Abstract
Objectives:
The aim of this study was to evaluate the effect of serum triglycerides on the development of multiple or persistent organ failure in patients with acute pancreatitis.
Methods:
A retrospective cohort study was conducted among patients hospitalized for acute pancreatitis between 2006 and 2013. Triglyceride levels measured before and within 72 hours of admission were compared. In addition, the effect of triglyceride levels on the development of multiple or persistent organ failure during hospitalization for acute pancreatitis was assessed.
Results:
Among 2519 patients, 267 patients (10.6%) developed organ failure, of which 75 patients developed multiple system organ failure and 82 patients developed persistent organ failure. Triglyceride levels in patients who developed organ failure were initially much higher than in patients who did not develop organ failure, but by 72 hours into admission, approached levels of patients who did not develop organ failure. Approximately 8% of patients had triglyceride levels greater than 500 mg/dL, the majority of which had similarly high levels before admission.
Conclusions:
Increased triglyceride levels were associated with the development of multiple or persistent organ failure among patients hospitalized with acute pancreatitis. Patients with high triglyceride levels at the time of admission were likely to have high triglyceride levels before admission.
Keywords: cohort, pancreatitis, triglycerides
Acute pancreatitis is the most common gastrointestinal disease requiring hospitalization, accounting for more than 270,000 hospitalizations on an annual basis in the United States.1 The clinical course of acute pancreatitis can vary from a relatively mild, self-limited illness to a more severe disease complicated by persistent or multisystem organ failure.
Elevated triglyceride levels are an established risk factor for acute pancreatitis, with hypertriglyceridemia accounting for 1% to 10% of all cases of acute pancreatitis.2,3 The incidence of acute pancreatitis has been shown to increase by the level of baseline hypertriglyceridemia.4,5 However, the relationship between hypertriglyceridemia and severity of the clinical course of acute pancreatitis remains controversial. Several studies have indicated a relationship between hypertriglyceridemia and pancreatitis-associated complications.6–9 However, not all studies have shown a difference in clinical course by level of serum triglycerides.10,11
The purpose of this study was to further characterize the relationship between serum triglyceride levels and clinical outcomes in a large cohort of patients admitted for acute pancreatitis in a regional community–based population. Our specific study aims were to (1) evaluate the relationship between baseline serum triglycerides and levels measured in the hospital setting and to(2) determine the relationship between elevated serum triglycerides and pancreatitis-related complications (multiple or persistent organ failure).
MATERIALS AND METHODS
Study Design and Setting
A retrospective cohort study was conducted using data from the Kaiser Permanente Southern California (KPSC) health care system and Research Data Warehouse. Kaiser Permanente comprises 7 regions, of which Southern California is one of the largest, including 14 acute care hospitals, more than 200 medical offices, and over 4 million health plan members. The institutional review board of KPSC approved this study.
Study Population
Kaiser Permanente Southern California patients aged 18 years or older who were hospitalized due to acute pancreatitis, defined using the International Classification of Diseases, Ninth Revision code (577.0), between January 1, 2006 and December 31, 2013, were identified. For patients with multiple admissions during the study period, only the first admission was selected. Patients were excluded if they (1) did not have serum lipase levels greater than or equal to 3 times the upper limit of the reference range during the index hospitalization or (2) were missing a measurement of serum triglycerides during the index hospitalization.
Definition of Variables
Baseline (prehospitalization) serum triglyceride level was defined as the most recent serum triglyceride measured within the year before the development of acute pancreatitis. The inpatient triglyceride level was defined as the initial serum triglyceride measurement obtained during the first 72 hours of the index hospitalization.
Serum triglyceride levels were analyzed initially as a continuous variable and subsequently as a categorical variable in multivariable analyses using thresholds established by the Adult Treatment Panel III guidelines (normal, <150 mg/dL; borderline high, 150–199 mg/dL; high, 200–499 mg/dL; and very high, ≥500 mg/dL).12
Outcome Assessment
Organ failure was defined according to the Modified Marshall Criteria.13 In addition to the established criteria, organ failure was also defined by hypoxemia (oxygen saturation: <90%, measured on 2 occasions within a 6-hour period) or hypotension (systolic blood pressure: <90 mm Hg, measured on 2 occasions within a 6-hour period).
