Abstract
Context
The antipsychotic drug haloperidol has antiproliferative and growth-inhibiting properties on prostate cancer cell lines in vitro by binding the sigma 1 protein. Evidence is needed regarding a possible preventive association in men.
Objective
To examine whether our epidemiologic data support an inverse association of haloperidol use with risk of prostate cancer.
Design
These case-control analyses used conditional logistic regression to estimate relative risk by odds ratios (ORs) adjusting for race/ethnicity and aspects of medical care related to detection of prostate cancer. We tested 3 other commonly used antipsychotic drugs, risperidone, quetiapine, and olanzapine, for sigma 1 protein binding and inhibition of clonogenic growth of prostate cancer cells. Use of any of these by men was considered use of a comparator drug.
Main Outcome Measures
1) association of haloperidol with prostate cancer; 2) sigma 1 binding and clonogenic growth.
Results
Probably owing to small numbers of haloperidol recipients, evidence of a preventive association was inconsistent, depending on the definition of long-term use. If duration of use was greater than 1 year, the odds ratio (OR) was 0.38 (95% confidence interval (CI) = 0.14–1.01) for haloperidol and 0.80 (95% CI = 0.66–0.98) for the comparator drug; if the duration of use was greater than 2 years, the OR was 0.66 (95% CI = 0.24–1.76) for haloperidol and 0.84 (95% CI = 0.66–1.08) for the comparator drug. Unlike haloperidol, risperidone, quetiapine, and olanzapine did not bind sigma 1 or inhibit clonogenic growth.
Conclusion
Given the laboratory evidence, our ambiguous epidemiologic findings should encourage more epidemiologic evaluation of haloperidol use and risk of prostate cancer. Finding a negative association could be a scientific advance in prostate cancer prevention but would not be sufficient basis for recommending the prescription of haloperidol for that purpose.
Keywords: haloperidol, pharmacology, prevention, prostate cancer
LABORATORY EVIDENCE SUGGESTS THE HYPOTHESIS THAT THE ANTIPSYCHOTIC DRUG HALOPERIDOL MAY BE ASSOCIATED WITH REDUCED RISK OF DEVELOPING PROSTATE CANCER
Cancer prevention is a prominent component of the recent interest in repurposing drugs for beneficial effects other than their initial indications.1 Part of this effort has been focused on molecular mechanisms that may work in opposite directions in the development of mental illness and cancer.2,3 Genes and genetic pathways have been studied in this regard.3 Also of interest are polypharmacologic drugs and drugs with multifunctional targets. Among the latter, the putative sigma receptors are emerging as multifunctional drug targets at the interface or intersection of diverse physiologic and pathophysiologic pathways.4,5 The sigma proteins have been divided into 2 categories, sigma 1 and sigma 2, primarily on the basis of ligand-binding studies.4–6 Whereas both proteins have been implicated in neurologic diseases and disorders as well as cancer,4,5 sigma 1 was cloned more than 20 years ago and thus is the more extensively studied binding site.4,5 Most publications regarding sigma 1 describe its neuropharmacology5,7,8; however, a number of publications have described a potential role for sigma 1 in cancer biology.4
The antipsychotic drug haloperidol, whose pharmacologic mechanism of action is defined by its dopamine D2 receptor antagonism, also binds sigma 1 and, with essentially equal affinity, Ki of approximately 2 nM for both.4,9 Haloperidol and its metabolites have been categorized as sigma 1 antagonists and inhibitors and have been reported to have antiproliferative and growth-inhibiting properties on cancer cell lines in vitro.4,10–12 We demonstrated a role for sigma 1 in prostate cancer and antitumor efficacy of compounds derived from haloperidol in vivo and in vitro.10,11,13,14 Therefore, we investigated whether haloperidol use may be inversely associated with risk of prostate cancer in men.
