribute to greater reproductive success, including faster engagement in copulation. A circuit that consists of serotonergic NSM and HSN neurons signaling via the mod-1 receptor and of an opposing pigment dispersing factor (PDF) signaling is required to mediate the effects of asc#10 on both physiology and exploratory behavior (Aprison and Ruvinsky, this issue).
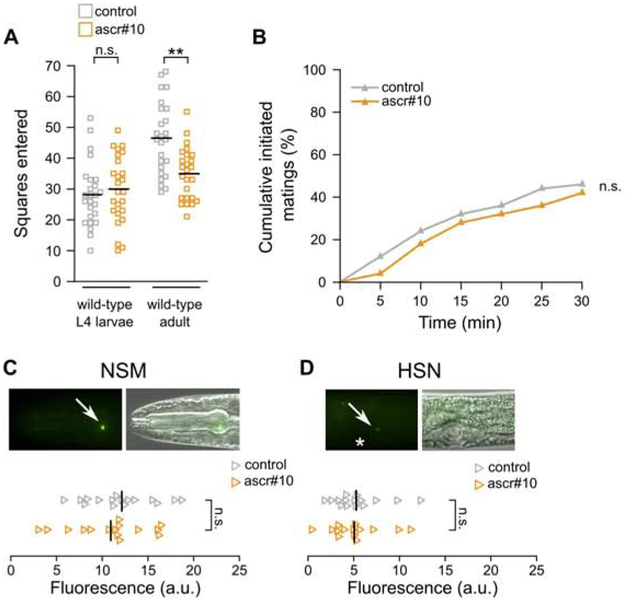
In contrast to adult hermaphrodites in which these pheromone responses were originally described, late larval (L4) hermaphrodites do not decrease exploratory movement in the presence of ascr#10 (Figure 1A). They also do not show decreased latency to engage in mating (Figure 1B), do not show increased expression of the serotonin-producing enzyme tph-1 in NSM and HSN neurons (Figure 1C,D), and do not show the characteristic physiological response in the germline (Figure S1). A parsimonious interpretation of these results is that only sexually mature adult hermaphrodites are competent to respond to the ascr#10 pheromone.
Figure 1. Larvae do not respond to ascr#10.
(A) Exploratory behavior of sexually immature L4 larval hermaphrodites compared to young adults. (B) Latency of mating initiation with sexually immature L4 larval hermaphrodites. (C) Expression of tph-1::YFP in NSM and (D) HSN neurons in L4 larval hermaphrodites. Fluorescence and fluorescence overlaid with differential contrast images. Arrows point to cell bodies. An asterisk marks the position of the developing vulva. Anterior is to the left and ventral is down. In A, each square represents one animal, in C and D, each triangle represents one imaged neuron. ** p<0.01. See Figure S1 for additional related results and Data S1 for primary data and details of statistical analyses.
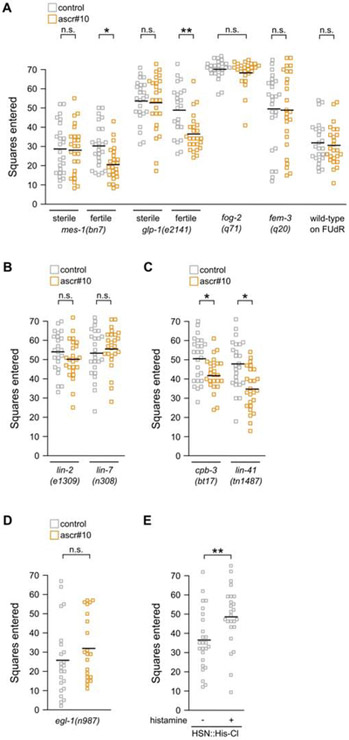
Perhaps the most notable difference between late larvae and adults is the presence in the latter of a mature and functional reproductive system, including the germline. Suggestively, one of the two pairs of serotonergic neurons that are required for hermaphrodite response to ascr#10, HSNs, are located in the mid-body next to the vulva and are thought to be motorneurons that control egg-laying [7]; their role in regulating exploratory behavior is somewhat puzzling [8]. We hypothesized that one possible role of HSNs may be to report on an aspect of the reproductive state that is relevant for exploratory behavior. To test this idea, we examined behavioral response to ascr#10 in animals with compromised reproduction. Four categories of self-sterile adult hermaphrodites did not reduce exploration in the presence of ascr#10 – a) mutants lacking germline (partially penetrant allele of mes-1 [9]), b) mutants with severely reduced numbers of germline cells (conditional allele of glp-1 [10]), c) mutants that produced only one type of gametes, either oocytes (fog-2 [11]) or sperm (fem-3 gain-of-function [12]), and thus no self-offspring, and d) animals chemically sterilized with FUdR (Figure 2A, Figure S2A). These germline defects, however, are unlikely to directly cause the inability to respond to ascr#10 because self-fertile but vulvaless [13] animals also did not reduce exploration in the presence of the pheromone (Figure 2B), whereas strains with even minimal egg-laying showed behavioral response (Figure 2C, Figure S2B). We therefore hypothesized that active egg-laying was required to permit decreased exploration in the presence of ascr#10.