The primary study outcome was a composite of either multiple organ failure (organ failure involving 2 or more organ systems during the hospitalization) or persistent organ failure (organ failure lasting ≥48 hours or the need for mechanical ventilation or hemodialysis), because both are important markers of disease severity in the Revised Atlanta Classification system.14 Secondary study outcomes included length of stay and in-hospital mortality.
Additional Covariates
Other variables included established etiologies of acute pancreatitis ([1] alcohol use, either self-reported from previous outpatient visits or any reported history of alcohol use in the year before or the year after index hospitalization; [2] gallstone disease according to the International Classification of Diseases, Ninth Revision code 574.x in the year before or year after index hospitalization; and [3] cholecystectomy [Current Procedural Terminology codes 47562, 47563, 47600, 47605] in the 180 days after index hospitalization), demographic variables including age and sex, as well as the following additional potential confounders: smoking, body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), and diabetes.
Data Analysis
Categorical variables are presented as counts and percentages, whereas continuous variables are reported as means and standard deviations. The demographic and clinical characteristics of patients with or without multiple/persistent organ failure were compared using a χ2 or Fisher’s exact test for categorical variables and a t-test or Wilcoxon rank-sum test for continuous variables. Shapiro-Wilk normality test was used to test the parametric assumption for testing on continuous variables.
The relationship between inpatient triglyceride level and the composite outcome (multiple or persistent organ failure) was examined using univariate and multivariable robust Poisson regression.15,16 The crude and adjusted relative risks (RRs) of developing the composite outcome and the 95% confidence intervals (CIs) were estimated to evaluate the effect of inpatient triglyceride level on the outcome, adjusting for age and sex. Although a logistic regression is well suited for the outcome analyzed, the application of a robust Poisson model allowed the direct estimation of RRs.
The relationship between the baseline triglyceride measure and the inpatient measure was examined by Spearman correlation coefficient and displayed with a scatterplot. To examine the patterns of serum triglyceride measures in patients with and without the outcome, we took the maximum serum triglyceride level within each 12-hour window (up to 72 hours after hospitalization) per patient and plotted the mean maximum measure among patients with and without the composite outcome.
All reported P values are two-sided with a significance level of 0.05. All statistical analyses were conducted in Statistical Analysis System (SAS) version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
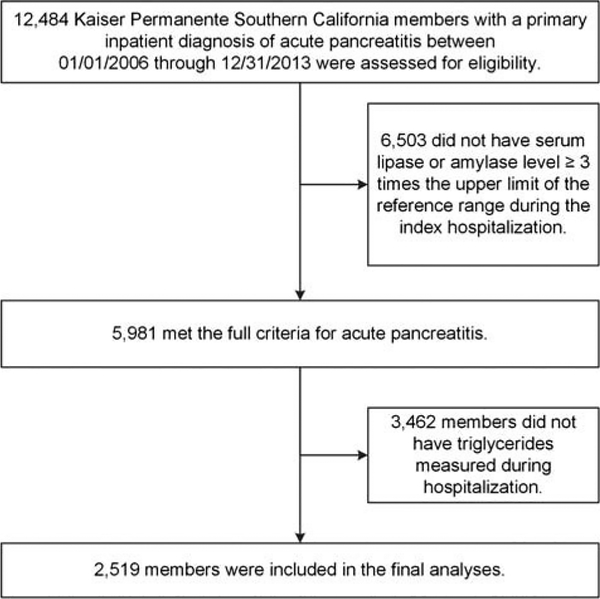
A total of 5981 patients met criteria for hospitalization for acute pancreatitis during the study period. Of these, 2519 patients (42.1% of total hospitalized patients) had serum triglycerides measured at least once during hospitalization, and all further study analyses were based on this sample of 2519 patients (Fig. 1). For comparison purposes, study population characteristics of the analytic sample are presented alongside those patients who did not have serum triglycerides measured (see Supplemental Table 1, http://links.lww.com/MPA/A596).
FIGURE 1.
Study population selection flow chart for a cohort of patients with acute pancreatitis at Kaiser Permanente Southern California, 2006–2013.
