Abstract
BACKGROUND
Data regarding the efficacy of treatment with ibrutinib–rituximab, as compared with standard chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab, in patients with previously untreated chronic lymphocytic leukemia (CLL) have been limited.
METHODS
In a phase 3 trial, we randomly assigned (in a 2:1 ratio) patients 70 years of age or younger with previously untreated CLL to receive either ibrutinib and rituximab for six cycles (after a single cycle of ibrutinib alone), followed by ibrutinib until disease progression, or six cycles of chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab. The primary end point was progression-free survival, and overall survival was a secondary end point. We report the results of a planned interim analysis.
RESULTS
A total of 529 patients underwent randomization (354 patients to the ibrutinib–rituximab group, and 175 to the chemoimmunotherapy group). At a median follow-up of 33.6 months, the results of the analysis of progression-free survival favored ibrutinib–rituximab over chemoimmunotherapy (89.4% vs. 72.9% at 3 years; hazard ratio for progression or death, 0.35; 95% confidence interval [CI], 0.22 to 0.56; P<0.001), and the results met the protocol-defined efficacy threshold for the interim analysis. The results of the analysis of overall survival also favored ibrutinib–rituximab over chemoimmunotherapy (98.8% vs. 91.5% at 3 years; hazard ratio for death, 0.17; 95% CI, 0.05 to 0.54; P<0.001). In a subgroup analysis involving patients without immunoglobulin heavy-chain variable region (IGHV) mutation, ibrutinib–rituximab resulted in better progression-free survival than chemoimmunotherapy (90.7% vs. 62.5% at 3 years; hazard ratio for progression or death, 0.26; 95% CI, 0.14 to 0.50). The 3-year progression-free survival among patients with IGHV mutation was 87.7% in the ibrutinib–rituximab group and 88.0% in the chemoimmunotherapy group (hazard ratio for progression or death, 0.44; 95% CI, 0.14 to 1.36). The incidence of adverse events of grade 3 or higher (regardless of attribution) was similar in the two groups (in 282 of 352 patients [80.1%] who received ibrutinib–rituximab and in 126 of 158 [79.7%] who received chemoimmunotherapy), whereas infectious complications of grade 3 or higher were less common with ibrutinib–rituximab than with chemoimmunotherapy (in 37 patients [10.5%] vs. 32 [20.3%], P<0.001).
CONCLUSIONS
The ibrutinib–rituximab regimen resulted in progression-free survival and overall survival that were superior to those with a standard chemoimmunotherapy regimen among patients 70 years of age or younger with previously untreated CLL. (Funded by the National Cancer Institute and Pharmacyclics; E1912 ClinicalTrials.gov number, .)
CHRONIC LYMPHOCYTIC LEUKEMIA (CLL) is one of the most common lymphoid cancers, accounting for approximately 11% of hematologic neoplasms. A marked improvement in progression-free survival and overall survival among patients with CLL has been realized with the addition of anti-CD20 monoclonal antibodies to purine nucleoside analogue or alkylator-based chemotherapy.1,2 Phase 3 trials have established the chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as the standard first-line treatment for patients with CLL who are 70 years of age or younger and who are suitable candidates for such therapy after consideration of organ function and side-effect risks.1,3 The fludarabine–cyclophosphamide–rituximab combination appears to be particularly effective in patients with immunoglobulin heavy-chain variable region (IGHV)– mutated CLL, with roughly half the patients in that subgroup remaining free from progression up to 8 years after initial treatment.4–7 Although fludarabine–cyclophosphamide–rituximab therapy is efficacious, it is associated with substantial toxic effects, including severe myelosup-pression, a small risk of treatment-related myelodysplasia, and infectious complications, including opportunistic infections due to T-cell immunosuppression.8
In parallel with advances in chemoimmunotherapy, elucidation of CLL B-cell biology has identified new therapeutic targets.9 The interruption of leukemia proliferative signals mediated through the B-cell receptor appears to be one of the most promising approaches.10–12 Major effects have been seen with ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase (BTK), a B-cell signaling protein involved in B-cell development, differentiation, proliferation, and survival.10,13
Ibrutinib was initially found to have durable efficacy in patients who had relapsed or refractory CLL.13,14 Subsequent phase 3 trials involving previously untreated patients with progressive CLL who were too frail to receive aggressive therapy showed superior progression-free survival and overall survival with ibrutinib as compared with chlorambucil, which led to approval of the drug by the Food and Drug Administration (FDA) as a first-line treatment option.15 However, data regarding the efficacy of ibrutinib as a first-line treatment for patients 70 years of age or younger with CLL, as compared with the efficacious chemoimmunotherapy regimen of fludarabine–cyclophosphamide–rituximab, have been limited. We conducted a multicenter, open-label, randomized, phase 3 trial (E1912) through the National Clinical Trials Network (NCTN) to evaluate the efficacy and safety of treatment with ibrutinib in combination with six cycles of rituximab, as compared with fludarabine–cyclophosphamide–rituximab, in previously untreated patients with CLL who were 70 years of age or younger.
