Abstract
After experiences are encoded into memory, post-encoding reactivation mechanisms are proposed to mediate long-term memory stabilization and transformation. The spontaneous reactivation of hippocampal representations, along with hippocampal-cortical interactions, are leading candidate mechanisms for promoting systems-level memory strengthening and reorganization. While the replay of spatial representations has been extensively studied in rodents, here we review recent fMRI work that provides evidence for spontaneous reactivation of non-spatial, episodic event representations in the human hippocampus and cortex, as well as for experience-dependent alterations in systems-level hippocampal connectivity. We focus on reactivation during awake post-encoding periods, relationships between reactivation and subsequent behavior, how reactivation is modulated by factors that influence consolidation, and the implications of persistent reactivation for biasing ongoing perception and cognition.
Keywords: Memory consolidation, replay, reactivation, rest, hippocampus, hippocampal interactions
Reactivation as a memory consolidation mechanism
The hippocampus and surrounding medial temporal lobe (MTL) structures were first discovered to be instrumental in episodic memory after the groundbreaking report of severe anterograde amnesia in patient H.M. [1]. While bilateral resection of these structures prevented H.M. from encoding new information into long-term memory, H.M. demonstrated intact memory for events and knowledge obtained during his distant past, providing the intriguing clue that remote memories are stored in cortical networks outside of the MTL [1–3]. Subsequent studies conducted across multiple species confirmed that the hippocampus is pivotal in the acquisition of novel episodic memories, while revealing that over time, these memory representations undergo a process of transformation and reorganization both within the hippocampus and across hippocampal-cortical networks [4–6]. Although there is debate whether detailed episodic memories ever become fully supported by extra-hippocampal networks [7–9], it is clear that the brain networks that are active and support memory retrieval do indeed change over time and become increasingly distributed across hippocampal-cortical networks.
How do memory representations become distributed across hippocampal-cortical networks over time? The current leading mechanism thought to support memory trace distribution is one that involves repeated memory reactivation. Early computational models suggest that each experience is initially encoded in an ensemble of hippocampal neurons which is then repeatedly reactivated during post-encoding time periods [10,11]. Post-encoding hippocampal reactivation is ideally suited to strengthen the coherence of hippocampal ‘event’ ensembles but it has also been hypothesized that reactivation extends into cortical circuits and can gradually strengthen the representation of ‘event’ patterns in and across cortical networks [6,10–12].
The first empirical evidence for post-encoding hippocampal reactivation came from recordings of neural ensembles in rodents during sleep. This early work demonstrated that the hippocampus exhibits spontaneous reactivation of neural ensembles that were active during recent experiences [13–15]. Specifically, sequences of hippocampal place cells that were activated as an animal traversed a spatial trajectory later spontaneously ‘replayed’ in roughly the same (or reverse) temporal order as during navigational behavior [15–18]. This form of reactivation (i.e. sequential replay) primarily occurs during brief (100–200ms) sharp-wave ripple (SWR) events in the hippocampal local field potential, both during post-encoding sleep (non-rapid-eye-movement, NREM sleep) and post-encoding awake brain states [15–20]. Awake SWRs and reactivation typically occur during pauses in ongoing behavior or quiescent periods [14–17,21] and this awake but ‘offline’ (not task-related) reactivation is the current leading mechanism thought to promote both the strengthening of memory event ensembles and the integration of these events across hippocampal-cortical networks [22–25].
It is important to highlight that several features of ‘replay’ make it an attractive mechanism for supporting memory consolidation. Replay events during SWRs are temporally compressed compared to active behavior, such that cell pair co-firing during SWRs falls within a time window that is conducive to inducing synaptic plasticity [26,27] which would then further strengthen the connections between the co-activated neurons [28,29]. Moreover, hippocampal replay is accompanied by robust hippocampal-cortical interactions [30–34], providing a basis for the post-encoding reorganization of memory representations across hippocampal-cortical networks. Most importantly, several studies have demonstrated the functional importance of rodent hippocampal replay/SWR events by linking their occurrence with later memory [20,35–37], changes in neural representations over time [38,39], and prior learning or novelty [40–42]. For example, the interruption of post-encoding SWRs impairs learning across days [35,36] and trials [43], while degrading the fidelity of subsequent hippocampal representations [39,44]. These features make reactivation/SWR events a primary mechanism underlying the persistence and distribution of memory representations.
While providing critical background, this work characterizing rodent replay has primarily been limited to spatial representations in the dorsal hippocampus and contributions of SWRs to navigational behavior. Thus, recent work has not only established that post-encoding reactivation occurs in the human brain, but has expanded the purview by assessing reactivation of non-spatial episodic experiences in the hippocampus and extra-hippocampal structures. While previous reviews have focused on the importance of sleep in memory consolidation [45,46], here, we review current evidence that event representations are spontaneously reactivated during awake time periods, and this reactivation evidence, as well as systems-level hippocampal-cortical interactions, is related to memory strengthening and integration into cortical networks.
Post-encoding awake reactivation in humans
There are challenges to translating rodent hippocampal replay into humans. First and foremost, is the difference between the fine-scale spatial and temporal resolution information present in neurophysiological recordings compared to coarser information present in neuroimaging methods, such as fMRI. However, it is important to highlight that reactivation should manifest at the level of the blood-oxygen-level-dependent (BOLD) fMRI signal for several reasons. First, despite the short duration of SWR events (on the order of 50ms in primates [47,48]), it has been verified that SWRs are accompanied by robust and brain-wide modulations of the BOLD signal in macaques (including, but not limited to the hippocampus) [47]. As expected, the temporal profile of BOLD signal changes associated with single SWR events resemble a canonical hemodynamic response lasting for several seconds [47]. Furthermore, even though individual replay events are brief, they have been shown to be associated with changes in the correlation structure of cell-pair co-firing, even when correlations are measured over long timescales such as minutes [49–51]. Thus, temporally extended measures of the neural correlation structure are sensitive to underlying reactivation. These findings highlight the plausibility of measuring reactivation in humans, since it should manifest in both detectable changes in the BOLD signal, as well as the correlation structure or patterns of functional connectivity measured over timescales of minutes.
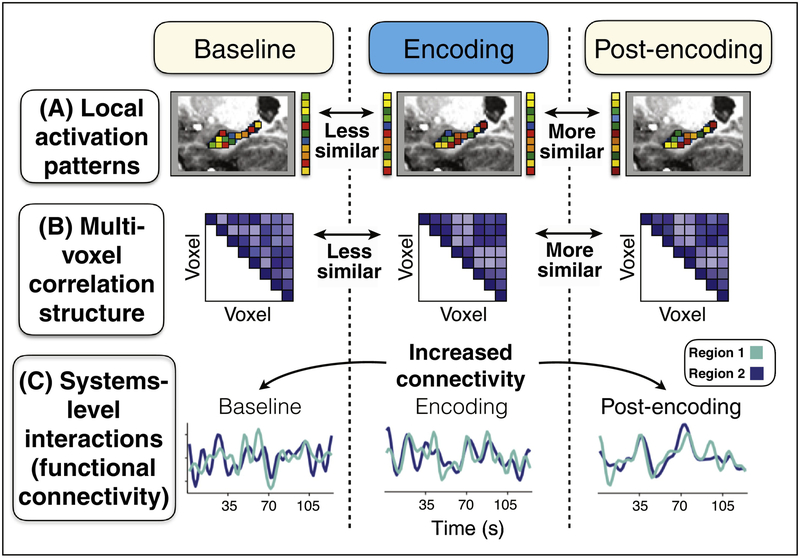
In the past few years, two primary fMRI approaches have been used to measure spontaneous memory reactivation during awake periods immediately after learning: the analysis of multi-voxel patterns to probe the reactivation of specific event patterns locally in a single brain region (Fig. 1A,B) and inter-regional correlations in the BOLD signal over time (i.e. functional connectivity) to measure systems-level hippocampal interactions (Fig. 1C). From a methodological standpoint, the clearest evidence for reactivation can be found when data is acquired during both a pre-encoding ‘baseline’ time period and a post-encoding time period when memory reactivation is expected to take place. This design provides face validity as it contrasts evidence for event reactivation when it is expected to occur (the post-encoding period) compared to the pre-experience baseline period, ensuring that it is related to encoding, per se, and is not an intrinsic property of the system or patterns being studied [52,53].
