Abstract
Purpose
Recently, a new marker protein for microglial cells in the brain was postulated, transmembrane protein 119 (TMEM119), raising the hope for a new opportunity to reliably and unambiguously detect microglial cells in histologic sections. It was of interest whether TMEM119 also was a reliable microglial marker in the retina.
Methods
Anti-TMEM119 antibodies of two providers were used to label microglia in the murine retina, and labeling properties were compared to those of antibodies against Iba1 and CD11b. As an example of a pathologic situation, labeling for TMEM119 was also performed in eyes treated by an argon laser as an experimental model for choroidal neovascularization.
Results
TMEM119 immunoreactivity (IR) was found on microglial cells in the naïve retina. However, specificity and sensitivity of TMEM119 IR varied clearly depending on the source of the antibody, age of the mouse, and location of retinal microglia. After laser treatment, however, microglial cells lost their IR for TMEM119 at the site of the laser spot. Moreover, other cells became positive for TMEM119; for example, Müller cells.
Conclusions
TMEM119 is a useful marker for the microglia in the brain. However, retinal microglia shows variable IR for TMEM119, and the microglia is not the only cell showing TMEM IR. Therefore, TMEM119 appears not to be applicable as a general marker for the retinal microglia in pathologic situations.
Translational Relevance
Reliable detection and quantification of microglial cells is of high importance to study disease mechanisms and effects of therapeutic approaches in the retina.
Keywords: TMEM119, microglia, immunohistochemistry, retina
Introduction
In the healthy mammalian retina, microglial cells are located in the ganglion cell, inner plexiform, and outer plexiform layers where they permanently survey the status of the nervous tissue. In case of an injury or disease, microglial cells switch into an activated state, can release a big variety of cytokines and other compounds, and phagocytose debris and damaged cells.1–4 In research on diseases of the central nervous system, including ocular diseases affecting the retina, it is of great importance to detect microglial cells reliably in the tissue. Antibodies against several microglial markers are in use to date, in particular against CD11b and Iba1. As long as integrity of the blood–retina barrier is not disturbed, it can be taken for granted that retinal cells labeled for CD11b or Iba1 are, in fact, resident retinal microglial cells. The situation becomes more complicated in pathologic situations when peripheral immune cells may invade the retina, as many of them also are positive for microglial markers, and vice versa. For a real distinction, labeling must be performed against different markers. As an example, the microglia shows little expression of CD11c or CD45, while these markers can be found on all nucleated hematopoietic cells, such as macrophages, T cells, B cells, or dendritic cells.
In this context, transmembrane protein 119 (TMEM119) became interesting. TMEM119 is a member of a family of transmembrane proteins that recently was described on osteosarcoma cells.5 Reports exist that microglial cells in the brain were immunohistochemically positive for TMEM119 (TMEM119+) and peripheral immune cells were not; thus, enabling distinction between these two cell populations.6,7 In particular, TMEM119 was expressed by the microglia in the brain in case of neurodegenerative diseases, such as Alzheimer's disease, whereas invading peripheral monocytes in case of inflammatory diseases were not TMEM119+.7 Recently, Haage et al.8 investigated so-called differentially expressed genes (DEGs) to distinguish microglia from peripheral monocytes, and they identified TMEM119 as one of the top DEGs in the microglia. They then confirmed in a series of experiments that in murine brain TMEM119 is expressed only by the resident microglia and not by peripheral monocytes.8 The function of TMEM119 remains unknown to date. Attaai et al.9 found that TMEM119 expression was increased by the growth factor TGFβ1, an important mediator of microglial maturation.
Almost all studies regarding TMEM119 expression by the microglia to date were performed in the brain. As an unambiguous identification of microglial cells in the retina also is of importance, in particular in pathologic situations, we checked the microglia in the murine retina on its immunoreactivity (IR) for TMEM119, using two different commercially available anti-TMEM119 antibodies. Moreover, it was noteworthy whether TMEM119 IR was really confined to retinal microglia, or if also other retinal cells were TMEM119+. To identify TMEM119+ cells, we performed double labeling of the retinal samples against CD11b and/or Iba1, glutamine synthetase (GS) and other markers.
Methods
Animals
We used healthy C57BL/6J mice of two different ages (approximately four or 21 months, designated as “young” and “old” mice, respectively). All tissue samples used in this study were obtained in the framework of a research project approved by the local authorities (LANUV, Recklinghausen, Germany, file number 84-02.04.2016.A395). All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the EU directive 2010/63/EU. Mice were held in ventilated cages at a 12 hours/12 hours light/dark cycle with standard food and drinking water ad libitum.
Mouse Model of Laser-Induced CNV
Mice were anesthetized by an intraperitoneal injection of a mixture of 130 mg/kg ketamine and 2.7 mg/kg xylazine. The pupils of mice were fully dilated with 1% tropicamide eye drops and 5% neosynephrine eye drops. Before placed in front of the slit-lamp for laser treatment, the cornea was anesthetized with 0.5% proparacaine eye drops. Both eyes of the mouse were treated with an argon green laser (532 nm) through dilated pupils with a coverslip over the cornea to create breaks in Bruch's membrane with a central bubble formation. The laser spots were placed on the retina around the optic nerve head between the large vessels. There were five laser spots in each eye. The laser beam had a 75 μm diameter, pulse duration 100 ms, and pulse energy 200 mW. After the laser treatment, mice were brought back into their cage and allowed to recover.