Figure 2. Functional egg-laying apparatus regulates behavioral response to ascr#10.
(A) Exploratory behavior of hermaphrodites with compromised reproduction. (B) Exploratory behavior of fertile, but vulvaless animals. (C) Exploratory behavior of fertile animals that have dramatically reduced brood sizes (~3 in cpb-3, ~2 in lin-41 vs. ~13 in N2). (D) Exploratory behavior in mutant hermaphrodites lacking HSN neurons. (E) Exploratory behavior in adult hermaphrodites in which HSN has been chemogenetically silenced. Each square represents one animal. * p<0.05, ** p<0.01. See Figure S2 for additional related results and Data S1 for primary data and details of statistical analyses.
The role of vulva muscles in reporting the egg-laying state
HSN neurons stimulate egg-laying by exciting the vulva muscles [14]. Supporting the role of HSN neurons in behavioral response to ascr#10, we found that egl-1(dm) mutants in which HSNs undergo inappropriate cell death [15] did not reduce exploration in the presence of ascr#10 (Figure 2D). Animals in which HSNs were chemogenetically silenced using histamine-gated chloride channel (HisCl) [16] under control of an appropriate tissue-restricted promoter [17], explored more (Figure 2E) even though egg-laying was not blocked completely (Figure S2C). This finding supports the idea that signaling from HSN neurons normally inhibits exploratory movement (Aprison and Ruvinsky, this issue).
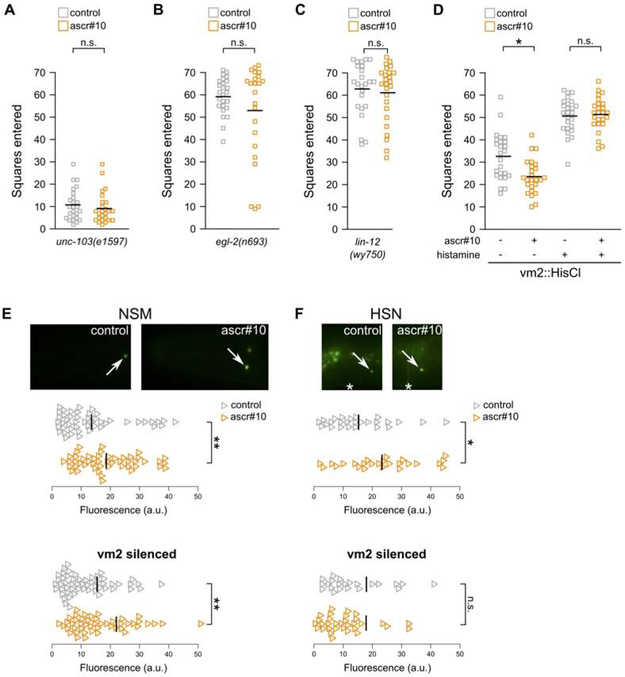
To further investigate the role of vulva muscle cells in behavioral response to ascr#10, we examined mutants – egl-2, egl-19, and unc-103 – that alter muscle excitability. In all examined cases, we found lack of behavioral (Figure 3A,B, Figure S3A) and germline (Figure S3B,C) responses to acr#10. However, these genes are broadly expressed [18-20] and therefore likely have complex pleiotropic effects on behavior and physiology. For this reason, we focused on vm2 vulval muscles because they receive direct synaptic input from HSN neurons [21] and because there exists a mutant – lin-12(wy750) – in which outgrowth of postsynaptic vm2 muscle arms is disrupted [22], effectively severing HSN-vm2 synaptic communication. These animals showed neither behavioral (Figure 3C) nor germline (Figure S3D) response to ascr#10, although they continued to lay eggs. These results demonstrated that the act of egg-laying per se is not alone sufficient to permit responses to ascr#10, which additionally require sensing the passage of embryos and communication between components of the egg-laying machinery.
Figure 3. The role of vm2 vulva muscles in regulating behavioral response to ascr#10.
(A,B) Exploratory behavior of mutant hermaphrodites with defective muscle excitability. (C) Exploratory behavior in a mutant hermaphrodite in which vm2 vulva muscles do not synapse with HSN neurons. (D) Exploratory behavior in hermaphrodites with functional or silenced vm2 vulva muscles. In this experiment silencing commenced prior to initiation of egg-laying. Comparison of the two grey data series demonstrates that silencing of vm2 muscles increases exploratory movement. (E) Expression of tph-1::YFP in NSM and (F) HSN neurons in adults with functional or silenced vm2 vulva muscles. Arrows mark cell bodies. Anterior is to the left and ventral is down. In A-D, each square represents one animal. In E,F, each triangle represents one neuron. * p<0.05, ** p<0.01. See Figure S3 for additional related results and Data S1 for primary data and details of statistical analyses.