Methods
Trial Participants
Eligible participants were previously untreated patients with CLL or the small lymphocytic lymphoma (SLL) subtype of CLL who were 70 years of age or younger and who would be appropriate candidates for treatment according to the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) Working Group criteria (see the Supplementary Appendix, available with the full text of this article at NEJM.org).16 Comprehensive eligibility criteria are listed in the Supplementary Appendix. Patients with chromosome 17p13 deletion were excluded from the trial because of the poor response of CLL in these patients to fludarabine–cyclophosphamide–rituximab therapy.1,3,17
Trial Oversight
This trial was designed and coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG– ACRIN) Cancer Research Group in collaboration with the other NCTN Cooperative Groups. The trial protocol (available at NEJM.org) was approved by the National Cancer Institute (NCI) central institutional review board and local institutional review boards as required by participating institutions. The trial was conducted in accordance with the principles of the Declaration of Helsinki. Data that were gathered and analyzed by trial investigators were entered into an electronic database maintained by the ECOG– ACRIN Biostatistical and Operations Office. The trial was monitored twice annually by a standing data and safety monitoring board that included persons from both within and outside ECOG– ACRIN. The authors reviewed and approved the manuscript for submission for publication and vouch for the accuracy and completeness of the data and for the adherence of the trial to the protocol. Ibrutinib was provided by Pharmacyclics (a subsidiary of AbbVie) under a cooperative research and development agreement with the NCI.
Randomization and Treatment
After providing written informed consent, eligible participants underwent randomization, which was stratified according to the age of the patients (<60 years vs. 60 to 70 years), ECOG performance-status score (0 or 1 vs. ≥2; scores are on a 5-point scale, with higher numbers indicating greater disability), Rai stage (0 to II [low or intermediate risk] vs. III or IV [high risk]), and the presence or absence of chromosome 11q22.3 deletion on fluorescence in situ hybridization analysis. Patients were randomly assigned in a 2:1 ratio to receive either ibrutinib–rituximab or chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab.
Patients who were randomly assigned to the ibrutinib–rituximab group received ibrutinib (at a dose of 420 mg per day until disease progression or an unacceptable level of side effects occurred) and rituximab (50 mg per square meter of body-surface area on day 1 of cycle 2; 325 mg per square meter on day 2 of cycle 2; and 500 mg per square meter on day 1 of cycles 3 through 7); each cycle was 28 days. Patients who were randomly assigned to the chemoimmunotherapy group received six cycles of intravenous fludarabine (at a dose of 25 mg per square meter) and cyclophosphamide (250 mg per square meter) on days 1 through 3 with rituximab (50 mg per square meter on day 1 of cycle 1; 325 mg per square meter on day 2 of cycle 1; and 500 mg per square meter on day 1 of cycles 2 through 6) every 28 days. Full treatment information, including instructions regarding dose delays and modifications, is provided in the Supplementary Appendix.
Prophylaxis against Pneumocystis jirovecii (tri-methoprim–sulfamethoxazole or alternative) and herpes zoster (valacyclovir or alternative) was given to patients in each group for 1 year after treatment initiation. All patients received allopurinol (300 mg orally once daily) on days 1 through 14 of cycle 1. Patients treated with ibrutinib–rituximab also received allopurinol with treatment cycle 2 (the first cycle that included rituximab). Growth factor support was permitted per guidelines of the American Society of Clinical Oncology (see the Supplementary Appendix).18
Assessments of Safety and Response
All adverse events were graded according to the NCI Common Toxicity Criteria, version 4. For the purposes of dose modification, adverse events involving platelet count and hemoglobin level were graded according to the IWCLL CLL Working Group grading scale (see the Supplementary Appendix).16
Responses were graded according to the 2008 IWCLL Working Group criteria that were in effect at the start of the trial16 along with the 2018 modification that included the category of complete response with incomplete bone marrow recovery19 (see the Supplementary Appendix). Isolated treatment-related lymphocytosis in the absence of disease progression according to other criteria was not considered to indicate progression until a nadir lymphocyte count was achieved.19,20 Bone marrow biopsies were performed at enrollment and at 12 months to assess response, with central review by an expert hematopathologist (see the Supplementary Appendix). Computed tomographic (CT) scans of the chest, abdomen, and pelvis were performed in all patients at enrollment and at the evaluation for response at 12 months. Minimal residual disease (MRD) was assessed by means of peripheral-blood flow cytometry with a sensitivity greater than 1 CLL cell per 10,000 leukocytes (see the Supplementary Appendix). MRD assays were performed at the time of the 12-month response evaluation and at 24 and 36 months after randomization.
Statistical Analysis
The primary end point was progression-free survival, which was defined as the time from randomization to documented CLL progression or death without documented progression. Patients alive without documented progression had their data censored at the last disease assessment. The trial was designed to have 80% power to detect a hazard ratio for progression or death (in the analysis of progression-free survival) of 0.67 (ibrutinib–rituximab vs. chemoimmunotherapy) or less with the use of a stratified log-rank test at the one-sided alpha level of 2.5%. The planned enrollment was 519 patients, to be randomly assigned in a 2:1 ratio to receive ibrutinib–rituximab or chemoimmunotherapy.
