Abstract
TWIK related K+ channel (TREK-1) is a mechano- and anesthetic sensitive channel that when activated attenuates pain and causes anesthesia. Recently the enzyme phospholipase D2 (PLD2) was shown to bind to the channel and generate a local high concentration of phosphatidic acid (PA), an anionic signaling lipid that gates TREK-1. In a biological membrane, the cell harnesses lipid heterogeneity (lipid compartments) to control gating of TREK-1 using palmitate-mediated localization of PLD2. Here we discuss the ability of mechanical force and anesthetics to disrupt palmitate-mediated localization of PLD2 giving rise to TREK-1’s mechano- and anesthetic-sensitive properties. The likely consequences of this indirect lipid-based mechanism of activation are discussed in terms of a putative model for excitatory and inhibitory mechano-effectors and anesthetic sensitive ion channels in a biological context. Lastly, we discuss the ability of locally generated PA to reach mM concentrations near TREK-1 and the biophysics of localized signaling. Palmitate-mediated localization of PLD2 emerges as a central control mechanism of TREK-1 responding to mechanical force and anesthetic action.
Keywords: anesthesia, mechanosensation, rafts, palmitoylation, super resolution microscopy, lipid
Introduction:
TWIK related K+ channel (TREK-1) is a mechano- and anesthetic-sensitive member of the two-pore-domain potassium (K2P) family of channels [1]. Inhaled anesthetics (e.g. isoflurane, chloroform, diethyl ether) activate TREK-1 and genetic deletion of TREK-1 decreases anesthesia sensitivity in mice [2–4]. Mechanical force also activates TREK-1 [5–9], making TREK-1 an inhibitory mechanosensitive channel (i.e. attenuates mechanosensation) and a permissive anesthetic channel (i.e. facilitates anesthesia).
Initial theories for TREK-1 activation by anesthetics and force assumed distinct direct interactions [10–12]. This assumption was based on the widely accepted theory that anesthetics bind directly to the transmembrane domains of channels [13–15], and it is consistent with computational modeling of anesthetics [3,16] to TREK-1. Additionally, the ‘force from lipid’ [17] mechanism (which requires changes in the membrane to directly lead to channel gating) was assumed to primarily regulate force in cellular membranes based on force induced opening of TREK-1 in purified lipid vesicles [10,11].
Recently indirect (non-canonical) mechanisms for force transduction have emerged based on disruption of protein-protein and protein-lipid interaction[18–20]. For example cells sense disassembly of caveolae and disruption of G-protein interactions [19,20]. Another mechanisms relies on palmitate mediated sequestration of the enzyme phospholipase D2 (PLD2) with monosialotetrahexosylganglioside1 (GM1) lipids (sometimes called lipid rafts or lipid domains) away from its substrate [18,21]. Disruption of lipids by mechanical force disrupts PLD2 localization and leads to activation. This PLD2-based mechanosensation was found to be the tension sensor for endocytosis [22] and appears to function upstream of force transduction through mTOR signaling [23]. PLD2 is also important for TREK-1 since PLD2 activates the channel by local production of the signaling lipid phosphatidic acid (PA)[24]. And similar to endocytosis and mTOR, this has led to a two-step mechanism for TREK-1 mechanosensation in a biological membrane [25].
Anesthetics also disrupt ordered membranes [26–29] and shift their melting temperature [30–33]. Recently we showed inhaled anesthetics activate PLD2 by disruption of palmitate-mediated localization and substrate presentation giving rise to anesthetic sensitivity in TREK-1 [28] (preprint).
This review discusses the evidence for palmitate-based clustering and its similarities and differences between mechanical force and anesthetics in TREK-1 activation. We contrast the induced de-clustering of PLD2 and its causative effect on TREK-1 channel activation through lipid binding and competition [34,35]. The molecular underpinnings of the two-step membrane mechanism discussed here for TREK-1 is conceptually very distinct from canonical models of ion channel activation. Much work needs to be done to fully appreciate its extent in other biological systems. The review starts by summarizing recent advancements in lipid domains and their organization of select proteins. Raft disruption and palmitate-mediated localization is then discussed as a regulator of PLD2 and the central pathway for membrane-mediated activation of TREK-1 by anesthetics and mechanical force. Challenges for establishing localization-based mechanisms conclude the review. Details on TREK-1 specific cellular responses to anesthetics and mechanical force are only briefly discussed as they have been reviewed extensively elsewhere; see [2,36,37] for details on canonical TREK-1 anesthesia and [38] for canonical mechanosensation.
Lipid domains and cholesterol-induced palmitoyl de-clustering.
The membrane is a heterogenous mixture of fluid and gel-like lipids densely packed with proteins [39,40]. Proteins, including cytoskeletal proteins, compartmentalize the membrane and restrict lateral diffusion [41–44]. Membrane regions enriched in a particular type of lipid are called lipid domains, clusters, or rafts and they are also thought to reduce lateral movement [40,43]. The best characterized domains contain sphingomyelin (GM1) and are functionally modulated by cholesterol [21,45].
Cells contain a second class of lipid heterogeneity comprised of the anionic lipids phosphatidyl inositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5 triphosphate (PIP3). Early studies in detergent resistant membranes found PIP2 associated with GM1 lipids [46–48]. However, mass spectrometry showed PIP2 is mostly unsaturated [49–51] which is inconsistent with GM1 localization [52]. And recent development of super resolution technology [21] (e.g., direct stochastical optical reconstruction microscopy (dSTORM) and stimulated emission depletion (STED)[53–56])) found PIP2 and PIP3 cluster separate from GM1 lipids [57–59] and from each other [18,60,61]. For all raft types, the imaging limited their putative size to less than 100 nm [62]; however, the exact composition of lipid clusters, in particular their exact size and transient existence, are still heavily debated [40,63].
Palmitoylation is a post translational modification that covalently attaches a saturated lipid (e.g., a 16 carbon palmitate) to a protein [64]. Among other roles, palmitoylation is the primary signal that traffics and targets proteins to lipid rafts [65–67]. Numerous channels and regulatory proteins are palmitoylated [68–70] including PLD2. PLD2 is palmitoylated at cysteines near its pleckstrin homology (PH) domain, and these palmitoylations are confirmed to localize PLD2 to lipid rafts [71–74]. The PH domain also binds PIP2 which opposes the localization by palmitoylation [21,75]. Absent substrate, sequestration to the GM1-enriched domain renders PLD2 inactive [18]. This process by which a palmitoylated enzyme is sequestered to a GM1 domain and then released to its substrate is termed ‘substrate presentation’ [18].
Super resolution imaging shows that removal of cholesterol with methyl-β-cyclodextrin (MβCD) disrupts clustering of cholera toxin B (CTxB) labeled GM1 lipids in a cellular membrane [18,63]. The role of lipid raft disruption has been widely studied in particular for immune cell regulation [40,76–81]. In C2C12 myoblastoma muscle cells, disruption was identified as a decrease in both cluster size and number [18]. The result is complicated by a technical difficulty to quantify a cluster size and the fact that CTxB is pentadentate (binds five GM1 lipids) resulting in CTxB potentially inducing artificial clustering (see below for a detailed discussion on the caveats of super resolution imaging)[63]. Nonetheless, a change in size is an indicator of some type of perturbation in the biological membrane.
Cholesterol-dependent de-clustering of GM1 lipids is consistent with de-clustering of PLD2 [76]. In C2C12 cells and in neuroblastoma 2a (N2a) cells, super resolution imaging showed that cholesterol depletion with MβCD led to translocation of PLD2 out of GM1 lipids [18,28]. The PLD2 then translocated to PIP2 lipids near its substrate phosphatidylcholine (PC) [18]. A live cell PLD2 assay confirmed the translocation causes PC hydrolyzes, further establishing a mechanism of substrate presentation for its enhanced activity [18]. PLD2 activation by substrate presentation is further supported by the observation that PLD2 products are unsaturated [82]. Unsaturated lipids reside in the disordered region with PIP2, not GM1 domains, so unsaturated PC is hydrolyzed by its localization with unsaturated PIP2. PLD2 is constitutively active in vivo and invitro [83], suggesting the many other signaling molecules that regulate PLD2 [84] may also work by enhancing translocation and substrate presentation. For example, binding of a prenylated regulatory factor would alter PLD2 localization and increase access to substrate.