Figure 1. Approaches for measuring post-encoding reactivation using fMRI.
(A) Data are acquired during a baseline time period (cream box), an encoding experience or learning task (blue box), and a post-experience time period, when reactivation is expected to take place (cream box). To examine the reactivation of multi-voxel representations or patterns, template patterns are defined from the encoding data (center column), which can be activation patterns (A) or functional connectivity patterns (B). Template patterns are then compared to the baseline and post-experience data and their similarity is measured. Reactivation evidence is operationalized as greater levels of similarity between encoding representations and the post-encoding data, as compared to the similarity between encoding patterns and the baseline data. (C) Systems-level interactions can be examined by measuring the level of correlation, or functional connectivity, of the BOLD signal between regions of interest (e.g. hippocampus and cortical regions). Experience-dependent changes in functional connectivity, from pre- to post-experience time periods, serves as an index of systems-level interactions that may be related to memory consolidation.
Multi-voxel pattern analysis (MVPA), a powerful and widely-used tool for characterizing neural representations [54], is well-suited to studying event reactivation using fMRI. First, template patterns that characterize particular encoding experiences are defined. These patterns can be representations of specific stimuli or events (Fig. 1A), or connectivity patterns corresponding to particular encoding contexts or tasks (Fig. 1B). Reactivation is typically operationalized as greater levels of similarity between multi-voxel patterns measured during an experience (encoding time period) and those present during the post-encoding time period (as compared to the preceding baseline time period) (Fig. 1 A,B). Using this approach, reactivation evidence (i.e. increased similarity of encoding patterns with post-encoding versus baseline periods) has been revealed during awake rest in the human hippocampus [55–58] and visual cortical regions [59]. Specifically, several recent studies have examined the reactivation of encoding representations at a variety of ‘levels’: activation patterns corresponding to category-level reinstatement [56,59,60], events experienced over multiple trials [58,61,62], or even individual trials or episodes [63]. These studies typically estimate the dynamic, timepoint-by-timepoint similarity of the data during rest with template encoding patterns, which queries reactivation evidence in a time varying fashion. Other work has shown that more temporally extensive brain ‘states’ as defined by the connectivity patterns across voxels (multi-voxel correlation structure) and measured over specific tasks or time periods (e.g. minutes) also are reinstated in future time windows [55,57]. Importantly, variance in the extent of post-encoding reactivation in hippocampus [55–57] and cortex [61,63] has been demonstrated to be related to later memory for reactivated representations. Thus, reactivation is not simply obligatory or epi-phenomenal but, rather, is related to the ongoing strengthening of learning experiences as evidenced in later behavioral outcomes. Whether such reactivation evidence reflects underlying replay of sequential information is discussed in Box 1, and the timescale of reactivation is considered in Box 2.
BOX 1. ‘Replay’ versus ‘reactivation’ – what’s the difference?
Early work in rodents coined the term ‘replay’ -- referring to neural activity elicited during awake behavior that is re-expressed during subsequent sleep. This was initially demonstrated at the level of firing rates of individual place cells, such that placing a rat in a specific spatial location elevated the firing rate of neurons with place fields in that location during subsequent sleep [13]. With the ability to simultaneously record groups of neurons, later work examined finer grained patterns of co-activity across cell pairs, or the neural correlation structure. This revealed that co-activation patterns expressed during active behavior were evident in the spontaneous correlation structure after navigational behavior [14,49,50] (see [55,57] for adoption of this approach to multi-voxel fMRI patterns). As the number of simultaneously recorded neurons expanded, it was possible to record extended sequences of hippocampal neurons that represent specific spatial trajectories and probe high fidelity ‘replay’ more directly. Initial work used template matching to examine the re-expression of temporally ordered sequences [15,142]. Modern approaches use decoding to take advantage of the entire ensemble of recorded hippocampal activity, ‘re-playing’ or providing a time-varying estimate of spatial location during SWR events [18,131].
Following the history of advances in the rodent literature, the term ‘replay’ is now typically used to refer to high fidelity decoding of prior experience based on the activation of sequences of neurons. In contrast, the term ‘reactivation’ refers to evidence of non-sequential neural reinstatement or the re-expression of activity patterns (see [23]). According to these definitions, the majority of the fMRI evidence reviewed here does not provide evidence for sequential ‘replay’ directly, given that sequentially ordered reinstatement has not been probed until very recently [58]. This work demonstrated that multi-voxel hippocampal patterns representing sequentially experienced events (or ‘task-states’) tended to be re-expressed in a temporally clustered and ordered fashion during post-learning rest, consistent with the notion that recently experienced sequences were reactivated within a short time window. Although this study did not query the notion of sequences beyond the expression of pair-wise patterns (i.e. evidence for reactivation of triplets or higher order sequences), it provides an important step towards probing the limits of the temporal structure that can be observed with BOLD fMRI. Moreover, the broader evidence reviewed here is consistent with the notion of post-encoding reactivation and ‘replay’, especially when reactivation evidence is compared between a post-encoding and pre-encoding time period (see Box 2 for further consideration of this issue). The use of multiple measurements in future work will likely be fruitful to understand the potential similarity and differences in ‘replay’-like phenomenon across species and measurement approaches. For example, which measures of reactivation or systems-level interactions are most consistently predictive of memory retention across species? In addition to commonly relating neuronal phenomenon to behavior, other homologous approaches hold promise to understanding reactivation and its contribution to behavior across species, such as examining SWR events [48,143,144], parallel uses of targeted memory reactivation across species [34,145,146], and relationships to subsequent cognition beyond memory retention per se.
BOX 2. What is the temporal nature of memory reactivation in humans?
Much of the human fMRI work reviewed here was motivated by observations of temporally compressed hippocampal replay observed during brief (100–200ms) SWR events in rodents. In the section “Post-encoding awake reactivation in humans” we outline the rationale that, if SWR reactivation events occur during typical post-encoding rest scans [48], in principle, they should manifest in detectable and systematic changes in BOLD fMRI. While the reviewed evidence is indeed consistent with, and could theoretically be driven by, brief reactivation events during SWRs, these fMRI observations could also be driven by neural events and mechanisms distinct from SWRs. For example, in contrast to temporally compressed SWR replay (~10x behavioral speeds), there is evidence that hippocampal sequences are reactivated at behavioral timescales (without temporal compression) in REM sleep during theta-like states [142]. Additionally, the general persistence or ‘reverberation’ of correlated neural firing patterns has been described in hippocampus and cortical regions [50,51,147]; such reverberation could be driven by both discrete reactivation events as well as Hebbian mechanisms that modify the neural correlation structure in a temporally broad manner [14,50,147].
Considering that there may be reactivation mechanisms at different timescales, which ones drive post-encoding reactivation evidence seen in human fMRI? It is difficult to answer this question at present, as fMRI approaches are typically agnostic to the underlying timescale of putative reactivation. On the surface, functional connectivity or correlations of the BOLD signal over extended time scales (Fig. 1B, C) are the most temporally coarse, and so could theoretically be driven by multiple mechanisms or underlying timescales of reactivation. As reviewed above, analysis of individual time points provides time-varying reactivation evidence, speaking against ‘reverberation’ or Hebbian-like mechanisms being the only drivers of fMRI measures. However, it is important to note that reactivation within single fMRI time points may possibly reflect multiple reactivation events that occur in close temporal succession, as well as the fidelity or strength of reactivated patterns. In principle, it is possible that BOLD fMRI in combination with clever experimental paradigms could be used to distinguish between reactivation unfolding at compressed versus slower (behavioral) speeds: temporally compressed reactivation occurring within SWRs should result in brief, highly clustered reactivation evidence, whereas reactivation unfolding at behavioral speeds should result in extended reactivation evidence across longer durations. Future studies that combine fMRI reactivation measures with faster timescale information, either in a simultaneous fashion (EEG) [148] or in separate, parallel measurements (MEG, intracranial recordings) should help to shed light on the underlying temporal nature of human memory reactivation.