With input from the FDA, interim analyses for progression-free survival were planned to start at 24 to 27 months after full enrollment and annually until either the efficacy boundary was crossed or full information (203 events of progression or death) was reached. The prespecified boundary for the first interim analysis of progression-free survival was 2.807 on the z-statistic scale, which corresponded to a one-sided P value of 0.0025. A truncated version of the O’Brien–Fleming boundary was to be used subsequently.21 Futility rules for harm and inefficacy were included (see the Supplementary Appendix).
Overall survival, which was defined as time from randomization to death from any cause, was a secondary end point and was to be tested only if the result in the progression-free survival analysis crossed the efficacy boundary. Patients who were alive had their data censored at the last date of contact. Interim analyses for overall survival were to start when the efficacy boundary for progression-free survival was crossed and to continue annually until early stopping criteria were met or full information (125 deaths) was reached. Critical values at each interim analysis were to be determined with the use of the truncated O’Brien–Fleming boundary.
Stratified log-rank tests22 were used to compare time-to-event distributions. Hazard ratios were estimated with the use of stratified Cox proportional-hazards models.23 The frequency of response and the incidence of adverse events were compared between the two groups with the use of Fisher’s exact test. Descriptive statistics were used to summarize the characteristics of the patients. Time-to-event distributions were estimated with the use of the Kaplan–Meier method. The primary analysis was conducted in the intention-to-treat population, which included all the patients who had undergone randomization, regardless of eligibility or treatment status. P values are two-sided, and 95% confidence intervals are presented.
Results
Patients
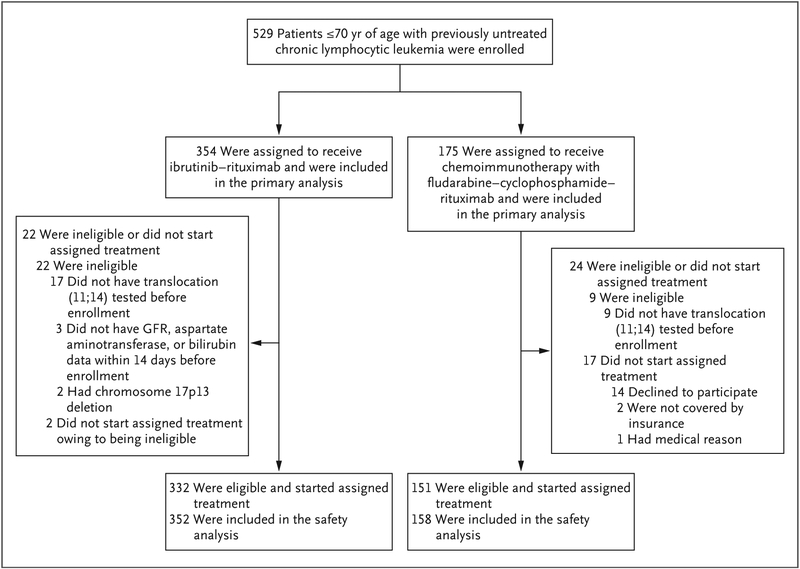
From March 2014 through June 2016, the trial enrolled 529 patients (Table 1). A total of 354 patients were assigned to the ibrutinib–rituximab group, and 175 to the chemoimmunotherapy group. A total of 19 patients did not start the protocol-specified therapy (Fig. 1).
Table 1.