Overall inpatient mortality in the study cohort was 31 (1.2%) and median length of stay was 5 days (interquartile range [IQR], 3–8). Baseline demographic and clinical characteristics of the study population classified by organ failure status are presented in Table 1. A total of 790 patients (31.4%) had an elevated triglyceride level (≥150 mg/dL), including 231 patients with a triglyceride level greater than or equal to 500 mg/dL. There were a total of 267 patients (10.6%) who developed any organ failure. Among these, 75 patients (28.1%) developed multiple system organ failure and 82 patients (30.7%) developed persistent organ failure. Among patients with persistent organ failure, 54 (65.8%) had multiple organ system failure. Patients who developed organ failure were more likely to be older, male, former tobacco users, and have had a history of diabetes mellitus, compared with patients who did not develop organ failure.
TABLE 1.
Study Population Characteristics of a Cohort of Patients With Acute Pancreatitis at Kaiser Permanente Southern California (n = 2519) by the Development of Organ Failure, 2006–2013
| No Organ Failure | Transient Organ Failure | MOP | Total | ||
|---|---|---|---|---|---|
| 2252 (89.4) | 164 (6.5) | 103 (4.1) | 2519 | P | |
| Age at hospitalization, mean (SD), y | 51.1 (17.7) | 60.1 (20.2) | 57.3 (18.5) | 51.9(18.1) | <0.001 |
| Sex, female | 1140 (50.6) | 73 (44.5) | 32 (31.1) | 1245 (49.4) | <0.001 |
| Race/ethnicity | 0.079 | ||||
| White, non-Hispanic | 882 (39.2) | 73 (44.5) | 45 (43.7) | 1000 (39.7) | |
| Black, non-Hispanic | 217 (9.6) | 25 (15.2) | 11 (10.7) | 253 (10) | |
| Hispanic | 975 (43.3) | 53 (32.3) | 38 (36.9) | 1066 (42.3) | |
| Asian, non-Hispanic | 142 (6.3) | 10 (6.1) | 9 (8.7) | 161 (6.4) | |
| Other, non-Hispanic | 36 (1.6) | 3 (1.8) | 0 (0) | 39 (1.5) | |
| Etiology of acute pancreatitis | 0.655 | ||||
| Gallstone disorders | 600 (26.6) | 42 (25.6) | 33 (32) | 675 (26.8) | |
| Alcohol-related | 447 (19.8) | 37 (22.6) | 20 (19.4) | 504 (20) | |
| Other | 1205 (53.5) | 85 (51.8) | 50 (48.5) | 1340 (53.2) | |
| History of alcohol use | 574 (25.5) | 48 (29.3) | 27 (26.2) | 649 (25.8) | 0.483 |
| Current tobacco use | 295 (13.1) | 20 (12.2) | 16 (15.5) | 331 (13.1) | 0.016 |
| Former tobacco use | 390 (17.3) | 45 (27.4) | 17 (16.5) | 452 (17.9) | |
| History of diabetes mellitus | 391 (17.4) | 48 (29.3) | 21 (20.4) | 460 (18.3) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 29.5 (6.4) | 28.7 (6.9) | 28.9 (6.9) | 29.4 (6.5) | 0.317 |
| <18.5 (underweight) | 24 (1.7) | 5 (5.2) | 4 (6.6) | 33 (2.1) | |
| 18.5–24.9 (normal weight) | 305 (22.0) | 24 (25.0) | 14 (23) | 343 (22.2) | |
| 25.0–29.9 (overweight) | 472 (34.0) | 30 (31.3) | 19 (31.1) | 521 (33.7) | |
| ≥30.0 (obese) | 586 (42.2) | 37 (38.5) | 24 (39.3) | 647 (41.9) | |
| Initial hospital triglycerides, mg/dL | 0.115 | ||||
| <150 | 1560 (69.3) | 111 (67.7) | 58 (56.3) | 1729 (68.6) | |
| 150–199 | 218 (9.7) | 21 (12.8) | 12 (11.7) | 251 (10.0) | |
| 200–499 | 264 (11.7) | 24 (14.6) | 20 (19.4) | 308 (12.2) | |
| 500–999 | 73 (3.2) | 3 (1.8) | 6 (5.8) | 82 (3.3) | |
| ≥1000 | 137 (6.1) | 5 (3.0) | 7 (6.8) | 149 (5.9) |
Values presented as n (%) unless otherwise indicated.
