Abstract
B7x (B7-H4, B7S1, VTCN1) was discovered by us and others in 2003 as the seventh member of the B7 family. It is an inhibitory immune checkpoint with great significance to human diseases. Tissue-expressed B7x minimizes autoimmune and inflammatory responses. It is overexpressed in a broad spectrum of human cancers, where it suppresses anti-tumor immunity. Further, B7x and PD-L1 tend to have mutually-exclusive expression in cancer cells. Therapeutics targeting B7x are effective in animal models of cancers and autoimmune disorders, and early phase clinical trials are underway to determine the efficacy and safety of targeting B7x in human diseases. It took 15 years moving from the discovery of B7x to clinical trials. Further studies are needed to identify its receptors, to reveal its physiological functions in organs, and to combine therapies targeting B7x with other treatments.
Keywords: B7x, Immune Checkpoint, Immunotherapy, Cancer, Autoimmunity, Inflammation
The Immune Checkpoint B7x
The discovery of immune checkpoint pathways and the subsequent development of therapeutics that target these pathways have marked a revolution in the treatment of cancer and immunological diseases. In particular, immune checkpoint blockade (ICB) against the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and programmed death-1 (PD-1)/PD-L1 checkpoint pathways has seen significant clinical success for cancer therapy, which has in turn driven widespread and rapid adoption of these therapeutics for a variety of human cancers. Indeed, the 2018 Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuku Honjo in recognition of these milestones in immunotherapy. These successes have spurred interest in immune checkpoints that have relevance to human disease.
B7 homolog x (B7x) is a particularly promising, emerging target for immunotherapy of cancer and autoimmune disorders. It is also referred to as B7-H4 (B7 homolog 4), B7S1 (B7 superfamily member 1), or by its gene name V-set domain-containing T cell activation inhibitor 1(VTCN1) in literature, but all these terms refer to the same protein or gene. B7x was first characterized in 2003[1–3], classifying it as the seventh member of the B7 family of cell-signaling ligands. B7x has notable immunosuppressive roles, regulating cells of both the adaptive and innate immune systems. Further, B7x is frequently overexpressed by a diverse array of human cancers, where it mediates pathways that promote immune evasion. As a result, characterizing the B7x pathway and developing therapeutics to target it have been active areas of research.
In this review, we provide a detailed overview of the physiological functions and clinical significance of B7x. We describe the structure, expression pattern, and regulation of B7x. We will also discuss its immunological functions, detailing its roles in normal tissues, cancer, inflammatory diseases, and infectious diseases. Lastly, we detail the therapeutic strategies being developed to target it in the pre-clinical and clinical stages for cancer and autoimmune diseases.
Structure of B7x
The human B7x gene (annotated by the gene name VTCN1) is located on p12–13.1 of chromosome 1. Full length human B7x is a 282 amino acid protein that contains one signal peptide, two extracellular Ig (immunoglobulin) domains, one consecutive stalk region, one transmembrane domain and one very short cytoplasmic tail bearing no signaling motif[1, 2] (Figure 1A). B7x orthologs are found in other mammalian species, including non-human primates, canines, and rodents, and have high amino acid sequence conservation. In fact, 87% of the amino acids are conserved between human and mouse B7x[1]. B7x orthologs are also identified in the genomes of non-mammalian vertebrates such as birds, amphibians, and fishi [4].
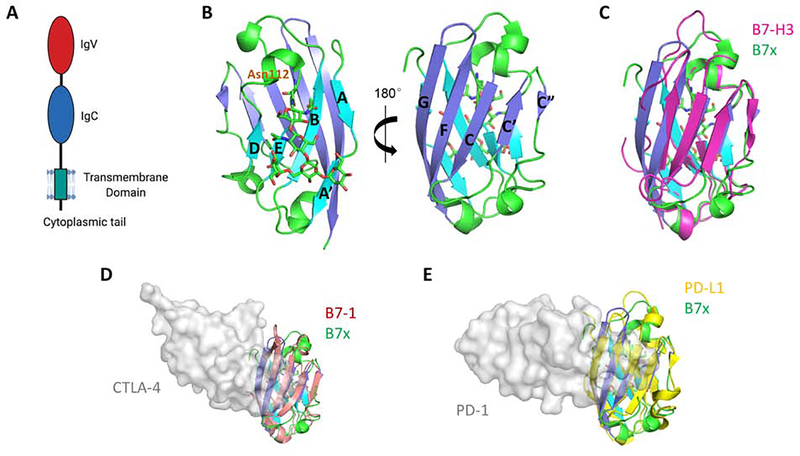
Figure 1. Structure of the Human B7x Protein and Its Comparison with Other B7 Ligands.
(A) Full length human B7x consists of the receptor-binding IgV domain, an IgC domain, a stalk region, a transmembrane domain, and a very short cytoplasmic tail with no signaling motif. (B) The crystal structure of the human B7x IgV domain is shown. The back-sheet, front-sheet and the connecting loops (PDB: 4GOS) are colored as cyan, blue and green respectively. Residue Asn112 and the linked glycan are shown as sticks. (C) Superimposition of human B7x with the reconstituted monomeric mouse B7-H3 model (colored as magenta) built from dimeric B7-H3 (PDB: 4I0K). (D) Superimposition of the IgV domain of human B7x with human B7–1 (colored as salmon). The receptor CTLA-4 is shown as grey surface to indicate the binding interface on B7–1 (PDB: 1I8L). (E) Superimposition of the IgV domain of human B7x with human PD-L1 (colored as yellow). The receptor PD-1 is shown as grey surface to indicate the binding interface on PD-L1 (PDB: 4ZQK), suggesting a possible interaction between B7x and its yet-unidentified receptor.
Human B7x is a cell membrane-bound ligand with most of its structure extending into the extracellular space, i.e. the ectodomain. The ectodomain of B7x is composed of one Ig variable (IgV) domain and one Ig constant (IgC) domain, similar to other B7-family ligands[2]. The crystal structure of the human B7x IgV domain has been determined (PDB: 4GOS, Figure 1B) and resembles the structural organization to the IgV domain of other B7-family ligands such as B7–1, B7–2, B7-H3, PD-L1 and PD-L2. Human B7x IgV adopts a beta sandwich fold composed of a back-sheet (AA’BED strands) and a front-sheet (CC’C”FG strands) which is stabilized by a disulfide bond formed between B and F strands. The B strand contains residues G52-F58 and F strand contains residues G126-T134. Notably, a single glycosylation site at Asparagine 112 has been identified on the back-sheet E strand, which is composed of residues N112-L117, marking the presence of a glycan group that is added post-translationally[5].
The front-sheets of the IgV domains of B7 ligands are critical for binding to their receptors. For example, human B7–1 and B7–2 utilize their front-sheets to bind to their receptor CTLA-4, as do PD-L1 and PD-L2 in their interaction with PD-1 (Figure 1D, E) [6]. Notably, the conserved N-glycosylation site of B7x is located at the back-sheet of the structure, and so does not pose steric conflict to the potential front-sheet interaction. Superimposition of human B7x with these ligands shows similar structural organization. Therefore, it is likely that B7x also engages its receptor on its IgV surface.