A second index of post-encoding reactivation may be evident in inter-regional systems-level functional connectivity, specifically between hippocampus and cortical regions. The notion is that brain regions that are initially engaged in a coordinated fashion during encoding should continue to exhibit elevated correlated activity in the post-encoding time periods (relative to a pre-encoding time period) if indeed these systems-level interactions promote some forms of memory consolidation. Thus, pre- to post-encoding functional connectivity changes provide an index of experience-dependent changes, or network-level plasticity, in systems-level interactions (Fig. 1C). In one of the earliest demonstrations of this, we queried hippocampal interactions with the lateral occipital (LO) cortex, a region that was engaged during the encoding of visual stimulus pairs. We found that hippocampal-LO functional connectivity increased from baseline to post-encoding rest that followed associative encoding of visual stimulus pairs [64]. Importantly, the increase in hippocampal-cortical connectivity from pre- to post-encoding rest was predictive of later memory for the recently encoded associations [64]. Several recent studies have similarly examined hippocampal interactions with category-selective visual cortical regions, showing evidence for experience-dependent, pre- to post-encoding hippocampal-cortical functional connectivity changes that are related to later memory for recently encoded stimuli [59,65]. These findings are consistent with the hypothesis that hippocampal event representations of a recent experience are replayed locally in the hippocampus and that this is associated with coordinated interactions across hippocampal-cortical networks. Importantly, some studies have additionally shown that trait-level connectivity in the same networks prior to encoding are not in and of themselves predictive of later memory for the upcoming experiences [64], further highlighting the selectivity of post-encoding time periods to the reactivation of preceding experiences to promote their strengthening and accessibility. Future work that causally manipulates awake post-encoding reactivation and connectivity will further help to further understand how these mechanisms directly support subsequent behavior (see Box 3).
BOX 3. Causal tests of post-encoding reactivation.
The work reviewed in the main text provides correlational evidence that signatures of persistent reactivation of encoding brain activity (reactivation and systems-level interactions), measured immediately after learning, are related to later memory and changes in memory over time. Although establishing these correlational links is critical, this approach leaves open the question of whether post-encoding reactivation plays a truly unique role, or whether post-encoding activity simply reflects a carry-over of encoding-related activity, which is in turn predictive of later memory. This has been partly addressed in several papers that have shown that a unique statistical contribution of post-encoding reactivation to levels of subsequent memory, above and beyond contributions during learning [56,65].
However, causal manipulations of post-encoding reactivation provide the most parsimonious way to directly test the contribution of reactivation to subsequent behavior. One popular approach, termed targeted memory reactivation (TMR), seeks to induce reactivation via the re-exposure of sensory cues previously associated with encoded information [145,149]. Although most work has used TMR to externally cue reactivation during sleep, we recently found that cued exposure during an immediate post-encoding awake time period enhanced the stability of associated memory representations [101]. Critically, the awake cueing was masked such that participants were mostly unaware of the content of the cue. Thus, awake cued reactivation can increase memory (see also [150]), in addition to benefits of TMR during sleep [151]. In the future, it will be important to better understand the conditions that bias whether awake cueing strengthens, updates, and/or weakens reactivated memories; what distinguishes the induction of reactivation versus reconsolidation [152]?
In addition to applying external cues to attempt to induce reactivation, another important step is to perform neural manipulations that may more directly influence awake post-encoding reactivation. This aim has been successful in rodent studies, where SWRs are monitored and real-time micro-stimulation or optogenetic silencing can be performed to interfere with reactivation events [35,39]. In humans, a variety of approaches have been used to modulate endogenous oscillations during sleep [153–155], which are in turn coupled with SWRs [144], and examine their influence on memory retention and integration [156]. However, little work has used stimulation to more directly manipulate awake post-encoding reactivation immediately after learning to test its importance for later memory. It will be important to fill this gap in future work, using stimulation approaches which have successfully modulated other phases of memory [157–159].
In addition to hippocampal-cortical interactions which are thought to foster systems-level memory reorganization, recent work has expanded these findings and identified interactions between the dopaminergic ventral tegmental area (VTA) and MTL structures during immediate post-encoding periods, based on the importance of dopamine and reward in facilitating long-lasting memory retention [66,67]. These studies find evidence that experience-dependent changes in VTA-hippocampal and VTA-perirhinal cortex functional connectivity are related to later memory for recently encoded associations [56,65,68] and items [68]. Together, this work indicates that post-encoding systems-level interactions involving dopaminergic structures are also important for facilitating memory consolidation and retention.
Reactivation: functional relevance beyond strengthening memory
The work summarized above provides foundational evidence in humans for post-encoding reactivation of event representations that facilitates the strengthening of those memory representations. Having established that it is indeed possible to measure post-encoding reactivation, researchers have begun to explore some of the contexts that modulate the extent of reactivation, determine what gets reactivated and how reactivation might relate not only to strengthening individual memories [55,57–59] but also to the integration of new memories into cortical circuits [60,61]. In this way, it is fruitful to consider that post-encoding time periods may provide the additional benefit of allowing the memory system to selectively enhance or suppress new event representations based on several factors, including their value [66,69], affective salience [70], relevance to future behavior [71], and congruence with prior knowledge [72,73].
Given that memory retention is strongly influenced by the salience of newly learned representations, it is logical to consider whether post-encoding reactivation is modulated by salience. Recent work has examined how reward, a key factor that enhances memory retention [66,74], influences awake reactivation. Indeed, it has been shown that representations associated with greater levels of reward are preferentially reactivated in the hippocampus after encoding [56]. Furthermore, experience-dependent changes in hippocampal-cortical [65] and hippocampal-VTA functional connectivity [56] selectively predict later memory for information associated with high reward. Recent findings suggest that anterior and posterior hippocampal interactions with cortical representational regions may preferentially track the consolidation of high versus low value information [65]. Reward is not the only modulator of reactivation. Using similar methods, recent work has also demonstrated that stimulus categories associated with shock during fear conditioning are preferentially reactivated during awake rest after conditioning [59]. Another modulator of memory retention, active choice during encoding (versus passive learning), has also been related to resting post-encoding hippocampal-cortical interactions [75]. Taken together, this work suggests that the relative salience of experiences biases the content of immediate post-encoding reactivation and hippocampal interactions (see also related rodent work [20,76,77]). Beyond value learning, other factors known to bias memory retention, such as emotional arousal [70,78] and relevance for future behavior [71,79], are also likely to influence post-encoding memory reactivation.
Post-encoding reactivation and other memory consolidation processes not only serve to strengthen important experiences in memory to make those events more accessible later on, but also may distribute memory representations across hippocampal-cortical networks. This form of systems-level consolidation is thought to promote the extraction and integration of knowledge acquired across experiences, facilitating generalization and abstraction across memories [10]. Supporting this notion, recent findings have shown that post-encoding reactivation in ventral temporal cortex, as well as levels of hippocampal-cortical functional connectivity, positively relate to memory integration as measured in behavioral assays [60,80]. Moreover, hippocampal-cortical resting connectivity immediately after learning is predictive of the reorganization of cortical memory representations across shared experiences one week later [81]. On the flip side, other work has shown that the presence of prior knowledge during new learning is also associated with increased post-encoding hippocampal-cortical connectivity [73,82], consistent with a role of prior experience in modulating post-encoding systems-level interactions. Together, this work provides compelling evidence that post-encoding reactivation serves to not only strengthen recent experiences in memory, but also reflects the selection of relevant experiences and the promotion of memory integration both behaviorally and via the emergence of integrated, or schematic, cortical representations [81]. Notably, reactivation which facilitates integration occurs in an apparent spontaneous manner during post-encoding rest; it is currently unclear how resting reactivation is similar or distinct from incidental or intentional reactivation during the learning of overlapping events [83,84].
‘Optimal’ brain states for memory reactivation and consolidation?
