Abstract
Cyanobacteria and associated heterotrophic bacteria hold key roles in carbon as well as nitrogen fixation and cycling in the Baltic Sea due to massive cyanobacterial blooms each summer. The species specific activities of different cyanobacterial species as well as the N- and C-exchange of associated heterotrophic bacteria in these processes, however, are widely unknown. Within one time series experiment we tested the cycling in a natural, late stage cyanobacterial bloom by adding 13C bi-carbonate and 15N2, and performed sampling after 10 min, 30 min, 1 h, 6 h and 24 h in order to determine the fixing species as well as the fate of the fixed carbon and nitrogen in the associations. Uptake of 15N and 13C isotopes by the most abundant cyanobacterial species as well as the most abundant associated heterotrophic bacterial groups was then analysed by NanoSIMS. Overall, the filamentous, heterocystous species Dolichospermum sp., Nodularia sp., and Aphanizomenon sp. revealed no or erratic uptake of carbon and nitrogen, indicating mostly inactive cells. In contrary, non-heterocystous Pseudanabaena sp. dominated the nitrogen and carbon fixation, with uptake rates up to 1.49 ± 0.47 nmol N h-1 l-1 and 2.55 ± 0.91 nmol C h-1 l-1. Associated heterotrophic bacteria dominated the subsequent nitrogen remineralization with uptake rates up to 1.2 ± 1.93 fmol N h-1 cell -1, but were also indicative for fixation of di-nitrogen.
Introduction
Cyanobacterial blooms are a worldwide phenomenon in limnic, brackish and marine systems. In the Baltic Sea, blooms occur regularly during summer [1], and due to their high biomasses they significantly add to eutrophication [2,3]. Start of blooms is promoted by rising water temperatures and low N:P ratios after N-depletion due to the capability of atmospheric nitrogen fixation by several cyanobacterial species [1,3,4]. Total cyanobacterial nitrogen fixation in the Baltic Sea was estimated at 370 kt yr-1 [2], and may contribute up to 55% of total nitrogen input [5,6]. Furthermore, filamentous cyanobacteria may contribute up to 44% of the community primary production [7]. The major part of nitrogen and carbon fixation is performed in the early summer, followed by a peak in biomass, and ultimately the decay of the bloom in which predominantly recycling processes occur [6,8].
Cyanobacteria live in close associations with heterotrophic bacteria, and interactions between them may range from symbiosis to competition [9,10]. These interactions strongly influence carbon and nutrient cycling and thereby the stability of aquatic food webs [11,12]. In phytoplankton blooms, heterotrophic bacteria may provide macronutrients via recycling (or fixation) but may be also competitors for inorganic nutrients [11]. Especially at the late stages of cyanobacterial blooms, the associated heterotrophic bacteria may be responsible for a significant share of elemental cycling and fluxes, i.e. for the input of nutrients and organic matter in the ecosystem due to remineralization. Studies on the role of associated bacteria at these late cyanobacterial bloom stages, however, are lacking.
The predominant cyanobacterial genera in Baltic Sea blooms are Aphanizomenon, Nodularia, Dolichospermum, Pseudanabaena and Synechococcus, whereby the dominant groups and species may differ between years and stage of the bloom [12]. The first three mentioned genera are filamentous and heterocystous, and may form dense surface scums [1]. Baltic Sea Synechococcus sp. and Pseudanabaena sp. are supposed to be incapable of nitrogen fixation [13,14], even though nitrogenase genes occur in Pseudanabaena [14,15]. Thus, Aphanizomenon, Nodularia, and Dolichospermum are thought to dominate the biological nitrogen input into the Baltic Sea [14]. Recently, however, heterotrophic bacteria were shown to be capable of nitrogen fixation at depth in the central Baltic Sea [16] and may even be the principle N2 fixing organism in a Baltic Sea estuary [17]. However, studies that examined carbon and nitrogen fixation in cyanobacterial blooms and associated heterotrophic bacteria mostly focussed on single cyanobacterial species [7,18], or neglected associated bacteria as well as the fate of the fixed carbon and nitrogen in the associations [14]. In the present study, we incubated a natural late stage Baltic Sea cyanobacterial bloom with 13C bi-carbonate and 15N2, and followed the uptake over time by means of NanoSIMS. Thereby, we aimed at unravel the specific contribution of different cyanobacterial species and associated heterotrophic bacteria in carbon and nitrogen fixation as well as the fate of the fixed carbon and nitrogen in the associations.
Material and methods
Incubation experiments
A natural cyanobacterial bloom was sampled at station TransA (58°43.8`N, 18°01.9`E, Fig 1) on 13.08.2015. Positive phototactic zooplankton was removed by means of a light trap and bloom samples were concentrated until a cyanobacterial chl. a concentration of 9 μg l-1 was reached (measured with a PHYTO-PAM, Heinz Walz GmbH). At Askö laboratory (ca. 1 h transfer), five 176 mL opaque Nalgene bottles were filled with the concentrated bloom till overflowing and sealed with septum caps enabling addition and retrieval of liquids with syringes.
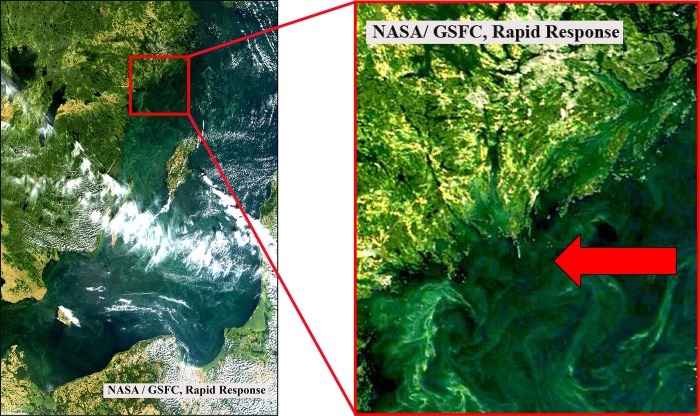
Fig 1. True color satellite image of a cyanobacterial bloom in the Baltic Sea on August 13, 2015 derived from MODIS/Terra (NASA/GSFC, Rapid Response).
The arrow in the zoom image on the right side points towards the sampling station TransA.
For 15N addition, 1 mL of the sample was removed and subsequently 1 mL 99% pure 15N2 gas injected with a syringe, resulting in 31.68 atom % 15N. For amending 13C, 5 ml sample were removed with a syringe and subsequently 5 ml F/2 medium [19] without nitrogen source, adjusted to 8 PSU and spiked with 0.4 g NaH13CO3 added (final concentration 108.36 atom % 13C). Incubation times were 10 min, 30 min, 1 h, 6 h and 24 h. Bottles were incubated in an incubation chamber at 16.5 ± 0.5°C at approximately 60 μmol photons s-1 m-2 (delivered from ROHS 36W 840 light bulbs), resembling the natural conditions of sampling under constant light (S1 Table).
Sampling
Each sample was fixed with formaldehyde (2% final concentration) for 3 h in the dark at room temperature, and filtered gently onto 3 μm pore width (we only aimed at the directly attached heterotrophic bacterial fraction) polycarbonate filters for later inspection with CARD-FISH and NanoSIMS. Before start of the incubation, 80 mL of the stock sample were filtered onto 3 μm pore width polycarbonate filter for DNA extraction of the associated bacterial fraction. For phytoplankton counting, a 100 mL subsample was fixed with an acidic Lugol solution [20] and counted according to the Utermöhl technique. To determine biomass percentages, the carbon content (μg l−1) of each species was calculated using the official PEG Biovolume Report 2016 (International Council for the Exploration of the Sea) for phytoplankton species and the carbon content per counting unit for the respective size class.
DNA extraction
DNA was extracted as described in [21] with modifications. Briefly, the filters were cut into pieces and mixed with sterilized zirconium beads, 500 μl of phenol/chloroform mix, and 500 μl of SLS extraction buffer. After centrifugation of the mixture, the supernatant was transferred to another tube and the process was repeated. DNA was precipitated overnight at −20°C. The pellet was washed with ethanol, dried, and resolved in autoclaved DEPC-treated water.
PCR and sequencing
For PCRs, 10 ng of DNA was added to autoclaved DEPC-treated water, 10× PCR buffer, BSA, MgCl2, dNTPs, forward and reverse primers, and native Taq polymerase. Bacterial DNA was amplified using the primers 341f and 805r [22], under the following conditions: 30 cycles of denaturation for 40 s at 95°C, 40 s of annealing at 53°C, and 1 min of elongation at 72°C. PCR products were cleaned with the Nucleospin kit following the manufacturer’s instructions and shipped to LGC Genomics GmbH (Berlin). Illumina MiSeq V3 sequencing with 300 bp paired-end reads was performed using the 16S primers 341F and 785R. The forward and reverse reads were deposited at the European Nucleotide Archive under the accession number PRJEB23316 (sample B15_3). Taxonomic identification of the associated bacterial community, was performed as described in [23] with the NGS analysis pipeline of the SILVA rRNA gene database project (SILVAngs 1.3).