Because in C. elegans Notch signaling is known to be involved in multiple aspects of development and function of the nervous system [23-26], it is possible that lin-12(wy750) has defects beyond HSN-vm2 communication. For this reason, we also tested whether chemogenetic silencing of vm2 muscles [17] alters response to ascr#10. We found that hermaphrodites with silenced vm2s did not lay eggs (Figure S3E), did not reduce exploratory behavior in the presence of ascr#10 (Figure 3D), and did not respond in the germline (Figure S3F). Notably, silencing of vm2 muscles had an effect on the upregulation of tph-1 expression in response to ascr#10. Unlike in hermaphrodites with functional vulva muscles that upregulated tph-1 expression in both NSM and HSN neurons, animals with silenced vm2s showed increased tph-1 expression only in NSM, but not in HSN neurons (Figure 3E,F, Figure S3G,H). We concluded that functional vm2 muscles synaptically connected to HSN neurons are required for appropriate ascr#10 response. We expect future work to elucidate which aspect of vm2 function needs to be communicated to HSNs, as well as the specific signal(s) that mediate this communication, to allow adult-like hermaphrodite behavior in the presence of ascr#10.
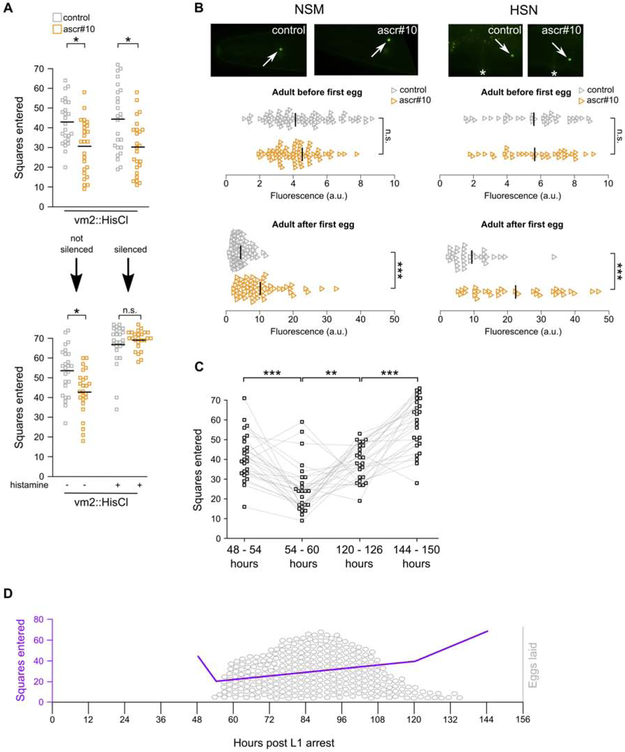
In the experiments shown in Figure 3D-F, vm2 muscles were continuously silenced since before the age when the first eggs were supposed to be laid. It therefore remained formally possible that hermaphrodites could display appropriate reduction of exploratory behavior as long as they initiated egg-laying. To test this hypothesis, we silenced vm2 muscles after the onset of egg-laying and found that this treatment acutely eliminated the ability of hermaphrodites to alter exploratory behavior on ascr#10 (Figure 4A). This result demonstrated that continuous input from the reproductive system is required to permit adult-like behavior.
Figure 4. The role of active reproductive state in regulating exploratory behavior.
(A) Exploratory behavior in hermaphrodites before and after chemogenetic silencing of vm2 vulva muscles. Note, top of this panels shows exploratory behavior in hermaphrodites after they have laid their first egg. The experiment in top portion of the panel was carried out on animals that were between 48 and 60h after release from L1 arrest. In the bottom, the same animals between 60 and 72h. Comparing grey data series between animals before and after vm2 silencing (right portion of the panel) demonstrates that silencing of vm2 increases exploratory movement. (B) Expression of tph-1::YFP in NSM and HSN neurons in adults exposed to ascr#10 before they have laid their first egg (48 to 54 hours after release from L1 arrest) and after they have laid their first egg (54 to 60 hours after release from L1 arrest). Arrows point to the cell body. Anterior is to the left and ventral is down. (C) Exploratory behavior of individual hermaphrodites during four episodes (~6 hours each) that encompass key events across the reproductive span. First and second episodes were carried out immediately before and immediately after the onset of egg-laying, respectively. In the third time window hermaphrodites had few remaining self-sperm, while in the fourth they exhausted supplies of self-sperm. The same animals were tested across these four time windows – grey traces connect exploratory movements of the same animal. Age is expressed as hours after release from L1 arrest. (D) A model showing exploratory behavior superimposed on the egg-laying schedule. Purple trace represents average performance from Figure 4C, egg-laying data (represented as grey ovals) are from [38]. In A and C, each square represents one animal. In B, each triangle is one neuron. *p<0.05, ** p<0. 01, *** p<0.001. See Figure S4 for additional related results and Data S1 for primary data and details of statistical analyses.
There appears to be a qualitative difference between the states of the nervous system before and after the onset of egg-laying. For example, exposure to ascr#10 upregulates tph-1 expression only in animals that have initiated reproduction, but not in adult animals of very similar age that are yet to lay their first egg (Figure 4B). In the absence of externally applied male pheromone, hermaphrodites also appear to display an inverse relationship between egg-laying and exploratory movement (see below).