In addition to its cell membrane-bound form, B7x can also be found intracellularly and can be secreted in soluble forms. Human B7x contains a nuclear localization sequence near the C-terminus, which allows it to translocate to the nucleus. In cancer cells, this may allow it to promote oncogenic signaling pathways, such as suppressing apoptosis and promoting cancer progression[7]. Soluble B7x increases in the serum in certain disease states. Elevated soluble B7x can be detected in the sera of ovarian cancer[8] and renal cell carcinoma[9] patients. It also increases in women with elevated risk of preeclampsia in the first trimester of pregnancy[10], and in patients with rheumatoid arthritis[11]. The diverse array of diseases in which soluble B7x is found suggests that it can have different functions, acting to either activate the B7x pathway and suppress immunity in cancer, or to act as a decoy to block the B7x-receptor in inflammatory diseases. However, the mechanistic details of how intracellular or soluble B7x function are not well understood, and will require further studies to elucidate.
Expression of B7x in Normal Tissues
Early reports in mice indicated that B7x transcripts could be widely detected in a variety of tissues[1–3]. Subsequent immunohistochemistry studies found that the B7x protein has much more restricted distribution in both mice and humans, being predominantly expressed in the epithelial tissues, such as those of the trachea, lung, gynecologic tract (uterus, ovary), breast, pancreas, and kidney[12–14]. It is also expressed on some non-epithelial tissues, such as bone marrow-derived mesenchymal stem cells[15]. In contrast, tissues such as muscle, intestine, and lymphoid tissues are largely negative for B7x protein despite having detectable mRNA[12]. This dichotomy between B7x mRNA and protein suggests translational or post-translational regulation of B7x expression, although the precise mechanisms remain unclear.
Whether B7x is normally expressed on cells of hematopoietic origin is controversial. As with solid tissues, B7x mRNA is widely detectable on mouse and human immune cells[1–3]. However, flow cytometric analyses of B7x protein expression on immune cells are contradictory. Initial studies on murine cells found limited expression of B7x on T cells, but high expression on B cells and macrophages[3]. Another early report on human cells found that although resting T cells, B cells, monocytes, and dendritic cells expressed little B7x, they could be induced to express it with in vitro stimulation[2]. However, another study did not detect B7x on either human or mouse immune cells, with or without in vitro stimulation[14]. Although there is no clear consensus on which immune cells express B7x in the context of normal tissue homeostasis, the pathological microenvironment within cancerous tissues induces B7x expression in tumor-infiltrating immune cells. In particular, tumor-associated macrophages (TAMs) express B7x, contributing to the immunosuppressive tumor microenvironment[16–18].
Physiological Functions of B7x
B7x has been consistently demonstrated to have inhibitory functions in the immune system. It is best known for its suppressive effects on CD4 and CD8 T cells. In vitro studies demonstrated its ability to suppress T cell effector functions, including inflammatory cytokine production and cytolytic activity[1–3]. More specifically, B7x reduces the production of at least 11 cytokines from human T cells: interferonγ ( IFNγ), tumor necrosis factor α (TNF)α, interleukin (IL)-5, IL-13, IL-2, IL-9, IL-10, IL-17A, IL-4, IL-21, and IL-22[19]. Further, it inhibits the proliferation of T cells by arresting their progression through the cell cycle at the G0/G1 phase[2]. However, B7x regulates different subtypes of T cells in distinct ways. While B7x suppresses proinflammatory functions of effector CD4 T cells, it conversely promotes the activity of natural regulatory T cells (Treg), a population of immunosuppressive CD4 T cells[20] (Figure 2A). Further, it modulates the polarization of naïve CD4 T cells into specific helper subtypes, inhibiting conversion to the inflammatory TH1 and TH17 subtypes, and promoting conversion to induced Tregs. In this way, B7x alters the inflammation-tolerance balance within a given tissue by shifting the population of T cells toward an immunosuppressive milieu.
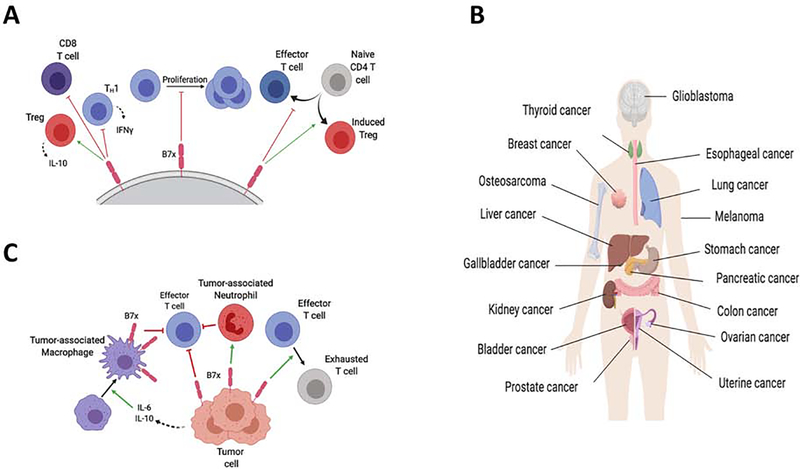
Figure 2. B7x Mediates Immunosuppressive Pathways and Promotes Tumor Immune Evasion.
(A) B7x mediates multiple immunosuppressive mechanisms by modulating T cell functions. B7x blocks the inflammatory functions of effector CD4 and CD8 T cells, reducing the production of cytokines such as IFNγ, as well as inhibiting their proliferation. Conversely, B7x promotes Treg function, including their secretion of immunosuppressive factors such as IL-10. B7x also regulates the differentiation of T cells, inhibiting their polarization into effector T cells and inhibiting differentiation into Tregs. (B) B7x is expressed by a wide variety of cancer types. In general, tumor-expressed B7x correlates with advanced disease progression and poorer prognosis. (C) B7x is expressed by tumor cells and tumor-associated macrophages to mediate the immune escape of tumors. B7x expressed by tumor cells inhibits the activity of anti-tumor T cells, and promotes an exhausted, dysfunctional state in these T cells. Further, B7x promotes tumor-associated neutrophils (myeloid-derived suppressor cells). In ovarian cancer and glioma, tumor cells secrete cytokines such as IL-6 and IL-10, which drives expression of B7x in tumor-associated macrophages
In additions to its effects on T cells, B7x also regulates cells of the neutrophil lineage. B7x inhibits the production of neutrophils from bone-marrow progenitors, thus regulating neutrophil-mediated immunity against bacterial infection[21]. In addition, B7x also appears to bind to tumor-infiltrating neutrophils, indicating these cells have a receptor for it[22]. These findings indicate that B7x-mediated regulation extend to the innate as well as the adaptive immune systems.