CARD-FISH analyses
The Illumina runs mostly yielded Alphaproteobacteria and Cytophaga/Bacteroidetes, and probes Alf968 [24] and CF968 [25] were chosen for analyses of associated heterotrophic bacteria. CARD FISH analyses were computed as described in [26] with modifications: Filter pieces were doused in 0.2% fluid agarose, dried, and subsequently incubated for 60 min at 37°C in 10 mg ml-1 lysozym solution and thereafter for 15 min at 37°C with achromopeptidase (180 U ml-1). For inactivation, filter pieces were doused subsequently to 1x PBS, autoclaved MilliQ and 99% ethanol and following placed for 10 min in 0.01 M HCl at room temperature. Hybridization with horseradish peroxidase labeled 16S rRNA probes Alf968 and CF968 were carried out at 35°C with 55% formamide for 3.5 and 4 h, respectively. Signal amplification was achieved with Oregon green 488-X bound to tyramide as described in [27]. After hybridization, filter pieces were stained with 4,6-diamidin-2-phenylindol (DAPI) solution for unspecific counter-staining of all cells.
Laser-Scanning Microscope, Scanning electron microscope and sputtering
Spots of interest were determined by fluorescence microscopy and subsequently laser marked with a laser microdissectional microscope. For confirmation of associated bacteria and cyanobacterial species, SEM analyses were performed. Therefore, filter pieces were covered with approximately 8 nm gold in a sputter coater (Cressington108 auto-sputter coater). Samples were analyzed with a Scanning electron microscope (Zeiss Merlin VP compact) with the Zeiss Smart SEM Software. Before NanoSIMS analyses, filter pieces were covered with ca. 30 nm additional gold with a sputter coater (see above).
NanoSIMS measurements
SIMS imaging was performed using a NanoSIMS 50L instrument (Cameca, France). A 133Cs+ primary ion beam was used to erode and ionize atoms of the sample. Among the received secondary ions, images of 12C-, 13C-, 12C14N- and 12C15N- were recorded simultaneously for cells at the laser microdissectional (LMD)-marked spots using electron multipliers as detectors. The mass resolving power was adjusted to suppress interferences at all masses allowing, e.g. the separation of 12C15N- from interfering ions such as 13C14N-. Prior to the analysis, sample areas of 30×30 μm were sputtered for 2 min with 600 pA to erode the gold and reach the steady state of secondary ion formation. The primary ion beam current during the analysis was 1 pA; the scanning parameters were 512×512 px for areas of 20–30 μm, with a dwell time of 250 μs per pixel.
Analyses of NanoSIMS measurements
All NanoSIMS measurements were analysed with the Matlab based program look@nanosims [28]. Briefly, the 60 measured planes were checked for inconsistencies and all usable planes accumulated, regions of interest (i.e. cells of cyanobacterial filaments, associated bacterial cells and filter regions without organic material for background measurements) defined based on 12C14N mass pictures, and 13C/12C as well as 15N/14N ratios calculated from the ion signals for each region of interest. Measurements of heterocysts in Aphanizomenon sp., Dolichospermum sp., and Nodularia sp. were avoided due to rapid transfer of fixed nitrogen. For analyses of each measurement, first the means of background measurements were determined, and this mean factorized for theoretical background values (0.11 for 13C/12C and 0.00367 for 15N/14N). These factors were applied to all non-background regions of interest in the same measurement. For each time-point, values for each species (or bacterial group for the associated bacteria) were pooled (i.e. different cells in one measurement as well as different measurements) and means for each species (or bacterial group for the associated bacteria) for each time-point calculated. Work flow for an example spot from Card-FISH to NanoSIMS analyses is illustrated in Fig 2. The numbers of measured cells per species/group and time point, as well as overall measured areas per time point are given in S2 Table, the outcomes of all NanoSIMS analyses are given in S3 Table.
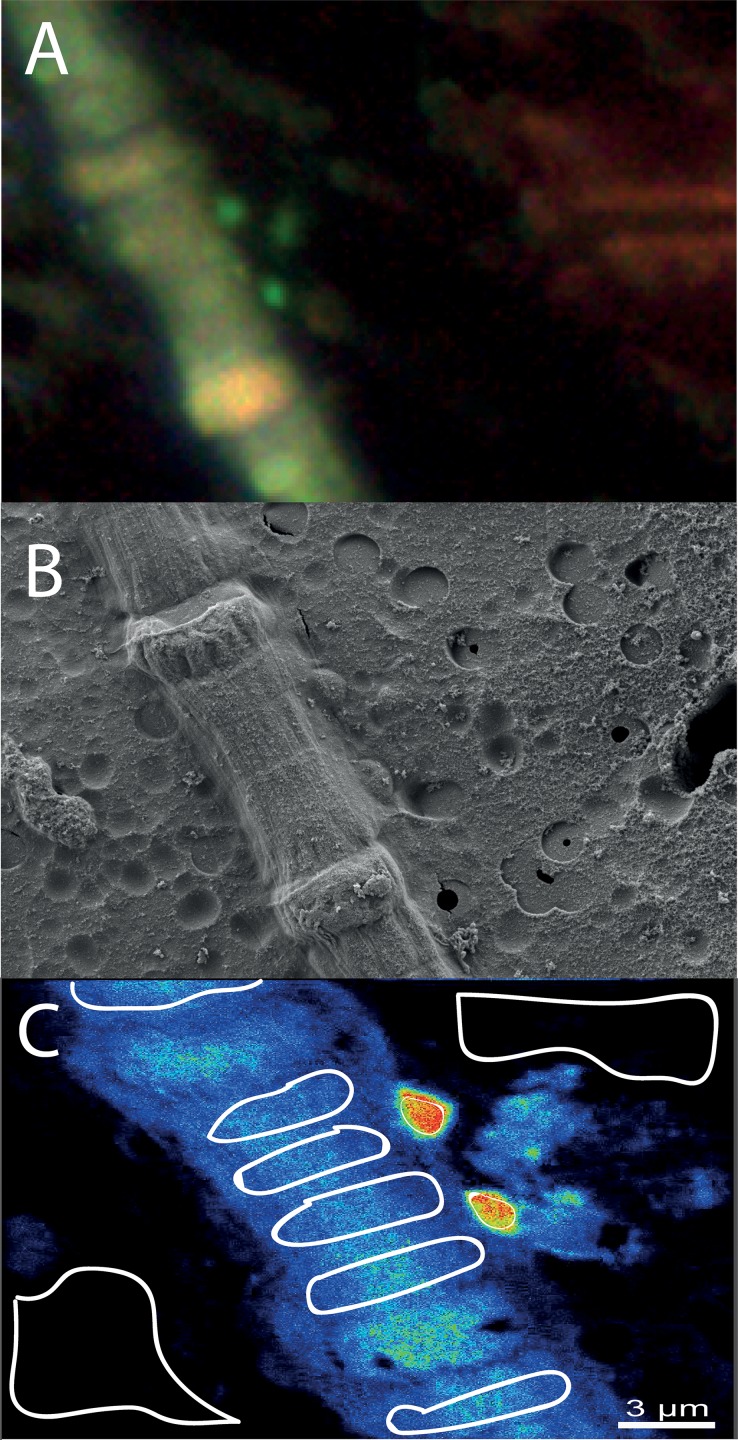
Fig 2. Work flow for analyses of cyanobacteria and their associated heterotrophic bacteria.
A: Card-FISH image of a Nodularia sp. filament with two associated Alphaproteobacteria taken with a laser microdissectional microscope. The marking arrow can be seen at the right side. B: Scanning electron microscope image of the same spot for confirmation of associated bacteria (middle-right side of the filament) and identification of Nodularia sp. The tip of the marking arrow can be seen at the right side of the image. C: accumulated NanoSIMS images of the same spot with blue (low) to red (high) 15N signal (as example). The circled areas display the regions of interest, where 13C/12C and 15N/14N ratios were calculated. Control (filter without cyanobacteria or heterotrophic bacteria) regions can be seen at the top-right and down-left, Nodularia sp. regions are displayed in the bluish part, and the associated Alphaproteobacteria by the smaller regions in the reddish part of the image.