Similar to cholesterol depletion, shear force de-localizes PLD2 with GM-1 domains [18,25]. Shear force (3 dynes/cm2) applied to a cell membrane caused PLD2 to translocate from GM1 to PIP2 domains where the enzyme mixed with its substrate phosphatidylcholine (PC) to produce PA [25], very similar to the process of cholesterol depletion[25]. In addition to binding PIP2, PLD2 also binds directly to the C-terminus of TREK-1 and activates the channel through local production of phosphatidic acid [24,35], presumably in the disordered region of the cell membrane [18]. This is significant since previous work also implicated TREK-1 C-terminus in mechanosensitivity [85].
Similar to mechanical force, super resolution imaging shows inhaled anesthetics disrupt lipid rafts. Inhaled anesthetics are a chemically diverse collection of compounds [86]. In a live cell PLD2 assay, activation was pronounced across all classes of inhaled anesthetics tested (diethyl ether, chloroform, isoflurane, and xenon) [28]. Consistent with this result and substrate presentation, anesthetics caused a pronounced translocation of PLD2 out of GM1 domains almost identical to mechanical force [28,87]. However, the effect of anesthetics to GM1 domain structure is opposite from mechanical force, anesthetics increased the size and number of GM1 domains; force reduces the size and number. Hence the size of GM1 domain structure is not the key intermediary for anesthesia activation of PLD2. Rather palmitate-mediated localization of the enzyme is the key intermediary [28].
Shared molecular pathway of force and anesthesia signaling to TREK-1.
Palmitate-mediated localization is the first step central to both anesthetic and force activation of TREK-1. This was further established using electrophysiology experiments. The addition of a catalytically dead PLD2 mutant completely blocked the effects of force and anesthetic to TREK-1 channels [25,28]. Importantly the anesthetic sensitivity could be transferred to an anesthetic insensitive channel TRAAK by transferring the PLD2 binding site to TRAAK.
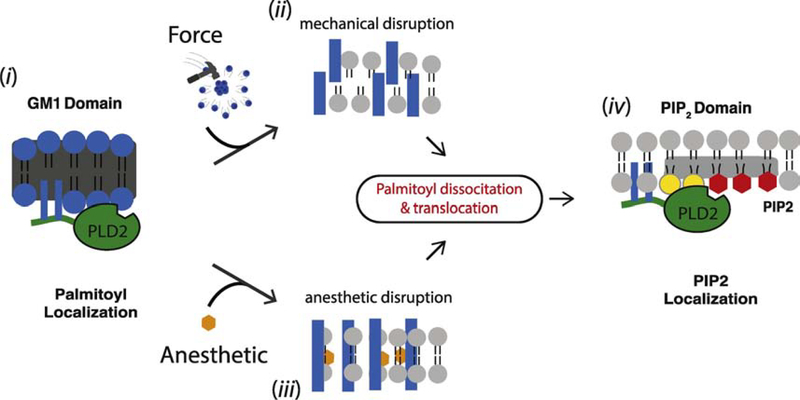
Figure 1 shows a cartoon representation of the two PLD2 pathways (anesthetic and mechanical) for TREK-1 activation. First anesthetic or force perturb GM1 domain structure. For mechanosensation the rafts are degraded; for anesthetics the rafts remain intact and expand. In the second step, regardless of its structural state, the perturbations lead to identical disruption of palmitate-mediated localization and PLD2 translocation. The size and number of GM1 domains are either of no consequence to PLD2 mediated anesthesia and force transduction, or their contribution is insignificant compared to palmitate-mediated localization of PLD2.
Figure 1. Palmitate-mediated localization, a central component of TREK-1 mechanosensation and anesthesia.
(i) Phospholipase D2 (PLD2, green) is shown with two palmitoylation sites that localize the enzyme to a GM1 lipids (blue spheres). (ii) Mechanical force disrupts lipid domains resulting in smaller dispersed domains within the plasma membrane (indicated by disordered blue bars). (iii) Anesthetics (orange hexagon) disrupt lipid domains causing them to expand and become larger while remaining relatively ordered (indicated by intact blue rectangles). Both types of raft disruption perturb palmitate mediated localization of PLD2 to GM1 lipids resulting in PLD2 translocation, PLD2 binding to PIP2 lipids (red hexagon), substrate (phosphatidylcholine, grey) presentation, and phosphatidic acid (PA, yellow) production. PA then activates TREK-1 (not shown). The horizontal grey rectangle indicates clustered lipids on the inner leaflet (PIP2 domain).
The molecular basis as to why both disruption (mechanical force) and expansion (anesthetics) lead to PLD2 translocation is unclear. Among the more probable reasons include that the density changes or high-order packing energetics are being perturbed by the addition of these compounds and force. The expansion of rafts by anesthetics is consistent with studies in artificial membranes—anesthetics expand artificial membranes [29,88]. The requirement for PLD2 to translocate does not rule out anesthetics indirectly affecting PLD2 or TREK-1 through a lipid independent pathway, e.g. phosphorylation, or direct anesthetic binding, but these other pathways appear secondary to PLD2 translocation. Further study is needed to better explain the biophysical changes which lead to PLD translocation.
Local anesthetics also disrupt palmitate-mediated localization of PLD2 to GM1 domains [87], yet they inhibit TREK-1 [89]. Surprisingly the mechanism of inhibition was shown to be primarily through PLD2. Local anesthetics directly bind to and potently inhibit PLD2 [87]. Hence PLD2 translocates to its substrate and binds to the C-terminus of TREK-1, but the direct inhibition of the enzymes blocks PA production leading to TREK-1 inactivation [87]. This showed for the first time how the disordered C-terminus could regulate a channel despite having no structure or a hydrophobic binding site suitable for an anesthetic.
Lipid signaling and inhibitory and excitatory mechano- and anesthetic-effectors.
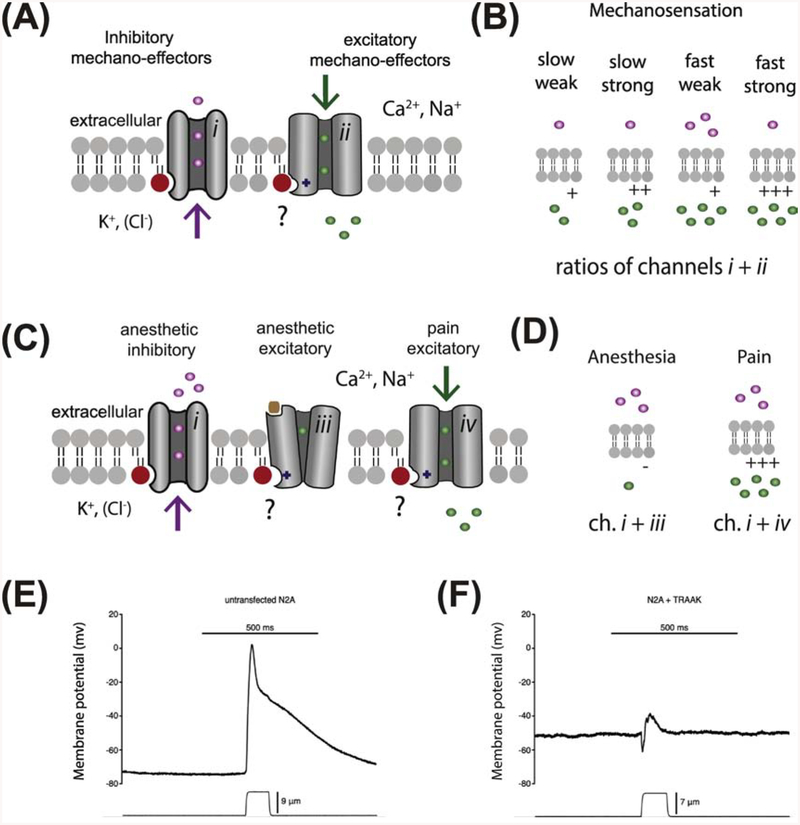
The shared mechano- and anesthetic-pathway for TREK-1 activation yields potentially novel insights into how this process could generate a physiological response (see Fig 2A–D). TREK-1 is an inhibitory mechanosensor or an inhibitory effector of mechanical force. When activated, TREK-1 inhibits neuronal firing by hyperpolarizing the cell (Figure 2A, channel type i) [90]. Combining an inhibitory mechanosensor with an excitatory mechanosensor (Figure 2A, channel type ii) would attenuate the signal depending on the ratio of inhibitory to excitatory mechano-effectors. This principle was shown experimentally by Brohawn et al. by overexpressing TRAAK, a mechanosensitive homolog of TREK-1, with endogenously expressed Piezo in neuroblastoma N2a cells [11]. Mechanical activation of over-expressed TRAAK counteracted almost all of the Piezo specific current (Figure 2E–F) [11].