B7x-mediated regulation of adaptive and innate immune cells has significant implications in autoimmunity and inflammation. Decreased expression or function of B7x has been implicated in rheumatoid arthritis, Type 1 diabetes (T1D), nephritis, and juvenile idiopathic arthritis in humans[11, 23–25]. In mouse models, B7x deficiency worsens the pathophysiology of autoimmune diabetes, autoimmune nephritis, and systemic lupus erythematosus[14, 26, 27]. In the pancreas, B7x is expressed on the islet β-cells, and several studies have demonstrated the role of B7x in the prevention of autoimmune diabetes[14, 28, 29]. B7x expressed on pancreatic islet cells inhibits CD4 and CD8 T cell-mediated autoimmunity and prevents progression of diabetes[14, 28]. Conversely, overexpression of B7x on islet cell transplants reduced allograft rejection and prolonged their survival[30]. B7x-mediated inhibition of autoimmune disease serves as a rationale for using B7x-agonists as therapeutics. Yet although B7x-knockout mice develop more severe phenotypes in induced disease models, B7x-knockout mice do not spontaneously develop any inflammatory or autoimmune disease[31]. This suggests that in normal tissue homeostasis, other immune checkpoint pathways are able to compensate for a deficiency in B7x.
Like other B7-family ligands, B7x plays a role in the immune response to microbial infection. B7x expression on bronchial epithelial cells dampens lung immunity, as B7x-knockout mice are resistant to pulmonary infection of Streptococcus Pneumoniae[13]. Similarly, B7x-knockout mice are resistant to infection by Leishmania and Listeria[21, 31]. Intriguingly, B7x is involved in the pathogenesis of viral infections as well. Infection by human cytomegalovirus (CMV) upregulates B7x in monocytes, contributing to an immunosuppressive phenotype that promotes viral latency[32]. Another study found that human immunodeficiency virus (HIV) infection promotes the development of monocytic myeloid derived cells that overexpress B7x, that in turn suppress the immune response against secondary infections by CMV[33].
Expression and Clinical Significance of B7x in Cancer
Although B7x expression is limited in normal tissues, it is often overexpressed in a wide array of solid malignancies including cancers of the breast[34, 35], kidney[36], ovary[37], prostate[38], stomach[39], skin[40], pancreas[41], brain[16], liver[17, 42] and lung[19, 43, 44] (Figure 2B). In general, B7x expression in tumors is associated with greater disease progression and poorer clinical prognosis. Interestingly, the role of B7x in cancer seems to be conserved between mammalian species, as expression of B7x is also associated with worse prognosis in canine bladder cancer[45]. Table 1 summarizes the studies on the association between B7x protein expression and its clinical significance in human cancers.
Table 1.
Expression and Clinical Significance of B7x in Human Cancers
| Cancer type | Samples | Prevalence | Pathological Correlates | Clinical Outcomes | Reference |
|---|---|---|---|---|---|
| Prostate cancer | 823 | 80% | More extracapsular extension, seminal vesicle invasion and nonorgan-confined disease | Increased risk of cancer recurrence and cancer-specific death | [38] |
| Non-small cell lung cancer | 201 (cohort 1) 350 (cohort 2) |
13% (cohort 1), 23% (cohort 2) | More squamous cell histology | No correlation | [43] |
| Non-small cell lung cancer | 195 (discovery cohort) 197 (validation cohort) |
69%(discovery cohort) and 68% (validation cohort) | More adeno histology and more Asian race (only in discovery cohort, not in validation cohort) | No correlation | [19] |
| Renal cell carcinoma | 259 | 59% | More constitutional symptoms, tumor necrosis, advanced tumor size, stage, grade. | Poorer OS | [46] |
| Glioma | 138 (for expression) 70 (for survival) | Unclear | More glioma progression | Poorer PFS and OS | [16] |
| Breast cancer | 155 invasive ductal carcinomas; 18 lobular carcinoma; |
95% ductal carcinoma; 100% lobular carcinoma |
Intensity: more negative progesterone receptor status, history of neoadjuvant chemotherapy Proportion: more negative progesterone receptor and negative HER-2/neu |
No correlation | [35] |
| Gastric cancer | 156 | 45% | More myometrial invasion, lymph node metastasis and recurrence | Poorer OS | [39] |
| Osteosarcoma | 104 | 70% | More advanced tumor and distant metastasis | Poorer OS | [47] |
| Intrahepatic cholangiocarcinoma | 140 | Unclear | More lymph node metastasis, advanced TNM stage, poor tumor differentiation | Poorer OS and DFS | [42] |
| Small cell lung cancer | 90 | 2.6% | No correlation | Poorer OS | [44] |
| Ovarian cancer | 70 | Unclear | No correlation | Higher intensity of macrophage B7x is correlated with shorter survival | [48] |
| Thyroid Cancer | 64 | 95% | Advanced TNM stages and more extrathyroidal extension | Poorer OS | [49] |
| Pancreatic cancer | 63 | 62% | Larger tumors, more lymph-node metastasis, more invasion depth | Poorer survival | [41] |
| Bladder cancer | 62 | 76% | More advanced pathological stage | Poorer OS | [50] |
| Colorectal carcinoma | 56 | 48% high expression | Deeper infiltration depth and more lymph node metastasis | Unclear | [51] |
| Melanoma | 26 | Primary: 97% Metastases: 90% |
No correlation | Poorer OS | [40] |
Abbreviations used: OS: overall survival, PS: progression-free survival, DFS: disease-free survival
Aberrant expression of B7x is associated with greater disease progression and more severe pathology. For example, in a study regarding B7x expression in renal cell carcinoma (RCC), B7x expression was associated with tumor necrosis, advanced tumor size, stage, and grade[36]. In another study in intrahepatic cholangiocarcinoma patients, high B7x expression was significantly correlated to malignant phenotype, such as lymph node metastasis, high tumor stage, and poor differentiation[42]. Similarly, a large cohort study of patients with prostate cancer found that tumors with strong intensity for B7x were significantly more likely to have extracapsular extension, seminal vesicle invasion, and non-organ-confined disease compared with patients without strong intensity[42].
Due to its association with advanced disease progression, B7x is a useful prognostic tool for patient survival. In the above study of renal cell carcinoma, patients with tumors expressing B7x were three times more likely to die from RCC compared with patients lacking B7x[36]. In prostate cancer, patients with strong intensity for B7x were at increased risk of clinical cancer recurrence and cancer-specific death[52]. In glioma, there is an inverse correlation of tumor cell B7x expression levels and survival in terms of progression-free survival (PFS, see Glossary) and overall survival (OS), and higher B7x expression correlated with a worse prognosis following dendritic cell-based vaccination therapy[16]. In addition to B7x expressed on the cell membrane, soluble B7x in the serum can also serve as a biomarker. Serum B7x levels were elevated by as much as 100-fold in patients with ovarian cancer. In early-stage patients, the sensitivity at 97% specificity increased from 52% for CA125 alone to 65% when used in combination with B7x[8]. Similarly, soluble B7x is significantly higher in the serum of renal cell carcinoma patients than in sex and age-matched healthy blood donors, and is associated with advanced stage[9].
