Abstract
To understand the effect of type 1 diabetes (T1D) on bone mineral content (BMC) and bone density (BMD), we studied 125 T1D adolescents and 80 pubertal stage matched controls. T1D was associated with lower whole-body BMC and BMD compared to controls, even when adjusted for age, sex and sex hormones.
Keywords: Bone mineral density, Bone mineral content, Type 1 diabetes, Adolescents, Fracture risk, Bone health
1. Introduction
Osteoporosis is generally considered a disease of postmenopausal women. However, in type 1 diabetes (T1D), osteoporotic fractures are common in both men and women and risk is increased even in children and adolescents.1–3
The effects of T1D on bone metabolism are not well understood. Insulin deficiency, low insulin-like growth factor-1 (IGF-1), increased urinary calcium excretion and elevated inflammatory cytokines are thought to be responsible for low bone mineral density (BMD) in patients with T1D.4
The incidence of T1D peaks between the ages of 10 and 12 years.5 Development of T1D at a young age may reduce bone accrual, resulting in lower bone mass.6 However, studies on the effects of T1D on bone mass and/or BMD in youth are conflicting.7–10 Therefore, we aimed to investigate differences in whole-body bone mineral content (BMC) and BMD between adolescents with T1D and pubertal stage matched healthy peers.
2. Methods
Adolescents (12–19 years) with T1D (n = 125) and pubertal stage frequency-matched controls (n = 80) with whole-body BMC and BMD dual x-ray absorptiometry (DXA) available were included from two ongoing studies: RESistance to InSulin in Type 1 And Type 2 diabetes (RESISTANT) and Effects of MEtformin on CardiovasculaR Function in AdoLescents with Type 1 Diabetes (EMERALD).11,12 T1D was defined per the American Diabetes Association criteria plus ≥1 diabetes-associated antibodies.11,12 Controls were healthy adolescents without diabetes or a family history of diabetes; absence of diabetes was confirmed by a 2-h, 75-g oral glucose tolerance test. Height, weight and body mass index (BMI) were measured as described previously.11,12 Pubertal development was assessed by a pediatric endocrinologist using Tanner and Marshall criteria. Exclusion criteria were Tanner stage <2, hypertension (blood pressure 003E 140/90 mmHg), hemoglobin <9%, serum creatinine >1.5 mg/dl, HbA1c ≥12%, smoking and receiving steroid or oral hypoglycemic agents. Both studies were approved by the Colorado Multiple Institutional Review Board (COMIRB) and all participants and/or parents provided written informed assent and consent.
Laboratory testing was performed in the AM after a 12-h overnight fast. HbA1c was measured by DCCT-calibrated, ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA). Serum creatinine, estradiol, testosterone and c-peptide were measured by standard methods at the Clinical and Translational Research Center (CTRC) Laboratory. Whole-body BMC, BMD, and body composition were assessed by DXA (Hologic, Waltham, MA).
All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). Variables were checked for the distributional of normality. Continuous variables were compared using unpaired t-tests and categorical variables were compared using chi-square tests. Multiple regression models were used to test the association between BMC, BMD and T1D while adjusting for age, sex, ethnicity and sex hormones (testosterone and/or estradiol). All tests performed were two-sided and a p-value <0.05 was considered statistically significant.
3. Results
Of 205 adolescents, 125 had T1D and 80 were controls. Due to known sex differences in BMC and BMD, Table 1 presents baseline characteristics by diabetes status and sex. Adolescents with T1D were older and had lower fat mass than controls. As expected, HbA1c was higher and C-peptide lower in adolescents with T1D compared to controls.
Table 1.
Baseline participant characteristics.
| Male | Female | |||
|---|---|---|---|---|
| T1D (n = 60) | Control (n = 28) | T1D (n = 65) | Control (n = 52) | |
| Age (years)* | 15.6 | 14.4 | 15.9 | 14.8 |
| Duration of diabetes (years) | 6.8 | N/A | 6.9 | N/A |
| Tanner stages (N, %) | ||||
| II | 3 (5) | 4 (14) | 1 (2) | 0 (0) |
| III | 7 (13) | 5 (18) | 4 (6) | 2 (4) |
| IV | 19 (35) | 13 (46) | 10 (15) | 6 (12) |
| V | 26 (47) | 6 (21) | 50 (77) | 44 (85) |
| BMI (kg/m2)* | 23.60 | 25.50 | 23.94 | 27.26 |
| Ethnicity* (N, %) | ||||
| White | 47 (78) | 16 (57) | 60 (92) | 24 (46) |
| Hispanic | 6 (10) | 10 (36) | 3 (5) | 15 (29) |
| Other | 7 (12) | 2 (7) | 2 (3) | 13 (25) |
| HbA1c (%)* | 8.56 | 5.13 | 8.61 | 5.25 |
| C-peptide (ng/mL)* | 0.14 | 2.14 | 0.13 | 2.48 |
| Estradiol (pg/mL)* | - | - | 85.5 | 57.28 |
| Testosterone (nmol/L)* | 448.23 | 320.12 | - | - |
| Fat mass (kg)* | 18.25 | 22.81 | 21.15 | 27.22 |
| Lean mass (kg) | 50.52 | 49.42 | 41.28 | 41.47 |
p < 0.05 T1D; type 1 diabetes, BMI; body mass index.