Figure 2. A putative role for anionic lipid regulation in mechanosensation and anesthesia.
(AB) A model of graded mechanical response based on lipid activation. (A) A lipid (red circle) is shown activating inhibitory K+ channel (grey cylinders) (i) and excitatory Ca2+ and Na+ channels (ii). Inhibitory K+ ions (purple spheres) are shown flowing out of the cell. Inhibitory Cl− ions flow into the cell (not shown). Excitatory Ca2+ and Na+ (green spheres) are shown flowing into and depolarizing the cell. A ‘?’ indicates ion channels that are known to be regulated by an anionic signaling lipid but the role of the lipid has not been directly linked to the mechano- or anesthetic-sensitivity of the channel. (B) Four putative ratios of inhibitory and excitatory mechano-effector channels. The amount of conductance for each ion is indicated by the number spheres (1–5). Mechanosensory currents are elicited when more excitatory effectors are active than inhibitory. Having many channels present and open leads to faster and stronger firing. (C-D) A model of an anesthetic response based on lipid activation. An anionic signaling lipid, produced in response to anesthetic, is shown activating a potassium channel (e.g. TREK-1, i) and inhibiting neurotransmitter-gated (brown square) Ca2+ and Na+ channels (iii) (all channels are shown as grey cylinders. A second class of anesthetic sensitive excitatory channels are shown activated by anesthetics (e.g. TRPV1, iv). (D) A lipid driven binary response to anesthetics is shown where lipid dependent external K+ is high and internal Ca2+ and Na+ remains low resulting in anesthesia. (D) Lipid activation of a Ca2+ and Na+ conducting pain channel by anesthetic is shown counteracting lipid activation of K+ channels. (E-F) Traces of membrane potentials from neuroblastoma 2a (N2a) cells measured under current clamp with mechanical poke by a glass rod. The application and distance of the mechanical poke is shown underneath the trace. Data are taken from Brohawn et. al., PNAS, 111 (2014) pg. 3614–9. (F) The same conditions in (E) with over expressed TRAAK channel, from the same source.
Figure 2C–D shows a model of signaling lipids (e.g., PA and PIP2) and putative combinations of inhibitory and excitatory mechano-effectors tuning a graded response to force. The model is based on the limited information that mechanical force tends to activate both inhibitory and excitatory mechanosensitive channels (Figure 2A, channel types i and ii respectively). In Figure 2B, the model shows a rapid activation by lipids of both channel types and the ratios of surface expression (inhibitory vs. excitatory) dictating the overall rate and strength of depolarization. Surface expression levels of channels are known to regulate excitability of a synapse [91]. In addition to channel surface expression, the concentration of anionic lipid could dictate the effective number of open channels. If the addition of anionic signaling lipids proves pivotal to mechanosensation, it would add a layer of complexity allowing for fast regulation and dynamic control of channels within the membrane compartment [52].
TREK-1’s physiological role in anesthesia [2–4] and the dependence of TREK-1 on the lipid membrane for anesthetic action [28] suggests the plasma membrane is a target for anesthetics and it plays a role in anesthesia. For now, this role is only conclusively established for TREK-1 [28]. However, the ability to transfer anesthetic sensitivity from TREK-1 to anesthetic insensitive TRAAK channels using the PLD2 binding domain suggests that an indirect, lipid-based anesthetic sensitivity can be modular and transferable [28]. Other anesthetic channels with potential to be influenced by anesthetic disruption of lipid regulation include the nicotinic acetylcholine receptor (PA activates), GABAA (PIP2 bound in a cryoEM structure), and NMDA (PIP2 regulates), among others [92–94].
In contrast to the apparent activation of mechanical channels, anesthetics both activate and inhibit anesthetic-sensitive channels. Rather strikingly, almost all classes of anesthetics have the opposite effect on excitatory and inhibitory channels [15]. They activate inhibitory channels and inhibit excitatory channels. Most of the inhibited channels are neurotransmitter-gated [15]. One explanation for this opposite regulation would be if an intermediary, such as a lipid, has the opposite regulatory effect on the channel types (Figure 2C) and then the anesthetics are affecting the regulatory molecule. An intermediary would better explain the observed action on ion channels compared to direct binding of a ligand to allosteric sites which one would expect a more random distribution of activation vs. inhibition among inhibitory and excitatory channel types respectively.
Due to the added inhibitory contribution to anesthesia compared to mechanosensation, we expect a slightly different model. A speculative model for lipid regulation in anesthesia is shown in Figure 2D. Lipids and/or direct binding of anesthetic are shown potentiating potassium and chloride channels (e.g. TREK-1 and GABAA respectively, Figure 2D channel type i) and inhibiting calcium and sodium channels (Figure 2D channel type iii). Only TREK-1 has been shown to work almost entirely through indirect lipid activation [28]. For other anesthetic channels (e.g. GABAA, nAChR, and NMDA) that bind lipids [92–94] it is not known if any of the anesthetic sensitivity is transmitted to the channel through the lipid or how a lipid would inactivate a channel.
The combined activation and inhibition of excitatory and inhibitory channels respectively would, in theory, lead to a much more binary response compared to the gradation expected from the ratio of mechanical effectors (Figure 2C–D). The finding that the levels of anionic lipid are modulated by anesthetics strongly suggests there will be some influence from indirect lipid binding. But, data for these models is extremely limited and much work is needed to elucidate the roles of anionic signaling lipids in anesthesia through ion channels.
Paradoxically, anesthetics can cause pain when applied to a pain fiber [95,96]. Anesthetics are known to activate excitatory Ca2+ conducting transient receptor potential (TRP) channels (Figure 2C channel type iv) contributing to pain [97,98]. The channels are highly regulated by signaling lipids, but it is not known to what degree the lipid transmits the anesthetic sensitivity. Assuming a major contribution of the lipid similar to TREK-1, then in nociceptive membranes with both types of channels, the ratio of lipid-activated channel likely contributes to the outcome of excitable pain (Figure 2D). In tissues with lipid activating TRP channels, the anesthetic induced potassium/chloride current would be insufficient to counteract TRP and the pain nerve would fire. Supporting a role for lipids in pain, PA increases the threshold for pain sensitivity in flies [25], the signaling lipid sphingosine 1-phosphate decreases the threshold in mice [99], and PIP2 lowed the mechanical sensitivity threshold in B-cells [100].
Localized signaling
The demonstration that a local production of PA could activate TREK-1 [24] convincingly established the existence of lipid gradients within the membrane [21]. These gradients depend on restricted movement of lipids. Live-imaging of the GM1 domains, which regulates TREK-1 through PLD2 sequestration, showed the domains to be dynamic but stable over long timescales (minutes) [18]. The partitioning of lipids is thought to involve both lipid and protein-mediated partitioning (e.g. phase separation and cytoskeletal ‘picket fence’ respectively) [39]. This level of control at nanoscale distances allows for an estimation of the time and concentration it would take to initiate a signaling event through TREK-1 in response to the mechanical disruption of lipid domains.