Mechanistically, tumors exploit the inhibitory functions of B7x to suppress and evade T cell-mediated immunity. In murine models, B7x expressed on both tumor and host cells can promote tumor immune evasion[22, 53]. It does this by reducing the activation and subsequent effector functions of tumor-infiltrating CD4 and CD8 T cells, including inflammatory cytokine production and cytolytic activity[17, 22, 53]. Further, B7x induces “exhaustion” in effector T cells, a dysfunctional state marked by the co-expression of markers such as PD-1 and Tim-3[17, 22, 53]. In addition to dampening the activity of effector T cells, B7x also promotes immunosuppressive cells within the tumor microenvironment, including Tregs, myeloid derived suppressor cells, and macrophages[22, 53]. It is not clear whether B7x directly promotes the development or recruitment of these cell types within the tumor microenvironment, or if this is an indirect consequence of suppressing proinflammatory T cells. Regardless, by inhibiting effector T cells and promoting immunosuppressive cells, B7x induces an overall immunologically tolerant and immunosuppressive tumor microenvironment.
Interestingly, the expression pattern of B7x in human cancers appears to be distinct from that of other immune checkpoints such as PD-L1. In non-small cell lung carcinoma, B7x and PD-L1 are rarely co-expressed[19, 43]. Instead, B7x is more commonly co-expressed with other immune checkpoints such as HHLA2[19]. Whereas PD-L1 expression correlates with increased immune cell infiltration, i.e. “hot tumors”, no such association is observed with B7x expression[43], suggesting that B7x may mark immunologically “cold” tumors. Similarly, triple-negative breast carcinomas are also stratified into distinct B7x- and PD-L1-positive tumors. PD-L1 expression in breast cancer was associated with greater CD8 T cell infiltration, while B7x-expressing tumors had little CD8 T cell infiltration, essentially forming “immune deserts.”[54] The differential expression patterns of B7x and PD-L1 has relevance to checkpoint blockade therapy, as identifying which pathway is active in a given tumor may have predictive value for the efficacy of PD-1/PD-L1 blockade.
As compared to solid tumors, B7x may play a different role in hematologic malignancies such as acute-myeloid leukemia (AML). B7x is expressed on leukemia-initiating cells (LICs)-enriched CD34+ AML cells. In silico analysis from the Leukemia Gene Atlas showed that B7x expression level was positively correlated with the overall survival of AML patients[55]. Thus, B7x may play a tumor suppressor role in AML, analogous to the PD-1 pathway in T cell lymphoma[56].
B7x expression in human cancers is not limited to the tumor cells themselves but is also found on TAMs (Figure 2C). TAMs are a major immunosuppressive population in many tumor types, and one of the mechanisms by which they mediate suppression is the expression of B7x. B7x-expressing TAMs are observed in many cancer types, including glioblastoma, hepatocellular carcinoma, and ovarian cancer[16–18]. In glioma, increased percentage of B7x-expressing macrophages/microglia correlates with higher grades and poorer prognoses[16]. In ovarian cancer, B7x-expressing macrophages suppress anti-tumor antigen-specific T cell immunity, and are prognostic of poorer patient outcomes[18, 48]. Expression of B7x on macrophages but not tumor cells correlated with a worse patient prognosis in ovarian cancer[48], indicating that macrophage-expressed B7x may play the dominant role in certain tumor types. Similarly, expression of B7x in hepatocellular carcinoma seemed to predominate in antigen-presenting cells as opposed to tumor cells, suggesting that macrophage-expressed B7x is clinically significant in various cancer types[17].
The roles of B7x on tumor-infiltrating immune cells are clear, but some groups report that it also mediates cell-intrinsic, oncogenic signaling pathways. B7x protein can be localized intracellularly, where it mediates pathways that promote cell proliferation.[7] B7x overexpression inhibits apoptosis in vitro and increases tumor formation in a xenograft model [34]. Further, high B7x expression can promote tumor progression through induction of epithelial-to-mesenchymal transitions (EMT). Knockdown of B7x in cholangiocarcinoma cells results in the increased expression of epithelial marker E-cadherin and decrease in the mesenchymal marker Vimentin [42]. Increased B7x expression has been associated with stemness, marked by expression of stem markers such as CD133, Lgr5, Sox9, and CD44[16, 57, 58].
Regulation of B7x Expression
The mechanisms regulating the expression of B7x in normal tissues are not well understood, but studies in neoplastic cells and TAMs reveal transcriptional regulation of B7x in response to a variety of stimuli. In vitro, human cancer cell lines increase B7x expression in response to hypoxia and transforming growth factorβ1 (TGFβ1), both of which are factors found in tumor microenvironments. B7x is transcriptionally upregulated through hypoxia inducible factor1α (HIF1α) and TGFβ1-driven Smad3/4 signaling[59, 60] (Figure 3A, left panel). Further, tumor cells can induce B7x expression in infiltrating myeloid cells by secreting IL-6 and IL-10[16, 18]. IL-6 and IL-10 induce JAK signaling in macrophages, which in turn activates Stat3 and drives B7x transcription[16]. Similarly, induction of the NF-κB-family transcription factor RelA drives B7x expression in tumor-infiltrating macrophages and allows suppression of CD8 T cells[61] (Figure 3A, right panel)
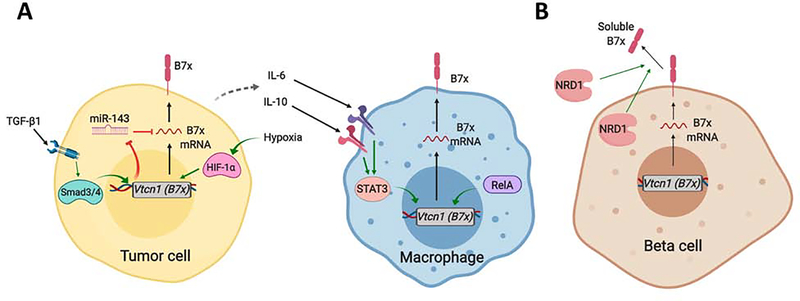
Figure 3. Regulatory Mechanisms of B7x Expression.
(A) Factors in the tumor microenvironment drive the expression of B7x in tumor cells and macrophages. TGFβ1 and hypoxia drive B7x expression in tumor cells through Smad and HIF1α signaling, respectively. Smad3/4 signaling in tumor cells downregulates the miRNA-mediated inhibition of B7x mRNA translation. Further, tumor cells secrete IL-6 and IL-10 to drive B7x expression in macrophages through the Jak-STAT3 pathway. The NFκB (RelA) pathway also promotes transcription of B7x in tumor-associated macrophages. (B) B7x is expressed on pancreatic beta cells and antigen-presenting cells (APCs) in pancreatic islets. Loss of B7x in these cells is associated with immune-mediated killing of beta cells, and subsequent development of autoimmune diabetes. In pancreatic islet cells and islet-APCs, B7x is lost through proteolytic cleavage of the B7x protein. The metalloprotease Nardilysin (NRD1) cleaves the membrane-bound B7x, releasing it as a less-functional, soluble form.