MOP indicates multiple or persistent organ failure.
Baseline Versus Inpatient Serum Triglyceride Levels
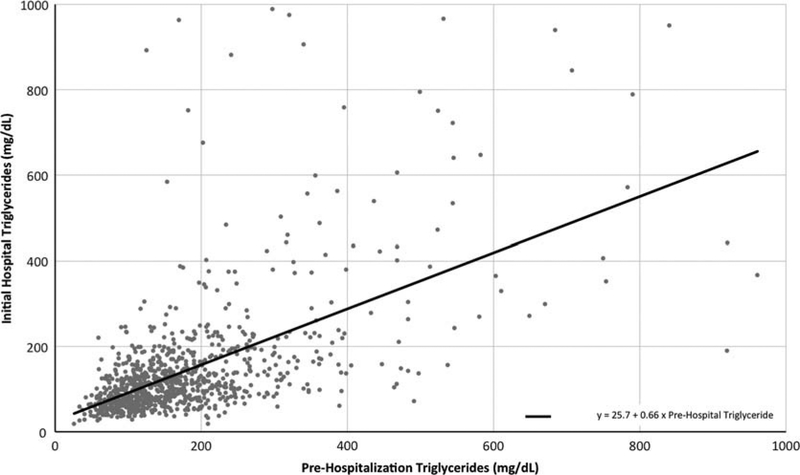
There were 1009 patients (40.1%) with both baseline (prehospitalization) and inpatient triglycerides available for comparison. As shown in Figure 2, within this subgroup there was a significant correlation between peak baseline triglyceride level and initial inpatient triglyceride level (Spearman rank correlation: 0.612, P < 0.01). Table 2 provides the distribution of patients with a baseline triglyceride of less than 500 mg/dL or 500 mg/dL or higher, measured in the year before hospitalization, by categories of inpatient serum triglyceride levels. Approximately 8% of patients had very high inpatient serum triglyceride levels (>500 mg/dL). The majority of these patients (59.5%) also had a very high previous measurement in the year before hospitalization.
FIGURE 2.
Scatterplot with regression line of highest prehospitalization serum triglyceride measurement with initial hospital serum triglyceride measurement (n = 1009) among patients with both measurements in a cohort of patients with acute pancreatitis at Kaiser Permanente Southern California, 2006–2013.
TABLE 2.
Prehospitalization Serum Triglyceride Levels by Initial Hospitalization Serum Triglyceride Levels Within a Cohort of Patients With Acute Pancreatitis at Kaiser Permanente Southern California (n = 1009)*, 2006–2013
| Initial Hospitalization Triglyceride Levels, mg/dL | |||||
|---|---|---|---|---|---|
| <150 | 150–200 | 200–500 | ≥500 | Total | |
| 695 (68.9) | 116 (11.5) | 114 (11.3) | 84 (8.3) | 1009 | |
| Prehospitalization triglyceride level, mean (SD), mg/dL† | 144 (78) | 240 (400) | 378 (625) | 1211 (1378) | 271 (555) |
| <500 | 694 (73.8) | 113 (12) | 99 (10.5) | 34 (3.6) | 940 |
| ≥500 | 1 (1.4) | 3 (4.3) | 15 (21.7) | 50 (72.5) | 69 |
Values presented as n (%) unless otherwise indicated.
This table includes patients who had both prehospitalization (baseline) and initial hospitalization serum triglyceride levels available.
Prehospitalization triglyceride level is the most recent value measured within 1 year before admission.