Tissue Processing and Fluorescent Immunohistochemistry
For brain tissue preparation, the skull of mice was opened. The brain was isolated, and half of the brain was fixed in 4% paraformaldehyde (PFA) for 4 hours at room temperature. Eyes were isolated and fixed in 4% PFA for 1 hour at room temperature. Eyes of laser-treated treated C57BL/6 mice were isolated from animals of approximately 4 months. Tissue samples (eyes and brain) of at least two female and two male animals were used from each group, and typical examples are shown in the Results section. All tissue samples were washed 2× in PBS pH 7.4 for 5 minutes, incubated in 30% sucrose for 1 hour, in 30% sucrose plus NEG-50 (Thermo Fisher Scientific, Waltham, MA) 1:1 for 1 hour, and in pure NEG-50 for 1 hour. Afterwards, the tissues were frozen in NEG-50. Cryosections (thickness 10 μm) were cut using a Cryostar NX70 cryostat (Thermo Fisher Scientific), placed on Starfrost Advanced Adhesive glass slides (Engelbrecht, Edermünde, Germany) and were stored at −20 °C until used for immunohistochemistry.
Sections were dried at room temperature for 30 minutes to 1 hour and then washed in 0.05% Tween in PBS twice for 5 minutes and once for 5 minutes. Sections were blocked with Power Block reagent (HK085-5K; BioGenex San Ramon, CA) at room temperature for 6 minutes, then washed three times with 0.1 M PBS and incubated overnight with primary antibodies at 4°C. The sections then were washed three times with 0.1 M PBS and incubated with appropriate secondary antibodies for 1 hour at room temperature. The nuclei were counterstained with 4′6′-diamidino-2-phenylindole dihydrochloride (DAPI) diluted with pure water 1:300 for 7 minutes at room temperature. Finally, sections were washed three times with 0.1 M PBS and mounted under a glass coverslip using mounting medium (Immu Mount TM; Thermo Scientific). The primary antibodies were diluted with 5% bovine serum albumin (BSA) containing 0.3% Triton X-100, and secondary antibodies were diluted with 1% BSA.
We optimized dilutions of antibodies for best specific labeling and lowest possible background fluorescence. For all secondary antibodies, so-called “negative controls” were performed; that is, labeling procedures were performed where primary antibodies were omitted. Nonspecific background labeling was not seen in any case. All antibodies and their dilution used for immunohistochemistry are listed in Table 1.
Table 1.
Antibodies Used in This Study
| Antibodies |
Specificity |
Host |
Dye |
Supplier |
Catalogue No. |
Dilution |
| Primary antibodies | CD8 | Rat | Serotec, Oxford, United Kingdom | MCA1768T | 1:500 | |
| CD11b | Rat | Serotec | MCA711 | 1:60 | ||
| CD11c | Hamster | Abcam | ab33483 | 1:20 | ||
| CD45 | Rat | Santa Cruz Biotechnology, Dallas, TX | sc-59071 | 1:20 | ||
| GS | Guinea Pig | Synaptic Systems | 367005 | 1:400 | ||
| GFAP | Mouse | Sigma Aldrich Corp., St. Louis, MO | G3893 | 1:500 | ||
| Iba1 | Guinea Pig | Synaptic Systems | 234 003 | 1:500 | ||
| NeuN | Guinea Pig | Synaptic Systems | 266 004 | 1:400 | ||
| RPE65 | Mouse | Abcam | ab13826 | 1:50 | ||
| TMEM119 | Rabbit | Abcam | ab209064 | 1:80 | ||
| TMEM119 | Rabbit | Synaptic Systems | 400 002 | 1:500 | ||
| Vimentin | Chicken | Abcam | ab39376 | 1:125 | ||
| Secondary antibodies | Chicken | Goat | Texas Red | Life Technologies, Carlsbad, CA | A11008 | 1:100 |
| Guinea Pig | Goat | Alexa Fluor 488 | Abcam | ab150185 | 1:400 | |
| Hamster | Goat | Texas Red | Abcam | ab5743 | 1:200 | |
| Mouse | Goat | Alexa Fluor 594 | Thermo Fisher | A-11032 | 1:600 | |
| Mouse | Donkey | Alexa Fluor 647 | Invitrogen, Carlsbad, CA | A31571 | 1:200 | |
| Rabbit | Donkey | Alexa Fluor 647 | Invitrogen | A31573 | 1:200 | |
| Rat | Goat | Texas Red | Life Technologies | A11007 | 1:200 | |
| Rat | Rabbit | Texas Red | Abcam | ab6732 | 1:200 |
For digital imaging, an epifluorescence microscope (EVOS fl; Advanced Microscopy Group, Bothell, WA) was used to acquire images. Moreover, confocal microscopy was done using the Zeiss ELYRA/LSM 780 microscope (Carl Zeiss Microscopy, Jena, Germany) with ×40 and ×63 plan-apochromat oil-immersion objectives, a four-channel filter set (BP 420-488, BP 495-575, BP 570-650, LP 655) and an electron-multiplying CCD camera. Images were processed with ZEN imaging software. We made sure that filters in both microscopes showed only fluorescence of the appropriate fluorescent dye.