B7x mRNA is broadly found in normal tissues despite its limited protein expression, pointing to mechanisms of translational or post-translational regulation. Several miRNA have been identified to negatively regulate B7x expression, including miR-125b-5p in macrophages[62], and miR-155/miR-143 in colon cancer cell lines[60]. In pancreatic islet cells, B7x protein levels are maintained under an intriguing post-translational mechanism; the metalloproteinase nardilysin (NRD1) proteolytically cleaves the B7x protein from the cell membrane, releasing it in a soluble, inactive state[25, 63] (Figure 3B). Indeed, upregulation of Nrd1 and elevated soluble B7x is characteristic of patients with T1D but not T2D[25], suggesting that loss of B7x plays a role in the pathogenesis of autoimmune diabetes and is not a product of the associated metabolic alterations.
The mechanisms that underlie expression of B7x in tumors appear to be independent of those that regulate other immune checkpoints such as PD-L1. Inflammatory cytokines that drive PD-L1 expression such as IFNγ and TNFα do not increase B7x expression on tumor cell lines[53], whereas B7x can respond to the immunosuppressive cytokines TGFβ1 and IL-10[16, 60]. Such distinct regulatory mechanisms are likely the cause of why human lung and breast cancers rarely coexpress PD-L1 or B7x, but instead differentially express one or the other depending on the cytokine milieu in the tumor microenvironment[18, 19, 54]. Certain stimuli such as hypoxia signaling through HIF1α have been shown to induce PD-L1 [64] as well as B7x [59]in tumor cells in vitro, but why tumors preferentially express one or the other in vivo is not clear. Integration of a combination of signals in the tumor microenvironment may cause the distinct expression patterns of PD-L1 or B7x by tumor cells in vivo.
Receptors of B7x
The identity of the receptor for B7x is not yet certain. But although its identity is not clear, its existence on specific cell types can be shown experimentally through in vitro functional assays and cell-surface staining with recombinant B7x-Ig fusion protein. B7x-Ig has been shown to bind to activated T cells[3], tumor-infiltrating T cells[17], and tumor-associated neutrophils[22], consistent with the populations that have been shown to functionally respond to B7x stimulation in vitro.
The transmembrane glycoprotein neuropilin-1 (Nrp1) has been proposed to be a receptor for B7x for thymic-derived Tregs, although a subsequent study was not able to find any direct interaction between B7x and Nrp1[53]. More recent evidence suggests that the Nrp1-B7x interaction relies on the additional binding partners Semaphorin-3a and Plexin A4[20]. However, whether B7x indeed binds to Nrp1 remains controversial. Moreover, since Nrp1 is mostly restricted to thymic derived Tregs, the receptor through which B7x regulates other T cell subsets remains yet unknown.
Expression of coinhibitory receptors such as PD-1, Tim-3, and Lag-3 have been shown to mark “exhausted” or dysfunctional phenotypes in effector T cells, particularly in tumor-infiltrating T cells[65]. Interestingly, expression of the B7x-receptor by tumor-infiltrating CD8 T cells occurs at an earlier stage than PD-1 or Tim-3, and cells that co-express PD-1 and B7x-Receptor are relatively more activated than cells that express PD-1 and Tim-3[17]. These findings indicate that expression of the B7x-receptor is dynamic and occurs distinctly from the exhaustion mechanisms that induce PD-1 and Tim-3 expression.
Immunotherapies Targeting B7x: Pre-Clinical Models
ICB with monoclonal antibodies has proven to be an effective means of therapy for many cancer types, as seen with ICB targeting CTLA-4, PD-1, and PD-L1. Since B7x mediates immune evasion in tumors, blocking this pathway would restore anti-tumor immunity. In fact, anti-B7x therapy has demonstrated significant therapeutic efficacy in a variety of syngeneic murine models. B7x blockade reduces the metastatic capacity of tumor cells in an intravenous metastasis model, reducing the capacity for B7x-expressing tumor cells to form metastatic nodules in the lung[5]. Similarly, B7x-blockade reduces primary tumor growth of orthotopic breast tumors and a variety of subcutaneous tumors as well[5, 17]. Inhibition of B7x has also been shown to be synergistic with existing therapies, such as PD-1 blockade[17] and chemotherapy[66], showing its utility in combination treatment.
Due to frequency of expression of B7x in tumors, it can be targeted for cytotoxic therapy or immune-mediated killing. The aforementioned blocking antibodies can mediate cell killing through antibody-dependent cytotoxicity (ADCC) [5]. A more direct cytotoxic strategy is the use of antibody-drug conjugates: anti-B7x antibody conjugated to the microtubule-disrupting compound MC-vc-PAB-MMAE was demonstrated to have therapeutic efficacy in orthotopic xenograft breast tumor models[67]. More recently, a bispecific B7x-CD3 antibody was also shown to be an effective strategy, successfully crosslinking B7x on tumor cells with the T cell receptor on human CD4 and CD8 T cells, directing the T cells to lyse B7x-expressing tumor cells[68] (Figure 4A).
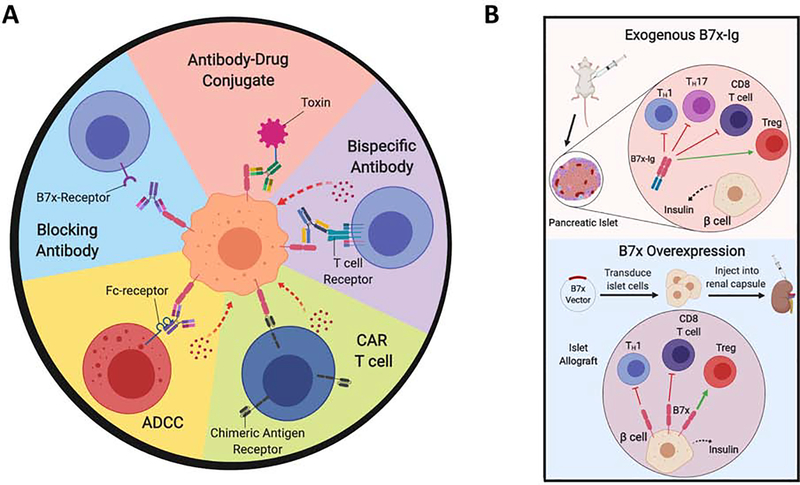
Figure 4. Therapeutics Targeting the B7x Pathway.