An activity-dependent mechanism that licenses adult-like functions of the nervous system
Adult animals display behavioral repertoires that facilitate reproduction. These most notably include mating-related performance, but also foraging and food preferences [27]. Here we presented evidence that only adult C. elegans hermaphrodites respond to male sex pheromone ascr#10 by altering exploratory behavior and aspects of germline physiology, whereas larvae and pre-reproductive adults do not. In the companion paper (Aprison and Ruvinsky, this issue) we showed that hermaphrodite responses to ascr#10 facilitate reproductive success.
Progression of sexual maturation in the nervous system constitutes a developmental program that is regulated by several genes in the heterochronic pathway [28, 29]. Our findings reveal an additional layer of regulation because simply reaching adulthood is not sufficient for hermaphrodites to respond to male sex pheromone. At least three categories of adults are not capable of altering exploratory behavior in the presence of ascr#10 – sterile individuals, those fertile but unable to lay eggs (due to obstruction of the vulva) or egg-laying adults with defects in HSN neurons or connectivity between HSN and vm2 muscles. We propose that in all three of these cases the underlying cause of behavioral defects is the lack of signal from vm2 via HSN to other neurons (including AIY and RIF, see Aprison and Ruvinsky, this issue) that egg-laying, this most adult of behaviors, is taking place.
The idea of a feedback between components of the egg-laying machinery has been proposed previously [30]. HSN neurons serve as command motorneurons of egg-laying by stimulating vm2 muscles [7], whereas vm2 muscles provide retrograde signal that modulates HSN activity [17]. Our results support this notion of a feedback because chemogenetic silencing of vm2 muscles prevented upregulation of tph-1 expression in HSN, but not NSM neurons (Figure 3E,F). HSN neurons are born in the embryo and their axonal outgrowth and synaptogenesis occur during larval development [31], culminating in the acquisition of adult-specific activity patterns coincident with sexual maturity [17]. These facts are sufficient to explain the inability of juveniles and pre-reproductive adults to respond to ascr#10. Following the onset of adulthood, hermaphrodites that possess enough germline to produce offspring, unobstructed passage through the vulva, and functional HSN neurons and vm2 muscles, initiate egg-laying cycles. In addition to stimulating egg-laying, HSN and vm2 also continuously certify ongoing reproduction, thus licensing age-appropriate reproductive behaviors and physiology, each of which may have additional regulatory inputs. This model explains the previously puzzling [8] involvement of HSN neurons in exploratory behavior and stresses that signals from the reproductive system are continuously required to license adult-specific behaviors.
On the physiological relevance of coordinating reproductive and exploratory behaviors
Our results offer two lines of evidence suggesting an antagonistic relationship between reproduction and exploratory behavior. First, silencing of vm2 muscles in hermaphrodites, either before or after the onset of reproduction, resulted in cessation of egg-laying and a concomitant increase in exploratory movement (Figure 3D, Figure 4A). Second, given the relationship we established between egg-laying and adult-specific behavioral response to ascr#10, it may be expected that hermaphrodites would alter ways in which they explore their habitat depending on their reproductive status, even in the absence of external pheromones. To test this idea, we compared the extent of exploratory movement (over periods of ~6 hours) by singled hermaphrodites just before vs. just after the onset of egg-laying. We found a notable decrease in movement in reproductively active individuals (Figure 4C). Consistent with the inverse relationship between reproduction and movement, animals at the end of the self-fertile period moved more than at the beginning of the reproductive phase, and even more once self-sperm were exhausted (Figure 4C). We interpret these results as an indication of alternative states – one optimized for reproductive performance, while the other focused on habitat exploration (Figure 4D). Organizing behavior in alternative states may be an efficient way of resolving the trade-off between somewhat mutually exclusive requirements on organismal performance imposed by reproduction and exploration (also see Aprison and Ruvinsky, this issue).
A relationship between egg-laying and locomotion in C. elegans has been noted previously. Hardaker et al. have demonstrated that hermaphrodites tend to transiently increase a) movement velocity in the immediate (<30 s) anticipation of egg-laying episodes and b) propensity for changing movement direction immediately (<60 s) following egg-laying [32]. Both of these locomotory behaviors associated with reproduction require HSN neurons and serotonergic signaling [32], as do the responses we described here. It remains to be determined whether changes in movement seen on ~10 s time scales (described by Hardaker et al. [32]) are alone sufficient to account for the dramatically decreased exploration seen during the several days of the reproductive period (Figure 4D). In either case, the circuit described in Aprison and Ruvinsky (this issue) together with vm2 muscle cells appear to play an important role in shaping internal states that probabilistically control and ultimately coordinate locomotion and egg-laying.
Finally, several elements of the circuit that regulates exploratory behavior are conserved among deeply diverged animal lineages [8, 33-36]. There are similarities between HSN neurons in C. elegans and raphe serotonergic neurons in mammals [37]. It is thus tempting to speculate that at least some aspects of the mechanism we described here may act in other species to provide activity-dependent input from the reproductive system in the regulation of adult-specific patterns of exploratory or other behaviors.