Uptake rates of 13C and 15N
Uptake rates for nitrogen and carbon were calculated as described in [29] according to the equation:
where AN2 is the 15N or 13C enrichment of the N or C available for fixation; APN0 the 15N or 13C enrichment of particulate N or C at the start of the experiment; APNf, the15N or 13C enrichment of particulate N or C at the end of the experiment; and
where PX is the concentration of N or C for the respective cyanobacterial species in the incubation bottles, or the cellular N or C content for the associated bacteria. The solubility of N and C was calculated using the Excel Sheet provided by Joe Montoya, based on [30] for CO2 and [31] for N2. For cyanobacteria gross uptake rates could be calculated per volume and time (absolute numbers were known). For the associated bacteria uptake rates were calculated per cell and time, because no absolute numbers of associated bacteria were existent. The C:N ratios in the cyanobacteria were assumed with 6.3 [14,32]. The size of the associated bacteria was assumed with 2 x1 (length x width) μm (SEM analyses), the carbon content of 0.35 pg C μm-3 [33], and the C:N ratio of 5:1 [34]. We are aware that the used “bubble-method” for injection of N2 gas assumes an instantaneous equilibrium between the 15N2 gas bubble and the N2 dissolved in water, which in fact may be time-delayed [35], and ultimately leads to an underestimation of fixation rates. Thus, especially at the early measuring points (10 and 30 min), the calculated rates should be considered as proxy values with percentage errors up to 70% [36].
Data analyses
All data were analysed with R [37] and R studio [38]. To test for differences in stable isotope ratios between species/groups or between different time-points in the same group/species, ANOVAS (analyses of variance) with subsequent Tukey HSD posthoc tests with the package agricolae [39] were performed. Likewise, the impact of the host species on the stable isotope uptake of the associated bacteria was tested with ANOVAs, by comparing associated bacterial cells from different hosts. Possible cell-to-cell transfer of 13C and 15N between host and associated bacteria were tested by calculating linear models of 13C/12C and 15N/14N ratios between the host cells and the associated bacterial cells for each incubation period. To test for correlations between 13C and 15N uptake, linear models were calculated with the lm function. To test for differences in relations of 13C to 15N uptake between species/groups, dissimilarity matrices (horn distances) were calculated with a xy (x = 13C/12C, y = 15N/14N) system, and subsequently ANOSIM analyses performed with the vegan package [40]. To test for differences in 13C/15N uptake relations between functional groups, ANCOVAs with and without interactions between the factor and the co-variable were calculated with linear models. Here, 13C uptake was set as dependent variable, 15N uptake as co-variable, and the functional group as factor. Next, ANOVAs were calculated for both ANCOVAs to test for differences in the slopes of the linear models.
Results
Community composition of the phytoplankton and associated bacteria
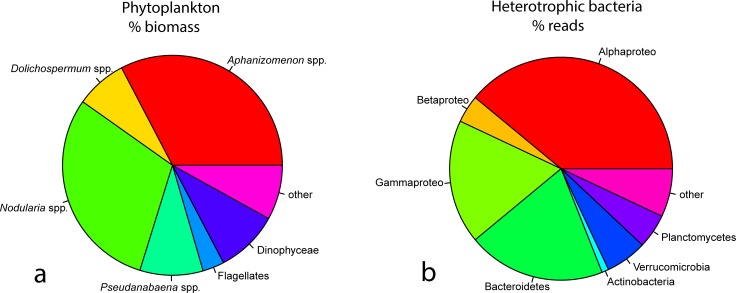
The phytoplankton community was dominated by the cyanobacteria Aphanizomenon sp. (33% biomass), Nodularia sp. (30% biomass), Pseudanabaena sp. (9% biomass) and Dolichospermum sp. (8% biomass), which together accounted for 80% of the total biomass (Fig 3A). The most abundant associated bacteria belonged to Alphaproteobacteria (39%), Cytophaga/Bacteroidetes (20%), Gammaproteobacteria (18%), Verrucomicrobia (6%), Planctomycetes (5%), Betaproteobacteria (4%) and Actinobacteria (1%, Fig 3B).
Fig 3.
A: Pie chart for the most abundant phytoplankton groups (left side, in % biomass). B: Pie chart for the most abundant bacterial groups (right side, in % of sequencing reads).
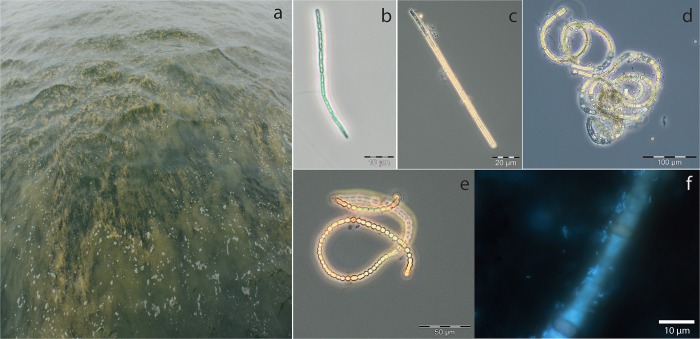
The general appearance of the bloom (Fig 4A), and microscopy of cyanobacteria (Fig 4B–4E) both indicated a late stage of the bloom (especially the “curly” appearance of Nodularia sp.), with many associated bacteria to the heterocystous species (Fig 4F).
Fig 4.
Appearance of the bloom at the day of sampling (a), and microscopic images of Pseudanabaena sp. (b), Aphanizomenon sp. (c), Nodularia sp. (d), Dolichospermum sp. (e), and a DAPI stained sample with Nodularia sp. and associated bacteria.
Bi-carbonate uptake of cyanobacteria and associated heterotrophic bacteria
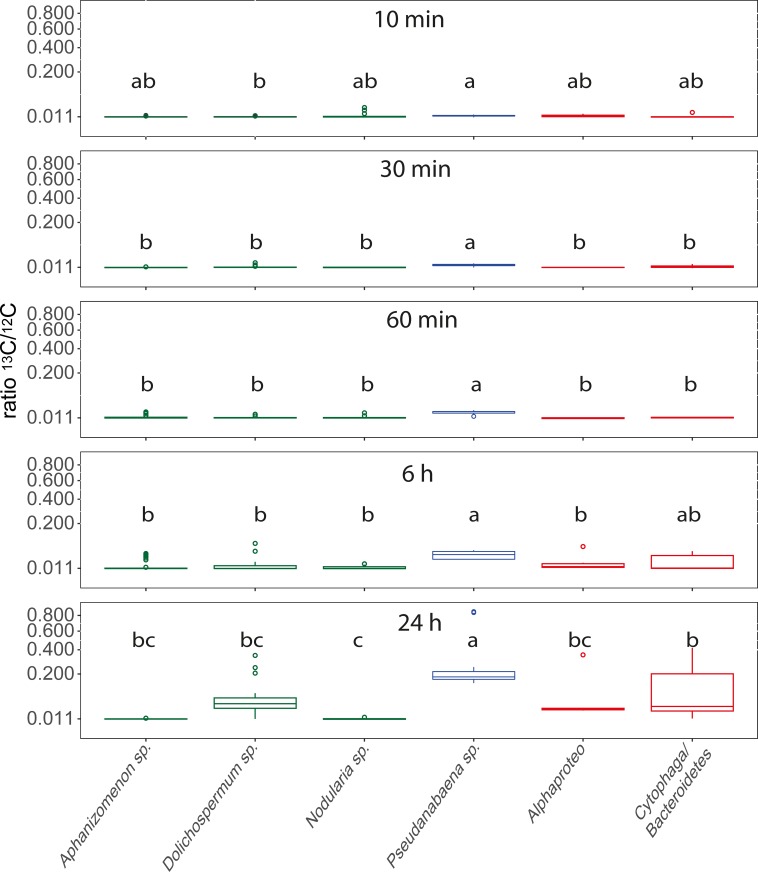
Significant differences in the 13C incorporation between the bacterial groups were observed at all sampling points (Fig 5). Pseudanabaena sp. showed the highest 13C/12C ratios at all sampling points with continuously increasing incorporation of 13C over time. At the early time points (10, 30 and 60 min), all other species/groups displayed a 13C/12C ratio close to the natural occurring value of 0.011 (Fig 5). After 6 and 24 h of incubation, however, Cytophaga/Bacteroidetes revealed the second highest 13C/12C ratios, corresponding to significant 13C enhancements with a more than two- and ten-fold increase of the natural occurring ratio after 6 and 24 h, respectively (Fig 5). Mentionable, the filamentous cyanobacteria Aphanizomenon sp., Dolichospermum sp. and Nodularia sp. did not display elevated 13C/12C ratios over the whole 24 h incubation period with two exceptions: Aphanizomenon sp. revealed enhanced ratios after 6 h and Dolichospermum sp. after 24 h of incubation (Fig 5, S4 Table). To test for a possible impact of the host-species on 13C uptake of the associated bacteria, we compared the 13C/12C ratios obtained from Alphaproteo- and Cytophaga/Bacteroidetes bacteria from different host species. In most cases, however, no significant differences occurred between the hosts (S4 Table). Especially in the 6 and 24 h exposures, where increased 13C/12C ratios were obtained for both of the associated bacterial groups (Fig 5), no impact of the host species could be seen (S4 Table). Linear models on 13C uptake between the host cells and the associated bacterial cells did not suggest cell-cell transfer of 13C except for the 60 min incubation (R2 = -0.05, 0.12, 0.24, -0.03, -0.05; p = 0.84, 0.24, 0.01, 0.48, 0.75, for 10 min, 30 min, 60 min, 6 h and 24 h incubation, respectively). The calculated uptake rates of the cyanobacteria were highest for Pseudanabaena sp. after 60 min with 2.55 ± 0.91 nmol C h-1 l-1, and from the associated bacteria for Cytophaga/Bacteroidetes bacteria with 0.31 ± 0.34 fmol C h-1 cell-1 after 24 h of incubation (Table 1).