Profile of Initial Serum Triglyceride Levels in Acute Pancreatitis
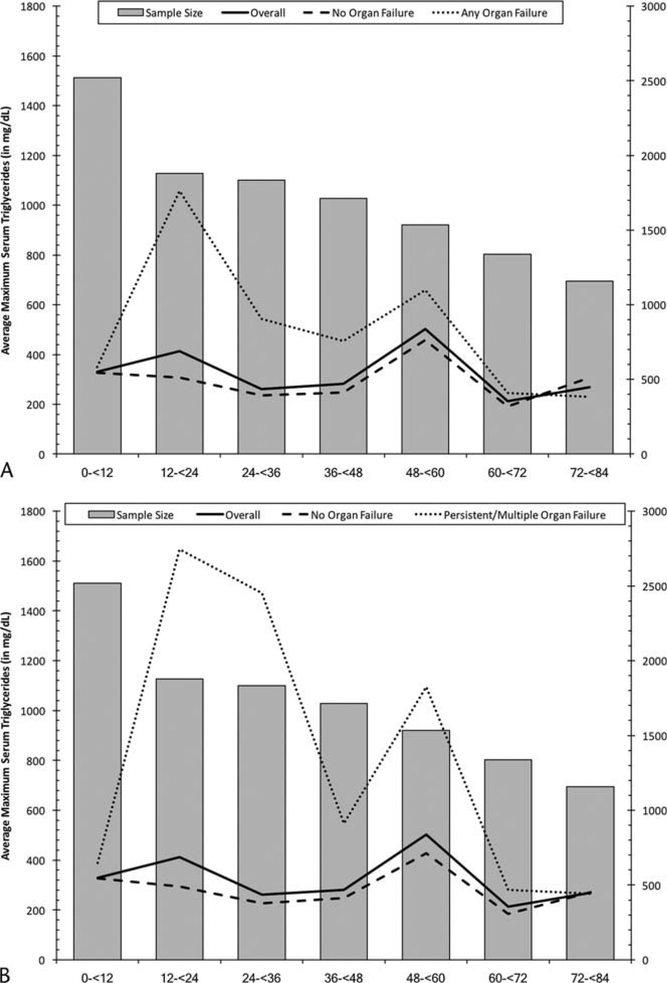
Within the study sample, 96% of initial serum triglyceride levels were collected within 72 hours (92.3% within 24 hours, 94.5% within 48 hours, and 96% within 72 hours). Serum triglyceride values stratified by organ failure status in the first 72 hours of admission are presented in Figure 3A, B. Within the first 12 hours of admission, there was an initial difference in triglyceride levels between patients who would later develop organ failure, compared with those who did not. However, by 72 hours into admission, serum triglyceride levels seemed to converge across the various groups.
FIGURE 3.
Average maximum serum triglyceride levels in each 12-hour window of admission time by any organ failure (A) or MOP (B) status in a cohort of patients with acute pancreatitis at Kaiser Permanente Southern California, 2006–2013.
Relationship Between Triglyceride Level and Clinical Outcomes
In a univariate analysis, increased initial serum triglyceride level was associated with elevated risk of the primary study outcome (multiple or persistent organ failure [MOP]). Median initial triglyceride levels were 135.0 mg/dL for patients with MOP and 111.0 mg/dL in those without any organ failure (P = 0.003). Increased initial serum triglyceride level was not associated with increased length of stay (median [IQR] length of stay for triglyceride level ≥200 was 5 [3–7] days vs 5 [3–8] days for triglyceride level <200, P = 0.06) or with in-hospital mortality (median [IQR] of serum triglyceride level for survivors was 107 [72–177] vs 118 [86–180] for nonsurvivors, P = 0.37).
In multivariable robust Poisson regression adjusting for age and sex, both high (200–499 mg/dL) and very high (>500 mg/dL) initial triglyceride levels were associated with increased risk of MOP (RR, 2.16; 95% CI, 1.28–3.65 and RR, 2.13; 95% CI, 1.11–4.07, respectively) when compared with patients with normal baseline levels (Table 3).
Table 3.