STAR Methods
Lead Contact and materials availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ilya Ruvinsky (ilya.ruvinsky@northwestern.edu). This study did not generate new unique reagents.
Experimental Model and Subject Details
The strains MIA71, MIA116, and MIA194 were a gift from K. Collins (University of Miami). The remaining strains were obtained from the Caenorhabditis Genetics Center. Most strains were maintained at 20°C on OP50 under standard nematode growth conditions [39]. The following strains were maintained at 15°C: SS149 mes-1(bn7), CB4037 glp-1(e2141), JK816 fem-3(q20), JK1973 fem-3(q96), YM19 cpb-3(bt17), DG3784 lin-41(tn1487), DR730 dpy-13(e184); ama-1(m118m238). Synchronized populations of larvae of all strains were prepared by hypochlorite treatment of gravid hermaphrodites [40]. The liberated eggs were allowed to hatch overnight in M9 buffer. The following morning arrested L1 larvae were deposited onto lawn plates of OP50 at a density of 30-60 larvae per plate. 48 hours after release from larval arrest was designated as Day 1 of adulthood based on our previous experience staging N2 hermaphrodites [41, 42]. Some strains were slightly delayed (2-3 hours) in their development and timing of experiments was adjusted to account for this delay. Strain DR730 dpy-13(e184); ama-1(m118m238) was delayed 24 hours. In addition, one strain, MIA194 lin-12(wy750), developed normally but was delayed in the onset of egg-laying. This strain was singled to control or ascr#10 plates at 48 hours and transferred to fresh plates for the exploratory behavior experiment 12 hours later. On Day 1 of adulthood, hermaphrodites were transferred to either control plates or ascaroside treatment plates and housed in small populations of 30 worms per plate. Adult hermaphrodites were transferred every other day to fresh plates.
Method Details
Conditioning plates with ascarosides.
Synthetic ascaroside ascr#10 was provided by F. C. Schroeder (Cornell University). Concentrated solutions of ascarosides in ethanol were kept at −20°C. These stocks were diluted further with water and a total of 100μL (for 60mm plates) or 50μL (for 35mm plates) of ascaroside solution (for a total of 2.2 or 1.1 femtograms per plate, respectively) was applied to the surface of the agar and distributed evenly with a glass rod. The plates were incubated at 20°C overnight to allow the ascaroside to absorb into the agar. The following day, the plates were seeded with a 20μL spot (for experiments counting GPCs) or 50μL evenly spread (for behavioral experiments) of 1:10 dilution of an overnight culture of OP50 and were incubated at 20°C for 24 hours. Control plates without ascarosides were prepared in the same manner, except 100μL (or 50μL) of water contained no synthetic ascaroside.
Exploration assays.
The exploration behavior of hermaphrodites was measured using the assay described in Flavell et al. [8]. 35mm control or treatment plates were prepared with a uniformly seeded OP50 lawn. Day 1 adult hermaphrodites were singled to each plate and allowed to explore the plate for 6-16 hours at 20°C. After the exploratory period, the hermaphrodite was removed and the plate was laid over a grid of 86 squares. The number of squares entered by the worm tracks was counted. Each experiment was run in parallel with its matched control. mes-1(bn7) is a partially-penetrant allele that results in ~50% of animals lacking the germline at 20°C. The number of GPCs in the gonads of unaffected fertile mes-1 hermaphrodites are similar to those of N2. glp-1(e2141) hermaphrodites were shifted from 15°C to the non-permissive temperature (25°C) at the end of the L1 larval stage, which prevented the normal proliferation of germline cells. YM19, DG3784, and DR730 had dramatically reduced brood sizes – they produced ~2-3 offspring during the ~12 hours of exploratory behavior assays, compared to >13 in the wild-type N2 strain. In the experiment displayed in Figure 4C, exploratory movement was measured in a cohort of hermaphrodites at 48-54 h (immediately before the onset of reproduction), at 54-60 h (immediately after the onset of reproduction), at 120-126 h (when most animals had very few remaining self-sperm), and at 144-150 h (when hermaphrodites exhausted self-sperm and were thus self-sterile). In all cases, reproductive status of each individual was verified by visual inspection. Age represents hours post release from L1 arrest. Animals were moved to fresh plates at the onset of each experimental episode, allowing us to trace exploratory performance of individual animals across the entirety of their reproductive span.
Mating initiation assay.
The ability of male/hermaphrodite pairs to initiate mating was determined using an assay described in Fagan et al. [43]. ascr#10-conditioned and control 35mm plates were prepared as above. The evening before the assays were performed, small populations of N2 males at the L4 larval stage were segregated onto separate plates. Similarly, small populations of young adult hermaphrodites were kept on separate plates. The ascr#10 and control plates were seeded with a 20μL drop of a 1:10 dilution of OP50 overnight culture. This amount yielded a bacterial spot ~7.5mm in diameter. To start the assay, a single L4 hermaphrodite (~41 hours post release from L1 arrest) was placed in the center of the bacterial spot and a single male was placed on the outer edge of the spot. Plates were checked every 5 minutes for signs of mating initiation – the placement of the ventral side of the male tail against the hermaphrodite [44]. For every initiated mating, we noted the time since the start of the experiment and removed the plate from further consideration. Plates were monitored for up to 30 minutes.