Fig 5. Boxplots of 13C/12C ratios for Aphanizomenon sp., Dolichospermum sp., Nodularia sp., Pseudanabaena sp., Alphaproteobacteria and Cytophaga/Bacteroidetes bacteria over time, with square root transformed y axis.
Values originate from pooled data for the respective species from different measurements and cells (S2 Table). Lower case letters above the boxplots refer to different groups of Tukey HSD Post-Hoc tests. Heterocystous cyanobacteria are displayed in green, non-heterocystous cyanobacteria in blue, and associated heterotrophic bacteria in red.
Table 1. Carbon and nitrogen uptake rates ± standard deviation given in nmol C or N h-1 l-1 for cyanobacteria, and fmol C or N h-1 cell-1 for associated bacteria.
| 10 min | 30 min | 60 min | 6 h | 24 h | |
|---|---|---|---|---|---|
| 13C uptake nmol C h-1 l-1 (fmol C h-1 cell -1 for associated bacteria) | |||||
| Aphanizomenon sp. | 0.3 ± 3.52 | 0.00 ± 0.57 | 1.06 ± 2.69 | 0.41 ± 1.35 | 0.00 ± 0.02 |
| Dolichospermum sp. | 0.06 ± 0.76 | 0.25 ± 0.71 | 0.05 ± 0.2 | 0.07 ± 0.13 | 0.27 ± 0.18 |
| Nodularia sp. | 5.88 ± 20.28 | 0.00 ± 0.95 | 0.4 ± 1.5 | 0.19 ± 0.4 | 0.00 ± 0.03 |
| Pseudanabaena sp. | 2.48 ± 1.5 | 1.98 ± 0.89 | 2.55 ± 0.91 | 1.13 ± 0.72 | 1.87 ± 1.08 |
| Alphaproteo* | 0.59 ± 0.9 | 0.00 ± 0.04 | 0.00 ± 0.07 | 0.13 ± 0.23 | 0.2 ± 0.32 |
| Cytophaga/Bacteroidetes* | 0.12 ± 0.09 | 0.24 ± 0.45 | 0.03 ± 0.06 | 0.19 ± 0.27 | 0.31 ± 0.34 |
| 15N uptake nmol N h-1 l-1 (fmol N h-1 cell -1 for associated bacteria) | |||||
| Aphanizomenon sp. | 1.03 ± 1.6 | 0.00 ± 1.27 | 0.28 ± 0.42 | 0.17 ± 0.4 | 0.00 ± 0.02 |
| Dolichospermum sp. | 0.73 ± 0.45 | 0.17 ± 0.3 | 0.05 ± 0.08 | 0.09 ± 0.17 | 0.04 ± 0.03 |
| Nodularia sp. | 8.07 ± 18.4 | 0.03 ± 0.87 | 0.18 ± 0.53 | 0.19 ± 0.23 | 0.00 ± 0.02 |
| Pseudanabaena sp. | 1.49 ± 0,47 | 0.8 ± 0.56 | 0.84 ± 0.17 | 0.48 ± 0.31 | 0.17 ± 0.05 |
| Alphaproteo* | 0.00 ± 0.08 | 0.2 ± 0.73 | 0.31 ± 0.76 | 1.15 ± 1.29 | 0.34 ± 0.2 |
| Cytophaga/Bacteroidetes* | 0.95 ± 1.01 | 0.36 ± 0.53 | 1.2 ± 1.93 | 0.67 ± 0.92 | 0.25 ± 0.17 |
Lines with rates of the associated bacteria are indicated with an asterisk.
15N2 uptake of cyanobacteria and associated heterotrophic bacteria
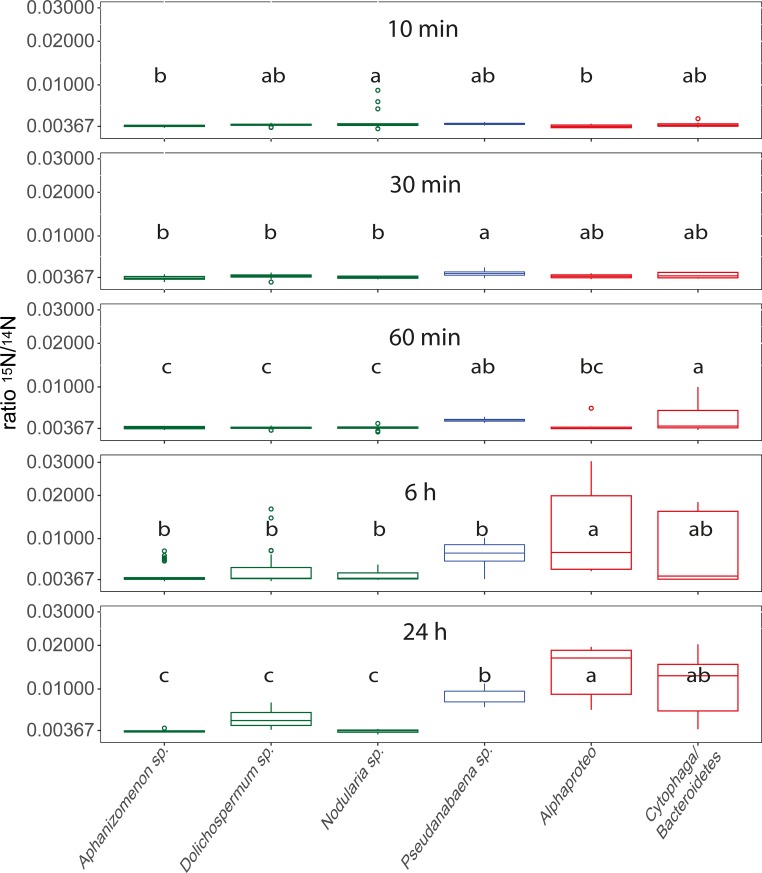
For all time points, significant differences of 15N incorporation between the species/groups occurred (Fig 6). After 30 min Pseudanabaena sp. (which reveals the highest 15N incorporation), and the associated heterotrophic bacteria showed enhanced 15N/14N ratios (Fig 6). Between 1 and 6 h of incubation especially the Alphaproteobacteria increased their 15N/14N ratios, and after 24 h of incubation pronounced differences between the species occurred, with associated Alphaproteobacteria showing the highest 15N incorporation (mean = 0.0143 ± 0.0059, almost 4-times increased 15N/14N ratios compared to the natural ratio). In general, after 24 h of incubation the associated bacteria revealed the highest ratios, followed by Pseudanabaena sp., whereas the heterocystous cyanobacteria displayed even after 24 h of incubation 15N/14N ratios close to the natural value (Fig 6).
Fig 6. Boxplots of 15N/14N ratios for Aphanizomenon sp., Dolichospermum sp., Nodularia sp., Pseudanabaena sp., Alphaproteobacteria and Cytophaga/Bacteroidetes bacteria over time with square root transformed y-axis.
Values originate from pooled data for the respective species from different spots and cells (S2 Table). Lower case letters above the boxplots refer to different groups of Tukey HSD Post-Hoc tests. Heterocystous cyanobacteria are displayed in green, non-heterocystous cyanobacteria in blue, and associated heterotrophic bacteria in red.