Counting Cells
Cells showing immunoreactivity for TMEM119 and other antigens were counted in digital images of at least three different samples using Adobe Photoshop (Adobe, San Jose, CA), on an area of 15,000 μm2 in brain sections and at a length of 400 μm in retina sections, and we evaluated extent of colocalization of the different antigens. Results of cell counting are given as medians with median absolute deviation.
Results
TMEM119 IR in the Brain
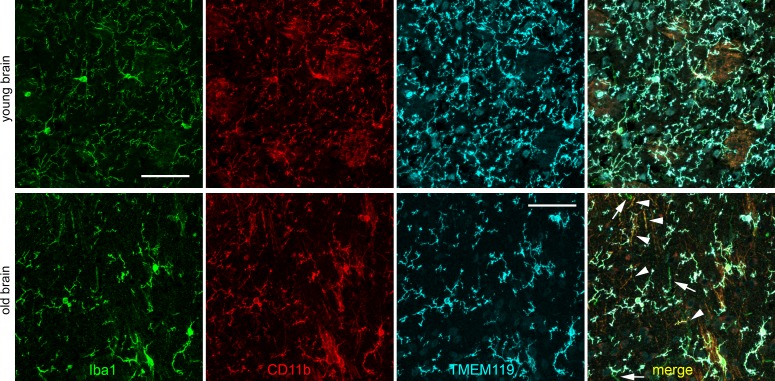
We first checked IR for TMEM119 in cryosections of mouse brain (Fig. 1). We found that the vast majority of microglial cells showed IR for Iba1, CD11b, and TMEM119. In the brain of young mice, intensity levels of IR showed only little differences in individual cells. Most of the cells exhibited similar IR for the three markers, and a few other cells were positive mainly for one of the three markers. In old mice, IR for TMEM119 was reduced, and some microglial cells showed IR almost just for Iba1 and CD11b (white arrowheads in Fig. 1) or even almost just for Iba1 (white arrows in Fig. 1). In some cases, TMEM119 IR displayed different intensity within a single microglial cell; in particular, it sometimes was weaker in the processes of the microglial cells.
Figure 1.
Confocal images of immunohistochemical labeling of Iba1 (green), CD11b (red), and TMEM119 (turquoise, antibody by Synaptic Systems) as indicated in brain cryosections obtained from a young (upper row) and old (lower row) mouse. In the merged image of the old mouse, white arrows point to cells that display almost only Iba1 IR, and white arrowheads to cells with Iba1 IR and CD11b IR and almost no TMEM119 IR. Scale bars: 50 μm.
We compared anti-TMEM119 antibodies by two different suppliers, Abcam (the antibody used in the original studies of brain TMEM119 labeling; Cambridge, United Kingdom) and Synaptic Systems (Goettingen, Germany), on brain sections from young and old mice. Procedures for labeling with the Abcam antibody were very similar to the original work, with some slight changes in fixation time, detergent, and blocking agent used. Nevertheless, these changes did not prevent strong labeling in brain sections of young and old mice. In general, we saw only small differences between these two antibodies regarding amount and intensity of microglia labeling, indicating that they are both similarly suitable on brain sections. As an example, 81.2 ± 6.6% of TMEM119+ cells and 82.4 ± 10.3 of Iba1+ cells showed colocalization for both markers in the old mouse with the antibody by Abcam, and 85.4 ± 14.6% of TMEM119+ cells and 75.6 ± 21.9 of Iba1+ cells with the antibody by Synaptic Systems.
As may be deduced from the appearance of labeling in Figure 1, the number of cells showing IR for Iba1 and TMEM119 was smaller in the brain of old mice. Indeed, counting of labeled cells revealed such a decrease. In the brain of young mice, we counted 74.5 ± 3.0 Iba+ cells and 72.5 ± 4.5 TMEM119+ cells, and in the brain of old mice 23.5 ± 1.7 Iba+ cells and 19.0 ± 5.0 TMEM119+ cells (antibody by Synaptic Systems).
TMEM119 IR in the Naïve Retina
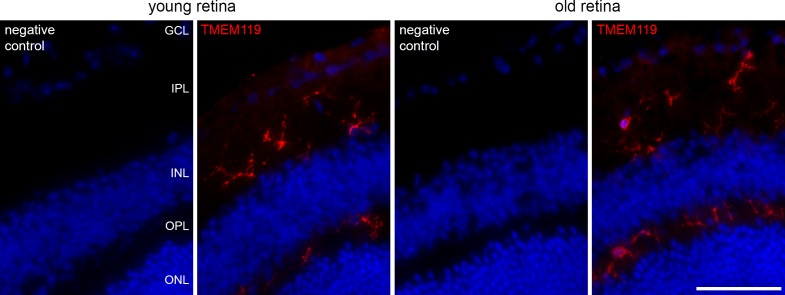
IR for TMEM119 also was found in the retina. In Figure 2, labeling of microglial cells by the anti-TMEM119 antibody by Synaptic Systems in the retina of young and old mice is shown, together with the corresponding negative controls. Labeled microglial cells can be seen in the inner and outer plexiform layers.
Figure 2.
Immunohistochemical labeling of microglial cells in retinal cryosections of a young and old mouse against TMEM119 (red, antibody by Synaptic Systems) as indicated. Corresponding negative controls are included. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bar: 50 μm.