Characteristics of the Patients at Baseline (Intention-to-Treat Population).*
| Characteristic | Ibrutinib-Rituximab Group (N = 354) | Chemoimmunotherapy Group (N = 175) | Total (N = 529) |
|---|---|---|---|
| Age | |||
| Mean | 56.7±7.5 | 56.7±7.2 | 56.7±7.4 |
| ≥60 yr — no. (%) | 145 (41.0) | 70 (40.0) | 215 (40.6) |
| Sex — no. (%) | |||
| Female | 118 (33.3) | 55 (31.4) | 173 (32.7) |
| Male | 236 (66.7) | 120 (68.6) | 356 (67.3) |
| Rai stage — no. (%) | |||
| Low risk, 0 | 11 (3.1) | 9 (5.1) | 20 (3.8) |
| Intermediate risk, I or II | 187 (52.8) | 94 (53.7) | 281 (53.1) |
| High risk, III or IV | 156 (44.1) | 72 (41.1) | 228 (43.1) |
| ECOG performance-status score — no. (%)† | |||
| 0 | 226 (63.8) | 109 (62.3) | 335 (63.3) |
| 1 | 119 (33.6) | 63 (36.0) | 182 (34.4) |
| 2 | 9 (2.5) | 3 (1.7) | 12 (2.3) |
| Beta2 microglobulin — mg/liter | |||
| Mean | 4.0±2.1 | 4.0±1.9 | 4.0±2.0 |
| Median | 3.6 | 3.4 | 3.6 |
| Interquartile range | 2.6–4.6 | 2.7–4.8 | 2.6–4.7 |
| Dohner classification — no. (%) | |||
| Chromosome 17p13 deletion‡ | 2 (0.6) | 0 | 2 (0.4) |
| Chromosome 11q22.3 deletion | 78 (22.0) | 39 (22.3) | 117 (22.1) |
| Trisomy 12 | 70 (19.8) | 27 (15.4) | 97 (18.3) |
| Normal | 69 (19.5) | 37 (21.1) | 106 (20.0) |
| Chromosome 13q deletion | 121 (34.2) | 58 (33.1) | 179 (33.8) |
| Other | 14 (4.0) | 14 (8.0) | 28 (5.3) |
| IGHV mutation status — no./total no. (%)§ | |||
| Mutated | 70/280 (25.0) | 44/115 (38.3) | 114/395 (28.9) |
| Unmutated | 210/280 (75.0) | 71/115 (61.7) | 281/395 (71.1) |
Plus–minus values are means ±SD. Patients were randomly assigned to receive ibrutinib–rituximab or chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab. Data include patients with small lymphocytic lymphoma (SLL); overall, 11.4% of the patients (11.7% in the ibrutinib–rituximab group and 10.9% in the chemoimmunotherapy group) had the SLL subtype of chronic lymphocytic lymphoma. Percentages may not total 100 because of rounding. More detailed information regarding the characteristics of the patients at baseline is provided in Table S1 in the Supplementary Appendix.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability.
Two patients with chromosome 17p13 deletion were enrolled and randomly assigned to the ibrutinib–rituximab group but were later found to be ineligible on the basis of fluorescence in situ hybridization analysis.
Immunoglobulin heavy-chain variable region (IGHV) mutation status was tested in the 436 patients (82.4% of the overall population) who agreed to participate in the correlative study component of the trial and who provided a research sample. Among the 436 patients who underwent testing, IGHV status could be determined in 395.
Figure 1. Enrollment, Randomization, and Follow-up.
All 529 patients who had been enrolled in the trial were included in the intention-to-treat analysis. Of the 529 patients who underwent randomization, 31 (5.9%) were determined to have not met the eligibility criteria and were excluded from the analysis of eligible patients who started assigned therapy (see the Supplementary Appendix). The safety analysis included 510 patients who started the assigned protocol therapy. GFR denotes glomerular filtration rate.
At a median follow-up of 33.6 months, 279 of the 354 patients (78.8%) who had been randomly assigned to receive ibrutinib–rituximab continued ibrutinib therapy, and 132 of the 175 patients (75.4%) who had been randomly assigned to receive chemoimmunotherapy continued to be monitored. The mean number of treatment cycles among all the patients in the chemoimmunotherapy group who started therapy was five; 67.1% of these patients (106 of 158 patients) received six cycles. At the interim analysis, the median duration of treatment in the ibrutinib– rituximab group was 33.0 months (range, 1.0 to 54.0); 16.8% of the patients discontinued therapy for reasons other than disease progression or death (Table S10 in the Supplementary Appendix).
Efficacy
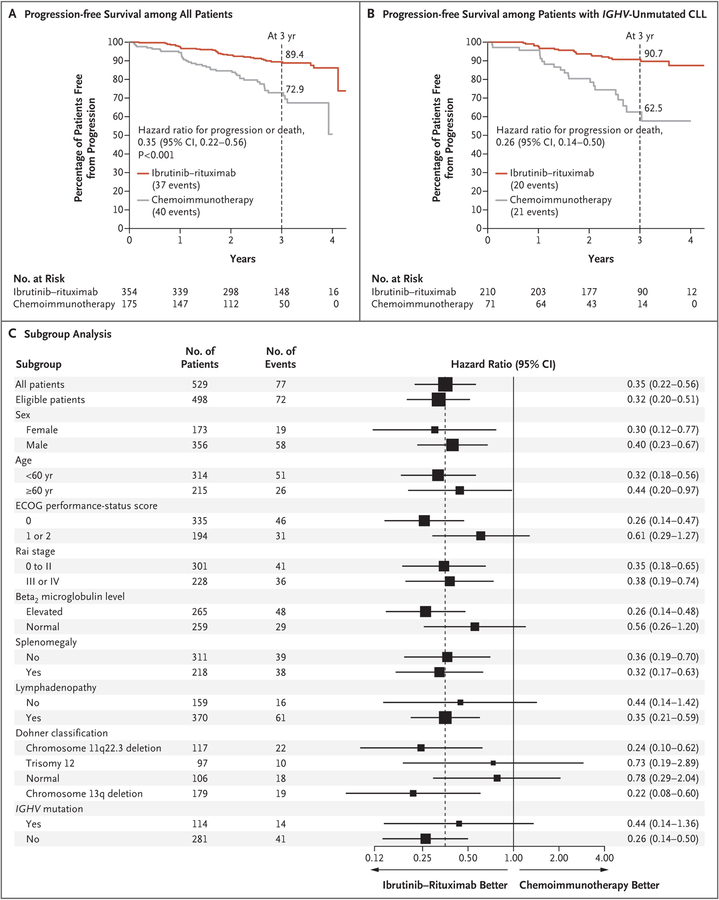
The first interim analysis was performed in September 2018. There were 77 events of progression or death and 14 deaths (8 without progression). The Kaplan–Meier estimates of progression-free survival and overall survival are shown in Figures 2A and 3, respectively. At 3 years, the percentage of patients with progression-free survival was 89.4% (95% confidence interval [CI], 86.0 to 93.0) in the ibrutinib–rituximab group, as compared with 72.9% (95% CI, 65.3 to 81.3) in the chemoimmunotherapy group. The difference in progression-free survival crossed the prespecified boundary (hazard ratio for progression or death, 0.35; 95% CI, 0.22 to 0.56; P<0.001).