(A) Several strategies are being pursued to target B7x in human cancers, and have demonstrated efficacy in pre-clinical murine models. Blocking the interaction of B7x with its receptor on immune cells with anti-B7x monoclonal antibodies eliminates this mechanism of immune escape. Further, anti-B7x antibodies can be used to target B7x-expressing tumor cells for cytotoxic or immune-mediated killing. Anti-B7x antibodies can mediate antibody-dependent cellular cytotoxicity through Fc-dependent killing. Further, anti-B7x/anti-CD3 bispecific antibodies crosslink B7x to the T cell receptor on T cells, allowing effector T cells to kill target tumor cells. Anti-B7x antibodies can be crosslinked with toxins such as MMAE to kill tumor cells. B7x-expressing tumor cells can also be targeted by cellular therapies such as chimeric antigen receptor CAR-T cells, where T cells are engineered to target B7x on tumor cells, and thus mediate immune killing. (B) Two strategies have been used to activate the B7x pathway for the treatment of autoimmune disease such as Type 1 Diabetes. The B7x-Ig fusion protein of the extracellular region of B7x and the immunoglobulin-Fc domain can be administered systemically, which binds to the B7x-receptor on T cells and thereby induces systemic immunosuppression. B7x-Ig inhibits the effector functions of effector CD4 (Th1, Th17 cells) and CD8 T cells, and promotes immunosuppressive Tregs. Alternatively, the B7x gene can be genetically overexpressed in a target tissue. For example, a B7x-overexpression vector is transduced into pancreatic islet cells, upon which they are transplanted into the host organism. The renal capsule is a clinically relevant site to engraft the islet cells. B7x-overexpression in these cells suppresses autoreactive T cells, promotes Tregs, and thereby prolongs survival of the islet allografts.
The emergence of cellular therapies represents a promising new approach for cancer therapy. The success of chimeric antigen receptor (CAR) T cell therapy against the CD19 antigen in B cell malignancies prompted a surge of interest in relevant targets for other cancer types. Being overexpressed by many solid tumors, B7x is one such promising target. Indeed, a CAR engineered to target human and mouse B7x successfully caused tumor regression in murine ovarian tumor xenograft models[12]. However, treated mice also experienced lethal toxicity due to off-tumor killing on normal tissues, reflecting a broader challenge in the use of CAR T cells in solid tumors. Methods to reduce toxicity, such as suicide-switches or masked receptors, would be needed for the B7x-targeting CAR strategy to be feasible in future studies.
For the treatment of autoimmune disease and transplant rejection, agonism of the B7x-pathway has been experimentally shown to effectively induce immune tolerance. To deliver B7x exogenously, the most commonly used strategy has been to administer B7x-Ig, a fusion protein of B7x and the IgG Fc region (Figure 4B, upper panel). B7x-Ig therapy has been effective in the treatment of mouse models of inflammatory and autoimmune diseases, including T1D [69, 70], lupus nephritis[26], acute hepatitis[71], and autoimmune encephalomyelitis[72]. In these models, B7x-Ig alleviates disease pathology by inhibiting the activation of effector T cells, promoting immunosuppressive Tregs, and increasing serum levels of anti-inflammatory cytokines.
In the treatment of T1D, autologous transplant of new islet cells to replace the lost tissue is a strategy that has received an enormous amount of attention. Extending the survival of these transplanted cells is a significant challenge and is a key step in making this strategy clinically viable. Pancreatic β-cells normally express B7x to prevent immune-mediated killing[28, 29], so overexpression of this pathway would prolong their survival in the context of transplantation (Figure 4B, lower panel). Indeed, a few studies have demonstrated that overexpression of B7x inhibits T cell-mediated graft rejection and prolongs the survival of β-cell transplants in murine autoimmune diabetes models[30, 73, 74]. This is consistent with findings in other inflammatory disease models; in an acute hepatic injury model, overexpression of B7x-Ig in the liver via hydrodynamic gene delivery protected mice from liver necrosis[71]. Although gene modification therapies are still in early stages for human diseases, activating the B7x pathway holds promise for suppressing inflammation and inducing tolerance to transplants.
Immunotherapies Targeting B7x: Clinical Trials
The potential for B7x-driven regulation of immune responses is now being explored in clinical trials for both autoimmune disease and cancer. Two Phase 1 trials have begun for AMP-110, a fusion protein of the extracellular domain of B7x and human IgG Fc. The first trial is a Phase 1 study where AMP-110 is being assessed for safety, tolerability, and pharmacokinetics in patients with rheumatoid arthritis (Clinical Trial Numberii: ). The second is a Phase 1b randomized, multi-dose, placebo-controlled study for the safety, tolerability, pharmacokinetics and clinical activity of AMP-110 in subjects with rheumatoid arthritis (Clinical Trial Number: ). Although both studies have been finished, the results have not yet been reported.
Recently, a Phase 1a/1b study of the anti-B7x antibody FPA150 began in patients with advanced solid tumors to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of FPA150 alone or in combination anti-PD1 (Clinical Trial Number: ). FPA150 is a fully humanized IgG1 κ monoclonal antibody that binds to B7x IgV ectodomain with high affinity (KD= 2nM) and provides T cell checkpoint blockade. In addition, it is afucosylated and demonstrates higher affinity binding to FcγRIIIA and enhanced ADCC activity. FPA150 monotherapy was well tolerated and evaluation of anti-tumor activity is ongoing[75].
Concluding Remarks and Future Perspectives
In this review, we explored the known roles of B7x in the immune system and its relevance to human disease. The immunosuppressive pathways mediated by B7x offer many opportunities for therapeutic intervention, whether as blockade for malignancy or agonism for autoimmune disease.
B7x plays many intriguing roles in immune regulation, but much about its functions and expression patterns remains to be understood. Perhaps the most pressing question on the B7x pathway is the identity of the receptor(s) through which B7x acts (see Outstanding Questions). Much remains unknown about the downstream signaling pathway within T cells and other immune cells after B7x binds to their cell surface, and a more complete understanding will require identification of the receptor. B7x has clear suppressive effects on the immunity and inflammation, which is critical in preventing autoimmune disease in tissues such as the pancreatic islets. Its role in other tissues where it is expressed at high levels, particularly the mammary gland and gynecological tract, are largely unknown. These aspects of the B7x signaling pathway will need to be elucidated in future studies.
Outstanding Questions.
Which receptor(s) does B7x act through, and which downstream signaling pathways does it activate in T cells and other immune cells?
What roles does B7x play in the tissues where it is normally expressed, such as the lung, breast, endometrium, and ovary?
What mechanisms drive B7x expression in “cold” tumors?
What factors in the tumor microenvironment determine the mutually-exclusive expression of B7x and PD-L1 in tumor cells?
What biomarkers predict the response to anti-B7x therapy?
Which currently available immunotherapies would be most effective in combination with B7x-targeting therapeutics?
The roles of B7x within the tumor microenvironment have been extensively studied, establishing it as a potent means of immune evasion. In many ways, it acts differently than other checkpoint pathways such as the PD-1/PD-L1 pathway. In particular, whereas PD-L1 expression is associated with immunologically “hot” tumors, the expression of B7x marks “cold” environments. Such “cold” tumors have markedly reduced immune infiltration and are thus considered poorer targets for immunotherapy. Therefore, B7x may serve as a useful biomarker for predicting the efficacy of immunotherapy, although further studies are needed to determine this.