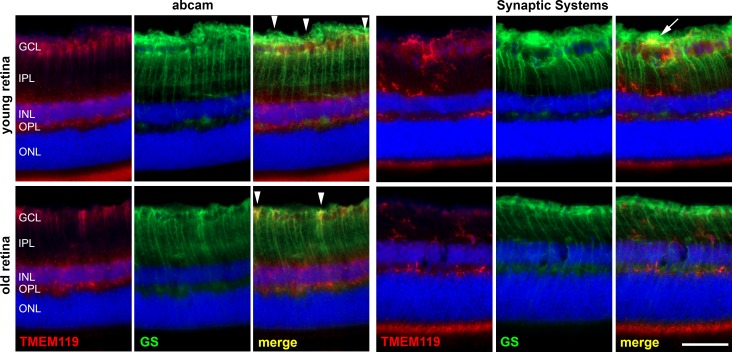
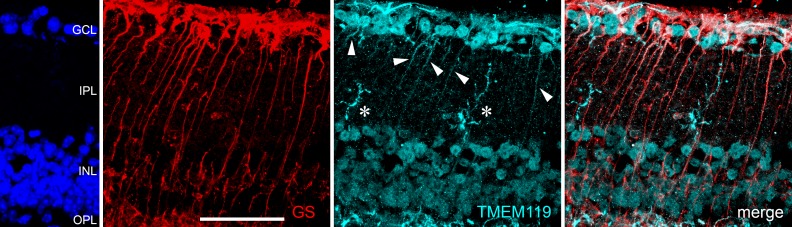
However, despite all attempts to optimize the protocols of eye fixation and staining, the Abcam antibody did not provide good labeling of the retinal microglia. This was in sharp contrast to the results obtained in brain samples. In the retina, we saw some stained microglial cells mainly in the outer plexiform layer. In the inner plexiform and ganglion cell layers, we found almost no TMEM+ cells that could be microglia with the Abcam antibody. In contrast, long stretched cells were labeled that had the shape of Müller cells. Therefore, we performed simultaneous labeling of a marker of Müller cells, GS, and we found some colocalization with TMEM119 IR (Fig. 3). In the retina of young mice, 77.8 ± 13.1% of TMEM119-positive cells also showed IR for GS, whereas only 43.4 ± 1.8% of TMEM119-positive cells also displayed IR for CD11b. These portions were 38.5 ± 11.5% and 83.3 ± 16.7%, respectively, in the retina of old mice.
Figure 3.
Immunohistochemical staining of a retinal cryosection from a young and old mouse against TMEM119 (red) and GS as indicated. Anti-TMEM119 antibodies were delivered by Abcam (left) and by Synaptic Systems (right). With the anti-TMEM119 antibody by Abcam, a poor labeling of microglial cell was achieved, and a clear IR of other cell populations in the retina; for example, the Müller cells, as demonstrated by GS IR. White arrowheads point to particularly obvious colocalization of TMEM119 IR and GS IR. White arrow points to an overlap of TMEM119 IR and GS IR without real colocalization. Scale bar: 50 μm.
When the antibody by Synaptic Systems was used, TMEM119 IR was seen for cells that had the same morphology and were located in the same layers as microglial cells, and no colocalization with GS IR was found (Fig. 3). At some places, there is a slight overlap of TMEM119 IR and GS IR in the ganglion cell layer and nerve fiber layer (arrow in Fig. 3). Nevertheless, this finding is caused by possible simultaneous presence of microglial cells and Müller cell end feet in these layers, and the different shapes of cells exhibiting corresponding IR indicated that there is no real colocalization. Therefore, we decided to use only the antibody by Synaptic Systems in all further experiments (Figs. 4–9).
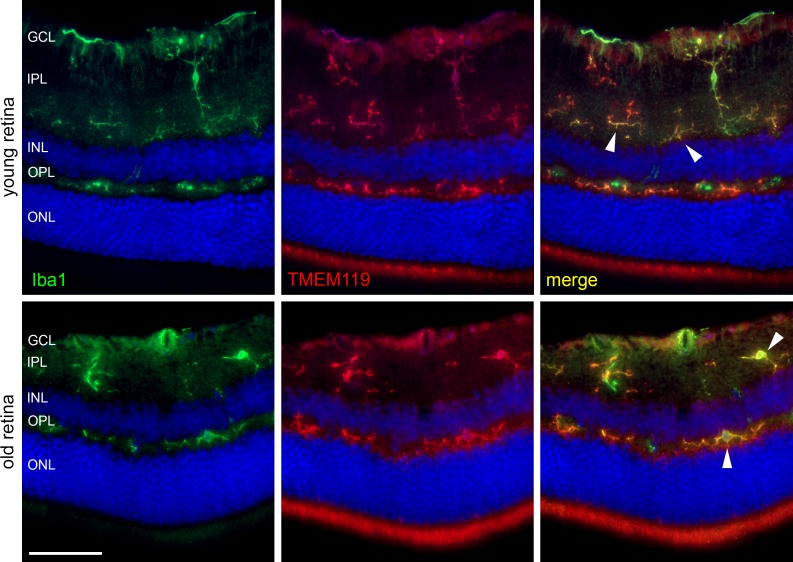
Figure 4.