WE SOUGHT EVIDENCE FOR OR AGAINST A NEGATIVE ASSOCIATION OF HALOPERIDOL USE AND RISK OF DEVELOPING PROSTATE IN MEN
In an initial analysis in our population-based screening of pharmaceutical drugs for associations with risk of cancer,15 men who had ever received haloperidol were at approximately half (odds ratio [OR], 0.55; 95% confidence interval = 0.41–0.72) the risk of developing prostate cancer compared with men who had never received it. This finding led us to conduct a more detailed epidemiologic study focused on evidence that would support or cast doubt on a negative association that might suggest a causal relationship. The main possible source of doubt would relate to ascertainment bias, the likelihood that the detection and diagnosis of prostate cancer would be reduced in patients with psychosis.16 We also assessed sigma 1 binding of haloperidol and 3 other frequently used antipsychotic drugs that served as comparators in our epidemiologic analysis.
OUR EPIDEMIOLOGIC METHOD
In this analysis, we assessed cancer risk in users of pharmaceuticals by case-control analysis of members of the Kaiser Permanente Northern California (KPNC) whose benefits include at least partial payments for prescription drugs in KPNC pharmacies. The period of study was January 1996 through December 2016. The current analyses were based on 39,872 men with prostate cancer (cases) recorded in KPNC’s Cancer Registry and up to 50 men per case without prostate cancer (controls), matched for age and calendar year of joining the program. The index dates were: For cases, the date of diagnosis, and for controls, the date that would give them equal follow-back time for ascertainment of drug dispensing recorded in the KPNC pharmacies. Initial conditional logistic analyses (initial model) were adjusted for race/ethnicity with HIV-positive men previously removed from the database. Subsequent analyses (full model) were controlled for characteristics that could affect the detection and diagnosis of prostate cancer, including medical clinic visits 1 to 2 years before the index date (0, 1, 2, ≥ 3), prostate-specific antigen (PSA) tests within 3 years before the index date (0, ≥ 1), and diagnosis of benign prostatic hyperplasia before the index date (yes vs no). Each of these was positively related to risk of prostate cancer. However, although among controls PSA testing was negatively related to haloperidol use as expected, the number of medical clinic visits was positively related. (These data and all others subsequently summarized but not shown are available from the authors on request.)
We calculated duration of use by totaling the days’ supply of all prescriptions and categorized duration as less than 1 year, 1 to less than 2 years, and 2 years or more as likely sufficient exposure to detect a preventive effect. The latter 2 categories were combined into 1 year or more. Comparing ORs for longer with shorter durations would also provide some evidence of dose response. We also looked at advanced prostate cancer (ie, with regional or distant metastases), which is unlikely to go undetected. A new-user analysis was also performed that focused on men who had been members for at least 1 year before their first recoded prescription of haloperidol.
Haloperidol is 1 of 4 antipsychotic drugs that were commonly dispensed from KPNC pharmacies and showed up in our screening of frequently used drugs by having had at least 25,000 recipients in the study cohort. The others are olanzapine, quetiapine, and risperidone. We treated the receipt of any of these as exposure to a comparator drug and included the comparator drug in a unified case-control analysis that would yield contrasts in risk of prostate cancer between use of haloperidol only and of comparator only. The 19 men who received both haloperidol and comparator were excluded from all analyses.
EPIDEMIOLOGIC RESULTS INDICATING THAT OUR DATA WERE INADEQUATE
In the initial and full models, there were moderate negative associations of similar magnitude for both haloperidol and comparator used for less than 1 year (Table 1). These were virtually unchanged for 1 or more years and 2 or more years of comparator use. There were only 4 patients with prostate cancer who had taken haloperidol for at least 1 year, and all 4 had taken it for at least 2 years, which yielded ambiguous results because of the differing numbers of controls available for comparison (576 for ≥ 1 years and 352 for ≥ 2 years of use). With at least 1 year of use, risk was approximately halved in both the initial and full models compared with less than 1 year of use with borderline statistical significance compared with nonuse in the full model. With at least 2 years of use, the difference decreased markedly, returning to virtually no difference in the full model (Table 1).