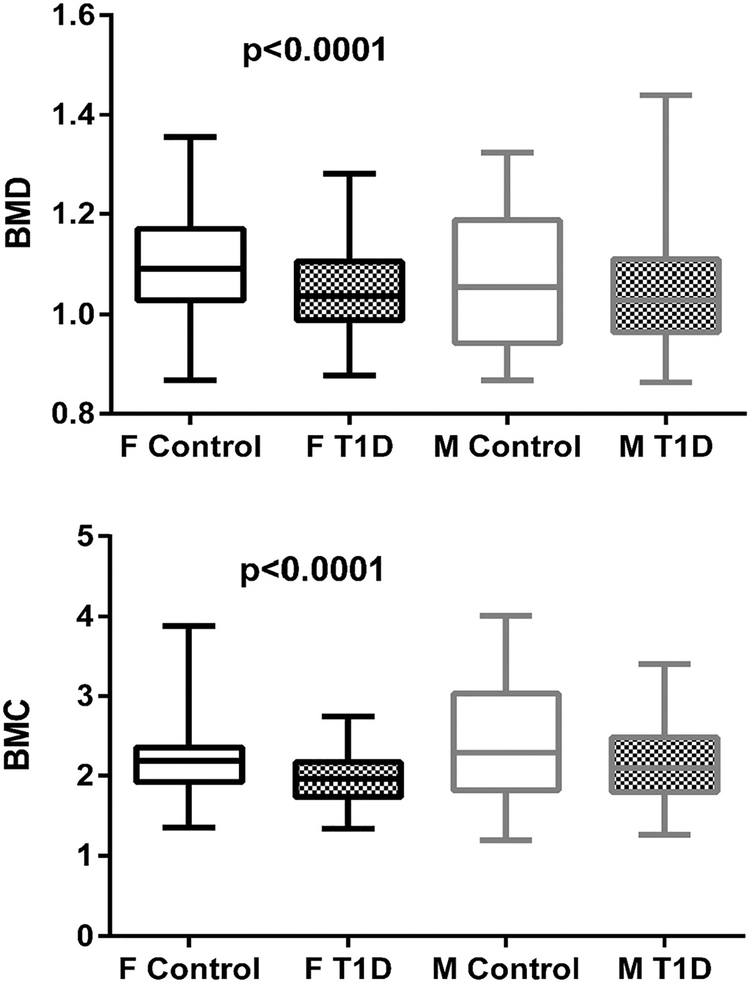
Total whole-body BMD (Least Squares (LS) mean T1D 1.03 ± 0.01, control 1.10 ± 0.01, p < 0.0001) and BMC (LS mean T1D 1.97 ± 0.05, control 2.34 ± 0.05, p < 0.0001) were lower in adolescents with T1D when adjusted for age, sex and ethnicity. T1D status was associated with lower whole-body BMD in males (LS mean T1D 1.02 ± 0.02, control 1.09 ± 0.02, p = 0.0028) and females (LS mean T1D 1.04 ± 0.02, control 1.10 ± 0.01, p = 0.0006) when adjusted for age and ethnicity (Fig. 1A and B). The differences in whole-body BMD and BMC remained significant after further adjustment for sex steroids (data not shown).
Fig. 1.
Whole body bone mineral density (A) and bone mineral content (B) by sex and diabetes status. # P-values are from models adjusted for age and race/ethnicity; F; females, M, males, T1D; Type 1 diabetes.
4. Discussion
T1D is common in children and adolescents,5 occurring during a crucial period for bone accrual, which may result in higher risk for fractures later in life. A study in children and adolescents with T1D showed reduced total BMC in T1D patients compared to controls.6 This difference was driven by significantly lower lumbar spine and total body BMC in girls with T1D than control girls (p = 0.002); no such difference was observed in boys.6 Similarly, most, but not all, studies in children and adolescents with T1D have shown lower BMD than controls.6–10 Our results agree with other studies showing reduced whole-body BMC and BMD among adolescents with T1D. However, our findings go beyond the limitations of reported studies by adjusting whole-body BMC and BMD for age, sex, ethnicity and sex steroid levels.
Age and fat mass have major influences on bone mineral accrual and density.13,14 Despite older age and lower fat mass, adolescents with T1D had lower whole body BMC and BMD compared to controls.
A recent meta-analysis of 16 studies showed a modest reduction in femoral neck BMD and no difference in spine BMD among adults with T1D compared to controls.9 Patients with T1D may have a normalization of BMD over time. Bechtold et al. evaluated bone size using peripheral quantitative computed tomography in children with T1D at baseline and after a mean of 5.6 years.15 They reported a normalization of total and cortical cross-sectional area at the non-dominant radius over that period.15 Similarly, normalization of BMD was reported in young women with T1D over 2 years.16 Therefore, lower BMC and/or BMD in children and adolescents with T1D alone may not fully explain the higher fracture risk observed among adults with T1D. Another possibility is that temporarily impaired bone accrual during childhood or adolescence results in an altered matrix to mineral ratio and impaired bone quality. However, no studies to-date have assessed the mineral to matrix ratio or collagen quality among children or adolescents with T1D.