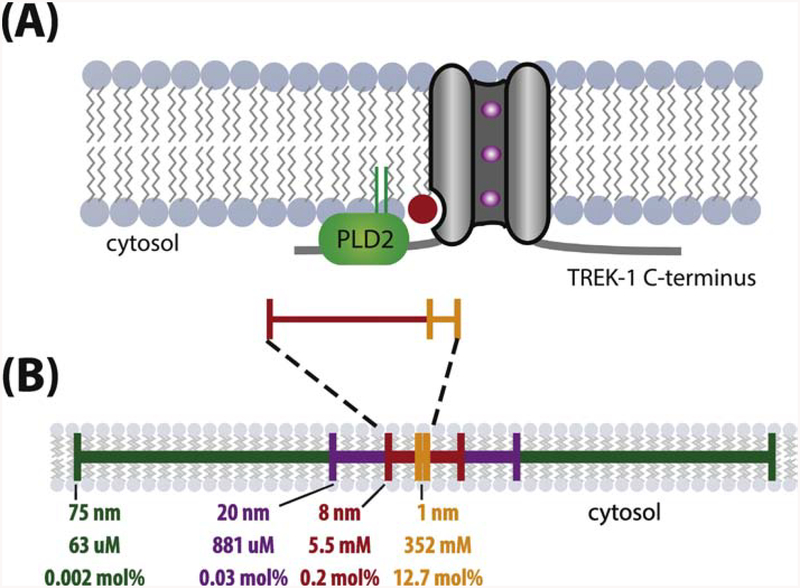
We find that the local production of PA through PLD2 allows for theoretical gating of TREK-1 on sub millisecond time scales, which is sufficiently fast to allow typical mechanosensitive signaling. We estimate a single PA molecule within 16 nm results in an effective concentration of ~5.5 mM, a concentration 275x the measured Kd (Figure 3) [35]. For PLD2 the rate of production is thought to be 1 PA molecule every ~45 ms [101] which is halved when considering that each TREK-1 protein contains two PLD2 binding sites. This means that it would only require an effective time of ~300 μs to reach a Kd on par with PA for TREK-1. Importantly, this rate applies to an unstimulated PLD2, the actual rate of PA production can be much higher if PLD2 is bound to PIP2 or some other activator. When added to the latency previously calculated for the activation of PLD2 from domain disruption [18] a conservative estimate of ~0.9 ms is sufficient to activate TREK-1 in a PLD2-dependent raft-disruption model of mechanosensation. This places mechanically-induced second-messenger signaling to TREK-1 well within the temporal limit previously thought to be only achieved by direct-to-channel mechanosensitive mechanisms.
Figure 3. Generation of mM lipid concentrations by local production. Generation of mM lipid concentrations by local production.
(A) Cartoon illustrating the proximity of phospholipase D2 (PLD2) and phosphatidic acid (PA) production (red lipid) to a TREK-1 channel (grey cylinder) in the plasma membrane. PLD2 bound to the C-terminus of TREK-1 will produce PA within 8 nm of the TREK-1 lipid binding site (red scale bar). Since TREK-1 is a homodimer, an identical setup would exist on the opposite side as well (not shown). (B) The effective molar concentration and mole percent of a single signaling lipid (PA) within select radii from the channel. The former is calculated by the formula 1/*πr2hNA, where NA is Avogadro’s number, the height, h, of a lipid leaflet is estimated to be 15 Å, and the distance, r, from TREK-1 is indicated. Mole percent were calculated using the assumption that each um2 of lipid contain 1×105 lipids**. Only at radii ~130 nm from the channel are concentrations estimated below the measured Kd of TREK-1 for PA (~20 μM).
Considerations of dSTORM imaging.
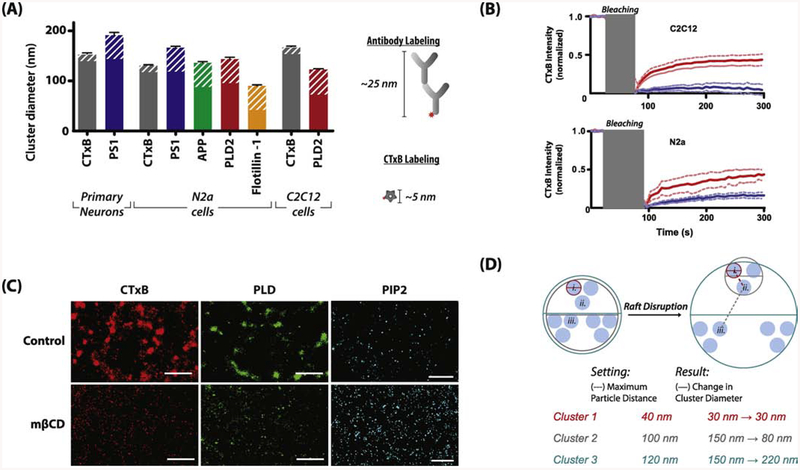
For dSTORM, a molecule of interest must be fluorescently labeled, and this introduces perturbations to the system. Fluorescently labeled CTxB is the most common label for GM1 lipids. Figure 4A shows differences in CTxB domain size from 3 different cells types, (primary mouse neurons, N2a, and C2C12). CTxB is pentadentate and clusters GM1 lipids in live cells [63,102,103]. In fixed cells the result is less clear. GM1 is a lipid and if the lipid is mobile after fixation then the CTxB could still induce artificial clustering.
Figure 4. Acquisition and analysis considerations of super-resolution images.
(A) Comparison of the size of CTxB labeled GM1 domains from three cell types with antibody labeled proteins known to localized within the GM1 domains. The potential added diameter from antibody or CTxB labeling is indicated by white stripes. Fixed proteins within the GM1 lipids form clusters approximately the same size as CTxB labeled GM1 clusters, suggesting CTxB does not cluster unfixed lipids after treatment. (Antibodies used: 13891, ab15272, ab41927, MAB5232; secondary Cy3b antibodies and CTxB, along with the protocols used can be found in [18,28]). (B) Fluorescence recovery after photobleaching (FRAP) quantification showing movement of CTxB labeled lipids after fixation. C2C12 cells show negligible recovery in fixed cells vs. live cells while N2a showed a slight increase indicated some diffusion of lipids in N2a cells. (C) Images from C2C12 cells showing changes in lipid and protein clustering upon application of the cholesterol-sequestering molecule methyl-β-cyclodextrin (mβCD). Removal of cholesterol leads to a reduction in diameter in CTxB (80 to 58 nm, red) and PIP2 clusters (73 to 57 nm, blue). Data taken from Petersen et al. Nature Comm. 7 (2016) 13873 with permission. (D) A hypothetical schematic showing how the selection of the maximum particle distance (MPD) parameter using DBSCAN can change the apparent diameter of a cluster in identical images or across treatments. In the two ‘samples’ shown, comparison of cluster sizes can lead to either shrinking (Cluster 2), enlargement (Cluster 3), or no apparent change (Cluster 1) in overall size in identical images. Care should be taken in both the selection of MPD used and the resulting interpretation of such data.
Fixing with either paraformaldehyde (PFA) and glutaraldehyde (GA) or osmium tetroxide reduces the movement of protein and lipids and the potential for artificial clusters[104]. Fluorescent recovery after photobleaching (FRAP) showed very little movement of sphingomyelin labeled lipid after osmium tetroxide treatment. This is consistent with our findings that 3% PFA and 0.1% GA also shows little movement of CTxB labeled lipid using FRAP (Figure 4B, data not previously published). However, single particle tracking for 100 ms showed substantial movement [105]. The movement was over a relatively small area, on the order of a 100 nm cluster (~0.01μm2). Combining these data, lipids in fixed membranes appear to have some mobility in very confined spaces but not over long distances.
Hence determining an absolute cluster or domain size is unlikely to be accurate below 100 nm but determining the distance between fixed domains and/or measuring the translocation of a molecule from one domain to the another is likely accurate and useful.
When quantifying the diameter of these small clusters, the length of the labeling molecules adds to the inherent error. For example, CTxB can add up to ~5 nm of distance to the measurement and antibodies (primary + secondary) up to ~25 nm (Figure 4A). Antibodies are bidentate meaning they can also cause increased clustering and artificial generation or enlargement of a domain or cluster (Figure 4A) to unfixed protein or lipids. For this reason, along with the technical inhibitions of accurately measuring domain size with many techniques, conclusions about changes in raft sizes should be limited to relative quantification between treated and control samples rather than absolute sizes measured, although with the proper instrumentation and controls these types of measurements may be possible [62].