Immunohistochemical labeling of microglial cells in retinal cryosections of a young and old mouse against Iba1 (green) and TMEM119 (red, antibody by Synaptic Systems) as indicated. Arrowheads point to cells that are similarly positive for Iba1 and TMEM119. Scale bar: 50 μm.
Figure 9.
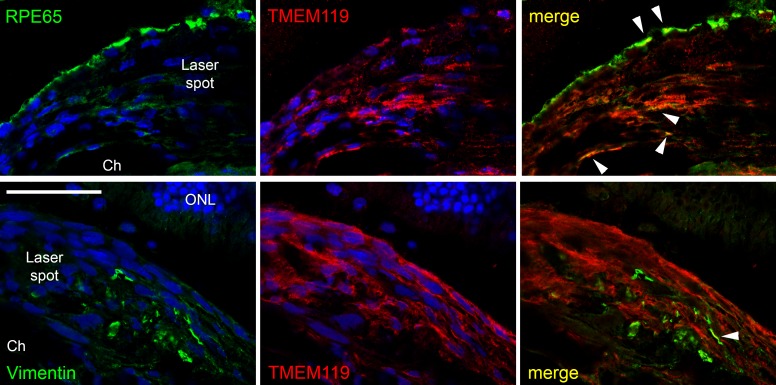
Confocal images of double labeling of the proliferation area in the laser spot for TMEM119 (red, antibody by Synaptic Systems) and RPE65 (green, upper row) and vimentin (green, lower row). Colocalization could be found only in very few cases (white arrowheads). ONL, outer nuclear layer; Ch, choroid. Scale bar: 50 μm.
In addition, a strong TMEM119 IR was seen in the layer of photoreceptor inner segments, in particular with the antibody by Synaptic Systems.
In Figure 4, an example of double labeling against TMEM119 and the typical microglial marker Iba1 is shown. Labeled microglial cells are located in the usual layers; that is, the ganglion cell and inner and outer plexiform layers. In general, there is a very good colocalization of TMEM119 IR with Iba1 IR. Nevertheless, there are differences in labeling intensity. Fluorescence intensity for TMEM119 is present in the inner plexiform and outer plexiform layers, whereas it seems weaker in the ganglion cell layer. Moreover, TMEM119 IR and Iba1 IR are distributed unevenly across some single microglial cells as noticed already in the brain. In addition, there is IR for TMEM119 with little or without colocalization for Iba1, and vice versa.
Counting of labeled cells in digital images revealed that 63.6.0 ± 10.3% of Iba1+ cells also were positive for TMEM119 in retinas of young mice, and this number increased to 85.0 ± 8.3% in retinas of old mice. On the other hand, 63.3 ± 9.5% of TMEM119+ cell were positive for Iba1 in young retinas, and 87.2 ± 7.9% in old retinas.
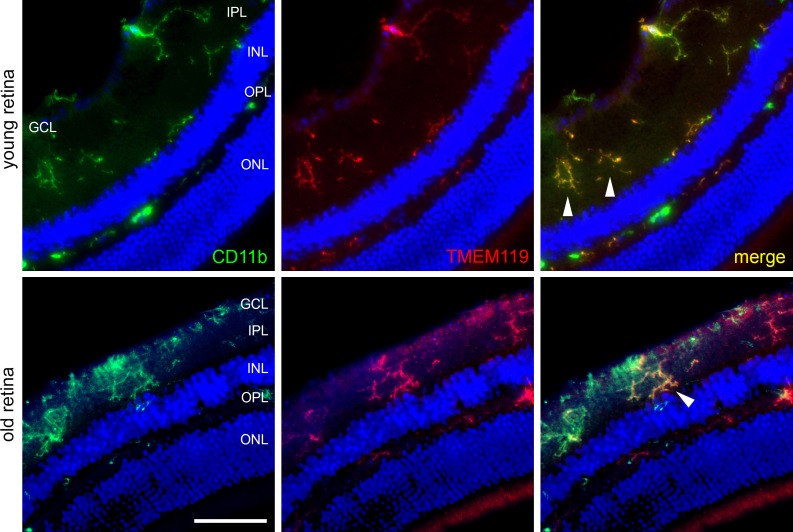
A similar picture is found when double labeling of TMEM119 and CD11b is performed (Fig. 5). Again, most microglial cells showed a colocalization of TMEM119 with microglial marker CD11b, and the extent of TMEM119 IR compared to CD11b IR showed clear variations between individual cells. Counting of stained cells in digital images revealed that 94.7 ± 5.3% of CD11b+ cells also were positive for TMEM119 in retinas of young mice, and this number decreased to 79.2 ± 1.4% in retinas of old mice. In our samples, every TMEM119+ cell displayed CD11b IR in at least a small part of the cell.
Figure 5.
Immunohistochemical labeling of microglial cells in retinal cryosections of a young and old mouse against CD11b (green) and TMEM119 (red, antibody by Synaptic Systems) as indicated. Arrowheads point to cells that are similarly positive for CD11b and TMEM119. Scale bar: 50 μm.