Limitations to the present study include the cross-sectional design, use of a convenience cohort from ongoing studies, and lack of site-specific BMD measurements.
In a summary, we found that whole-body BMC and BMD are lower among adolescents with T1D than controls, even when adjusting for sex, age, ethnicity, and sex steroids. Further research is needed to understand the influence of T1D on bone accrual and bone quality to reduce fragility fracture among adults with T1D.
Acknowledgment
We would like to thank the participants and their families, Children’s Hospital Colorado, The Barbara Davis Center for Diabetes and the University of Colorado CTRC nurses. VNS conceptualized the study, interpreted the data, edited and reviewed the manuscript. PJ, HK, SF prepared the first draft of the manuscript, JT and LP did statistical analysis. KJN reviewed the manuscript and provided feedback. KJN is the guarantor of the work and, as such, has full access to all the data in the study. All authors have seen and approved the final manuscript.
Funding
We would like to thank the following funding sources: ADA 7-11-CD-08, JDRF Award #11-2010-343, National Center for Research Resources (NCRR) K23-RR-020038-01, NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 1R56-DK-088971-01, JDRF 5-2008-291 and NIH/NCATS Colorado CTSI UL1 TR001082.
References
- 1.Shah VN, Shah CS, Snell-Bergeon JK. Risk for fracture in type 1 diabetes: a meta-analysis and review of the literature. Diabet Med 2015;32:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestergaard P Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int 2007;18:427–44. [DOI] [PubMed] [Google Scholar]
- 3.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care 2015;38:1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhaon P, Shah VN. Type 1 diabetes and osteoporosis: a review of literature. Indian J Endocrinol Metab 2014;18:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Chapter 1: epidemiology of type 1 diabetes. Endocrinol Metab Clin N Am 2010;39:481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Léger J, Marinovic D, Alberti C, Dorgeret S, Chevenne D, Marchal CL, et al. Lower bone mineral content in children with type 1 diabetes mellitus is linked to female sex, lowinsulin-like growth factor type I levels, and high insulin requirement. J Clin Endocrinol Metab 2006;91:3947–53. [DOI] [PubMed] [Google Scholar]
- 7.Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ. Alterations in bone characteristics associated with glycemic control in adolescents with type 1diabetes mellitus. J Pediatr 2004;144:56–62. [DOI] [PubMed] [Google Scholar]
- 8.Pan H, N W, Yang T, He W Association between bone mineral density and type 1 diabetes mellitus: a meta-analysis of cross-sectional studies. Diabetes/metabolism research and reviews. Diabetes Metab Res Rev 2014;30:531–42. [DOI] [PubMed] [Google Scholar]
- 9.Shah VN, Harrall KK, Joshee P, et al. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta-analysis and review of the literature. Osteoporos Int 2017;28:2601–10. [DOI] [PubMed] [Google Scholar]
- 10.Mosso C, Hodgson MI, Ortiz T, Reyes ML. Bone mineral density in young Chilean patients with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2016;29:731–6. [DOI] [PubMed] [Google Scholar]
- 11.Bjornstad P, Cree-Green M, Baumgartner A, Coe G, Reyes YG, Schäfer M, et al. ISPAD clinical guideline goals is associated with higher insulin sensitivity and cardiopulmonary fitness in adolescents with type 1 diabetes: results from RESistance to InSulin in type 1 ANd type 2 diabetes (RESISTANT) and Effects of MEtformin on cardiovasculaR function in AdoLescents with type 1 Diabetes (EMERALD) studies. Pediatr Diabetes 2018. May;19(3):436–42, 10.1111/pedi.12598. [Epub 2017 Oct 30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornstad P, Cree-Green M, Baumgartner A, Coe G, Reyes YG, Schafer M, et al. Leptin is associated with cardiopulmonary fitness independent of body-mass index and insulin sensitivity in adolescents with type 1 diabetes: a brief report from the EMERALD study. J Diabetes Complicat 2017;31:850–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marwaha RK, Garg MK, Bhadra K, Mahalle N, Mithal A, Tandon N. Assessment and relation of total and regional fat mass with bone mineral content among Indian urban adolescents. J Pediatr Endocrinol Metab 2015;28:1085–93. [DOI] [PubMed] [Google Scholar]
- 14.Clark EM, Ness AR, Tobias JH. The Avon longitudinal study of parents and children study. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab 2006;91:2534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bechtold S, Putzker S, Bonfig W, Fuchs O, Dirlenbach I, Schwarz HP. Bone size normalizes with age in children and adolescents with type 1 diabetes. Diabetes Care 2007;30:2046–50. [DOI] [PubMed] [Google Scholar]
- 16.Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care 2008;31:1729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]