The work reviewed here has identified signatures of post-encoding reactivation primarily during periods of rest, in which participants are either instructed to rest with their eyes closed [55,64,65] or open [56,60]. Such ‘offline’ brain states, or time periods in which the brain is not explicitly processing incoming stimuli from the external environment, are thought to promote dynamics which favor a state of memory consolidation and reactivation (permissible to SWRs and hippocampal-cortical interactions), in contrast to a state of external engagement which may be beneficial for encoding new information into memory [85,86]. In rodents, SWRs and reactivation occur predominantly during ‘offline’ brain states, during both wake and NREM sleep, with awake SWRs typically occurring during quiescence or pauses in ongoing behavior [16,17,19].
Consistent with the notion that rest periods may represent a ‘brain state’ that fosters reactivation and consolidation, recent behavioral studies have demonstrated that periods of rest after encoding lead to significantly better memory retention. In this work, participants are given an opportunity to rest versus perform another task after encoding, such as ‘spot the difference’ [87–90] or the game snood [91]. Rest periods, as compared to these other tasks, have been found to benefit memory retention in both younger [89–92] and older adults [87,88,93] (but see [94,95]). Interestingly, higher memory retention over the course of a distractor task was associated with increased reports of internally-oriented cognition (thinking about the past, future planning, meditating, etc.) and reduced task-oriented attention [91]. Although neural reactivation was not examined in these studies, they are consistent with the idea that reactivation is more likely to occur during rest or internally-oriented brain states, compared with externally-oriented tasks.
Nonetheless, it is also clear that mechanisms underlying memory consolidation do not only occur during periods of rest or disengagement from the external environment; memory-related post-encoding reactivation and systems-level interactions have also been reported while participants perform math [63,68] or other tasks [96]. Also, no behavioral benefit of rest on memory retention has been reported when rest is compared with a working memory task [94,95,97]. Thus, the current literature indicates that while an opportunity to rest may promote memory retention, putative consolidation mechanisms are not abolished by all externally-oriented tasks. Furthermore, cues that promote autobiographical retrieval or future planning, during an otherwise unfilled delay period, may block the benefit of rest on memory [92,95]. This impairment in memory retention may be due to an engagement of the hippocampus in retrieval during the post-encoding time period [98], which might, hypothetically, prevent the hippocampus from spontaneously reactivating information in the service of consolidation. Much work is needed to systematically manipulate behavioral demands during post-encoding time periods to help clarify which awake brain states may be optimal for supporting memory reactivation and consolidation.
In addition to examining the conditions that facilitate spontaneous reactivation, it may be fruitful to also consider when manipulations that aim to externally induce reactivation (targeted memory reactivation, TMR) most strongly modulate memory retention. While most prior work found no reliable influence of TMR on memory when it takes place during an attention-demanding working memory task [99,100], we recently found that masked memory cueing during a monotonous task designed to promote a ‘rest-like’ state did reliably enhance memory stability [101]. Intriguingly, in that study, we found that the extent of externally-oriented attention, as measured via response time to an external task immediately before memory cueing, was negatively related to the influence of TMR subsequent memory. This suggests a possible competitive relationship between externally-oriented attention and internal memory reactivation. Along with the work reviewed above, these findings are consistent with the notion that a more internally-oriented brain state, perhaps associated with lower levels of acetylcholine [85,102], may be optimal for memory cueing and awake consolidation.
Reactivation of prior brain states biases ongoing cognition
When specific memories or more global brain states are spontaneously reactivated after learning, how do they interact with ongoing perception and cognition? As discussed in Box 4, it seems unlikely that reactivation during post-encoding time periods is strongly driven by intentional or explicit rehearsal of recently encoded information. However, stimulus-related mentation does spontaneously occur during post-encoding rest periods [89,90] and this was initially described a century ago as the tendency of recently encountered information to ‘perseverate in consciousness’ [103]. It is possible, but currently unknown, whether dynamic changes in reactivation evidence during rest is linked with stimulus-related mentation or other spontaneous cognitive processes [104]. This possibility could be examined in future work using experience-sampling approaches [105], potentially with the aid of real-time fMRI analysis to detect reactivation events.
BOX 4. Is resting reactivation driven by rehearsal?
When studying memory reactivation during awake post-encoding time periods, it is important to consider whether reactivation may be driven by or related to intentional rehearsal of recently learned information. In order to reduce potential relationships between rehearsal and post-encoding reactivation, prior fMRI studies have sought to minimize rehearsal demands. This has been accomplished by using incidental rather than intentional encoding tasks (i.e. participants are not aware that their memory for stimuli would be later be tested) [55,64] or by using intentional encoding paradigms, but performing memory testing prior to the post-encoding time period of interest [65]. Post-scan questionnaires can be used to assess whether participants intentionally rehearse recently encoded information during post-encoding periods or expect subsequent memory testing [64,89,90]. When stimulus-related thoughts do emerge during post-encoding rest, they tend to be spontaneous in nature rather in the form of intentional rehearsal [89,90], making it unlikely that post-encoding reactivation is driven by explicit rehearsal. The frequency of stimulus-related mentation during post-encoding rest varies across studies, from occurring infrequently [64] to in approximately half of participants [89,90].
Several lines of behavioral evidence further suggest that intentional rehearsal is not linked with awake resting reactivation. Specifically, the behavioral benefit of rest on memory retention does not differ between stimuli that are amenable versus difficult to rehearse [88], is present when participants who report intentional rehearsal during rest are excluded [89,90], and is not related to the extent of spontaneous stimulus-related thoughts during rest [91]. Additionally, a recent study showed that the benefit of targeted memory reactivation on later memory is actually inversely related to explicit knowledge of which items were cued, suggesting that the benefit of awake cued reactivation is not driven by intentional cue-triggered retrieval processes [101]. Together, this work suggests that awake post-encoding reactivation is not clearly linked with or driven by intentional or willful retrieval from memory. Nonetheless, future work that directly contrasts conditions in which instructed rehearsal is manipulated may help to shed light on this issue.
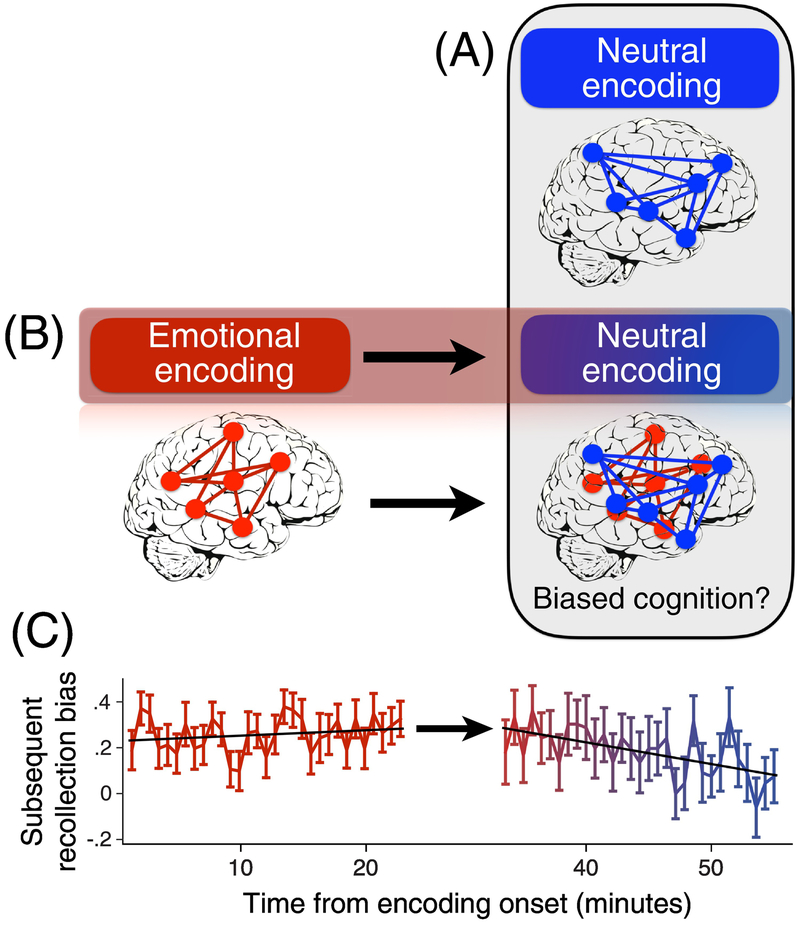
Beyond spontaneous reactivation leading to consciousness of the past, it is also conceivable that brain states associated with one context may persist or reactivate in other contexts and bias the way that new information is processed, attended and perceived (Fig. 2). In other words, spontaneous post-encoding reactivation can shape the way in which we experience and interact with the world. As a step towards addressing this possibility, in recent work, we found that patterns of activation and functional connectivity associated with prior emotional experiences spontaneously re-emerged during the subsequent encoding of distinct neutral stimuli, tens of minutes later [106] (Fig. 2B). The persistence of these emotional ‘brain state’ signatures resulted in the enhanced recollection of neutral stimuli encountered after emotional learning, a classic signature of the influence of emotional arousal on memory [107]. This bias towards enhanced recollection faded over the course of minutes during subsequent neutral encoding (Fig. 2B). Thus, exposure to emotional stimuli biased the way in which new and distinctive information was subsequently encoded into memory and later remembered. Relatedly, other recent studies have found that incidental reminder cues can reactivate neural representations of prior contexts and bias subsequent decision making [108–110]. In future work, it will be interesting to more broadly examine how memory reactivation influences the way in which new information is experienced, processed and acted upon.