Quantification of fluorescence.
Thirty L4 (~38 hours post release from L1 arrest in Figures 1C,D) or young adult hermaphrodites (Figures 3E,F and Figure 4B) were transferred to either ascr#10 plates or control plates for 6 hours. After 6 hours of exposure, the hermaphrodites were transferred to 2% agarose pads and anesthetized with 12.5mM levamisole (Vector Laboratories, Burlingame, CA) for imaging. An exposure time was selected to ensure that the pixel intensity of the fluorescent signal was in the linear range. Images were acquired on a Leica DM5000B microscope using a Retiga 2000R camera and the corrected total cell fluorescence was measured in ImageJ (NIH). The average of these values is presented in arbitrary units. OH12495 otIs517[tph-1(fosmid)::SL2::YFP::H2B + ttx-3::mCherry] is shown in Figures 1 and 4, and a hybrid between OH12495 and MIA71 in Figure 3.
Silencing HSN neurons and vulva muscles.
To reversibly silence HSN neurons and vm2 muscles, we used a transgenic strain expressing histamine-gated chloride channel (HisCl) [16] under control of an appropriate tissue-restricted promoter, as reported previously [17]. Experiments were carried out as previously described [16]. Histamine was added for a final concentration of 10mM to the NGM plates when they were poured. The strain MIA116 is developmentally delayed by about 24 hours. Transgenic worms were placed on either control or histamine-containing plates at 48 hours post release from L1 arrest (that is, prior to onset of egg-laying) and transferred to fresh control or histamine-containing plates for exploratory behavior testing from (71 to 80 hours post release from L1 arrest) or at ~60 hours (that is, soon after the onset of egg-laying).
Staining and counting germline precursor cells.
Hermaphrodites were aged as above in small populations of 30 per plate and stained with DAPI (4′,6-diamidino-2-phenylindole) as described previously [3] using a variation of the protocol by Pepper et al. [45]. Briefly, following washes in M9 and fixation with 95% ethanol, Day 5 adults were stained with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and mounted on 2% agarose pads for visualization. We counted the number of nuclei in the proliferative zone, as defined by Crittenden et al. [46]. In addition to mitotic nuclei, this population contains some nuclei in the early stages of meiosis [47]. In all animals, GPCs in only one of the two gonad arms were counted. In four cases, GPCs were counted earlier than on Day 5 (~150 hours) – in lin-12(wy750) and egl-2(n693) at 120 hours, in unc-103(e1597) at 96 hours, and in vm2::HisCl with silenced vm2 muscles at 72 hours due to increased internal hatching. In the experiment shown in Figure S1, exposure to ascr#10 commenced at the L1 stage and continued through the end of the L4 stage; GPC were counted in these animals.
Experiments involving FUdR.
35mm plates were prepared using NGM to which 5-fluoro-2’-deoxyuridine (Sigma) was added to yield a final concentration of 400μM as in Mitchell et al. [48]. 25 of these plates were conditioned with ascr#10 as above and 25 were kept as control plates. An overnight culture of OP50 was concentrated 20X and applied to these plates as a lawn. At about 46 hours post release from L1 arrest, 60 hermaphrodites in the L4.8 stage of vulval development as described by Mok et al. [49] were transferred to four additional plates containing FUdR. They were monitored during molting and all appeared to molt normally. At the 48-hour stage, worms were singled to the prepared lawn plates for the exploratory behavior experiment. Reduced exploration on FUdR has been noted previously [48].
Quantification and Statistical analysis
All experiments were compared against matched controls that were processed in parallel (Data S1). In the majority of instances, we used the Kolmogorov-Smirnov test to assess statistical significance of differences between treated and untreated animals (e.g., experiments like those shown in Figure 1A and 1C). This is a non-parametric alternative for comparing two samples that does not rely on the assumption of normal distribution of the underlying data. In several instances, our data did not appear to be normally distributed, justifying the choice. Analyses of data shown in Figures 1B relied on a log rank test. To evaluate significance of changes in exploratory behavior, as shown in Figure 4C, we conservatively asked whether a given animal explored less (when comparing 48–54 h window to 54–60 h window) or more (for the other two comparisons) than in the immediately previous sampled episode. We used a binomial test on the resultant categorical data. Numbers of worms, trials, p-values, and statistical tests applied are specified in Data S1.
Data and Code Availability
This study did not generate/analyze datasets.
Supplementary Material
Data S1. Summary of experiments in this study. Related to Figures 1-4 and STAR Methods.
A) Summary of experiments related to Figure 1.
B) Summary of experiments related to Figure 2.
C) Summary of experiments related to Figure 3.
D) Summary of experiments related to Figure 4.
E) Summary of experiments related to Figure S1.
F) Summary of experiments related to Figure S2.
G) Summary of experiments related to Figure S3.
H) Summary of experiments related to Figure S4.