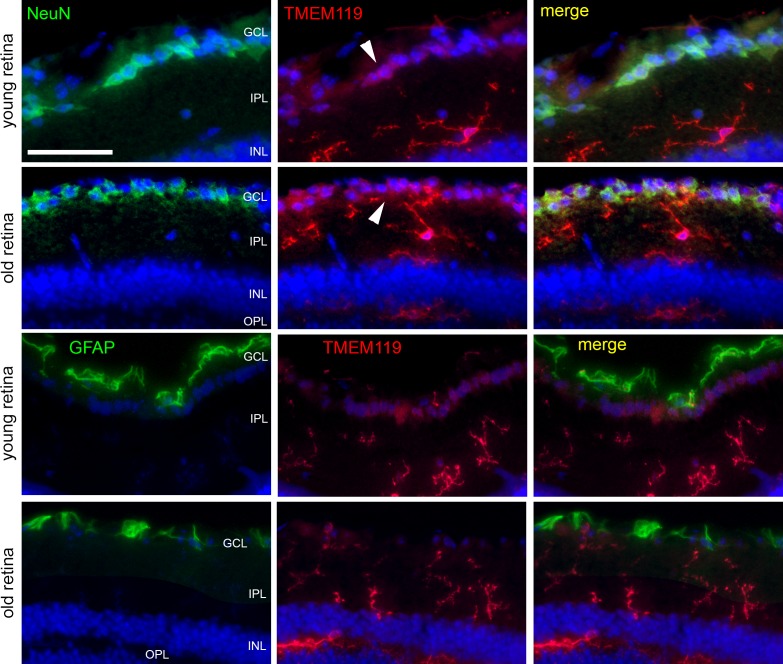
Despite its higher specificity on microglial cells, the anti-TMEM119 antibody by Synaptic Systems showed a slight IR for TMEM119 in the ganglion cell layer that could not be attributed to the microglia. Therefore, we performed double labeling for TMEM119 and markers for the two other major cell populations in the ganglion cell layer, neuronal marker NeuN for retinal ganglion cells, and glial fibrillary acidic protein (GFAP) for astrocytes (Fig. 6). In some retinal ganglion cells identified by NeuN IR, a slight IR for TMEM119 was seen. We found absolutely no colocalization of GFAP and TMEM119 IR in the samples.
Figure 6.
Immunohistochemical staining of retinal cryosections to label microglial cells (TMEM119, antibody by Synaptic Systems, red), ganglion cells (NeuN, green), and astrocytes (GFAP, green) in retinas of young and old mice, as indicated. Only inner layers of the retinas are shown. White arrowheads point to TMEM119-positive retinal ganglion cells. Scale bar: 50 μm.
TMEM119 IR in the Retina After Laser Treatment
It then was of interest to check IR for TMEM119 in the experimental model of laser-induced choroidal neovascularization (CNV). In this model, the retinal pigment epithelium (RPE) and the Bruch's membrane are destroyed by a focused laser pulse, which initiates a kind of wound healing accompanied by neovascularization. It is known that microglial cells become activated and migrate to the site of injury, with the highest activity at day 4 after laser treatment (see prior studies10,11).
We checked TMEM119 IR 4 days after laser treatment, and we found a changed pattern of TMEM119 IR. The first finding was that TMEM119 IR becomes more visible throughout other cells in the retina. In particular, numerous cells in the ganglion cell and inner nuclear layers display a distinct IR for TMEM119. Moreover, the number of retinal microglial cells with weaker TMEM119 IR seems to be larger than in the naïve retina. Closer inspection of TMEM119 IR in the retinas of laser-treated eyes revealed that also Müller cells became slightly TMEM119+, as demonstrated by labeling of GS (Fig. 7).
Figure 7.
Confocal images of immunohistochemical staining of a retinal cryosection from a young mouse 4 days after laser treatment by labeling of GS (red) and TMEM119 (turquoise, antibody by Synaptic Systems) as indicated. Retinal layers are indicated by DAPI staining on the left margin. Besides microglial cells (white asterisks) and cells in the ganglion cell layer and inner nuclear layer, also the tiny longish Müller cells show TMEM119 IR (white arrowheads). Scale bar: 50 μm.