Figure 2. Primary and Subgroup Analyses of Progression-free Survival.
Panel A shows the Kaplan–Meier estimate of progression-free survival among all the patients who underwent randomization. Panel B shows the Kaplan–Meier estimate of progression-free survival among patients whose chronic lymphocytic leukemia (CLL) did not have the immunoglobulin heavy-chain variable region (IGHV) mutation. Panel C shows a forest plot of hazard ratios for progression or death, according to prognostic sub-groups of patients with CLL. The subgroup analysis was conducted in the intention-to-treat population. Hazard ratios (ibrutinib–rituximab vs. chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab) were estimated with the use of univariable Cox proportional-hazards models stratified according to the four stratification factors. The size of the box is inversely proportional to the variance of the hazard ratio estimates. The dashed line indicates the overall hazard ratio among all patients who had undergone randomization. Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability. Rai stages of disease range from 0 (low risk) to I or II (intermediate risk) to III or IV (high risk).
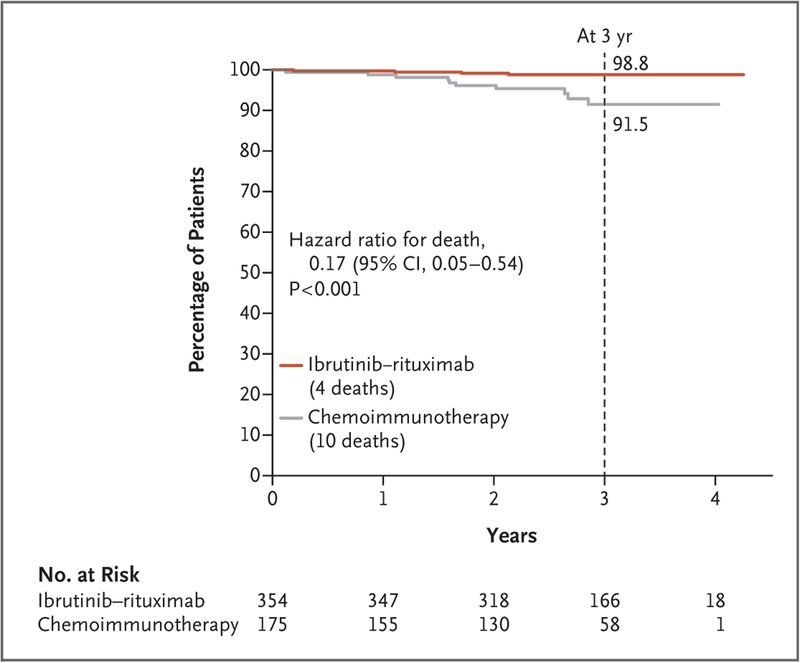
Figure 3.
Overall Survival (Intention-to-Treat Population).
Overall survival was higher in the ibrutinib–rituximab group than in the chemoimmunotherapy group (hazard ratio for death, 0.17; 95% CI, 0.05 to 0.54; P<0.001). Overall survival at 3 years was 98.8% (95% CI, 97.6 to 100) in the ibrutinib–rituximab group, as compared with 91.5% (95% CI, 86.2 to 97.0) in the chemoimmunotherapy group. Data regarding progression-free survival and overall survival among eligible patients who started the assigned treatment are provided in the Supplementary Appendix.
In prespecified subgroup analyses of progression-free survival, ibrutinib–rituximab was superior to chemoimmunotherapy independent of age, sex, or Rai stage and was also superior to chemoimmunotherapy in the subgroup of patients with chromosome 11q22.3 deletion (Fig. 2C). Ibrutinib–rituximab resulted in superior progression-free survival, as compared with chemoimmunotherapy, at 3 years among patients with IGHV-unmutated CLL (90.7% vs. 62.5%; hazard ratio for progression or death, 0.26; 95% CI, 0.14 to 0.50) (Fig. 2B). At the interim analysis, the difference in progression-free survival at 3 years among patients with IGHV-mutated CLL was not significant (87.7% in the ibrutinib–rituximab group and 88.0% in the chemoimmunotherapy group; hazard ratio, 0.44; 95% CI, 0.14 to 1.36) (Fig. S3A in the Supplementary Appendix).