The broad expression of B7x in human cancers and its roles in tumor immune evasion make it a compelling target for therapeutics. Whereas ICB against the PD-1/PD-L1 and CTLA-4 pathway has demonstrated clinical efficacy in a variety of cancers, the majority of patients do not respond. A major challenge for PD-1/PD-L1 blockade is that not all tumors rely on that pathway for immune evasion. B7x is independently expressed from PD-L1 in the vast majority of triple-negative breast and non-small cell lung carcinomas. Instead of using PD-1 or PD-L1 inhibitors against such B7x-expressing tumors, it may be more effective to use B7x-targeting therapeutics. Combination therapies of anti-B7x and other checkpoint inhibitors may also demonstrate synergy, as it has in pre-clinical models. In regards to safety profiles, anti-B7x antibodies with blocking and ADCC effects are well tolerated in both pre-clinical models and Phase 1 clinical studies. B7x-targeting CAR T cells are currently limited by lethal on-target, off-tumor toxicity, despite effectively causing tumor regression in vivo. Further, it is unclear what biomarkers, including tumor-associated factors and host factors, would determine response to B7x-targeting therapies. Future clinical studies are needed to determine the most effective and safe applications of therapeutics that target the B7x pathway.
Highlights.
B7x is a coinhibitory ligand of the B7 family that suppresses inflammatory functions of immune cells, particularly effector CD4 and CD8 T cells.
B7x is broadly overexpressed in many human cancers, where it suppresses the anti-tumor immune response and thereby mediates immune evasion.
In contrast to PD-L1 whose expression is associated with immunologically “hot” tumors, the expression of B7x marks “cold” environments.
Deficiencies in the B7x pathway in human have been associated with various autoimmune and inflammatory diseases, including Type 1 diabetes and rheumatoid arthritis.
Targeting the B7x pathway has been therapeutically effective in murine models of cancers and autoimmune and inflammatory diseases.
Early phase clinical trials are underway for B7x-targeting therapies for cancers and rheumatoid arthritis.
Acknowledgements
P.J. is supported by National Institutes of Health (NIH) F30CA224931. Research in the Zang lab is supported by NIH R01DK100525 and R01CA175495, Irma T.Hirschl/Monique Weill-Caulier Trust and Cancer Research Institute.
GLOSSARY
- Overall Survival (OS)
The length of time from randomization, date of diagnosis, or the start of treatment until that patient dies from any cause
- Progression-free survival (PFS)
The length of time from the start of treatment until the patient’s cancer grows or spreads further, or the patient dies from any cause
- Syngeneic
Tumor model where a model organism is engrafted with tumor cells of the same genetic background, e.g. C57BL/6 mice subcutaneously implanted with a C57BL/6-derived cancer cell line
- Xenograft Model
Tumor model where human cancer cells are engrafted into a model organism. The recipient host is immunocompromised to prevent immune rejection of the tumor
Footnotes
Conflict of Interest
X. Zang is an inventor on patent number US 9447186 covering cancer immunotherapy targeting B7x and a pending patent covering cardiovascular disease treatment and prevention targeting B7x.
https://www.ncbi.nlm.nih.gov/gene/79679/ortholog/?scope=117570 (Vtcn1 - V-set domain containing T cell activation inhibitor 1, accessed July 10 2019).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zang X et al. (2003) B7x: A widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 100, 10388–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sica GL et al. (2003) B7-H4, a molecule of the B7 family, negatively regulates T Cell immunity. Immunity 18, 849–861. [DOI] [PubMed] [Google Scholar]
- 3.Prasad DVR et al. (2003) B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 18, 863–873. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JD et al. (2009) The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol. Immunol 46, 457–472. [DOI] [PubMed] [Google Scholar]
- 5.Jeon H et al. (2014) Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 9, 10891098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazar-Molnar E et al. (2008) Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc. Natl. Acad. Sci. USA 105, 10483–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L et al. (2013) The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene 32, 5347–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon I et al. (2006) B7-H4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 66, 1570–1575. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH et al. (2008) Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 68, 6054–6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mach P et al. (2018) Soluble B7-H4 blood serum levels are elevated in women at high risk for preeclampsia in the first trimester, as well as in patients with confirmed preeclampsia. Am. J. Reprod. Immunol 80, e12988. [DOI] [PubMed] [Google Scholar]
- 11.Azuma T et al. (2009) Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS. Med. 6, e1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JB et al. (2016) Tumor regression and delayed onset toxicity following B7-H4 CAR T cell therapy. Mol. Ther 24, 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmeyer KA et al. (2012) Tissue-expressed B7x affects the immune response to and outcome of lethal pulmonary infection. J. Immunol 189, 3054–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS et al. (2012) B7x in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells. J. Immunol 189, 4165–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Q et al. (2010) The negative co-signaling molecule B7-H4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity. Stem Cells Dev. 19, 27–38. [DOI] [PubMed] [Google Scholar]
- 16.Yao Y et al. (2016) B7-H4(B7x)–mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin. Canc. Res 22, 2778–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J et al. (2018) Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8+ T Cells. Immunity 48, 1–14. [DOI] [PubMed] [Google Scholar]
- 18.Kryczek I et al. (2006) B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med 203, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H et al. (2018) Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1-negative human lung cancers. Clin. Cancer Res 24, 1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podojil JR et al. (2018) B7-H4 modulates regulatory CD4+ T cell induction and function via ligation of a Semaphorin 3a/Plexin A4/Neuropilin-1 complex. J. Immunol 201, 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu G et al. (2009) B7-H4–deficient mice display augmented neutrophil-mediated innate immunity. Blood 113, 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abadi YM et al. (2013) Host B7x promotes pulmonary metastasis of breast cancer. J. Immunol 190, 3806–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y et al. (2006) Expression of the novel co-stimulatory molecule B7-H4 by renal tubular epithelial cells. Kidney Int. 70, 2092–2099. [DOI] [PubMed] [Google Scholar]
- 24.Albers HM et al. (2014) Genetic variation in VTCN1 (B7-H4) is associated with course of disease in juvenile idiopathic arthritis. Ann. Rheum. Dis 73, 1198–1201. [DOI] [PubMed] [Google Scholar]
- 25.Radichev IA et al. (2014) Nardilysin-dependent proteolysis of cell-associated VTCN1 (B7-H4) marks type 1 diabetes development. Diabetes 63, 3470–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawar RD et al. (2015) B7x/B7-H4 modulates the adaptive immune response and ameliorates renal injury in antibody-mediated nephritis. Clin. Exp. Immunol 179, 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao ZX et al. (2017) Immunosuppressive effect of B7-H4 pathway in a murine systemic lupus erythematosus model. Front. Immunol 8, 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei J et al. (2011) Tissue-specific expression of B7x protects from CD4 T cell-mediated autoimmunity. J. Exp. Med 208, 1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung SS et al. (2014) B7-H4 expression in normal and diseased human islet beta cells. Pancreas 43, 128–134. [DOI] [PubMed] [Google Scholar]