Comparisons of the 15N/14N ratios in each species/group between different incubation times revealed significant 15N incorporation in most species/groups, but inconsistent 15N uptake in the heterocystous species (Fig 6). In general, the heterocystous cyanobacteria do not display pronounced 15N2 uptake over time. In contrast, Pseudanabaena sp. displays significantly enhanced 15N/14N ratios from 6 h of incubation onwards, with steadily increasing values over time and significant differences also between 6 and 24 h of incubation (Fig 6, S4 Table). Also Alphaproteobacteria and Cytophaga/Bacteroidetes reveal steadily increasing 15N/14N ratios over the 24 h incubation period, with significant differences between almost all incubation times (Fig 6, S4 Table). The separation of the obtained 15N/14N values of associated Alphaproteo- and Cytophaga/Bacteroidetes bacteria by the host species did not reveal differences between the host species (S4 Table). Linear models between the 15N/14N ratios of heterocystous cyanobacterial cells that carry associated bacteria and the associated bacteria did not suggest dependencies of 15N uptake between the host and the associated bacterium, with the exception of 30 min incubation (R2 = -0.02, 0.64, 0.08, -0.03, 0.1; p = 0.48, 0.02, 0.1, 0.39, 0.1 for 10 min, 30 min, 1h, 6 h, and 24 h incubation, respectively). The uptake rates were highest for Nodularia sp. with 8.07 ± 18.4 nmol N h-1 l-1 after 10 min of incubation. However, if excluding the 10 min incubation due to experimental uncertainties, Pseudanabaena sp. revealed the highest incorporation rates with 0.84 ± 0.17 nmol N h-1 l-1 after 1 h of incubation. For the associated bacteria Cytophaga/Bacteroidetes displayed the highest incorporation of 15N with 1.2 ± 1.93 fmol N h-1 cell-1 after 1 h incubation (Table 1).
Species- and group specific relations of 13C to 15N uptake
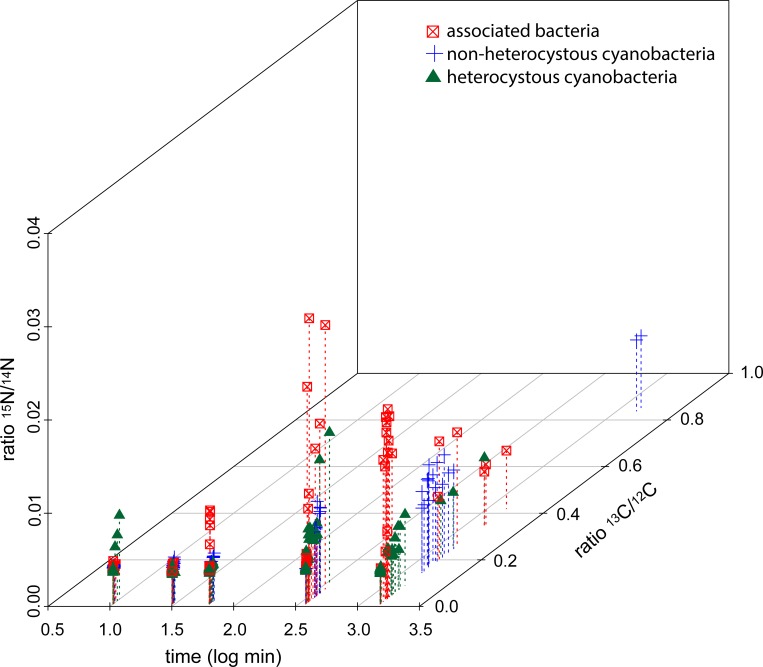
Significant differences between the species/bacterial groups occurred for all time points for relations of 13C against 15N uptake (ANOSIM, each p = 0.001), although different R values were obtained for different exposure times (R = 0.2387, 0.4203, 0.3098, 0.215, 0.585, for 10, 30, 60 min, 6 and 24 h exposure, respectively), indicating most pronounced differences in the relation of 13C to 15N uptake between the species/groups after 24 h of incubation. In general, Pseudanabaena sp. was the most noticeable species in the 13C uptake (starting with the 30 min exposure), and the associated Alphaproteo and Cytophaga/Bacteroidetes bacteria in the 15N uptake (starting after 60 min of exposure, Fig 7). The heterocystous cyanobacteria revealed a high patchiness with few cells displaying prominent 13C uptake (Fig 5), but mostly did not show obvious uptake of either 13C or 15N (Fig 7, Table 1). Pooling the different species (for bacteria groups) into the functional groups heterocystous cyanobacteria (Aphanizomenon sp., Dolichospermum sp., and Nodularia sp.), non-heterocystous cyanobacteria (Pseudanabaena sp.), and associated bacteria (Alphaproteo- and Cytophaga/Bacteroidetes bacteria), and plotting of the 13C/12C and 15N/14N ratios against time, revealed specific tasks of the functional groups (Fig 7, Table 2). The associated bacteria predominantly display enhanced 15N/14N ratios, with the highest ratios after 6 h incubation, whereas non-heterocystous cyanobacteria reveal the highest 13C/12C ratios with a time dependent increase. Controversially, only few heterocystous cyanobacteria show increased 13C/12C and/or 15N/14N ratios (Fig 7).
Fig 7. 13C/12C (z axis) and 15N/14N (y axis) ratios plotted against the exposure time (log transformed x axis) for the different functional groups (heterocystous cyanobacteria, non-heterocystous cyanobacteria, associated bacteria).
The color and symbol legend is given directly in the figure.
Table 2. Regression analyses of 13C over 15N uptake for the functional groups heterocystous cyanobacteria (Aphanizomenon sp., Dolichospermum sp., Nodularia sp.), associated bacteria (Alphaproteo and Cytophaga/Bacteroidetes bacteria), and non-heterocystous cyanobacteria (Pseudanabaena sp.) for the different incubation times.
| Incubation time | 10 min | 30 min | 60 min | 6 h | 24 h |
|---|---|---|---|---|---|
| Heterocystous cyanobacteria | Y = 0.001+0.246x, R2 = 0.88, p = 0.000 |
Y = 0.004–0.024x, R2 = 0.00, p = 0.308 |
Y = 0.001+0.03x, R2 = 0.16, p = 0.000 |
Y = 0.002+0.149x, R2 = 0.82, p = 0.000 |
Y = 0.00+0.01x, R2 = 0.56, p = 0.000 |
| Associated bacteria | Y = 0.004+0.018x, R2 = 0.02, p = 0.471 |
Y = 0.003+0.105x, R2 = 0.45, p = 0.06 |
Y = -0.02+1.94x, R2 = 0.52, p = 0.000 |
Y = 0.004+0.329x, R2 = 0.4, p = 0.007 |
Y = 0.015–0.022x, R2 = 0.24, p = 0.02 |
| Non-heterocystous cyanobacteria | Y = 0.004+0.028x, R2 = 0.04, p = 0.169 |
Y = 0.003+0.105x, R2 = 0.28, p = 0.02 |
Y = 0.004+0.05x, R2 = 0.72, p = 0.001 |
Y = 0.003+0.117x, R2 = 0.82, p = 0.000 |
Y = 0.009–0.002x, R2 = 0.02, p = 0.27 |
| Anova | F = 2.77, p = 0.066 | F = 5.69, p = 0.001 | F = 12.9, p = 0.000 | F = 60.42, p = 0.000 | F = 9.79, p = 0.000 |
Anova results display comparisons of regression slopes of the different functional groups (ANCOVAs with and without interactions between the factor (functional group) and the co-variable (15N uptake) were calculated with linear models, with 13C uptake set as dependent variable. ANOVAs were then calculated between both ANCOVAs to test for differences in the regression slopes).
Group specific behavior was corroborated by significantly different slopes between the functional groups in regression analyses of the 13C over 15N uptake for the different exposure times, despite the fact that significant correlations between 13C and 15N uptake occurred for all groups (Table 2). From 60 min of exposure onwards, the slopes of the associated bacteria are by far the steepest, corresponding to a predominant incorporation of 15N, whereas non-heterocystous cyanobacteria reveal flat slopes accompanying predominant incorporation of 13C (Table 2).
Discussion
The present study determined the specific contribution of four different cyanobacterial species and the two most abundant associated bacterial groups in carbon as well as nitrogen fixation and cycling in late stage cyanobacterial bloom associations. Altogether, the cyanobacterium Pseudanabaena spp. dominated the carbon assimilation as well as nitrogen fixation at the early time-points, and the associated Alphaproteo- and Cytophaga/Bacteroidetes bacteria the nitrogen cycling and possibly N2 fixation at the later time-points. The filamentous, heterocystous cyanobacteria Nodularia sp., Dolichospermum sp., and Aphanizomenon sp. on the other hand, either showed no or erratic carbon and nitrogen uptake. Among the associated heterotrophic bacteria Cytophaga/Bacteroidetes were more active in the carbon cycling, whereas Alphaproteobacteria revealed higher activity in nitrogen cycling. However, high intra-species variability was observed in all examined species, which partly impeded significant differences in isotope uptake between species and time points.