Table 1.
Risk of prostate cancer by cumulative days’ supply of haloperidol and comparator drug (excludes users of both haloperidol and comparator drugs)a
| Days’ supply | Haloperidol only | Comparator only | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of cases | No. of controls | Initial model OR 95% CI)b | Full model OR (95% CI)c | No. of cases | No. of controls | Initial model OR (95% CI)b | Full model OR (95% CI)c | |
| No use | 39,553 | 1,962,602 | 1 [Reference] | 1 [Reference] | 39,553 | 1,962,602 | 1 [Reference] | 1 [Reference] |
| < 1 y | 30 | 2008 | 0.69 (0.48–0.99) | 0.73 (0.51–1.05) | 166 | 8891 | 0.87 (0.75–1.02) | 0.83 (0.71–0.97) |
| At least 1 y | 4 | 576 | 0.32 (0.12–0.84) | 0.38 (0.14–1.01) | 100 | 5847 | 0.80 (0.66–0.98) | 0.75 (0.61–0.91) |
| At least 2 yd | 4 | 352 | 0.54 (0.20–1.44) | 0.66 (0.24–1.76) | 65 | 3639 | 0.84 (0.66–1.08) | 0.80 (0.62–1.02) |
At least 2 years is included in the at least 1-year group.
Conditional on matching factors (birth year, year of joining program, and follow-up time) and controlled for race/ethnicity.
Additionally controlled for number of medical clinic visits 1 to 2 years before the index date (1, 2, ≥ 3 vs 0), number of prostate-specific antigen tests within 3 years before the index date (≥ 1 vs 0), and diagnosis of benign prostatic hyperplasia before the index date (yes vs no).
There were no cases for the haloperidol-only users with 1 to 2 years of days’ supply.
CI = confidence interval; OR = odds ratio.
Neither the analysis of cases with advanced disease nor the new-user analysis was informative because of small numbers. Among users for at least 2 years, only 1 of the 4 haloperidol users and 8 of the 65 comparator users had advanced prostate cancer. There were 19 new users of haloperidol who developed prostate cancer, all of whom had received less than a 1-year supply. The new-user findings for the comparator were similar to those for all users.
LABORATORY METHODS AND FINDINGS
In the laboratory, we asked whether the differential association observed with haloperidol in contrast to the comparators in the epidemiologic study could be explained by haloperidol’s binding affinity for sigma 1. We performed sigma 1 selective radioligand ([3H](+)-pentazocine) binding competition assays and confirmed that although haloperidol binds sigma 1 as well as the dopamine D2 receptor with similarly high affinity, risperidone, quetiapine, and olanzapine did not displace the sigma 1 radioligand, indicating that these compounds do not bind sigma 1 (Table 2). The [3H](+)-pentazocine binding assay was performed as described elsewhere.13
Table 2.
Sigma 1 binding affinity of haloperidol, olanzapine, quetiapine, and risperidone
| Compound | Ki, nMa |
| Haloperidol | 2.4 |
| Olanzapine | > 1000 |
| Quetiapine | > 1000 |
| Risperidone | > 1000 |
Sigma 1 selective radioligand ([3H](+)-pentazocine) binding competition assays were performed as described in the article by Thomas et al.13
Ki = binding affinity.