Figure 2. Persistent brain states may bias future cognition.
(A) Depiction of a brain state, or activity pattern (shown in blue), associated with the encoding of neutral stimuli into memory. (B) Schematic depiction of the persistence of a brain state associated with emotional arousal (exposure to emotional stimuli, shown in red) into a subsequent time period in which neutral stimuli are encountered. The activity pattern during subsequent neutral encoding is a mixture of the two brain states or activity patterns, suggesting that cognitive processing may be biased by the persistence of prior brain states. (C) Example of biased memory formation for neutral stimuli encountered after emotional arousal (data adapted from Tambini, Rimmele, Phelps, Davachi, 2017). Emotional stimuli typically show greater levels of recollection during later memory testing (as opposed to being endorsed as familiar). This is operationalized here as the proportion of stimuli labeled as Remember (associated with the recollection of specific details) minus the proportion labeled as Know (familiarity without detailed recollection) during memory testing. A bias towards recollection is apparent for emotional stimuli (indicated by high levels of the red bars) and persists into and fades over time during neutral encoding, highlighting how memory for neutral information can be biased by prior experience.
Reactivation during SWR events has also been considered as a potential substrate for retrieval which may in turn contribute to ongoing processing, such as planning and decision making [23,111–113]. For example, interrupting ‘online’ SWR events has been reported to increase deliberation time at upcoming decision points [114] and when decision-making involves retrieving or maintaining information over short timescales, silencing ‘online’ SWRs impairs performance [115] while prolonging them improves memory [116]. Furthermore, the content and coordinated activity expressed during awake SWR events prospectively encodes immediate future navigational behavior [117–119] (see also [120]). Thus, sequences expressed during SWRs seem to be related to both aspects of future planning and decision making, as well as memory strengthening or consolidation [35,36]. Recent work suggests that awake ‘online’ (supporting immediate behavior) versus ‘offline’ (supporting memory consolidation) SWRs could be separated based on the time elapsed from prior task engagement [121], perhaps reflecting a shift from an externally to an internally-oriented brain state. It is thus possible that the fMRI evidence for post-encoding reactivation reviewed above, typically measured over extended time periods, may reflect both reactivations supporting memory consolidation and ongoing cognitive processing. We also speculate that memory-related signatures at stimulus offset reported in studies [122,123] may more closely resemble ‘online’ SWR-like activity. The use of techniques with higher temporal resolution [48,124] in future work may help to disambiguate human reactivation that supports ‘online’ cognition versus consolidation.
How is awake consolidation related to consolidation during sleep?
While much work has focused on the role of sleep in memory consolidation (often referred to as ‘sleep-dependent’ consolidation), the work reviewed here highlights the role of consolidation mechanisms during awake time periods immediately after learning. Considering post-encoding mechanisms across both sleep and awake time periods naturally leads to many questions for future research, such as whether and how awake post-encoding reactivation interacts with consolidation during subsequent sleep, and whether and how consolidation mechanisms across awake and sleep brain states differ (both neurally and in their contribution to subsequent behavior). While most studies separately examine one kind of post-encoding brain state (awake or sleep), studies examining consolidation across both time periods are necessary to gain a comprehensive and holistic understanding of the similarities and differences between mechanisms of consolidation during these distinct brain states.
Recent work has begun to provide a foothold into these questions. First, several studies have linked immediate post-encoding reactivation [57,59,65,68,81,101] or rest periods [87–89] with memory tested after one or more nights of sleep, indicating that at least some signatures of awake reactivation predict later memory after intervening sleep. An interesting study found that functional connectivity immediately after motor learning predicted subsequent overnight memory retention, suggesting a synergistic relationship between awake resting reactivation and consolidation during later sleep [125]. It is thus possible to speculate that memory strengthening via awake reactivation has the capacity to bias or ‘tag’ information for consolidation during subsequent sleep, which could influence the content of sleep reactivation and also interact with other mechanisms such as synaptic downscaling [25,126]. There is also evidence that SWR events may be homeostatically regulated [127], which could have implications for understanding potential interactions across brain states: could reactivation or consolidation during sleep compensate for a lack of awake reactivation?
In future work, it will also be interesting to examine whether and how consolidation during awake rest and sleep functionally differ, both mechanistically and in terms of their contribution to behavior. To the extent that similar brain or hippocampal ‘states’ that promote reactivation and consolidation [85,86] are present across wakeful periods and NREM sleep, some mechanistic similarities are likely to be present; however, it has also been proposed that distinct pathways may underlie hippocampal-cortical communication across these states [128]. It is also clear that NREM is fundamentally distinct from awake rest as it dominated by specific neurophysiological activity (slow wave activity and spindle events). Intriguingly, recent work indicates that power in the slow oscillatory frequency range during awake post-encoding rest is predictive of later memory [91], suggesting that, at least some consolidation-related oscillatory signatures may not be specific to sleep. Considering their contribution to behavior, separate literatures have implicated both awake and sleep consolidation mechanisms to later memory. Observations of higher fidelity reactivation during wake SWR events (versus sleep) [129–131] suggests that awake consolidation may be most important for memory strengthening and online behavior, whereas NREM sleep may preferentially support integrative and schematic representations [128,132]. For example, in separate human studies, awake rest periods promote accurate and detailed memory [133], whereas sleep may be optimal for memory extraction and generalization [134,135], perhaps suggesting distinct functional profiles. However, combined, within-study measures and comparisons of reactivation across brain states and their relationships with behavior will be helpful to signify their functional differences.
Concluding Remarks
In recent years, human neuroimaging studies across multiple labs have revealed key evidence for the occurrence of memory consolidation mechanisms during awake periods after encoding, including memory-related multi-voxel pattern reactivation and systems-level interactions (functional connectivity). These findings highlight the role of spontaneous, ‘offline’ mechanisms in supporting memory strengthening during wakefulness. This work importantly extends related findings in rodents by demonstrating reactivation of event representations beyond the spatial domain, including visual and conceptual representations of individual items [62], associations [58,61,63], stimulus categories [56,59,65], and tasks [55,57]. Most importantly, this work also provides many essential examples of links between reactivation evidence and features of future behavior, highlighting its functional importance. It is also important to consider that methods like fMRI can be viewed as advantageous because they provide broad coverage of activity across the entire brain. This permits the inquiry of reactivation across multiple brain regions (the hippocampus, cortical regions, and other structures of interest), systems-level functional interactions between a priori sets of brain regions, as well as the exploration of interactions across large-scale whole-brain networks [136].
Beyond being predictive of memory for recent experiences, signatures of post-encoding consolidation also track the preferential retention of salient information, predict transformations of memory representations across hippocampal-networks over time, and may bias the way in which new information is encountered and processed. Foundational evidence for these mechanisms, reviewed here, provides a new window into examining spontaneous memory-related processes in humans. While this review focused on hippocampal mechanisms in the context of episodic memory, similar awake post-encoding mechanisms likely support multiple domains of learning and memory [137–141].