Highlights:
Male pheromone promotes reproductive behavior in C. elegans hermaphrodites
Functional germline and ability to lay eggs are required for pheromone response
Retrograde signal from the vulva continuously affirms ongoing reproduction
Circuit ensures that only reproducing individuals behave in adult-specific manner
Acknowledgements
We thank R. Morimoto for generous hospitality and advice, M. Gallio, D. Greenstein, and E. Andersen for helpful comments, S. Favell for advice and discussion, K. Collins for strains and advice, and F. Schroeder for ascarosides. IR dedicates this paper to Sarah Ruvinsky for providing irrefutable proof that reproductive status impacts adult behavior. This work was funded in part by the NSF (IOS-1708518) and NIH (R01GM126125) grants to IR. We thank WormBase and the Caenorhabditis Genetics Center (CGC). WormBase is supported by grant U41 HG002223 from the National Human Genome Research Institute at the NIH, the UK Medical Research Council, and the UK Biotechnology and Biological Sciences Research Council. The CGC is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Declaration of interests
Some of the data reported here were used in a patent application 62/842,072.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wyatt TD (2014). Pheromones and Animal Behavior: Chemical Signals and Signatures, 2 Edition, (Cambridge University Press; ). [Google Scholar]
- 2.Aprison EZ, and Ruvinsky I (2015). Sex Pheromones of C. elegans Males Prime the Female Reproductive System and Ameliorate the Effects of Heat Stress. PLoS Genet 11, e1005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aprison EZ, and Ruvinsky I (2016). Sexually Antagonistic Male Signals Manipulate Germline and Soma of C. elegans Hermaphrodites. Curr Biol 26, 2827–2833. [DOI] [PubMed] [Google Scholar]
- 4.Aprison EZ, and Ruvinsky I (2017). Counteracting Ascarosides Act through Distinct Neurons to Determine the Sexual Identity of C. elegans Pheromones. Curr Biol 27, 2589–2599 e2583. [DOI] [PubMed] [Google Scholar]
- 5.Ludewig AH, Artyukhin AB, Aprison EZ, Rodrigues PR, Pulido DC, Burkhardt RN, Panda O, Zhang YK, Gudibanda P, Ruvinsky I, et al. (2019). An excreted small molecule promotes C. elegans reproductive development and aging. Nat Chem Biol 15, 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, von Reuss SH, Genoff MC, Sternberg PW, and Schroeder FC (2012). Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol 7, 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer WF (2006). Genetics of egg-laying in worms. Annu Rev Genet 40, 487–509. [DOI] [PubMed] [Google Scholar]
- 8.Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, and Bargmann CI (2013). Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strome S, Martin P, Schierenberg E, and Paulsen J (1995). Transformation of the germ line into muscle in mes-1 mutant embryos of C. elegans. Development 121, 2961–2972. [DOI] [PubMed] [Google Scholar]
- 10.Austin J, and Kimble J (1987). glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51, 589–599. [DOI] [PubMed] [Google Scholar]
- 11.Schedl T, and Kimble J (1988). fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton MK, Schedl TB, and Kimble J (1987). Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson EL, and Horvitz HR (1985). Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics 110, 17–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Chung SH, Fang-Yen C, Craig C, Kerr RA, Suzuki H, Samuel AD, Mazur E, and Schafer WR (2008). A self-regulating feed-forward circuit controlling C. elegans egg-laying behavior. Curr Biol 18, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conradt B, and Horvitz HR (1999). The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell 98, 317–327. [DOI] [PubMed] [Google Scholar]
- 16.Pokala N, Liu Q, Gordus A, and Bargmann CI (2014). Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proc Natl Acad Sci U S A 111, 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravi B, Garcia J, and Collins KM (2018). Homeostatic Feedback Modulates the Development of Two-State Patterned Activity in a Model Serotonin Motor Circuit in Caenorhabditis elegans. J Neurosci 38, 6283–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RY, Lobel L, Hengartner M, Horvitz HR, and Avery L (1997). Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J 16, 6066–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinshenker D, Wei A, Salkoff L, and Thomas JH (1999). Block of an ether-a-go-go-like K(+) channel by imipramine rescues egl-2 excitation defects in Caenorhabditis elegans. J Neurosci 19, 9831–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiner DJ, Weinshenker D, Tian H, Thomas JH, Nishiwaki K, Miwa J, Gruninger T, Leboeuf B, and Garcia LR (2006). Behavioral genetics of caenorhabditis elegans unc-103-encoded erg-like K(+) channel. J Neurogenet 20, 41–66. [DOI] [PubMed] [Google Scholar]
- 21.White JG, Southgate E, Thomson JN, and Brenner S (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314, 1–340. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Collins KM, Koelle MR, and Shen K (2013). LIN-12/Notch signaling instructs postsynaptic muscle arm development by regulating UNC-40/DCC and MADD-2 in Caenorhabditis elegans. Elife 2, e00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Bejjani R, and Hammarlund M (2012). Notch signaling inhibits axon regeneration. Neuron 73, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh K, Chao MY, Somers GA, Komatsu H, Corkins ME, Larkins-Ford J, Tucey T, Dionne HM, Walsh MB, Beaumont EK, et al. (2011). C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr Biol 21, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao MY, Larkins-Ford J, Tucey TM, and Hart AC (2005). lin-12 Notch functions in the adult nervous system of C. elegans. BMC Neurosci 6, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittenburg N, Eimer S, Lakowski B, Rohrig S, Rudolph C, and Baumeister R (2000). Presenilin is required for proper morphology and function of neurons in C. elegans. Nature 406, 306–309. [DOI] [PubMed] [Google Scholar]