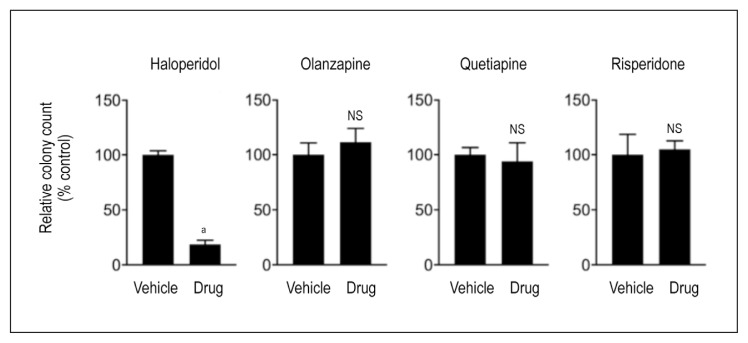
We subsequently tested the effect of treatment with these drugs on in vitro clonogenic growth and survival of LNCaP cells, a widely studied prostate cancer cell line that models hormone-sensitive disease. The clonogenic growth assay was performed as described elsewhere.13 We found that although haloperidol significantly inhibited colony formation and growth, risperidone, quetiapine, and olanzapine had no significant effect on clonogenic growth (Figure 1).
Figure 1.
Clonogenic growth of prostate adenocarcinoma cells in vitro. LNCaP prostate adenocarcinoma cells treated with 10 μM of haloperidol, olanzapine, quetiapine, or risperidone and compared with vehicle (dimethyl sulfoxide [DMSO]) control. Data are presented as the relative number of surviving cancer cell colonies after drug treatment compared with vehicle (DMSO) treated controls. An unpaired, 2-tailed t-test was used to compare the relative colony counts between vehicle and drug treated samples.
a p < .001.
NS = nonsignificant.
DISCUSSION
We have often found that epidemiologic evaluation does not support biologically plausible hypotheses regarding prevention of cancer by pharmaceuticals. This finding can occur simply as a failure to find the hypothesized negative association, for example, between norepinephrine antagonists and several cancers.17 Another reason for this finding is that adequate control for confounding factors that are related to use of a drug and risk of a cancer can greatly reduce or eliminate the hypothesized negative association, for example, between statins and primary liver cancer.18
In the present study, all the antipsychotics showed moderate reductions in risk with no clear dose response in the full model. The likelihood that patients with psychosis receive less primary medical care and screening for physical disease has been extensively reported and well summarized in the monograph by Lawrence et al.16 We attempted to reduce the resulting detection bias by including medical clinic (primary care) visits, receipt of PSA screening tests, and the diagnosis of benign prostatic hyperplasia in the full model. That users of the comparator had moderate negative associations with risk, even at relatively short durations, suggests that there was residual uncontrolled confounding in our analyses of haloperidol as well. Adding to the possibility of detection bias was our lack of data about the severity of mental illness being treated. Greater severity could have led to less contact with medical care and less prescribing of antipsychotic drugs.16
In the epidemiologic analysis, there were too few patients with long-term exposure to haloperidol to provide clear evidence for or against the biologically plausible hypothesis that use of haloperidol is associated with a reduced risk of developing prostate cancer in men. This analysis was not sufficiently powered, and the patients included do not represent a range of treatment times, doses, and ages. Evaluation of larger groups of patients who have taken haloperidol will likely provide more definitive findings. Of particular interest in this collaborative exploration is the contrast in the laboratory studies between the sigma 1 binding strength of haloperidol and its absence with the comparator antipsychotic drugs, chosen solely because of their frequent use by patients. Of note, laboratory-based studies do not evaluate prevention of prostate cancer in men. These studies simply demonstrate that among the 4 antipsychotic agents tested, only the drug with affinity for sigma 1 and with sigma 1 inhibitor and antagonist activity have anticancer properties.
We hope that this work will stimulate further epidemiologic evaluation of haloperidol and risk of prostate cancer, especially in settings where large numbers of men who take or have taken haloperidol can be followed-up for long periods. Also possible is that data from several smaller data sets can be included in meta-analyses, leading to more statistically stable conclusions. It would also be helpful if data were available on other risk factors for prostate cancer.
CONCLUSION
Establishing a negative association of haloperidol use with risk of developing prostate cancer could be a small but valuable scientific step in learning about possible means of preventing prostate cancer. However, it would not be a sufficient basis for recommending the prescription of haloperidol for this purpose.
Acknowledgments
This study was supported by Kaiser Foundation Hospitals Institutional and Planned Giving and grant R01 CA 098838 from the National Cancer Institute.