Outstanding questions.
How is hippocampal reactivation related to dynamic changes in whole-brain network interactions? Is memory consolidation associated with specific large-scale network configurations, or general signatures of large-scale functional connectivity? Does the hippocampus act as a ‘hub’ during post-encoding consolidation periods?
Is hippocampal reactivation, hippocampal-cortical interactions, and/or cortical reactivation most important for memory consolidation (predictive of subsequent behavior and memory transformations over time)?
How do reactivation and consolidation mechanisms across wake and sleep mechanistically and functionally differ?
How do reactivation and consolidation mechanisms interact between awake and sleep brain states? Are they independent or does awake reactivation bias the content of what gets reactivated during sleep?
Are there optimal (awake) brain states for spontaneous reactivation and memory consolidation? Are internal vs. externally oriented brain states optimal for reactivation and consolidation, based on differences in cholinergic tone across these brain states? Or, does the degree of externally-evoked hippocampal engagement determine whether the hippocampus is a mode supporting consolidation vs. other functions (encoding, cued retrieval, etc.)?
Is the benefit of rest periods on memory retention general, or preferential for certain kinds of information (e.g. associative memory)?
How does awake post-encoding reactivation bias the way in which new information is perceived and experienced?
Highlights.
Recent human fMRI studies provide evidence for spontaneous memory-related reactivation and hippocampal interactions during awake post-encoding time periods.
Post-encoding awake reactivation is modulated by factors that influence memory consolidation, such as salience and reward, and predicts behavioral memory integration and the reorganization of cortical memory representations.
Reactivation of prior brain states can bias the way in which new information is experienced, perceived and acted upon.
Several open questions remain, such as how awake reactivation is related to ongoing conscious experience, which awake brain states may be optimal for supporting reactivation and memory retention, and how reactivation during sleep and wake may interact to support memory consolidation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scoville WB and Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milner B et al. (1968) Further Analysis of Hippocampal Amnesic Syndrome - 14-Year Follow-up Study of Hm. Neuropsychologia 6, 215- [Google Scholar]
- 3.Corkin S (1984) Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Semin. Neurol 4, 249–259 [Google Scholar]
- 4.Squire LR and Alvarez P (1995) Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 5, 169–177 [DOI] [PubMed] [Google Scholar]
- 5.Winocur G et al. (2010) Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia 48, 2339–56 [DOI] [PubMed] [Google Scholar]
- 6.Frankland PW and Bontempi B (2005) The organization of recent and remote memories. Nat Rev Neurosci 6, 119–30 [DOI] [PubMed] [Google Scholar]
- 7.Winocur G and Moscovitch M (2011) Memory transformation and systems consolidation. J. Int. Neuropsychol. Soc 17, 766–80 [DOI] [PubMed] [Google Scholar]
- 8.Moscovitch M et al. (2016) Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annu. Rev. Psychol 67, 105–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonelinas AP et al. (2019) A contextual binding theory of episodic memory: Systems consolidation reconsidered. Nat. Rev. Neurosci DOI: 10.1038/s41583-019-0150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClelland JL et al. (1995) Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102, 419–457 [DOI] [PubMed] [Google Scholar]
- 11.Alvarez P and Squire LR (1994) Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A 91, 7041–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zola-Morgan S and Squire LR (1990) The primate hippocampal formation: evidence for a time-limited role in memory storage. Science (80-.). 250, 288–290 [DOI] [PubMed] [Google Scholar]
- 13.Pavlides C and Winson J (1989) Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci 9, 2907–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson MA and McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science (80-.). 265, 676–679 [DOI] [PubMed] [Google Scholar]
- 15.Lee AK and Wilson MA (2002) Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 [DOI] [PubMed] [Google Scholar]
- 16.Foster DJ and Wilson MA (2006) Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683 [DOI] [PubMed] [Google Scholar]
- 17.Diba K and Buzsáki G (2007) Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci 10, 1241–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson TJ et al. (2009) Hippocampal replay of extended experience. Neuron 63, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buzsáki G et al. (1983) Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 287, 139–71 [DOI] [PubMed] [Google Scholar]
- 20.Dupret D et al. (2010) The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13, 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzsáki G (1986) Hippocampal sharp waves: their origin and significance. Brain Res. 398, 242–52 [DOI] [PubMed] [Google Scholar]
- 22.Rasch B and Born J (2007) Maintaining memories by reactivation. Curr Opin Neurobiol 17, 698–703 [DOI] [PubMed] [Google Scholar]
- 23.Carr MF et al. (2011) Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci 14, 147–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neill J et al. (2010) Play it again: reactivation of waking experience and memory. Trends Neurosci. 33, 220–9 [DOI] [PubMed] [Google Scholar]
- 25.Diekelmann S and Born J (2010) The memory function of sleep. Nat Rev Neurosci 11, 114–26 [DOI] [PubMed] [Google Scholar]
- 26.Bi GQ and Poo MM (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci 18, 10464–10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzsáki G (1989) Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570 [DOI] [PubMed] [Google Scholar]
- 28.King C et al. (1999) Hebbian modification of a hippocampal population pattern in the rat. J. Physiol 521, 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizunuma M et al. (2014) Unbalanced excitability underlies offline reactivation of behaviorally activated neurons. Nat. Neurosci 17, 503–506 [DOI] [PubMed] [Google Scholar]
- 30.Ji D and Wilson MA (2007) Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10, 100–107 [DOI] [PubMed] [Google Scholar]
- 31.Ólafsdóttir HF et al. (2016) Coordinated grid and place cell replay during rest. Nat. Neurosci DOI: 10.1038/nn.4291 [DOI] [PubMed] [Google Scholar]
- 32.Siapas AG and Wilson MA (1998) Coordinated Interactions between Hippocampal Ripples and Cortical Spindles during Slow-Wave Sleep. Neuron 21, 1123–1128 [DOI] [PubMed] [Google Scholar]
- 33.Sirota A et al. (2003) Communication between neocortex and hippocampus. Proc Natl Acad Sci U S A 100, 2065–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothschild G et al. (2016) A cortical–hippocampal–cortical loop of information processing during memory consolidation. Nat. Neurosci 20, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ego-Stengel V and Wilson MA (2010) Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girardeau G et al. (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12, 1222–1223 [DOI] [PubMed] [Google Scholar]
- 37.Ramadan W et al. (2009) Hippocampal Sharp Wave/Ripples during Sleep for Consolidation of Associative Memory. PLoS One 4, e6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maingret N et al. (2016) Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci 19, 959–964 [DOI] [PubMed] [Google Scholar]
- 39.van de Ven GM et al. (2016) Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples. Neuron DOI: 10.1016/j.neuron.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyrache A et al. (2009) Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12, 919–926 [DOI] [PubMed] [Google Scholar]
- 41.Eschenko O et al. (2008) Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem 15, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng S and Frank LM (2008) New experiences enhance coordinated neural activity in the hippocampus. Neuron 57, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nokia MS et al. (2012) Disrupting neural activity related to awake-state sharp wave-ripple complexes prevents hippocampal learning. Front. Behav. Neurosci 6, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roux L et al. (2017) Sharp wave ripples during learning stabilize the hippocampal spatial map. Nat. Neurosci 20, 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feld GB and Born J (2017) Sculpting memory during sleep: concurrent consolidation and forgetting. Curr. Opin. Neurobiol 44, 20–27 [DOI] [PubMed] [Google Scholar]
- 46.Stickgold R and Walker MP (2013) Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci 16, 139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logothetis NK et al. (2012) Hippocampal-cortical interaction during periods of subcortical silence. Nature 491, 547–53 [DOI] [PubMed] [Google Scholar]
- 48.Axmacher N et al. (2008) Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131, 1806–17 [DOI] [PubMed] [Google Scholar]
- 49.Skaggs WE and McNaughton BL (1996) Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–3 [DOI] [PubMed] [Google Scholar]