The percentage of patients with an overall response as determined by physical examination was higher in the ibrutinib–rituximab group (95.8%; 95% CI, 93.1 to 97.6) than in the chemoimmunotherapy group (81.1%; 95% CI, 74.5 to 86.6). With the inclusion of CT scan results and central bone marrow review (when possible), the incidence of complete response with or without blood count normalization was lower in the ibrutinib–rituximab group (17.2%; 95% CI, 13.4 to 21.6) than in the chemoimmunotherapy group (30.3%; 95% CI, 23.6 to 37.7).
MRD in the peripheral blood at the 12-month response assessment was evaluated in 276 of 354 patients (78.0%) who were treated with ibrutinib–rituximab and in 103 of 175 patients (58.9%) who received chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab. A lower percentage of patients who were treated with ibrutinib–rituximab (8.3%; 95% CI, 5.4 to 12.2) than with chemoimmunotherapy (59.2%; 95% CI, 49.1 to 68.8) were MRD-negative at cycle 12 (Fig. S1 in the Supplementary Appendix).
Safety
All adverse events of grade 3 or higher that were reported in more than 2% of the patients in either group are summarized in Table 2. The incidence of adverse events of grade 3 or higher, independent of attribution, was similar in the two groups (in 282 of 352 patients [80.1%] treated with ibrutinib–rituximab and in 126 of 158 patients [79.7%] who received chemoimmunotherapy with fludarabine–cyclophosphamide– rituximab, P = 0.91). There was a lower incidence of grade 3 or 4 neutropenia in the ibrutinib– rituximab group than in the chemoimmunotherapy group (90 patients [25.6%] vs. 71 [44.9%], P<0.001), as well as a lower incidence of infectious complications, including neutropenic fever (37 patients [10.5%] vs. 32 [20.3%], P = 0.005). There was a higher incidence of grade 3 or 4 hypertension in the ibrutinib–rituximab group than in the chemoimmunotherapy group (66 patients [18.8%] vs. 13 [8.2%], P = 0.002). Hemorrhagic events of grade 3 or higher occurred in 4 patients (1.1%) in the ibrutinib–rituximab group (all grade 3 events) but in no patients in the chemoimmunotherapy group (P = 0.32).
Table 2.
Adverse Events of Grade 3 or Higher Reported in More Than 2% of Patients in Either Group.*
| Event | Ibrutinib-Rituximab Group (N = 352) | Chemoimmunotherapy Group (N = 158) | ||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| number of patients (percent) | ||||||
| Hematologic event | ||||||
| Anemia | 17 (4.8) | 0 | 0 | 17 (10.8) | 6 (3.8) | 0 |
| Hemolysis | 2 (0.6) | 0 | 0 | 3 (1.9) | 1 (0.6) | 0 |
| Leukocytosis | 61 (17.3) | 1 (0.3) | 0 | 12 (7.6) | 0 | 0 |
| Lymphocyte count decreased | 10 (2.8) | 0 | 0 | 43 (27.2) | 32 (20.3) | 0 |
| Lymphocyte count increased | 77 (21.9) | 0 | 0 | 12 (7.6) | 0 | 0 |
| Neutropenia | 38 (10.8) | 52 (14.8) | 0 | 35 (22.2) | 36 (22.8) | 0 |
| Platelet count decreased | 9 (2.6) | 6 (1.7) | 0 | 16 (10.1) | 8 (5.1) | 0 |
| White-cell count decreased | 7 (2.0) | 1 (0.3) | 0 | 35 (22.2) | 23 (14.6) | 0 |
| Nonhematologic event | ||||||
| Infection† | 28 (8.0) | 4 (1.1) | 1 (0.3) | 9 (5.7) | 5 (3.2) | 1 (0.6) |
| Febrile neutropenia | 8 (2.3) | 0 | 0 | 21 (13.3) | 4 (2.5) | 0 |
| Alanine aminotransferase increased | 6 (1.7) | 2 (0.6) | 0 | 1 (0.6) | 0 | 0 |
| Aspartate aminotransferase increased | 9 (2.6) | 0 | 0 | 2 (1.3) | 0 | 0 |
| Hyperglycemia | 12 (3.4) | 2 (0.6) | 0 | 8 (5.1) | 0 | 0 |
| Hyponatremia | 11 (3.1) | 0 | 0 | 3 (1.9) | 0 | 0 |
| Atrial fibrillation | 9 (2.6) | 2 (0.6) | 0 | 1 (0.6) | 1 (0.6) | 0 |
| Arthralgia | 17 (4.8) | 0 | 0 | 2 (1.3) | 0 | 0 |
| Hypertension | 65 (18.5) | 1 (0.3) | 0 | 13 (8.2) | 0 | 0 |
| Fatigue | 7 (2.0) | 0 | 0 | 4 (2.5) | 0 | 0 |
| Maculopapular rash | 11 (3.1) | 0 | 0 | 8 (5.1) | 0 | 0 |
| Diarrhea | 15 (4.3) | 0 | 0 | 2 (1.3) | 0 | 0 |
| Any event, according to worst grade | 204 (58.0) | 75 (21.3) | 3 (0.9) | 57 (36.1) | 67 (42.4) | 2 (1.3) |
Data include all adverse events of grade 3 or higher that occurred in more than 2% of the patients who started the assigned treatment in either group. Patients in the chemoimmunotherapy group received fludarabine–cyclophosphamide–rituximab.