- 27.Werner EE, and Gilliam JF (1984). The ontogenetic niche and species interactions in size-structured populations. Ann. Rev. Ecol. Syst 15, 393–425. [Google Scholar]
- 28.Lawson H, Vuong E, Miller RM, Kiontke K, Fitch DH, and Portman DS (2019). The Makorin lep-2 and the lncRNA lep-5 regulate lin-28 to schedule sexual maturation of the C. elegans nervous system. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira L, Aeschimann F, Wang C, Lawson H, Serrano-Saiz E, Portman DS, Grosshans H, and Hobert O (2019). Timing mechanism of sexually dimorphic nervous system differentiation. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins KM, Bode A, Fernandez RW, Tanis JE, Brewer JC, Creamer MS, and Koelle MR (2016). Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garriga G, Desai C, and Horvitz HR (1993). Cell interactions control the direction of outgrowth, branching and fasciculation of the HSN axons of Caenorhabditis elegans. Development 117, 1071–1087. [DOI] [PubMed] [Google Scholar]
- 32.Hardaker LA, Singer E, Kerr R, Zhou G, and Schafer WR (2001). Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J Neurobiol 49, 303–313. [DOI] [PubMed] [Google Scholar]
- 33.Pooryasin A, and Fiala A (2015). Identified Serotonin-Releasing Neurons Induce Behavioral Quiescence and Suppress Mating in Drosophila. J Neurosci 35, 12792–12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lottem E, Banerjee D, Vertechi P, Sarra D, Lohuis MO, and Mainen ZF (2018). Activation of serotonin neurons promotes active persistence in a probabilistic foraging task. Nat Commun 9, 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrios A, Ghosh R, Fang C, Emmons SW, and Barr MM (2012). PDF-1 neuropeptide signaling modulates a neural circuit for mate-searching behavior in C. elegans. Nat Neurosci 15, 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taghert PH, and Nitabach MN (2012). Peptide neuromodulation in invertebrate model systems. Neuron 76, 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloret-Fernandez C, Maicas M, Mora-Martinez C, Artacho A, Jimeno-Martin A, Chirivella L, Weinberg P, and Flames N (2018). A transcription factor collective defines the HSN serotonergic neuron regulatory landscape. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMullen PD, Aprison EZ, Winter PB, Amaral LA, Morimoto RI, and Ruvinsky I (2012). Macro-level modeling of the response of C. elegans reproduction to chronic heat stress. PLoS Comput Biol 8, e1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulston J, and Hodgkin J (1988). Methods In The Nematode Caenorhabditis elegans, Wood WB, ed. (Cold Spring Harbor Laboratory Press; ), pp. 587–606. [Google Scholar]
- 41.Aprison EZ, and Ruvinsky I (2014). Balanced trade-offs between alternative strategies shape the response of C. elegans reproduction to chronic heat stress. PLoS One 9, e105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouvea DY, Aprison EZ, and Ruvinsky I (2015). Experience Modulates the Reproductive Response to Heat Stress in C. elegans via Multiple Physiological Processes. PLoS One 10, e0145925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagan KA, Luo J, Lagoy RC, Schroeder FC, Albrecht DR, and Portman DS (2018). A Single-Neuron Chemosensory Switch Determines the Valence of a Sexually Dimorphic Sensory Behavior. Curr Biol 28, 902–914 e905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu KS, and Sternberg PW (1995). Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14, 79–89. [DOI] [PubMed] [Google Scholar]
- 45.Pepper AS, Killian DJ, and Hubbard EJ (2003). Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics 163, 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crittenden SL, Leonhard KA, Byrd DT, and Kimble J (2006). Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell 17, 3051–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox PM, Vought VE, Hanazawa M, Lee MH, Maine EM, and Schedl T (2011). Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle progression in the C. elegans germline. Development 138, 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell DH, Stiles JW, Santelli J, and Sanadi DR (1979). Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol 34, 28–36. [DOI] [PubMed] [Google Scholar]
- 49.Mok DZ, Sternberg PW, and Inoue T (2015). Morphologically defined sub-stages of C. elegans vulval development in the fourth larval stage. BMC Dev Biol 15, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Summary of experiments in this study. Related to Figures 1-4 and STAR Methods.
A) Summary of experiments related to Figure 1.
B) Summary of experiments related to Figure 2.
C) Summary of experiments related to Figure 3.
D) Summary of experiments related to Figure 4.
E) Summary of experiments related to Figure S1.
F) Summary of experiments related to Figure S2.
G) Summary of experiments related to Figure S3.
H) Summary of experiments related to Figure S4.
Data Availability Statement
This study did not generate/analyze datasets.