- 50.Kudrimoti HS et al. (1999) Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci 19, 4090–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman KL and McNaughton BL (2002) Coordinated reactivation of distributed memory traces in primate neocortex. Science (80-.). 297, 2070–2073 [DOI] [PubMed] [Google Scholar]
- 52.Dragoi G and Tonegawa S (2013) Selection of preconfigured cell assemblies for representation of novel spatial experiences. Philos. Trans. R. Soc. B Biol. Sci 369, 20120522–20120522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadeh T et al. (2019) Overlap between hippocampal pre-encoding and encoding patterns supports episodic memory. Hippocampus DOI: 10.1002/hipo.23079 [DOI] [PubMed] [Google Scholar]
- 54.Norman K. a. et al. (2006) Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci 10, 424–430 [DOI] [PubMed] [Google Scholar]
- 55.Tambini A and Davachi L (2013) Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc. Natl. Acad. Sci. U. S. A 110, 19591–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruber MJ et al. (2016) Post-learning Hippocampal Dynamics Promote Preferential Retention of Rewarding Events. Neuron 89, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hermans EJ et al. (2016) Persistence of Amygdala–Hippocampal Connectivity and Multi-Voxel Correlation Structures During Awake Rest After Fear Learning Predicts Long-Term Expression of Fear. Cereb. Cortex 27, bhw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuck NW and Niv Y (2019) Sequential replay of nonspatial task states in the human hippocampus. Science (80-.). 364, eaaw5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Voogd LD et al. (2016) Awake reactivation of emotional memory traces through hippocampal–neocortical interactions. Neuroimage DOI: 10.1016/j.neuroimage.2016.04.026 [DOI] [PubMed] [Google Scholar]
- 60.Schlichting ML and Preston AR (2014) Memory reactivation during rest supports upcoming learning of related content. Proc. Natl. Acad. Sci 2014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deuker L et al. (2013) Memory Consolidation by Replay of Stimulus-Specific Neural Activity. J. Neurosci 33, 19373–19383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schapiro AC et al. (2018) Human hippocampal replay during rest prioritizes weakly-learned information and predicts memory performance. Nat. Commun DOI: 10.1101/173021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staresina BP et al. (2013) Awake reactivation predicts memory in humans. Proc. Natl. Acad. Sci. U. S. A DOI: 10.1073/pnas.1311989110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tambini A et al. (2010) Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65, 280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murty VP et al. (2017) Selectivity in post-encoding connectivity with high-level visual cortex is associated with reward-motivated memory. J. Neurosci 37, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murayama K and Kitagami S (2013) Consolidation Power of Extrinsic Rewards: Reward Cues Enhance Long-Term Memory for Irrelevant Past Events. J. Exp. Psychol. Gen DOI: 10.1037/a0031992 [DOI] [PubMed] [Google Scholar]
- 67.Wang S-H and Morris RGM (2010) Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu. Rev. Psychol 61, 49–79, C1–4 [DOI] [PubMed] [Google Scholar]
- 68.Tompary A et al. (2015) Consolidation of Associative and Item Memory Is Related to Post-Encoding Functional Connectivity between the Ventral Tegmental Area and Different Medial Temporal Lobe Subregions during an Unrelated Task. J. Neurosci 35, 7326–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murayama K and Kuhbandner C (2011) Money enhances memory consolidation--but only for boring material. Cognition 119, 120–4 [DOI] [PubMed] [Google Scholar]
- 70.Sharot T and Phelps E. a (2004) How arousal modulates memory: disentangling the effects of attention and retention. Cogn. Affect. Behav. Neurosci 4, 294–306 [DOI] [PubMed] [Google Scholar]
- 71.Wilhelm I et al. (2011) Sleep selectively enhances memory expected to be of future relevance. J. Neurosci 31, 1563–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tse D et al. (2007) Schemas and memory consolidation. Science (80-.). 316, 76–82 [DOI] [PubMed] [Google Scholar]
- 73.van Kesteren MTR et al. (2010) Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A 107, 7550–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patil A et al. (2016) Reward retroactively enhances memory consolidation for related items. Learn. Mem 24, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murty VP et al. (2018) Decision-making Increases Episodic Memory via Postencoding Consolidation. J. Cogn. Neurosci 26, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singer AC and Frank LM (2009) Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64, 910–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McNamara CG et al. (2014) Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci 17, 1658–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGaugh JL (2018) Emotional arousal regulation of memory consolidation. Curr. Opin. Behav. Sci 19, 55–60 [Google Scholar]
- 79.Wamsley EJ et al. (2016) Test expectation enhances memory consolidation across both sleep and wake. PLoS One 11, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schlichting ML and Preston AR (2016) Hippocampal–medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiol. Learn. Mem 134, 91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tompary A and Davachi L (2017) Consolidation Promotes the Emergence of Representational Overlap in the Hippocampus and Medial Prefrontal Cortex. Neuron 96, 228–241.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Z-X et al. (2017) The effect of prior knowledge on post-encoding brain connectivity and its relation to subsequent memory. Neuroimage 167, 211–223 [DOI] [PubMed] [Google Scholar]
- 83.Kuhl BA et al. (2012) Neural reactivation reveals mechanisms for updating memory. J. Neurosci 32, 3453–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhl BA et al. (2013) Dissociable neural mechanisms for goal-directed versus incidental memory reactivation. J. Neurosci 33, 16099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hasselmo ME and McGaughy J (2004) High acetylcholine sets circuit dynamics for attention and encoding ; Low acetylcholine sets dynamics for consolidation. Prog. Brain Res. 02215, 1–50 [DOI] [PubMed] [Google Scholar]
- 86.Mednick SC et al. (2011) An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 34, 504–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dewar MT et al. (2012) Brief wakeful resting boosts new memories over the long term. Psychol. Sci 23, 955–60 [DOI] [PubMed] [Google Scholar]
- 88.Dewar MT et al. (2014) Boosting Long-Term Memory via Wakeful Rest: Intentional Rehearsal Is Not Necessary, Consolidation Is Sufficient. PLoS One 9, e109542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craig M et al. (2015) Rest Boosts the Long-term Retention of Spatial Associative and Temporal Order Information. Hippocampus DOI: 10.1002/hipo.22424 [DOI] [PubMed] [Google Scholar]
- 90.Craig M et al. (2016) Wakeful rest promotes the integration of spatial memories into accurate cognitive maps. Hippocampus 26, 185–193 [DOI] [PubMed] [Google Scholar]
- 91.Brokaw K et al. (2016) Resting state EEG correlates of memory consolidation. Neurobiol. Learn. Mem 130, 17–25 [DOI] [PubMed] [Google Scholar]
- 92.Craig M et al. (2014) Autobiographical thinking interferes with episodic memory consolidation. PLoS One 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Craig M et al. (2016) Comparable rest-related promotion of spatial memory consolidation in younger and older adults. Neurobiol. Aging 48, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varma S et al. (2017) Non-Interfering Effects of Active Post-Encoding Tasks on Episodic Memory Consolidation in Humans. Front. Behav. Neurosci 11, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Varma S et al. (2018) Promotion and suppression of autobiographical thinking differentially affect episodic memory consolidation. PLoS One 13, e0201780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peigneux P et al. (2006) Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol 4, e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Braun EK et al. (2018) Retroactive and graded prioritization of memory by reward. Nat. Commun 9, 4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schacter DL et al. (2012) The Future of Memory: Remembering, Imagining, and the Brain. Neuron 76, 677–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schreiner T and Rasch B (2014) Boosting Vocabulary Learning by Verbal Cueing During Sleep. Cereb. Cortex DOI: 10.1093/cercor/bhu139 [DOI] [PubMed] [Google Scholar]
- 100.Rudoy JD et al. (2009) Strengthening Individual Memories by Reactivating Them During Sleep. Science (80-.). 326, 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tambini A et al. (2017) Brief targeted memory reactivation during the awake state enhances memory stability and benefits the weakest memories. Sci. Rep 7, 1–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnold HM et al. (2002) Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 114, 451–460 [DOI] [PubMed] [Google Scholar]
- 103.Dewar MT et al. (2007) Forgetting Due to Retroactive Interference: A Fusion of Müller and Pilzecker’s (1900) Early Insights into Everyday Forgetting and Recent Research on Anterograde Amnesia. Cortex 43, 616–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kucyi A et al. (2018) Spontaneous cognitive processes and the behavioral validation of time-varying brain connectivity. Netw. Neurosci 2, 397–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christoff K et al. (2009) Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U. S. A 106, 8719–8724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tambini A et al. (2017) Emotional brain states carry over and enhance future memory formation. Nat. Neurosci 20, [DOI] [PubMed] [Google Scholar]