Infection included sepsis, sinusitis, skin infection, upper respiratory infection, urinary tract infection, infectious entero-colitis, lung infection, penile infection, scrotal infection, soft-tissue infection, lymph-gland infection, tooth infection, kidney infection, and catheter-related infection.
Cardiac toxic effects of grade 3 or higher occurred in 23 patients (6.5%) treated with ibrutinib–rituximab, including 13 cases of atrial fibrillation or atrial flutter, 3 cases of cardiac chest pain, 2 cases of supraventricular tachycardia, 2 cases of heart failure (nonfatal), 2 cases of pericardial effusion, 1 case of sinus bradycardia, 1 case of ventricular tachycardia, 1 case of cardiac arrest (nonfatal), and 1 case of myocardial infarction. Cardiac toxic effects of grade 3 or higher occurred in 3 patients who received chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab, including 2 cases of atrial fibrillation and 1 case of acute coronary syndrome. Atrial fibrillation of any grade occurred in 26 patients (7.4%) receiving ibrutinib–rituximab and in 5 patients (3.2%) receiving chemoimmunotherapy. One patient in the ibrutinib– rituximab group had atrial fibrillation at enrollment, which was exacerbated by ibrutinib during cycle 1; the patient had sudden death 21 days after the start of cycle 2 of ibrutinib–rituximab therapy. A detailed summary of all adverse events of grade 3 or higher that were attributed by the treating physician to trial treatment is provided in Table S7 in the Supplementary Appendix.
Causes of death are listed in Table S6 in the Supplementary Appendix. Among the 10 deaths in the chemoimmunotherapy group, the cause was CLL or therapy-related in the majority of patients (4 deaths due to CLL, 2 due to therapy-related acute myeloid leukemia, 1 due to lung cancer, 1 due to metastatic colon cancer, 1 due to drug overdose, and 1 due to infection). Among the 4 deaths in the ibrutinib–rituximab group, the cause was CLL in the minority of patients (1 death due to CLL, 1 due to lung adenocarcinoma with respiratory failure, 1 due to acute respiratory failure, and 1 sudden death in a patient with history of atrial fibrillation).
Discussion
In this randomized trial involving patients 70 years of age or younger who had previously untreated CLL or SLL and did not have chromosome 17p13 deletion, ibrutinib–rituximab treatment was superior to chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab with respect to progression-free survival and overall survival. Chemoimmunotherapy led to a higher frequency of complete response and rendered more patients MRD-negative than did ibrutinib– rituximab therapy. Nonetheless, the risk of progression or death was 65% lower and the risk of death was 83% lower with ibrutinib–rituximab than with chemoimmunotherapy. The proportion of patients who had an adverse event of grade 3 or higher was similar in the two groups.
Since fludarabine–cyclophosphamide–rituximab is widely accepted as an effective chemoimmunotherapy regimen for patients with CLL, the improvements in both progression-free survival and overall survival that were observed with six cycles of ibrutinib–rituximab followed by continuous ibrutinib therapy, as compared with the 6-month course of chemoimmunotherapy, are notable.1,3–7 Three previous trials have shown a survival advantage with one therapy over another among patients with previously untreated CLL.1,2,15 The advantage of six cycles of ibrutinib–rituximab followed by continuous ibrutinib therapy with regard to these end points exceeded the prespecified thresholds for superiority at the time of the first interim analysis, despite the fact that the progression-free survival and overall survival observed among patients treated with fludarabine–cyclophosphamide–rituximab was similar to or better than that anticipated on the basis of historical studies (Table S9 in the Supplementary Appendix).1,3 Since the current advantage with regard to overall survival was based on a limited number of events, the long-term follow-up for survival among the patients in this trial, as well as for the incidence of myelodysplastic syndrome or acute myeloid leukemia, second cancers, and infectious complications, will be important.
Ibrutinib–rituximab resulted in superior progression-free survival in high-risk subgroups, including patients with Rai stage III or IV disease, chromosome 11q22.3 deletion, and unmutated IGHV. Chemoimmunotherapy with fludarabine– cyclophosphamide–rituximab has proved to be particularly effective in patients with IGHV-mutated CLL,5,6 with roughly half the patients in this lower-risk subgroup remaining in remission 8 to 10 years after the initiation of treatment.7 Longer follow-up in this subgroup of patients will provide additional clinical insights.