PLD2 is a membrane associated protein which are not mobile after fixing [105]. In two of three cell types fixed PLD2 clusters were on average larger than CTxB clusters suggesting CTxB is not increases cluster size in those cell types, at least not in a way that is significant compared to inherent error of the measurement. Of course, the possibility of artificial clustering can never be completely ruled out for multivalent probes. Using monovalent probes would alleviate much of this problem.
Monovalent lipid binding domains are available and could be utilized for super resolution imaging to avoid induced clustering. For example, a 15 kDa toxin ostreolysin A (OlyA) labels sphingomyelin in both fixed and live cells [106]. And recently modified OlyA discriminated a cholesterol bound conformation of sphingomyelin from the unbound conformation[107,108]. These will likely be useful tools for imaging. Mutations creating mono and divalent CTxB also retain binding and partition with detergent resistant membranes, but with reduced affinity[109].
Lastly, some user-selected variables can affect the apparent size of a lipid domain. For example, density-based spatial clustering of applications with noise (DBSCAN), a commonly used clustering analysis algorithm, allows for user input on the maximum distance to consider a particle when defining a cluster. This distance can affect the apparent size of the resulting domain (see Figure 4D). Even when using the correct parameters, a measurement of an absolute size is uncertain. Rather one must compare two conditions and limit the comparison to a change in domain structure. For example, a relative shift in domain size with and without MβCD, force, anesthetic, etc. (Figure 4C) can indicate a perturbation to the system if similar preparations and internal controls (such as comparing the total number of localizations for dSTORM) are used. User-selected variables are unlikely a major factor for the observed decrease in CTxB and PLD2 domain size in Figure 4C due to the clear structural differences in the lipid domains. For PIP2 a reanalysis of the data with the more automated DBSCAN showed a decrease in size Figure 4C compared to the manual analysis which showed a slight increase [18].
For determining localization changes between two molecules, pair correlation offers a robust method to quantify these changes[110,111]. Pair correlation is largely unaffected by domain size and the overcounting common in dSTORM making it a preferred method of quantification for localization-based techniques. Pair correlation can identify and quantify relationship changes between two molecules before and after a stimuli. For example, the translocation of PLD2 out of GM1 domains to PIP2 domains is independent of domain size or number. Hence, observation of PLD2 translocation through changes in pair correlation is definitive evidence for anesthetic, mechanical, and cholesterol induced disruption of palmitate-mediated localization and domain disruption.
Perspective and Conclusions
Almost all mechanisms of TREK-1 regulation are proposed to depend on the carboxy C-terminus including mechanical force, temperature, pH, arachidonic acid (AA), and anesthetics[90]. This loop is disordered and has no obvious binding sites for hydrophobic molecules like AA or anesthetics nor an obvious structured mechanism for transmitting mechanical or thermal information to a putative gate in the transmembrane domain. Early TREK-1 studies[6,8,90] were unaware of PLD2 mechano and anesthetic sensitivity [18,28] and the direct interaction of PLD2 with TREK-1[24]. The role of PLD2 appears key for understanding regulation of TREK-1. Recently, the two step PLD2 mechanism nicely explained the role of the C-terminus for local anesthetic inhibition of TREK-1[87] showing the two step PLD2 model can account for both activation and inhibition of TREK-1 in specific cases.
As far as the authors are aware, no other models (apart from those described above) of ion channel activation have considered a multistep process for anesthesia or mechanosensation. Whether the properties found in TREK-1 extend to other lipid regulated channels is a question needing further investigation. Anesthetics have a minimal effect on the bulk (disordered) membranes [112] which has been the source of debate in the field. But these studies have not adequately considered the numerous potential lipid intermediaries. Many lipid modifying enzymes are similar to PLD2 and reside in the membrane; some of them undoubtedly bind disordered loops on channels producing local changes in lipid concentration. Any lipid molecule locally produced will have a very high local concentration and a potent effect on an associated channel or other protein. The local high concentration would likely mean a weak binding interaction making the identification of these putative lipid activators challenging.
The existence of stable domains based on lipid partitioning is not necessary for a disruption/mixing mechanism to function in the cell[44]. Much of the lipid heterogeneity could be based on gradients resulting from local production and small reductions in lateral mobility similar to local production of PA near TREK-1. This type of transient signaling through nanoclusters is thought to control cell growth and differentiation [44]. Furthermore, phase separation could simply alter the kinetics of localized signaling rather than form stable long lived domains. Recently phase separation has been observed for the soluble proteins in the cytosol [113] suggesting soluble ligands may also form gradients and activate ion channels through local high concentration. If the cell utilizes phase separation in the cytosol it seems likely the same properties would be utilized in the lipid membrane.
MβCD is not found natively in cells, but in the immune system apolipoproteins modulate raft function [114–116] and apolipoproteins are known to shuttle cholesterol between cells [117], a function that would require both the removal and addition of cholesterol to the membrane. Apolipoproteins could endogenously regulate PLD2 through cholesterol dependent palmitoyl-clustering similar to MβCD. One would expect increase cholesterol to decrease the effect of anesthetics and mechanical disruption, but this requires further study.
The broader implication of anesthetic and force induced de-clustering of palmitoylated proteins remain to be investigated. All cells must sense force [118–120] not just specialized sensory cells. The widespread production of GM1 lipids and expression of PLD2 suggest it may be the foundation of a suitable mechanism for detecting mechanical force in many unspecialized cells. There are many important palmitoylated proteins including most alpha subunit G-proteins[121] and numerous ion channels [68], many of which are anesthetic sensitive [15,122].
Highlights:
TREK-1 mechanosensation and anesthesia share a common pathway revealed by super resolution imaging.
Anesthetics and mechanical force disrupt palmitate mediated localization of phospholipase D2 to lipid domains.
Proposed models of anesthesia and mechanosensation regulation based on selective activation and inhibition of ion channels by lipids.
Caveats of super resolution imaging using polyvalent labels.
Acknowledgments
Work in the authors laboratories is supported by a Director’s New Innovator Award (1DP2NS087943-01) and an RO1 (1R01NS112534-01) from the National Institutes of Health and an Accelerating Innovation in Military Medicine Research award (W81XWH1810782) from the Department of Defense to S.B.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflicts of interests.