Bi-carbonate uptake of cyanobacteria and associated heterotrophic bacteria
Surprisingly, Pseudanabaena sp. and not the heterocystous cyanobacteria was the most prominent species in carbon assimilation (Fig 5), with fixation rates up to 2.55 nmol C h-1 l-1. Indeed, carbon fixation rates of Pseudanabaena sp. were much higher than the rates for the heterocystous species Aphanizomenon sp., Dolichospermum sp., and Nodularia sp. together (Table 1, exception after 10 min of incubation due to 3 extraordinary high measurements in Nodularia sp.). However, in combined measurements of June, July and August in the preceding seasons 2012 and 2013, the three heterocystous species together accounted for ca. 5–250 nmol C h-1 l-1 [14]. Thus, the heterocystous cyanobacteria still hold key roles in carbon fixation in the Baltic Sea [14,41], with much higher fixation rates compared to the estimated ones of Pseudanabaena sp. in the present study. In our case, the appearance of the bloom and the curly phenotype of Nodularia sp. suggested a late stage of the bloom (Fig 4), and the low activity of Aphanizomenon sp., Dolichospermum sp., and Nodularia sp. cells might be attributed to inactive cells at the late bloom stage. Pseudanabaena sp. was still active and may be adapted to this situation where phosphorus supply by degrading blooms may be granted.
Measurements of heterotrophic bacteria at the later incubation times also revealed enhanced 13C/12C ratios (Fig 5), and heterotrophic bacteria may also incorporate bi-carbonate [42]. However, the 13C signal in heterotrophic bacteria arises after 6 h incubation which may be related to recycled organic carbon released by Pseudanabaena sp. and other cells. The higher proportion of Cytophaga/Bacteroidetes bacteria in the incorporation of 13C compared to Alphaproteobacteria (Fig 5) fits the current knowledge on their ecology: Marine Cytophaga/Bacteroidetes are specialized in the degradation of high molecular weight compounds [43–45], which are especially exuded in high quantities in late stage and senescent blooms [46–48]. Alphaproteobacteria on the other hand preferentially use low molecular weight compounds such as amino acids [44] and may act complementary to Bacteroidetes/Cytophaga in cyanobacterial bloom associations [43]. Thus, the higher 13C incorporation in Cytophaga/Bacteroidetes bacteria may display the recycling of complex organic material whereas the lower signal in the Alphaproteobacteria account for the incorporation of low molecular weight exudates.
15N2 uptake of cyanobacteria and associated heterotrophic bacteria
Pseudanabaena sp. showed 15N2 incorporation after 30 min of incubation, and was the only species with significantly increased 15N/14N ratios at this time. Further, it was the species with the highest 15N/14N ratios after 60 min of incubation (Fig 6). Until now, the non-heterocystous Pseudanabaena sp. was not shown to be involved in fixation of atmospheric nitrogen in the Baltic Sea [13,14], despite the presence of nitrogenase genes [15]. However, picocyanobacteria and non-heterocystous filamentous species were suspected for nitrogen fixation under specific conditions before [14]. Taking into account that Pseudanabaena sp. was the only species with increased 15N/14N ratios at the early sampling points, our data suggest an active N2 fixation by Pseudanabaena sp., with fixation rates between 0.17 and 1.49 nmol N h-1 l-1 (Table 1). Thus, at this late stage of the bloom, Pseudanabaena sp. might have been responsible for the input of reactive nitrogen in the multi-species associations and ultimately into the nitrogen cycle of the Baltic Sea. Indeed, if converted to per cell rates, nitrogen fixation of Pseudanabaena sp. appears low with up to 0.07 fmol N cell -1 h-1 if compared to the heterocystous species Nodularia spumigena (11 fmol N cell-1 h-1, [18]) and Aphanizomenon sp. (1–11 fmol N cell-1 h-1, [7]). However, this difference might be attributed to the much smaller cell size of Pseudanabaena sp., and compensated by higher cell numbers. In a comparable study of a Baltic Sea cyanobacterial bloom, cumulative fixation rates for combined measurements of June, July and August of the heterocystous species Dolichospermum sp., Nodularia sp., and Aphanizomenon sp. were determined with ca. 0.5–80 nmol N l-1 h-1 [14], i.e. approximately one order of magnitude above that of Pseudanabaena sp. alone in the present study. Likewise to the carbon fixation, the inexistent nitrogen fixation of the heterocystous species in our study may be attributed to different stages of the blooms, with most cells of heterocystous species being inactive at the late stage of the bloom (Figs 5 and 6). Consistent with these results, early/mid-summer nitrogen fixation rates in the Baltic Sea were up to 30 times higher compared to late summer [8]. Thus, heterocystous cyanobacteria may still be the prime nitrogen fixers in the Baltic Sea [5,6], but the possible participation of Pseudanabaena spp. should not be neglected. If this temporal divided nitrogen fixation between different cyanobacterial species represents a general feature for the Baltic Sea needs to be investigated in consecutive studies.
The overall highest 15N/14N ratios by the associated bacteria after 6 and 24 h of exposure are surprising, taking the high abundance of diazotrophic cyanobacteria and the low 15N incorporation of the hosts into account. Indeed, one would expect the converse role allocation, where associated heterotrophic bacteria reveal lower 15N/14N ratios than their diazotrophic hosts [18,49]. However, our high 15N/14N ratios were obtained after 6 and 24 h of incubation, and thus, similar to the 13C incorporation, the associated bacteria may have used recycled nitrogen that was originally fixed by cyanobacteria. Supporting this assumption, heterotrophic microorganisms in cyanobacterial associations dominated by Aphanizomenon sp. relied on recycled nitrogen [49], and Aphanizomenon sp. was shown to release up to 35% of the fixed nitrogen as NH4+ [7]. However, direct cell to cell transmission between hosts and associated bacteria was not indicated (see 3.2 and the linear models), and release and transfer of newly fixed N2 was not indicated at a similar experiment during 12 h of incubation [14].
The role of heterotrophic bacteria in nitrogen fixation budgets for aquatic ecosystem were recently brought into focus [17,50], and might have been underestimated in preceding studies [51–53]. As examples, heterotrophic organisms dominated the nitrogen fixation in the South Pacific Gyre [52], and were also the principle nitrogen fixing organisms in a Baltic Sea estuary [17]. Indeed, there are hints that the associated bacteria in our study also performed nitrogen fixation themselves and not only used nitrogen released from other cells: First, if the associated bacteria would only recycle nitrogen that was fixed by other organisms, one would expect a dilution in the 15N/14N ratios from the primary fixer to the secondary user [8,14,49], which is not the case (Figs 6 and 7). Second, already after 30 min heterotrophic bacterial cells possessed the overall highest 15N/14N ratios (Fig 7), and this fast incorporation indicates active nitrogen fixation. Third, many Alphaproteobacteria [16,17,53] and Cytophaga/Bacteroidetes bacteria [54] possess nitrogenase genes, and are capable of nitrogen fixation. To validate heterotrophic nitrogen fixation we performed a gene functional analysis with the 16S data of the associated bacteria using paprica—PAthway PRediction by phylogenetIC plAcement [55]. In this analysis, however, only 1.2% of the associated bacteria yielded the full pathway (via ferredoxin) for nitrogen fixation (S5 Table) which does not support our assumption. Nevertheless, ecosystem key functions may be performed by low abundant bacteria [56,57], and the per cell fixation rates of the associated bacteria were more than one dimension higher compared to Pseudanabaena sp. (1.2 vs 0.07 fmol N h-1 cell-1), and in the same dimension as uptake rates for the much bigger heterocystous cyanobacteria (0.1–32.7 fmol N cell-1 h-1) in the Baltic Sea [14]. Thus, given the high abundances of associated bacteria, heterotrophic nitrogen fixation might contribute significantly to bulk fixation at this late stage bloom. At this stage of the bloom, senescent phytoplankton exhibit high exudation and leaking rates (e.g. [46]), and create an environment with high levels of labile DOC that fuels heterotrophic nitrogen fixation [51,58,59]. This is corroborated with the linear models, where bacteria associated to inactive, senescent hosts showed the highest 15N uptake (S6 Table). However, until now prerequisites and regulation of heterotrophic nitrogen fixation as well as principle contradictions as fixation in oxygenated waters and at high nitrate and ammonium concentrations are poorly understood [51], and should move into the focus of upcoming studies.