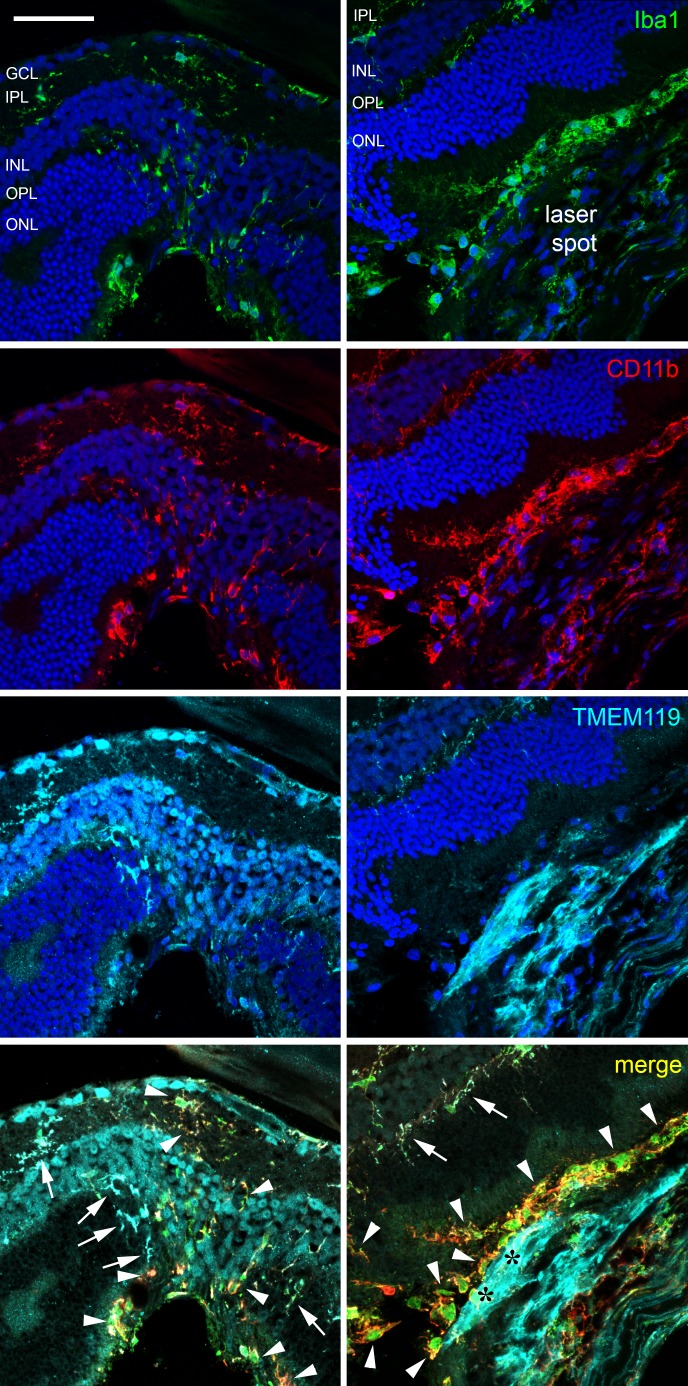
It must be mentioned that this behavior is present in the retina remote of the laser spots. Whereas microglial cells are clearly TMEM119+ more distant from the laser spot, no matter if in the inner or outer plexiform layer, most microglial cells that are CD11b+ and Iba1+ in the laser spot and in the track where the laser beam went through the inner retina displayed no IR for TMEM119 (Fig. 8).
Figure 8.
Confocal images of immunohistochemical staining of a retinal cryosection from a young mouse 4 days after laser treatment against Iba1 (green), CD11b (red), and TMEM119 (turquoise, antibody by Synaptic Systems) as indicated. DAPI staining is shown in the images of single channels and is not shown in the merged images for more clarity. In the left column, part of the inner retina above the laser spot is shown, while the laser spot including the proliferation area is shown in the right column. White arrows point to cells positive for all three microglial markers, whereas white arrowheads point to cells only positive for Iba1 and/or CD11b. Black asterisks indicate the proliferation area where cells are only TMEM+. Scale bar: 50 μm.
On the other hand, there are numerous TMEM119+ cells in the proliferation area of the laser spot. They do not have the typical shape of microglial cells or macrophages, display a stretched morphology and show no IR for Iba1 or CD11b (area with black asterisks in Fig. 8). The nature of these TMEM119+ cells is not clear yet, and we can speculate only at the moment. Only few of them show IR for vimentin, a marker for fibroblasts, and there is almost no colocalization between TMEM119 IR and RPE65 IR (Fig. 9).
We also checked IR for CD11c, CD45, and CD8 in the laser spot. However, we could not detect unambiguously immune cells positive for these markers in the laser spots, and a colocalization with TMEM119 was not clear (not shown).
Discussion
The reliable identification of microglial cells in the naïve adult retina is possible by their labeling with antibodies against Iba1 or CD11b, and they display a typical ramified shape and small cell bodies. Extent of IR for these two markers does not change notably during the life span of the mice. In pathologic situations, microglial cells get activated and migrate to the site of damage. An enhanced number of cells positive for CD11b and Iba1 can be found consequently at the sites of damage. In this context, it is disputed whether also peripheral immune cells, in particular macrophages, may be present, as they also may be positive for microglial markers. Bennet et al.6 introduced transmembrane molecule TMEM119 as a novel specific marker for microglial cells and stated that peripheral macrophages were not positive for TMEM119.
At first, as a kind of positive control, we checked IR for TMEM119 in sections of mouse brain. Similar to the findings reported by Satoh et al.,7 we found that most microglial cells were TMEM119+, and there was a big overlap of IR for TMEM119 and Iba1.
Moreover, the anti-TMEM119 antibodies by the two different suppliers we tested showed very similar labeling properties in the mouse brain. This is not the case in the mouse retina. Despite numerous attempts to optimize protocols of eye fixation and immunohistochemical staining, we found a less intense and a less specific labeling of microglial cells when the antibody by Abcam was used. The reason for the different staining behavior could be possibly the different immunogens used to produce the antibodies. The Abcam antibody was made using a recombinant fragment (GST-tag) within mouse TMEM119 amino acid 100 to the intracellular C-terminus, and the Synaptic Systems antibody was made using a recombinant protein corresponding to amino acids 189 to 280. To date, we do not know whether the TMEM119 molecule is expressed in different splice variants or other kinds of different subtypes in microglial cells in the brain and retina, or what else could explain the different behavior of the antibodies.