Risk of Organ Failure by Study Characteristics in a Cohort of Patients With Acute Pancreatitis at Kaiser Permanente Southern California (n = 2519), 2006–2013
| Any Organ Failure (n = 267; 10.6%) | Multiple/Persistent Organ Failure (n = 103; 4.1%) | |||
|---|---|---|---|---|
| Crude | Multivariable Adjusted | Crude | Multivariable Adjusted | |
| Serum triglycerides*, mg/dL | ||||
| <150 | Reference | Reference | ||
| 150–199 | 1.35 (0.93–1.95) | 1.45 (1.00–2.11) | 1.43 (0.77–2.65) | 1.52 (0.82–2.84) |
| 200–499 | 1.46 (1.05–2.04) | 1.70 (1.21–2.39) | 1.94 (1.16–3.22) | 2.16 (1.28–3.65) |
| ≥500 | 0.93 (0.59–1.46) | 1.30 (0.81–2.09) | 1.68 (0.92–3.06) | 2.13 (1.11–4.07) |
| Age at admission (5-unit increment) | 1.12 (1.08–1.16) | 1.14 (1.10–1.18) | 1.09 (1.03–1.15) | 1.13 (1.06–1.20) |
| Sex, male vs female | 1.51 (1.18–1.93) | 1.52 (1.19–1.95) | 2.17 (1.43–3.29) | 2.09 (1.37–3.19) |
Initial measurement of serum triglycerides during the first 72 hours of hospitalization.
DISCUSSION
In this regional, population–based, cross-sectional study of patients hospitalized for acute pancreatitis, we have confirmed that even modest elevations in initial serum triglyceride levels (>200 mg/dL) were associated with increased risk of MOP (composite outcome). The majority of patients with very high inpatient triglyceride levels (>500 mg/dL) also had a history of elevated triglyceride levels in the year before hospitalization. Interestingly, patients who developed organ failure had elevated serum triglyceride levels within the first 12 hours of hospitalization with subsequent decrease over time.
Elevated triglyceride levels are an established risk factor for acute pancreatitis, with hypertriglyceridemia accounting for 1% to 10% of all cases of acute pancreatitis.2,3 The incidence of acute pancreatitis has been shown to increase by the level of baseline hypertriglyceridemia.4,5 However, the relationship between hypertriglyceridemia and severity of the clinical course of acute pancreatitis remains controversial.
Findings from the present study support and extend previous work in this area. Several studies have indicated a relationship between hypertriglyceridemia and pancreatitis-associated complications.6–9 For example, a recent study of 400 patients with acute pancreatitis demonstrated an independent association between elevated triglyceride levels and persistent organ failure. The increased risk of persistent organ failure was proportional to the degree of hypertriglyceridemia within 72 hours of admission.9 However, not all studies have shown a consistent difference in clinical course by level of serum triglycerides.10,11 The findings presented here provide further support for the relationship between inpatient hypertriglyceridemia and risk of MOP in a regional population–based cohort of patients with acute pancreatitis.
Although it is established that severe elevations in serum triglyceride levels can lead to acute pancreatitis, the relationship between mild-to-moderate elevations of triglycerides with acute pancreatitis initiated by other primary factors (eg, gallstones, alcohol) is less well understood. Elevated serum triglycerides have been postulated to further pancreatic injury as a result of hydrolysis by pancreatic lipase, leading to formation of free fatty acids.17 Experimental evidence suggests that free fatty acids can induce direct injury to acinar cells through several potential mechanisms including impaired mitochondrial adenosine triphosphate activity18 or lipid peroxidation of the acinar cell membrane.19 It is not clear, however, whether elevated triglyceride levels consistently precede the development of acute pancreatitis or whether acute pancreatitis itself can lead to elevated triglyceride levels. In our study, patients who developed organ failure were more likely to be former tobacco smokers. We do not have a clear explanation for this finding, but speculate that perhaps chronic exposure may increase susceptibility of organs to inflammation, injury, or failure.
Interestingly, we noted an initial elevation of serum triglyceride levels (within 12 hours of hospitalization) among patients who developed organ failure. This was then followed by an overall decrease in triglyceride level during the subsequent hospital course, suggesting an initial proinflammatory/toxic effect leading to downstream complications even in the absence of persistent elevation of triglyceride levels.
Strengths of the present study include the relatively large number of consecutive patients with acute pancreatitis with inpatient as well as previous serum triglyceride levels available for analysis. In addition, the study setting within a regional community–based health care setting is in contrast to many of the previous studies that were conducted solely within academic referral centers and included a large proportion of patients transferred from other institutions. Finally, we were able to incorporate both administrative and clinical parameters to improve the accuracy for both case identification as well as outcome assessment.