Relation of 13C to 15N uptake
Significant correlations between 13C and 15N uptake occurred in most species and at most time points (Table 2), which is in accordance with similar studies from cyanobacterial blooms in the Baltic Sea (e.g. [14]). Nevertheless, relations between carbon and nitrogen uptake indicated specific tasks of functional groups (Fig 7, Table 2). Pseudanabaena sp. (non-hetercystous cyanobacterium) clearly dominated the 13C uptake (Fig 5) throughout the whole incubation period, but was also the first species with increased 15N signals (Fig 6). For the 15N/14N ratios, however, Pseudanabaena sp. was outpaced by the associated bacteria from 6 h incubation onwards (Fig 6), and revealed much lower per cell fixation rates (see above). Thus, the associated bacteria may have dominated the nitrogen cycling and possibly fixation at the later sampling points. This specification of functional groups was corroborated by significant different slopes in linear models calculated for correlations between 13C and 15N uptake (Table 2). The formation of distinct functional groups by different species in late stage bloom associations may ultimately result in the allocation of desired metabolic pathways to every member in the association, including members unable to perform these tasks [60,61]. The concerted action of diverse ecological functions by different functional groups was also proposed for a chlorophyte and its prokaryotic epiflora [62], and might be a general feature of multi-species associations.
Supporting information
Abiotic variables at the day of sampling at station TransA.
(DOCX)
A: Numbers of analysed cells per species/group as well as total analysed areas (which may contain different species/bacterial groups) for each incubation time. B: Numbers of analysed associated bacterial cells with the respective cyanobacterial host species for each incubation time.
(DOCX)
NanoSIMS analyses of 13C/12C and 15N/14N ratios for all measuring points and regions of interest (ROIs).
(XLSX)
ANOVAs and Post-Hoc analyses for differences in 13C and 15N uptake for the different species over time as well as differences in 13C and 15N uptake by the associated bacteria caused by different hosts.
(DOCX)
Outcome of the gene functional analysis with the 16S data of the associated bacteria using paprica—PAthway PRediction by phylogenetIC plAcement [55].
(XLSX)
13C/12C and 15N/14N ratios for host cells and associated bacterial cells.
(XLSX)
Acknowledgments
We thank Annett Grütmüller for NanoSIMS measurements, the field station Askö laboratory for provision of lab space and permission of experiments, Susanne Busch and Regina Hansen for phytoplankton counting, and Joe Montoya for the provision of the Excel Sheet for calculations of uptake rates.
Data Availability
The forward and reverse reads from the Illumina sequencing were deposited at the European Nucleotide Archive under the accession number PRJEB23316 (sample B15_3). All other data are in the paper and its Supporting Information files.
Funding Statement
This work was supported by Human Frontiers Science Program (RGP0020/2016) to MV. This work was also supported by the SIMS instrument was funded by the German Federal Ministry of Education and Research (BMBF), grant identifier 03F0626A to AV. And this work was also supported by Leibniz Association to FE and HS-V. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wasmund N. Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Int Rev der gesamten Hydrobiol und Hydrogr. 1997;82: 169–184. 10.1002/iroh.19970820205 [DOI] [Google Scholar]
- 2.Wasmund N, Voss M, Lochte K. Evidence of nitrogen fixation by non-heterocystous cyanobacteria in the Baltic Sea and re-calculation of a budget of nitrogen fixation. Mar Ecol Prog Ser. 2001;214: 1–14. 10.3354/meps214001 [DOI] [Google Scholar]
- 3.Stal LJ, Staal M, Villbrandt M. Nutrient control of cyanobacterial blooms in the Baltic Sea. Aquat Microb Ecol. 1999;18: 165–173. [Google Scholar]
- 4.Bianchi TS, Westman P, Rolff C. Cyanobacterial blooms in the Baltic Sea: Natural or human induced? Limnol Oceanogr. 2000;43: 716–726. [Google Scholar]
- 5.Stolte W, Balode M, Carlsson P, Grzebyk D, Janson S, Lips I, et al. Stimulation of nitrogen-fixing cyanobacteria in a Baltic Sea plankton community by land-derived organic matter or iron addition. Mar Ecol Prog Ser. 2006;327: 71–82. [Google Scholar]
- 6.Wasmund N, Nausch G, Schneider B, Nagel K, Voss M. Comparison of nitrogen fixation rates determined with different methods: a study in the Baltic Proper. Mar Ecol Prog Ser. 2005;297: 23–31. [Google Scholar]
- 7.Ploug H, Musat N, Adam B, Moraru CL, Lavik G, Vagner T, et al. Carbon and nitrogen fluxes associated with the cyanobacterium Aphanizomenon sp. in the Baltic Sea. ISME J. 2010;4: 1215–23. 10.1038/ismej.2010.53 [DOI] [PubMed] [Google Scholar]
- 8.Ohlendieck U, Stuhr A, Siegmund H. Nitrogen fixation by diazotrophic cyanobacteria in the Baltic Sea and transfer of the newly fixed nitrogen to picoplankton organisms. J Mar Syst. 2000;25: 213–219. 10.1016/S0924-7963(00)00016-6 [DOI] [Google Scholar]
- 9.Cole JJ. Interactions between bacteria and algae in aquatic ecosystems. Annu Rev Ecol Syst. 1982;13: 291–314. [Google Scholar]
- 10.Ramanan R, Kang Z, Kim B, Cho D, Jin L, Oh H, et al. Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. ALGAL. 2015;8: 140–144. 10.1016/j.algal.2015.02.003 [DOI] [Google Scholar]
- 11.Seymour JR, Amin SA, Raina J-B, Stocker R. Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat Microbiol. 2017;2: 17065 10.1038/nmicrobiol.2017.65 [DOI] [PubMed] [Google Scholar]
- 12.Walsby AE, Hayes PK, Boje R, Walsby AE, Hayes PK, Boje R. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur J Phycol. 1995;30: 87–94. 10.1080/09670269500650851 [DOI] [Google Scholar]
- 13.Bauersachs T, Schouten S, Compaore J, Wollenzien U, Stal LJ, Damste JSS. Nitrogen isotopic fractionation associated with growth on dinitrogen gas and nitrate by cyanobacteria. Limnol Oceanogr. 2009;54: 1403–1411. [Google Scholar]
- 14.Klawonn I, Nahar N, Walve J, Andersson B. Cell-specific nitrogen- and carbon-fixation of cyanobacteria in a temperate marine system (Baltic Sea). Environ Microbiol. 2016;18: 4596–4609. 10.1111/1462-2920.13557 [DOI] [PubMed] [Google Scholar]
- 15.Acinas SG, Haverkamp THA, Huisman J, Stal LJ. Phenotypic and genetic diversification of Pseudanabaena spp. (cyanobacteria). ISME J. 2009;3: 31–46. 10.1038/ismej.2008.78 [DOI] [PubMed] [Google Scholar]
- 16.Farnelid H, Bentzon-Tilia M, Andersson AF, Bertilsson S, Jost G, Labrenz M, et al. Active nitrogen-fixing heterotrophic bacteria at and below the chemocline of the central Baltic Sea. ISME J. 2013;7: 1413–23. 10.1038/ismej.2013.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentzon-Tilia M, Traving SJ, Mantikci M, Knudsen-Leerbeck H, Hansen JLS, Markager S, et al. Significant N2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J. 2015;9: 273–285. 10.1038/ismej.2014.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploug H, Adam B, Musat N, Kalvelage T, Lavik G, Wolf-Gladrow D, et al. Carbon, nitrogen and O(2) fluxes associated with the cyanobacterium Nodularia spumigena in the Baltic Sea. ISME J. 2011;5: 1549–58. 10.1038/ismej.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillard R. Culture of phytoplankton for feeding marine invertebrates In: Smith W, Chanley M, editors. Cultur of Marine Invertebrate Animals; 1975. pp. 26–60. [Google Scholar]
- 20.Willén T. Studies on the phytoplankton of some lakes connected with or recently isolated from the Baltic. Oikos. 1962;13: 169–199. 10.2307/3565084 [DOI] [Google Scholar]
- 21.Weinbauer MG, Fritz I, Wenderoth DF, Höfle MG. Simultaneous extraction from bacterioplankton of total RNA and DNA suitable for quantitative structure and function analyses. Appl Environ Microbiol. 2002;68: 1082–1087. 10.1128/AEM.68.3.1082-1087.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herlemann DP, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011;5: 1571–9. 10.1038/ismej.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eigemann F, Schulz-Vogt HN. Stable and labile associations of microorganisms with the cyanobacterium Nodularia spumigena. Aquat Microb Ecol. 2019; 3354 10.3354/ame01918 [DOI] [Google Scholar]