There is a change of TMEM119 IR with increasing age of the retina, and if the retina is damaged by laser treatment. The reasons and mechanisms of these changes are not clear to date. Expression analysis of microglial cells after laser treatment revealed that TMEM119 mRNA levels are clearly decreased at the site of the laser spot (Wieghofer, 2019, University of Leipzig, personal communication), which is corroborated by our immunohistochemical findings in the laser spot, where the microglial cells lost TMEM119 IR. The finding that microglial cells in the immediate vicinity of the laser spot were TMEM119+ demonstrates that the procedure of labeling worked at all.
On the other hand, a number of other retinal cells became positive for TMEM119, in particular Müller cells in the inner retina and to date unidentified cells in the proliferation area of the laser spots. Kaiser and Feng12 created transgenic mice by knocking-in EGFP, and they found that TMEM119 was not exclusively expressed by the microglia even in nontreated mice. Therefore, unambiguous identification of microglia remains a challenging task.
Several antigens can be found on microglial cells.4,13 They carry some macrophage markers, such as the integrin CD11b, surface glycoprotein F4/80, colony-stimulating factor-1 receptor, fractalkine receptor CX3CR1, and calcium-binding protein Iba1. As long as it can be ensured that no peripheral monocytes invade the retina, labeling of these markers can be used to detect microglial cells in the retina, and anti-Iba1 antibodies are used particularly often. Butovsky et al.14 described CD39 (ectonucleoside triphosphate diphosphohydrolase) as an antigen specific for brain microglia that does not occur in peripheral monocytes. Labeling of CD39 for the detection of microglial cells also has been performed in the retina (see Hu et al.15). However, blood vessels also are positive for CD39, which requires attention when judging histologic sections.
Recent studies have demonstrated that most immune cells in the laser spots are indeed resident microglia cells and not invading peripheral immune cells (Wieghofer, 2019, University of Leipzig, personal communication). Therefore, it is no surprise that we found almost no CD11c+ or CD45+ cells in the laser spot. As it was questionable if they display TMEM119 IR, we cannot conclude whether peripheral immune cells really do not show TMEM119 IR, as stated by Satoh et al.7 and Haage et al.8
In conclusion, more investigation of TMEM119 function and specific expression in the retina will be necessary; for example, by in situ hybridization or RNA scope, to better understand the importance of this protein and its role in the retinal microglia. TMEM119 may be a reliable microglial marker in the brain, in particular because invading peripheral monocytes do not express TMEM119. In the retina, the situation is more complicated, and it must be checked carefully whether the microglia retains TMEM119 expression in pathologic situations. We believe that Iba1 and CD11b are more useful markers for the retinal microglia than TMEM119, which also is important for research on therapeutic options in ocular diseases.
Acknowledgments
Supported in part by Henan Province Heatlh Ministry, PR China, project no. 2018061 (NS), and by a grant from the Helmut-Ecker-Stiftung (Ingolstadt, Germany), no. 02/17 (PH).
Disclosure: N. Su, None; S. März, None; T. Plagemann, None; J. Cao, None; G.-J. Schnittler, None; N. Eter, None; P. Heiduschka, None
References
- 1.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 2.Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: Insights from novel mouse models. Immunobiology. 2010;215:685–691. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Madeira MH, Boia R, Santos PF, Ambrósio AF, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat Inflamm. 2015. 673090. [DOI] [PMC free article] [PubMed]
- 4.Li L, Eter N, Heiduschka P. The microglia in healthy and diseased retina. Exp Eye Res. 2015;136:116–130. doi: 10.1016/j.exer.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Jiang ZH, Peng J, Yang HL, et al. Upregulation and biological function of transmembrane protein 119 in osteosarcoma. Exp Mol Med. 2017;49:e329. doi: 10.1038/emm.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh J, Kino Y, Asahina N, et al. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 2016;36:39–49. doi: 10.1111/neup.12235. [DOI] [PubMed] [Google Scholar]
- 8.Haage V, Semtner M, Vidal RO, et al. Comprehensive gene expression metaanalysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol Commun. 2019;7:20. doi: 10.1186/s40478-019-0665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attaai A, Neidert N, von Ehr A, Potru PS, Zöller T, Spittau B. Postnatal maturation of microglia is associated with alternative activation and activated TGFβ signaling. Glia. 2018;66:1695–1708. doi: 10.1002/glia.23332. [DOI] [PubMed] [Google Scholar]
- 10.Eter N, Engel DR, Meyer L, et al. In vivo visualization of dendritic cells, macrophages, and microglial cells responding to laser-induced damage in the fundus of the eye. Invest Ophthalmol Vis Sci. pp. 3649–3658. 200849. [DOI] [PubMed]
- 11.Li L, Heiduschka P, Alex AF, Niekämper D, Eter N. Behaviour of CD11b-positive cells in an animal model of laser-induced choroidal neovascularisation. Ophthalmologica. 2017;237:29–41. doi: 10.1159/000453550. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser T, Feng G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro. 2019;6 doi: 10.1523/ENEURO.0448-18.2019. 0448–18.20191-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol. 2015;7:a020537. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butovsky O, Siddiqui S, Gabriely G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu P, Hunt NH, Arfuso F, et al. Increased indoleamine 2,3-dioxygenase and quinolinic acid expression in microglia and müller cells of diabetic human and rodent retina. Invest Ophthalmol Vis Sci. 2017;58:5043–5055. doi: 10.1167/iovs.17-21654. [DOI] [PMC free article] [PubMed] [Google Scholar]