Our findings suggest an opportunity for primary prevention of hypertriglyceridemia-associated acute pancreatitis, because approximately 60% of patients with triglyceride levels greater than 500 mg/dL had similarly high magnitudes of hypertriglyceridemia in the year preceding hospitalization for acute pancreatitis. In addition, the prevalence of diabetes was higher among patients with transient, persistent, or multiple organ failure compared with those who did not develop organ failure. This suggests that future investigation into the role of the metabolic syndrome and its relationship to the mechanism of hypertriglyceridemia-associated acute pancreatitis may be worthwhile.
There are several limitations to the present study. Most notably, serum triglyceride levels were obtained in the context of routine clinical care at the discretion of the health care provider. As a result, the study cohort represents a selected subgroup of patients with acute pancreatitis. In addition, the overall frequency of organ failure in the present study cohort was lower than other series originating from tertiary referral centers.9,20 This likely reflects the community-based nature of the study cohort. Despite the overall study size, the relatively small number of clinical outcomes limited our ability to incorporate additional covariates into the multivariable analyses. In addition, there were relatively few patients with repeated measurement of serum triglycerides during their hospitalization, which limited our ability to evaluate the effect of changes in serum triglyceride levels on clinical outcomes. As more data are reported demonstrating the importance of triglyceride levels in the outcomes of patients with acute pancreatitis, perhaps more clinicians will start to include triglyceride levels in their assessments of these patients.
In summary, we have confirmed that hypertriglyceridemia during the early phase of acute pancreatitis is associated with increased risk of multisystem or persistent organ failure. Even modest levels of initial serum triglyceride elevation (≥200 mg/dL) were associated with increased risk of these outcomes. These findings indicate that elevated serum triglycerides may not only induce acute pancreatitis but also exacerbate the disease course, prompting a transition from mild interstitial disease to severe pancreatitis complicated by MOP.
Supplementary Material
Acknowledgments
This was an investigator-initiated study and was supported in part by Shire (Lexington, MA).
Footnotes
The authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewald N, Hardt PD, Kloer HU. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol. 2009;20:497–504. [DOI] [PubMed] [Google Scholar]
- 3.Tsuang W, Navaneethan U, Ruiz L, et al. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104: 984–991. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MJ, Sheng X, MacDonald TM, et al. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173:162–164. [DOI] [PubMed] [Google Scholar]
- 5.Scherer J, Singh VP, Pitchumoni CS, et al. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson F, Thomson SR, Clarke DL, et al. Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes. Pancreatology. 2009;9:252–257. [DOI] [PubMed] [Google Scholar]
- 7.Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14: 4558–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloret Linares C, Pelletier AL, Czernichow S, et al. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas. 2008;37:13–18. [DOI] [PubMed] [Google Scholar]
- 9.Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497–1503. [DOI] [PubMed] [Google Scholar]
- 10.Balachandra S, Virlos IT, King NK, et al. Hyperlipidaemia and outcome in acute pancreatitis. Int J Clin Pract. 2006;60:156–159. [DOI] [PubMed] [Google Scholar]
- 11.Hamada S, Masamune A, Kikuta K, et al. Clinical impact of elevated serum triglycerides in acute pancreatitis: validation from the nationwide epidemiological survey in Japan. Am J Gastroenterol. 2016;111: 575–576. [DOI] [PubMed] [Google Scholar]
- 12.Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. [DOI] [PubMed] [Google Scholar]
- 14.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Shi J, Qian L, et al. Comparison of robustness to outliers between robust Poisson models and log-binomial models when estimating relative risks for common binary outcomes: a simulation study. BMC Med Res Methodol. 2014;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Sternfeld L, Yang F, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58: 422–430. [DOI] [PubMed] [Google Scholar]
- 18.Criddle DN, Murphy J, Fistetto G, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. [DOI] [PubMed] [Google Scholar]
- 19.Morita Y, Yoshikawa T, Takeda S, et al. Involvement of lipid peroxidation in free fatty acid-induced isolated rat pancreatic acinar cell injury. Pancreas. 1998;17:383–389. [DOI] [PubMed] [Google Scholar]
- 20.Koutroumpakis E, Wu BU, Bakker OJ, et al. Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases. Am J Gastroenterol. 2015;110:1707–1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.