- 24.Glöckner FO, Fuchs BM, Glo FO, Amann R. Bacterioplankton compositions of lakes and oceans: a first comparison based on Fluorescence In Situ Hybridization. Appl Environ Microbiol. 1999;65: 3721–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acinas SG, Ferrera I, Sarmento H, Díez-Vives C, Forn I, Ruiz-González C, et al. Validation of a new catalysed reporter deposition-fluorescence in situ hybridization probe for the accurate quantification of marine Bacteroidetes populations. Environ Microbiol. 2015;17: 3557–3569. 10.1111/1462-2920.12517 [DOI] [PubMed] [Google Scholar]
- 26.Pernthaler A, Pernthaler J, Amann R. Fluorescence In Situ Hybridization and Catalyzed Reporter Deposition for the identification of marine bacteria. Appl Env Microbiol. 2002;68: 3094–3101. 10.1128/AEM.68.6.3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musat N, Foster R, Vagner T, Adam B, Kuypers MMM. Detecting metabolic activities in single cells, with emphasis on nanoSIMS. FEMS Microbiol Rev. 2012;36: 486–511. 10.1111/j.1574-6976.2011.00303.x [DOI] [PubMed] [Google Scholar]
- 28.Polerecky L, Adam B, Milucka J, Musat N, Vagner T, Kuypers MMM. Look@NanoSIMS—a tool for the analysis of nanoSIMS data in environmental microbiology. Environ Microbiol. 2012;14: 1009–1023. 10.1111/j.1462-2920.2011.02681.x [DOI] [PubMed] [Google Scholar]
- 29.Montoya JP, Voss M, Kahler P, Capone DG. A simple, high-precision, high-sensitivity tracer assay for N(2) fixation. Appl Environ Microbiol. 1996;62: 986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter J. New measurements of oxygen solubility in pure and natural water. Limnol Oceanogr. 1966;11: 264–277. [Google Scholar]
- 31.Weiss R. The solubility of nitrogen, oxygen and argon in water and seawater. Deep Res. 1970;17: 721–735. [Google Scholar]
- 32.Sveden JS, Adam B, Walve J, Nahar N, Sved JB, Musat N, et al. High cell-specific rates of nitrogen and carbon fixation by the cyanobacterium Aphanizomenon sp. at low temperatures in the Baltic Sea. FEMS Microbiol Ecol. 2015;91: 1–10. 10.1093/femsec/fiv131 [DOI] [PubMed] [Google Scholar]
- 33.Heinänen AP. Bacterial numbers, biomass and productivity in the Baltic Sea: a cruise study. Mar Ecol Prog Ser. 1991;70: 283–290. [Google Scholar]
- 34.Kroer N, Jorgensen NOG, Coffin RB. Utilization of dissolved nitrogen by heterotrophic bacterioplankton: A comparison of three ecosystems. Appl Environ Microbiol. 1994;60: 4116–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr W, Grosskopf T, Wallace DWR, LaRoche J. Methodological underestimation of oceanic nitrogen fixation rates. PLoS One. 2010;5: e12583 10.1371/journal.pone.0012583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wannicke N, Benavides M, Dalsgaard T, Dippner JW, Montoya JP, Voss M. New perspectives on nitrogen fixation measurements using 15N2 gas. Front Mar Sci. 2018;5: 1–10. 10.3389/fmars.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Development Core Team. R: A language and environment for statistical computing. 2008. Available: http://www.r-project.org/
- 38.RStudio Team. RStudio: Integrated Development for R. Boston, MA URL http//www.rstudio.com/. 2015.
- 39.De Mendiburu F. Una herramienta de analisis estadistico para la investigacion agricola. 2009. [Google Scholar]
- 40.Oksanen J, Blanchet GF, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community ecology package. 2019. [Google Scholar]
- 41.Stal LJ, Albertano P, Bergman B, Von Bröckel K, Gallon JR, Hayes PK, et al. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—Responses to a changing environment. Cont Shelf Res. 2003;23: 1695–1714. 10.1016/j.csr.2003.06.001 [DOI] [Google Scholar]
- 42.Hesselsoe M, Nielsen JL, Roslev P, Nielsen PH. Isotope labeling and microautoradiography of active heterotrophic bacteria on the basis of assimilation of 14CO2. Appl Environ Microbiol. 2005;71: 646–655. 10.1128/AEM.71.2.646-655.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández-Gómez B, Richter M, Schüler M, Pinhassi J, Acinas SG, González JM, et al. Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J. 2013;7: 1026–1037. 10.1038/ismej.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirchman DL. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol Ecol. 2002;39: 91–100. 10.1111/j.1574-6941.2002.tb00910.x [DOI] [PubMed] [Google Scholar]
- 45.Alonso C, Musat N, Adam B, Kuypers M, Amann R. HISH-SIMS analysis of bacterial uptake of algal-derived carbon in the Rio de la Plata estuary. Syst Appl Microbiol. 2012;35: 541–548. 10.1016/j.syapm.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 46.Mühlenbruch M, Grossart H-P, Eigemann F, Voss M. Phytoplankton-derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria. Environ Microbiol. 2018;20: 2671–2685. 10.1111/1462-2920.14302 [DOI] [PubMed] [Google Scholar]
- 47.Seymour JR, Ahmed T, Stocker R. Bacterial chemotaxis towards the extracellular products of the toxic phytoplankton Heterosigma akashiwo. J Plankton Res. 2009;31: 1557–1561. 10.1093/plankt/fbp093 [DOI] [Google Scholar]
- 48.Pinhassi J, Havskum H, Peters F, Malits A. Changes in bacterioplankton composition under different phytoplankton regimens. Appl Environ Microbiol. 2004;70: 6753–6766. 10.1128/AEM.70.11.6753-6766.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adam B, Klawonn I, Svedén JB, Bergkvist J, Nahar N, Walve J, et al. N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community. ISME J. 2016;10: 450–459. 10.1038/ismej.2015.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farnelid HM, Turk-Kubo KA, Zehr JP. Identification of associations between bacterioplankton and photosynthetic picoeukaryotes in coastal waters. Front Microbiol. 2016;7: 1–16. 10.3389/fmicb.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bombar D, Paerl RW, Riemann L. Marine non-cyanobacterial diazotrophs: Moving beyond molecular detection. Trends Microbiol. 2016;24: 916–927. 10.1016/j.tim.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 52.Halm H, Lam P, Ferdelman TG, Lavik G, Dittmar T, Laroche J, et al. Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. ISME J. 2012; 1238–1249. 10.1038/ismej.2011.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delmont TO, Quince C, Shaiber A, Esen ÖC, Lee ST, Rappé MS, et al. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat Microbiol. 2018;3: 804–813. 10.1038/s41564-018-0176-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue J, Oshima K, Suda W, Sakamoto M, Iino T, Noda S, et al. Distribution and evolution of nitrogen fixation genes in the phylum Bacteroidetes. Microbes Environ. 2015;30: 44–50. 10.1264/jsme2.ME14142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowman JS, Ducklow HW. Microbial communities can be described by metabolic structure: A general framework and application to a seasonally variable, depth-stratified microbial community from the coastal West Antarctic Peninsula. PLoS One. 2015; 1–18. 10.1371/journal.pone.0135868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawson W, Hör J, Egert M, Kleunen M Van, Pester M. A small number of low-abundance bacteria dominate plant species-specific responses during rhizosphere colonization. Front Microbiol. 2017;8: 1–13. 10.3389/fmicb.2017.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benjamino J, Lincoln S, Srivastava R, Graf J. Low-abundant bacteria drive compositional changes in the gut microbiota after dietary alteration. Microbiome. 2018; 1–13. 10.1186/s40168-017-0383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonnet S, Dekaezemacker J, Turk-kubo KA, Moutin T, Hamersley RM, Grosso O, et al. Aphotic N2 fixation in the eastern tropical South Pacific Ocean. PLos One. 2013;8: 1–14. 10.1371/journal.pone.0081265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moisander PH, Steppe TF, Hall NS, Kuparinen J, Paerl HW. Variability in nitrogen and phosphorus limitation for Baltic Sea phytoplankton during nitrogen-fixing cyanobacterial blooms. Mar Ecol Pro Ser. 2003;262: 81–95. [Google Scholar]
- 60.Graham LE, Knack JJ, Piotrowski MJ, Wilcox LW, Cook ME, Wellman CH, et al. Lacustrine Nostoc (Nostocales) and associated microbiome generate a new type of modern clotted microbialite. J Phycol. 2014;291: 280–291. 10.1111/jpy.12152 [DOI] [PubMed] [Google Scholar]
- 61.Frischkorn KR, Haley ST, Dyhrman ST. Coordinated gene expression between Trichodesmium and its microbiome over day-night cycles in the North Pacific Subtropical Gyre. ISME J. 2018;12: 997–1007. 10.1038/s41396-017-0041-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zulkifly S, Hanshew A, Young EB, Lee P, Graham ME, Graham ME, et al. The epiphytic microbiota of the globally widespread macroalga Cladophora glomerata (Chlorophyta, Cladophorales). Am J Bot. 2012;99: 1541–1552. 10.3732/ajb.1200161 [DOI] [PubMed] [Google Scholar]