References
- [1].Honoré E, The neuronal background K2P channels: focus on TREK1, Nat. Rev. Neurosci, 8 (2007) 251–261. [DOI] [PubMed] [Google Scholar]
- [2].Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M, TREK-1, a K+ channel involved in neuroprotection and general anesthesia, EMBO J, 23 (2004) 2684–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M, Inhalational anesthetics activate two-pore-domain background K+ channels, Nat. Neurosci, 2 (1999) 422–6. [DOI] [PubMed] [Google Scholar]
- [4].Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP, Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane, Mol Pharmacol, 65 (2004) 443–452. [DOI] [PubMed] [Google Scholar]
- [5].Dong YY, Pike ACW, Mackenzie A, Mcclenaghan C, Aryal P, Dong L, Quigley A, Grieben M, Goubin S, Mukhopadhyay S, Ruda GF, V Clausen M, Cao L, Brennan PE, a Burgess-brown N, Sansom MSP, Tucker SJ, Carpenter EP, K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac, Science, 347 (2015) 1256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Honoré E, Maingret F, Lazdunski M, Patel AJ, An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1, EMBO J, 21 (2002) 2968–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Honoré E, Patel AJ, Chemin J, Suchyna T, Sachs F, Desensitization of mechano-gated K2P channels, Proc. Natl. Acad. Sci. U. S. A, 103 (2006) 6859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chemin J, Patel AJ, Delmas P, Sachs F, Lazdunski M, Honore E, Regulation of the Mechano-Gated K2P Channel TREK-1 by Membrane Phospholipids, Curr. Top. Membr, 59 (2007) 155–70. [DOI] [PubMed] [Google Scholar]
- [9].Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honoré E, A phospholipid sensor controls mechanogating of the K+ channel TREK-1, EMBO J, 24 (2005) 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Berrier C, Pozza A, De Lacroix De Lavalette A, Chardonnet S, Mesneau A, Jaxel C, Le Maire M, Ghazi A, le Maire M, Ghazi A, The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension, J. Biol. Chem, 288 (2013) 27307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brohawn SG, Su Z, Mackinnon R, Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels, Proc. Natl. Acad. Sci. U. S. A, 111 (2014) 3614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E, Honoré E, Mechano- or Acid Stimulation, Two Interactive Modes of Activation of the TREK-1 Potassium Channel, J. Biol. Chem, 274 (1999) 26691–26696. [DOI] [PubMed] [Google Scholar]
- [13].Franks NP, Molecular targets underlying general anaesthesia, Br. J. Pharmacol, 147 (2006) 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hemmings HC, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL, Emerging molecular mechanisms of general anesthetic action, Trends Pharmacol. Sci, 26 (2005) 503–510. [DOI] [PubMed] [Google Scholar]
- [15].Krasowski MD, Harrison NL, General anaesthetic actions on ligand-gated ion channels, Cell. Mol. Life Sci, 55 (1999) 1278–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bertaccini EJ, Dickinson R, Trudell JR, Franks NP, Molecular modeling of a tandem two pore domain potassium channel reveals a putative binding site for general anesthetics, ACS Chem. Neurosci, 5 (2014) 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ridone P, Grage SL, Patkunarajah A, Battle AR, Ulrich AS, Martinac B, “Force-from-lipids” gating of mechanosensitive channels modulated by PUFAs, J. Mech. Behav. Biomed. Mater, 79 (2018) 158–167. [DOI] [PubMed] [Google Scholar]
- [18].Petersen EN, Chung H-W, Nayebosadri A, Hansen SB, Kinetic disruption of lipid rafts is a mechanosensor for phospholipase D, Nat. Commun, 7 (2016) 13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P, Cells respond to mechanical stress by rapid disassembly of caveolae, Cell, 144 (2011) 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Storch U, Mederos y Schnitzler M, Gudermann T, G protein-mediated stretch reception, Am. J. Physiol. Heart Circ. Physiol, 302 (2012) H1241–9. [DOI] [PubMed] [Google Scholar]
- [21].Robinson CV, Rohacs T, Hansen SB, Tools for Understanding Nanoscale Lipid Regulation of Ion Channels, Trends Biochem. Sci, 44 (2019) 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loh J, Chuang M-C, Lin S-S, Joseph J, Su Y-A, Hsieh T-L, Chang Y-C, Liu AP, Liu Y-W, Acute decrease in plasma membrane tension induces macropinocytosis via PLD2 activation, J. Cell Sci, (2019). [DOI] [PubMed] [Google Scholar]
- [23].Lin S-S, Liu Y-W, Mechanical Stretch Induces mTOR Recruitment and Activation at the Phosphatidic Acid-Enriched Macropinosome in Muscle Cell, Front. Cell Dev. Biol, 7 (2019) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Comoglio Y, Levitz J, Kienzler M. a., Lesage F, Isacoff EY, Sandoz G, Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid, Proc. Natl. Acad. Sci. U. S. A, 111 (2014) 13547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Petersen EN, Gudheti M, Pavel MA, Murphy KR, Ja WW, Jorgensen EM, Hansen SB, Phospholipase D Transduces Force to TREK-1 Channels in a Biological Membrane, BioRxiv, (2019) 758896. [Google Scholar]
- [26].Bandeiras C, Serro AP, Luzyanin K, Fernandes A, Saramago B, Anesthetics interacting with lipid rafts, Eur. J. Pharm. Sci, 48 (2013) 153–165. [DOI] [PubMed] [Google Scholar]
- [27].Weinrich M, Worcester DL, The actions of volatile anesthetics: a new perspective, Res. Pap. Acta Cryst, 74 (2018) 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pavel MA, Petersen EN, Lerner RA, Hansen SB, Studies on the mechanism of general anesthesia, BioRxiv, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weinrich M, Worcester DL, Xenon and other volatile anesthetics change domain structure in model lipid raft membranes, J. Phys. Chem. B, 117 (2013) 16141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gray E, Karslake J, Machta BB, Veatch SL, Liquid general anesthetics lower critical temperatures in plasma membrane vesicles, Biophys. J, 105 (2013) 2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Papahadjopoulos D, Jacobson K, Poste G, Shepherd G, Effects of local anesthetics on membrane properties I Changes in the fluidity of phospholipid bilayers, Biochim. Biophys. Acta, 394 (1975) 504–19. [DOI] [PubMed] [Google Scholar]
- [32].Lee AG, Model for action of local anaesthetics, Nature, 262 (1976) 545–8. [DOI] [PubMed] [Google Scholar]
- [33].Vanderkooi JM, Landesberg R, Selick H, McDonald GG, Interaction of general anesthetics with phospholipid vesicles and biological membranes, Biochim. Biophys. Acta, 464 (1977) 1–18. [DOI] [PubMed] [Google Scholar]
- [34].Chung H-W, Petersen EN, Cabanos C, Murphy KR, Pavel MA, Hansen AS, Ja WW, Hansen SB, A Molecular Target for an Alcohol Chain-Length Cutoff, J. Mol. Biol, 431 (2019) 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cabanos C, Wang M, Han X, Hansen SB, A Soluble Fluorescent Binding Assay Reveals PIP2 Antagonism of TREK-1 Channels, Cell Rep, 20 (2017) 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Franks NP, Honoré E, The TREK K2P channels and their role in general anaesthesia and neuroprotection, Trends Pharmacol. Sci, 25 (2004) 601–8. [DOI] [PubMed] [Google Scholar]
- [37].Steinberg EA, Wafford KA, Brickley SG, Franks NP, Wisden W, The role of K2P channels in anaesthesia and sleep, Pflügers Arch. - Eur. J. Physiol, 467 (2015) 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brohawn SG, How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2, Ann. N. Y. Acad. Sci, 1352 (2015) 20–32. [DOI] [PubMed] [Google Scholar]
- [39].Nicolson GL, The Fluid - Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years, Biochim. Biophys. Acta - Biomembr, 1838 (2014) 1451–1466. [DOI] [PubMed] [Google Scholar]
- [40].Sezgin E, Levental I, Mayor S, Eggeling C, The mystery of membrane organization: composition, regulation and roles of lipid rafts, Nat. Rev. Mol. Cell Biol, 18 (2017) 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Trimble WS, Grinstein S, Barriers to the free diffusion of proteins and lipids in the plasma membrane, J. Cell Biol, 208 (2015) 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mylvaganam SM, Grinstein S, Freeman SA, Picket-fences in the plasma membrane: functions in immune cells and phagocytosis, Semin. Immunopathol, 40 (2018) 605–615. [DOI] [PubMed] [Google Scholar]