Laura King, ELS, performed a primary copy edit.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Lee DK, Szabo E. Repurposing drugs for cancer prevention. Curr Topics Med Chem. 2016;16:2169–78. doi: 10.2174/1568026616666160216154946. [DOI] [PubMed] [Google Scholar]
- 2.Tabares-Seisdedos R, Rubenstein JL. Inverse cancer comorbidity: A serendipitous opportunity to gain insight into CNS disorders. Nature Rev Neurosci. 2013 Apr;14(4):293–304. doi: 10.1038/nrn3464. [DOI] [PubMed] [Google Scholar]
- 3.Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 2014 Feb;10(2):e1004173. doi: 10.1371/journal.pgen.1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim FJ, Maher CM. Sigma 1 pharmacology in the context of cancer. Handb Exp Pharmacol. 2017;244:237–308. doi: 10.1007/164_2017_38. [DOI] [PubMed] [Google Scholar]
- 5.Kim FJ. Introduction to sigma proteins: Evolution of the concept of sigma receptors. Handb Exp Pharmacol. 2017;244:1–11. doi: 10.1007/164_2017_41. [DOI] [PubMed] [Google Scholar]
- 6.Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: Decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990 Sep;527(2):244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 7.Cobos EJ, Entrena JM, Nieto FR, Cendán CM, Del Pozo E. Pharmacology and therapeutic potential of sigma1 receptor ligands. Curr Neuropharmacol. 2008 Dec;6(4):344–66. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009 Nov;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Janssen PA. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1988 Nov;247(2):661–70. [PubMed] [Google Scholar]
- 10.Schrock JM, Spino CM, Longen CG, et al. Sequential cytoprotective responses to Sigma1 ligand-induced endoplasmic reticulum stress. Mol Pharmacol. 2013 Nov;84(5):751–62. doi: 10.1124/mol.113.087809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim FJ, Schrock JM, Spino CM, Marino JC, Pasternak GW. Inhibition of tumor cell growth by sigma1 ligand mediated translational repression. Biochem Biophys Res Commun. 2012 Sep;426(2):177–182. doi: 10.1016/j.bbrc.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spruce BA, Campbell LA, McTavish N, et al. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004 Jul;64(14):4875–86. doi: 10.1158/0008-5472.can-03-3180. [DOI] [PubMed] [Google Scholar]
- 13.Thomas JD, Longen CG, Oyer HM, et al. Sigma1 targeting to suppress aberrant androgen receptor signaling in prostate cancer. Cancer Res. 2017 May;77(9):2439–52. doi: 10.1158/0008-5472.can-16-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvino JM, Srikanth YVV, Lou R, Oyer HM, Chen N, Kim FJ. Novel small molecule guanidine Sigma1 inhibitors for advanced prostate cancer. Bioorg Med Chem Lett. 2017 May;27(10):2216–20. doi: 10.1016/j.bmcl.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman GD, Udaltsova N, Chan J, Quesenberry CP, Jr, Habel LA. Screening pharmaceuticals for possible carcinogenic effects: Initial positive results for drugs not previously screened. Cancer Causes Control. 2009 Dec;20(10):1821–35. doi: 10.1007/s10552-009-9375-2/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence D, Holman DA, Jablensky A. Preventable physical illness in people with mental illness. Perth, Australia: The Unversity of Western Australia; 2001. [Google Scholar]
- 17.Friedman GD, Udaltsova N, Habel LA. Norepinephrine antagonists and cancer risk. Int J Cancer. 2011 Feb;128(3):737–38. doi: 10.1002/ijc.25351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman GD, Achacoso N, Fireman B, Habel LA. Statins and reduced risk of liver cancer: Evidence for confounding. J Natl Cancer Inst. 2016 Jul;108(10) doi: 10.1093/jnci/djw109. [DOI] [PMC free article] [PubMed] [Google Scholar]