- 30.Wang X et al. (2009) Local expression of B7-H4 by recombinant adenovirus transduction in mouse islets prolongs allograft survival. Transplantation 87, 482–490. [DOI] [PubMed] [Google Scholar]
- 31.Suh WK et al. (2006) Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol. Cell Biol 26, 6403–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu D et al. (2018) Human cytomegalovirus reprogrammes haematopoietic progenitor cells into immunosuppressive monocytes to achieve latency. Nat. Microbiol 3, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg A et al. (2017) Human immunodeficiency virus type-1 myeloid derived suppressor cells inhibit cytomegalovirus inflammation through interleukin-27 and B7-H4. Sci. Rep 7, 44485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salceda S et al. (2005) The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res 306, 128–141. [DOI] [PubMed] [Google Scholar]
- 35.Tringler B et al. (2005) B7-H4 is highly expressed in ductal and lobular breast cancer. Clin. Cancer Res 11, 1842–1848. [DOI] [PubMed] [Google Scholar]
- 36.Krambeck AE et al. (2006) B7-H4 expression in renal cell carcinoma and tumor vasculature: Associations with cancer progression and survival. Proc. Natl. Acad. Sci. USA 103, 10391–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kryczek I et al. (2007) Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Research 67, 8900–8905. [DOI] [PubMed] [Google Scholar]
- 38.Zang X et al. (2007) B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 104, 19458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J et al. (2010) Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol. Immunother 59, 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quandt D et al. (2011) B7-H4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin. Cancer Res 17, 3100–3111. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y et al. (2014) The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco. Targets Ther 7, 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Xie N et al. (2017) Upregulation of B7-H4 promotes tumor progression of intrahepatic cholangiocarcinoma. Cell Death Dis. 8, 3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schalper KA et al. (2017) Differential expression and significance of PD-L1, IDO-1, and B7-H4 in human lung cancer. Clin. Cancer Res 23, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvajal-Hausdorf D et al. (2019) Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J. Immunother. Cancer 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chand D et al. (2019) Immune checkpoint B7x (B7-H4/B7S1/VTCN1) is over expressed in spontaneous canine bladder cancer: the first report and its implications in a preclinical model. Bladder Cancer 5, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krambeck AE et al. (2006) B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc. Natl. Acad. Sci. USA 103, 10391–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Q and Ma X (2015) B7-H4 expression is associated with tumor progression and prognosis in patients with osteosarcoma. Biomed. Res. Int 2015, 156432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kryczek I et al. (2007) Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 67, 8900–8905. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J et al. (2013) B7-H4 expression is associated with cancer progression and predicts patient survival in human thyroid cancer. Asian Pac. J. Cancer Prev 14, 3011–3015. [DOI] [PubMed] [Google Scholar]
- 50.Fan M et al. (2014) B7-H4 expression is correlated with tumor progression and clinical outcome in urothelial cell carcinoma. Int. J. Clin. Exp. Pathol 7, 6768–6775. [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao LW et al. (2014) B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3+ regulatory T-cell infiltration. Acta Histochem. 116, 1163–1168. [DOI] [PubMed] [Google Scholar]
- 52.Zang X et al. (2007) B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 104, 19458–19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohaegbulam KC et al. (2017) Tumor-expressed immune checkpoint B7x promotes cancer progression and antigen-specific CD8 T cell exhaustion and suppressive innate immune cells. Oncotarget 8, 82740–82753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gruosso T et al. (2019) Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Invest 129, 1785–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia F et al. (2017) B7-H4 enhances the differentiation of murine leukemia-initiating cells via the PTEN/AKT/RCOR2/RUNX1 pathways. Leukemia 31, 2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wartewig T et al. (2017) PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 552, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang FB et al. (2017) B7-H4 overexpression is essential for early hepatocellular carcinoma progression and recurrence. Oncotarget 8, 80878–80888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piao L et al. (2018) B7H4 is associated with stemness and cancer progression in esophageal squamous cell carcinoma. Hum. Pathol 80, 152–162. [DOI] [PubMed] [Google Scholar]
- 59.Jeon YK et al. (2015) Cancer cell-associated cytoplasmic B7-H4 is induced by hypoxia through hypoxia-inducible factor-1α and promotes cancer cell proliferation. Biochem. Biophys. Res. Commun 459, 277–283. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X et al. (2016) TGF-β1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget 7, 67196–67211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L et al. (2018) NF-κB RelA renders tumor-associated macrophages resistant to and capable of directly suppressing CD8+ T cells for tumor promotion. Oncoimmunology 7, e1435250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diao W et al. (2017) MicroRNA-125b-5p modulates the inflammatory state of macrophages via targeting B7-H4. Biochem. Biophys. Res. Commun 491, 912–918. [DOI] [PubMed] [Google Scholar]
- 63.Radichev IA et al. (2016) Loss of peripheral protection in pancreatic islets by proteolysis-driven impairment of VTCN1 (B7-H4) presentation is associated with the development of autoimmune diabetes. J. Immunol 196, 1495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noman MZ et al. (2014) PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med 211, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hashimoto M et al. (2018) CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med 69, 301–318. [DOI] [PubMed] [Google Scholar]
- 66.Leung J et al. (2017) Synergistic effects of host B7-H4 deficiency and gemcitabine treatment on tumor regression and anti-tumor T cell immunity in a mouse model. Cancer Immunol. Immunother 66, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leong SR et al. (2015) An anti-B7-H4 antibody-drug conjugate for the treatment of breast cancer. Mol. Pharm 12, 1717–1729. [DOI] [PubMed] [Google Scholar]
- 68.Iizuka A et al. (2019) A T-cell-engaging B7-H4/CD3-bispecific Fab-scFv antibody targets human breast cancer. Clin. Cancer Res 25, 2925–2934. [DOI] [PubMed] [Google Scholar]
- 69.Wang X et al. (2011) Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes 60, 3246–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee IF et al. (2013) B7-H4.Ig inhibits the development of type 1 diabetes by regulating Th17 cells in NOD mice. Cell Immunol. 282, 1–8. [DOI] [PubMed] [Google Scholar]
- 71.Xu JF et al. (2010) Ectopic B7-H4-Ig expression attenuates concanavalin A-induced hepatic injury. Clin. Immunol 136, 30–41. [DOI] [PubMed] [Google Scholar]
- 72.Podojil JR et al. (2013) B7-H4Ig inhibits mouse and human T-cell function and treats EAE via IL-10/Treg-dependent mechanisms. J. Autoimm 44, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan CL et al. (2009) B7-H4 transfection prolongs beta-cell graft survival. Transpl. Immunol 21, 143–149. [DOI] [PubMed] [Google Scholar]
- 74.Wang X et al. (2013) Endogenous expression of B7-H4 improves long-term murine islet allograft survival. Transplantation 95, 94–99. [DOI] [PubMed] [Google Scholar]
- 75.Sachdev Jasgit C. et al. (2019) Phase 1a/1b study of first-in-class B7-H4 antibody, FPA150, as monotherapy in patients with advanced solid tumors., J. Clin. Onc p. 2529. [Google Scholar]