- [43].Lenne P-F, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo X-J, Rigneault H, He H-T, Marguet D, Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork, EMBO J, 25 (2006) 3245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Harding AS, Hancock JF, Using plasma membrane nanoclusters to build better signaling circuits, Trends Cell Biol, 18 (2008) 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lingwood D, Simons K, Lipid rafts as a membrane-organizing principle, Science, 327 (2010) 46–50. [DOI] [PubMed] [Google Scholar]
- [46].Liu Y, Casey L, Pike LJ, Compartmentalization of Phosphatidylinositol 4,5-Bisphosphate in Low-Density Membrane Domains in the Absence of Caveolin, Biochem. Biophys. Res. Commun, 245 (1998) 684–690. [DOI] [PubMed] [Google Scholar]
- [47].Johnson CM, Rodgers W, Spatial Segregation of Phosphatidylinositol 4,5-Bisphosphate (PIP(2)) Signaling in Immune Cell Functions, Immunol. Endocr. Metab. Agents Med. Chem, 8 (2008) 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pike LJ, Casey L, Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains, J. Biol. Chem, 271 (1996) 26453–26456. [DOI] [PubMed] [Google Scholar]
- [49].Wenk MR, Lucast L, Di Paolo G, Romanelli AJ, Suchy SF, Nussbaum RL, Cline GW, Shulman GI, Mcmurray W, De Camilli P, Di Paolo G, De Camilli P, Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry, Nat. Biotechnol, 21 (2003) 813–7. [DOI] [PubMed] [Google Scholar]
- [50].Milne SB, Ivanova PT, DeCamp D, Hsueh RC, Brown HA, A targeted mass spectrometric analysis of phosphatidylinositol phosphate species, J. Lipid Res, 46 (2005) 1796–802. [DOI] [PubMed] [Google Scholar]
- [51].Haag M, Schmidt A, Sachsenheimer T, Brügger B, Quantification of Signaling Lipids by Nano-Electrospray Ionization Tandem Mass Spectrometry (Nano-ESI MS/MS), Metabolites, 2 (2012) 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hansen SB, Lipid agonism: The PIP2 paradigm of ligand-gated ion channels, Biochim. Biophys. Acta, 1851 (2015) 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].a Jones S, Shim S-H, He J, Zhuang X, Fast, three-dimensional super-resolution imaging of live cells, Nat. Methods, 8 (2011) 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huang B, Wang W, Bates M, Zhuang X, Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy, Science, 319 (2008) 810–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF, Imaging intracellular fluorescent proteins at nanometer resolution, Science, 313 (2006) 1642–1645. [DOI] [PubMed] [Google Scholar]
- [56].Hess ST, Girirajan TPKK, Mason MD, Ultra-high resolution imaging by fluorescence photoactivation localization microscopy, Biophys. J, 91 (2006) 4258–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Graber ZT, Shi Z, Baumgart T, Cations induce shape remodeling of negatively charged phospholipid membranes, Phys. Chem. Chem. Phys, 19 (2017) 15285–15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sarmento MJ, Coutinho A, Fedorov A, Prieto M, Fernandes F, Ca(2+) induces PI(4,5)P2 clusters on lipid bilayers at physiological PI(4,5)P2 and Ca(2+) concentrations, Biochim. Biophys. Acta, 1838 (2014) 822–30. [DOI] [PubMed] [Google Scholar]
- [59].Wang Yu-hsiu; Collins Agnieszka; Guo Lin; Smith-Dupont Kathryn B.; Gai Feng; Svitkina Tatyana; Janmey PA Divalent cation-induced cluster formation by polyphosphoinosited in model membranes, J Am Chem Soc, 134 (2012) 3387–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmüller H, Diederichsen U, Jahn R, Membrane protein sequestering by ionic protein-lipid interactions, Nature, 479 (2011) 552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang J, a Richards D, Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane, Biol. Open, 1 (2012) 857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Honigmann A, Mueller V, Ta H, Schoenle A, Sezgin E, Hell SW, Eggeling C, Scanning STED-FcS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells, Nat. Commun, 5 (2014). [DOI] [PubMed] [Google Scholar]
- [63].Moon S, Yan R, Kenny SJ, Shyu Y, Xiang L, Li W, Xu K, Spectrally Resolved, Functional Super-Resolution Microscopy Reveals Nanoscale Compositional Heterogeneity in Live-Cell Membranes, J. Am. Chem. Soc, 139 (2017) 10944–10947. [DOI] [PubMed] [Google Scholar]
- [64].Smotrys JE, Linder ME, Palmitoylation of intracellular signaling proteins: regulation and function, Annu. Rev. Biochem, 73 (2004) 559–87. [DOI] [PubMed] [Google Scholar]
- [65].Levental I, Lingwood D, Grzybek M, Coskun U, Simons K, Palmitoylation regulates raft affinity for the majority of integral raft proteins, Proc. Natl. Acad. Sci, 107 (2010) 22050–22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lorent JH, Levental I, Structural determinants of protein partitioning into ordered membrane domains and lipid rafts, Chem. Phys. Lipids, 192 (2015) 23–32. [DOI] [PubMed] [Google Scholar]
- [67].Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoè-Pognetto M, Lüscher B, The gamma2 Subunit of GABAA Receptors Is a Substrate for Palmitoylation by GODZ, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shipston MJ, Ion channel regulation by protein palmitoylation, J. Biol. Chem, 286 (2011) 8709–8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kleuss C, Krause E, Galpha(s) is palmitoylated at the N-terminal glycine, EMBO J, 22 (2003) 826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Greaves J, Chamberlain LH, Palmitoylation-dependent protein sorting J. Cell Biol, 176 (2007) 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McDermott M, Wakelam MJO, Morris AJ, Phospholipase D, Biochem. Cell Biol, 82 (2004) 225–53. [DOI] [PubMed] [Google Scholar]
- [72].Xie Z, Ho WT, Exton JH, Functional implications of post-translational modifications of phospholipases D1 and D2, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids, 1580 (2002) 9–21. [DOI] [PubMed] [Google Scholar]
- [73].Han JM, Kim Y, Lee JS, Lee CS, Lee BD, Ohba M, Kuroki T, Suh P-G, Ryu SH, Localization of Phospholipase D1 to Caveolin-enriched Membrane via Palmitoylation: Implications for Epidermal Growth Factor Signaling, Mol. Biol. Cell, 13 (2002) 3976–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Czarny M, Fiucci G, Lavie Y, Banno Y, Nozawa Y, Liscovitch M, Phospholipase D2: Functional interaction with caveolin in low-density membrane microdomains, FEBS Lett, 467 (2000) 326–332. [DOI] [PubMed] [Google Scholar]
- [75].Lopez I, Arnold RS, Lambeth JD, Cloning and initial characterization of a human phospholipase D2 (hPLD2): ADP-ribosylation factor regulates hPLD2, J. Biol. Chem, 273 (1998) 12846–12852. [DOI] [PubMed] [Google Scholar]
- [76].Diaz O, Mébarek-Azzam S, Benzaria A, Dubois M, Lagarde M, Némoz G, Prigent A-F, Disruption of lipid rafts stimulates phospholipase d activity in human lymphocytes: implication in the regulation of immune function, J. Immunol, 175 (2005) 8077–86. [DOI] [PubMed] [Google Scholar]
- [77].Singh H, Wray N, Schappi JM, Rasenick MM, Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds, Neuropsychopharmacology, (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [78].Xia F, Gao X, Kwan E, Lam PPL, Chan L, Sy K, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG, Disruption of pancreatic beta-cell lipid rafts modifies Kv21 channel gating and insulin exocytosis, J. Biol. Chem, 279 (2004) 24685–91. [DOI] [PubMed] [Google Scholar]
- [79].Agarwal SR, Yang P-CC, Rice M, a Singer C, Nikolaev VO, Lohse MJ, Clancy CE, Harvey RD, Role of membrane microdomains in compartmentation of cAMP signaling, PLoS One,9 (2014) e95835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Szoke É, Börzsei R, Tóth DM, Lengl O, Helyes Z, Sándor Z, Szolcsányi J, Szoke E, Börzsei R, Tóth DM, Lengl O, Helyes Z, Sándor Z, Szolcsányi J, Effect of lipid raft disruption on TRPV1 receptor activation of trigeminal sensory neurons and transfected cell line, Eur. J. Pharmacol, 628 (2010) 67–74. [DOI] [PubMed] [Google Scholar]