- 107.Phelps EA and Sharot T (2008) How (and why) emotion enhances the subjective sense of recollection. Curr. Dir. Psychol. Sci 17, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wimmer GE and Buchel C (2016) Reactivation of Reward-Related Patterns from Single Past Episodes Supports Memory-Based Decision Making. J. Neurosci 36, 2868–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bornstein AM and Norman KA (2017) Reinstated episodic context guides sampling-based decisions for reward. Nat. Neurosci 20, 997–1003 [DOI] [PubMed] [Google Scholar]
- 110.Hoskin AN et al. (2018) Refresh my memory: Episodic memory reinstatements intrude on working memory maintenance. Cogn. Affect. Behav. Neurosci DOI: 10.3758/s13415-018-00674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ólafsdóttir HF et al. (2018) The Role of Hippocampal Replay in Memory and Planning. Curr. Biol 28, R37–R50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu JY and Frank LM (2015) Hippocampal-cortical interaction in decision making. Neurobiol. Learn. Mem 117, 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vaz AP et al. (2019) Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science (80-.). 363, 975–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Papale AE et al. (2016) Interplay between Hippocampal Sharp-Wave-Ripple Events and Vicarious Trial and Error Behaviors in Decision Making. Neuron 92, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jadhav SP et al. (2012) Awake hippocampal sharp-wave ripples support spatial memory. Science (80-.). 336, 1454–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernández-Ruiz A et al. (2019) Long-duration hippocampal sharp wave ripples improve memory. Science (80-.). 364, 1082–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pfeiffer BE and Foster DJ (2013) Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singer AC et al. (2013) Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77, 1163–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wikenheiser AM and Redish AD (2014) Decoding the cognitive map: Ensemble hippocampal sequences and decision making. Curr. Opin. Neurobiol 32, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown TI et al. (2016) Prospective representation of navigational goals in the human hippocampus. Science (80-.). 352, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 121.Ólafsdóttir HF et al. (2017) Task Demands Predict a Dynamic Switch in the Content of Awake Hippocampal Replay. Neuron DOI: 10.1016/j.neuron.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ben-Yakov A et al. (2013) Hippocampal immediate poststimulus activity in the encoding of consecutive naturalistic episodes. J. Exp. Psychol. Gen 142, 1255–1263 [DOI] [PubMed] [Google Scholar]
- 123.Sols I et al. (2017) Event Boundaries Trigger Rapid Memory Reinstatement of the Prior Events to Promote Their Representation in Long-Term Memory. Curr. Biol 27, 3499–3504.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kurth-Nelson Z et al. (2016) Fast Sequences of Non-spatial State Representations in Humans. Neuron 91, 194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gregory MD et al. (2014) Resting state connectivity immediately following learning correlates with subsequent sleep-dependent enhancement of motor task performance. Neuroimage 102 Pt 2, 666–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lewis PA and Durrant SJ (2011) Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn. Sci 15, 343–51 [DOI] [PubMed] [Google Scholar]
- 127.Girardeau G et al. (2014) Learning-Induced Plasticity Regulates Hippocampal Sharp Wave-Ripple Drive. J. Neurosci 34, 5176–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tang W and Jadhav SP (2019) Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states. Neurobiol. Learn. Mem 160, 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gomperts SN et al. (2015) VTA neurons coordinate with the hippocampal reactivation of spatial experience. Elife 4, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang W et al. (2017) Hippocampal-prefrontal reactivation during learning is stronger in awake as compared to sleep states. J. Neurosci 37, 11789–11805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karlsson MP and Frank LM (2009) Awake replay of remote experiences in the hippocampus. Nat Neurosci 12, 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roumis DK and Frank LM (2015) Hippocampal sharp-wave ripples in waking and sleeping states. Curr. Opin. Neurobiol 35, 6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Craig M and Dewar MT (2018) Rest-related consolidation protects the fine detail of new memories. Sci. Rep DOI: 10.1038/s41598-018-25313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diekelmann S et al. (2010) Sleep enhances false memories depending on general memory performance. Behav. Brain Res. 208, 425–429 [DOI] [PubMed] [Google Scholar]
- 135.Wagner U et al. (2004) Sleep inspires insight. Nature 427, 352–5 [DOI] [PubMed] [Google Scholar]
- 136.Sami S and Miall RC (2013) Graph network analysis of immediate motor-learning induced changes in resting state BOLD. Front. Hum. Neurosci 7, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewis CM et al. (2009) Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A 106, 17558–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sami S et al. (2014) The Time Course of Task-Specific Memory Consolidation Effects in Resting State Networks. J. Neurosci 34, 3982–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guidotti R et al. (2015) Visual Learning Induces Changes in Resting-State fMRI Multivariate Pattern of Information. J. Neurosci 35, 9786–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bang JW et al. (2018) Feature-Specific Awake Reactivation in Human V1 after Visual Training. J. Neurosci 38, 9648–9657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harmelech T et al. (2013) The Day-After Effect: Long Term, Hebbian-Like Restructuring of Resting-State fMRI Patterns Induced by a Single Epoch of Cortical Activation. J. Neurosci 33, 9488–9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Louie K and Wilson MA (2001) Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29, 145–56 [DOI] [PubMed] [Google Scholar]
- 143.Leonard TK et al. (2015) Sharp Wave Ripples during Visual Exploration in the Primate Hippocampus. J. Neurosci 35, 14771–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Staresina BP et al. (2015) Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci DOI: 10.1038/nn.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oudiette D and Paller KA (2013) Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci 17, 142–9 [DOI] [PubMed] [Google Scholar]
- 146.Bendor D and Wilson MA (2012) Biasing the content of hippocampal replay during sleep. Nat. Neurosci 15, 1439–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ribeiro S et al. (2004) Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2, E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bergmann TO et al. (2012) Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59, 2733–42 [DOI] [PubMed] [Google Scholar]
- 149.Rasch B et al. (2007) Odor cues during slow-wave sleep prompt declarative memory consolidation. Science (80-.). 315, 1426–1429 [DOI] [PubMed] [Google Scholar]
- 150.Oudiette D et al. (2013) The role of memory reactivation during wakefulness and sleep in determining which memories endure. J Neurosci 33, 6672–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paller KA (2017) Sleeping in a Brave New World : Opportunities for Improving Learning and Clinical Outcomes through Targeted Memory Reactivation. Curr. Dir. Psychol. Sci DOI: 10.1177/0963721417716928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schreiner T et al. (2015) Auditory feedback blocks memory benefits of cueing during sleep. Nat. Commun 6, 8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lustenberger C et al. (2016) Feedback-Controlled Transcranial Alternating Current Stimulation Reveals a Functional Role of Sleep Spindles in Motor Memory Consolidation. Curr Biol DOI: 10.1016/j.cub.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ngo H-VV et al. (2013) Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–53 [DOI] [PubMed] [Google Scholar]
- 155.Papalambros NA et al. (2017) Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults. Front. Hum. Neurosci 11, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ketz N et al. (2018) Closed-loop slow-wave tACS improves sleep dependent long-term memory generalization by modulating endogenous oscillations. J. Neurosci DOI: 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Censor N et al. (2014) Interference with Existing Memories Alters Offline Intrinsic Functional Brain Connectivity. Neuron 81, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tambini A et al. (2018) Hippocampal-targeted Theta-burst Stimulation Enhances Associative Memory Formation. J. Cogn. Neurosci 26, 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ezzyat Y et al. (2017) Direct Brain Stimulation Modulates Encoding States and Memory Performance in Humans. Curr. Biol 27, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]