- [81].Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J, Dynamics of putative raft-associated proteins at the cell surface, J. Cell Biol, 165 (2004) 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim T-W, Duff KE, Wenk MR, Arancio O, Di Paolo G, Phospholipase d2 ablation ameliorates Alzheimer’s disease-linked synaptic dysfunction and cognitive deficits, J. Neurosci, 30 (2010) 16419–16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gomez-Cambronero J, The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (And a surprise discovery: PLD2 is a GEF), Cell. Signal, 23 (2011) 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Exton JH, Regulation of phospholipase D, Biochim. Biophys. Acta, 1439 (1999) 121–33. [DOI] [PubMed] [Google Scholar]
- [85].Chemin J, Patel AJ, Delmas P, Sachs F, Lazdunski M, Honore E, Regulation of the Mechano-Gated K 2P Channel TREK-1 by Membrane Phospholipids, (2007). [DOI] [PubMed] [Google Scholar]
- [86].Kopp Lugli A, Yost CS, Kindler CH, Anaesthetic mechanisms: update on the challenge of unravelling the mystery of anaesthesia, Eur. J. Anaesthesiol, 26 (2009) 807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pavel MA, Chung H, Petersen EN, Hansen SB, Polymodal Mechanism for TWIK-Related K+ Channel Inhibition by Local Anesthetic, Anesth. Analg, (in press) (2019). [DOI] [PubMed] [Google Scholar]
- [88].Mori T, Matubayasi N, Ueda I, Membrane expansion and inhalation anesthetics Mean excess volume hypothesis, Mol. Pharmacol, 25 (1984) 123–30. [PubMed] [Google Scholar]
- [89].Shin HW, Soh JS, Kim HZ, Hong J, Woo DH, Heo JY, Hwang EM, Park J-Y, Lee CJ, The inhibitory effects of bupivacaine, levobupivacaine, and ropivacaine on K2P (two-pore domain potassium) channel TREK-1, J. Anesth, 28 (2014) 81–6. [DOI] [PubMed] [Google Scholar]
- [90].Enyedi P, Czirják G, Molecular background of leak K+ currents: two-pore domain potassium channels, Physiol. Rev, 90 (2010) 559–605. [DOI] [PubMed] [Google Scholar]
- [91].Diering GH, Huganir RL, The AMPA Receptor Code of Synaptic Plasticity, Neuron, 100 (2018) 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Laverty D, Desai R, Uchański T, Masiulis S, Stec WJ, Malinauskas T, Zivanov J, Pardon E, Steyaert J, Miller KW, Aricescu AR, Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer, Nature, 565 (2019) 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Michailidis IE, Helton TD, Petrou VI, Mirshahi T, Ehlers MD, Logothetis DE, Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin, J. Neurosci, 27 (2007) 5523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hamouda AK, Sanghvi M, Sauls D, Machu TK, Blanton MP, Assessing the lipid requirements of the Torpedo californica nicotinic acetylcholine receptor, Biochemistry, 45 (2006) 4327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P, Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1, Nat. Neurosci, 5 (2002) 546–51. [DOI] [PubMed] [Google Scholar]
- [96].Martin S, Jones JS, Wynn BN, Does warming local anesthetic reduce the pain of subcutaneous injection?, Am. J. Emerg. Med, 14 (1996) 10–12. [DOI] [PubMed] [Google Scholar]
- [97].Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C, The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons, J. Clin. Invest, 118 (2008) 763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Fischer MJM, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C, The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors, J. Biol. Chem, 285 (2010) 34781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hill RZ, Hoffman BU, Morita T, Campos SM, Lumpkin EA, Brem RB, Bautista DM, The signaling lipid sphingosine 1-phosphate regulates mechanical pain, Elife, 7 (2018) e33285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wan Z, Xu C, Chen X, Xie H, Li Z, Wang J, Ji X, Chen H, Ji Q, Shaheen S, Xu Y, Wang F, Tang Z, Zheng J-S, Chen W, Lou J, Liu W, PI(4,5) P2 determines the threshold of mechanical force-induced B cell activation, J. Cell Biol, (2018) jcb.201711055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Okamura S, Yamashita S, Purification and characterization of phosphatidylcholine phospholipase D from pig lung, J. Biol. Chem, 269 (1994) 31207–13. [PubMed] [Google Scholar]
- [102].Merritt EA,’, Sarfaty S,’ Van Den Akker Focco,’ L’hoir Cecile, Martial JA,’ And, Hol WGJ’, Crystal structure of cholera toxin B-pentamer bound to receptor GMl pentasaccharide, Cambridge University Press, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Day CA, Kenworthy AK, Mechanisms Underlying the Confined Diffusion of Cholera Toxin B-Subunit in Intact Cell Membranes, PLoS One, 7 (2012) e34923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Endapally S, Frias D, Grzemska M, Gay A, Tomchick DR, Radhakrishnan A, Molecular Discrimination between Two Conformations of Sphingomyelin in Plasma Membranes, Cell, 176 (2019) 1040–1053.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tanaka KAK, Suzuki KGN, Shirai YM, Shibutani ST, Miyahara MSH, Tsuboi H, Yahara M, Yoshimura A, Mayor S, Fujiwara TK, Kusumi A, Membrane molecules mobile even after chemical fixation, Nat. Methods, 7 (2010) 865–866. [DOI] [PubMed] [Google Scholar]
- [106].Skočaj M, Resnik N, Grundner M, Ota K, Rojko N, Hodnik V, Anderluh G, Sobota A, Maček P, Veranič P, Sepčić K, Tracking Cholesterol/Sphingomyelin-Rich Membrane Domains with the Ostreolysin A-mCherry Protein, PLoS One, 9 (2014) e92783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A, Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis, Elife, 2014 (2014) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Infante RE, Radhakrishnan A, Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol, Elife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wolf AA, Jobling MG, Saslowsky DE, Kern E, Drake KR, Kenworthy AK, Holmes RK, Lencer WI, Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster ganglioside GM1 molecules, Infect. Immun, 76 (2008) 1476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sengupta P, Jovanovic-Talisman T, Skoko D, Renz M, Veatch SL, Lippincott-Schwartz J, Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis, Nat. Methods, 8 (2011) 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sengupta P, Jovanovic-Talisman T, Lippincott-Schwartz J, Quantifying spatial organization in point-localization superresolution images using pair correlation analysis, Nat. Protoc, 8 (2013) 345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Herold KF, Sanford RL, Lee W, Andersen OS, Hemmings HC, Clinical concentrations of chemically diverse general anesthetics minimally affect lipid bilayer properties, Proc. Natl. Acad. Sci. U. S. A, (2017) 201611717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hyman AA, Weber CA, Jülicher F, Liquid-liquid phase separation in biology, Annu. Rev. Cell Dev. Biol, 30 (2014) 39–58. [DOI] [PubMed] [Google Scholar]
- [114].Wang S, Yuan S, Peng D, Zhao S, HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells, Atherosclerosis, 225 (2012) 105–14. [DOI] [PubMed] [Google Scholar]
- [115].Cheng AM, Handa P, Tateya S, Schwartz J, Tang C, Mitra P, Oram JF, Chait A, Kim F, Apolipoprotein A-I attenuates palmitate-mediated NF-κB activation by reducing Toll-like receptor-4 recruitment into lipid rafts, PLoS One, 7 (2012) e33917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yin K, Chen W-J, Zhou Z-G, Zhao G-J, Lv Y-C, Ouyang X-P, Yu X-H, Fu Y, Jiang Z-S, Tang C-K, Apolipoprotein A-I Inhibits CD40 Proinflammatory Signaling via ATP-Binding Cassette Transporter A1-Mediated Modulation of Lipid Raft in Macrophages, J. Atheroscler. Thromb, 19 (2012) 823–836. [DOI] [PubMed] [Google Scholar]
- [117].Liu C-CC-C, Liu C-CC-C, Kanekiyo T, Xu H, Bu G, Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy, Nat. Rev. Neurol, 9 (2013) 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ranade SS, Syeda R, Patapoutian A, Mechanically Activated Ion Channels, Neuron, 87 (2015) 1162–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Julius D, Molecular mechanisms of nociception, Nature, 413 (2001) 203–210. [DOI] [PubMed] [Google Scholar]
- [120].Hahn C, Schwartz MA, Mechanotransduction in vascular physiology and atherogenesis, Nat Rev Mol Cell Biol, 10 (2009) 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wedegaertner PB, Wilson PT, Bourne HR, Lipid modifications of trimeric G proteins, J. Biol. Chem, 270 (1995) 503–6. [DOI] [PubMed] [Google Scholar]
- [122].Minami K, Uezono Y, The recent progress in research on effects of anesthetics and analgesics on G protein-coupled receptors, J. Anesth, 27 (2013) 284–292. [DOI] [PubMed] [Google Scholar]