The safety results regarding ibrutinib–rituximab therapy in the E1912 trial were consistent with the results of previous trials of ibrutinib-based therapy.13–15 The risk of atrial fibrillation of any grade was 7.4% in the ibrutinib–rituximab group. The recently reported Alliance 041202 trial, which tested first-line ibrutinib–rituximab therapy and ibrutinib monotherapy, showed that the incidence of atrial fibrillation in these two groups was 14% and 17%, respectively, among patients with CLL who were 65 years of age or older (median age, 71 years).24 An age of 65 years or older, history of hypertension, and history of atrial fibrillation are risk factors for the development of atrial fibrillation during ibrutinib treatment,25 which suggests that the lower incidence of cardiac complications in the E1912 trial may be due to the younger patient population (median age, 58 years) relative to a pooled analysis of patients participating in other trials (median age, 67 years).25 Unexplained or unwitnessed death, which can be due to cardiac arrhythmias, was observed in only 1 patient in the E1912 trial, as compared with 11 of 361 patients (3%) treated with ibrutinib in the Alliance 041202 trial.24 Grade 3 or 4 hypertension in patients who were treated with ibrutinib–rituximab in the E1912 trial also occurred at a lower frequency than that seen among patients in the Alliance 041202 trial (18.8% vs. 34%).24 Major hemorrhagic events (all grade 3) occurred in 1.1% of the patients in the ibrutinib–rituximab group, with no fatal events. As of the interim analysis, one case of nonfatal cardiac arrest has been observed in the E1912 trial. Given the long-term, indefinite use of ibrutinib, we continue to monitor this end point closely.
The superior overall survival with ibrutinib-based therapy that was observed in the E1912 trial but not in the Alliance 041202 trial is notable because the chemoimmunotherapy that was used in the E1912 trial is more efficacious than the chemoimmunotherapy used in the Alliance trial.3 Several possible factors may contribute to these findings. First, more efficacious therapy may contribute more to survival prospects in younger patients with CLL with fewer coexisting conditions, who are less likely than older patients to die from unrelated causes. Second, more severe treatment-related toxic effects, including an increased risk of sudden death among older patients, may offset decreases in disease-related mortality among older patients. The higher mortality among patients receiving active treatment in the Alliance trial (7% of patients treated with ibrutinib–rituximab) relative to the participants in the E1912 trial (1%) and reported outcomes in a study in non-Hodgkin’s lymphoma subtypes support this notion.26
Although all the ibrutinib-treated patients in the E1912 trial also received rituximab, the benefits of combining rituximab with ibrutinib are unclear. No differences in progression-free survival or overall survival between the ibrutinib-alone group and the ibrutinib–rituximab group in the Alliance 041202 trial have been observed to date,24 a finding that is consistent with the results of a randomized comparison of ibrutinib alone and ibrutinib–rituximab in a trial predominantly involving patients with relapsed CLL.27 It is unclear whether these findings can be extrapolated to patients 70 years of age or younger with previously untreated CLL.
In conclusion, among patients 70 years of age or younger with previously untreated CLL, the combination of six cycles of ibrutinib–rituximab therapy followed by ibrutinib given continuously until relapse resulted in progression-free survival and overall survival that were superior to those with 6 months of chemoimmunotherapy with fludarabine–cyclophosphamide–rituximab. However, indefinite use of ibrutinib therapy has been associated with substantial expense28 and the potential for long-term toxic effects and may increase the risk of clonal selection leading to drug resistance.29,30
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute of the NIH (award numbers, CA193541, CA180820, CA180794, CA189828, CA180790, CA180791, CA180816, CA180833, CA189863, CA190140, CA180821, CA180888, CA189821, CA180816, CA180867, and CA180855) and in part by Pharmacyclics (a subsidiary of AbbVie).
Dr. Shanafelt reports receiving grant support from Pharmacyclics, AbbVie, Genentech, Celgene, Cephalon, Hospira, GlaxoSmithKline, Polyphenon E International, and Merck and holding a patent (US14/292,075) on green tea extract epigallocatechin gallate in combination with chemotherapy for chronic lymphocytic leukemia; Dr. Kay, receiving fees for serving on a data and safety monitoring board, paid to his institution, from Morpho-Sys, Infinity Pharmaceuticals, Celgene, Agios, and Rigel Pharmaceuticals, fees for serving on a data and safety monitoring board, paid to his institution, and advisory board fees, paid to his institution, from CytomX Therapeutics, advisory board fees, paid to his institution, from AstraZeneca, DAVA Pharmaceuticals, and Juno Therapeutics, grant support, paid to his institution, and advisory board fees, paid to his institution, from Pharmacyclics, Acerta Pharma, and Tolero Pharmaceuticals, and grant support, paid to his institution, from MEI Pharma; Dr. O’Brien, receiving consulting fees from Amgen, Astellas Pharma, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie, and Alexion, grant support from Kite Pharma, Regeneron, and Acerta Pharma, and grant support and consulting fees from Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis Pharmaceuticals; Dr. Barrientos, receiving grant support and advisory board fees from Pharmacyclics-AbbVie and lecture fees and travel support from Janssen, Gilead Sciences, and Genentech; Dr. Coutre, receiving grant support, advisory board fees, fees for education programs, and travel support from Pharmacyclics and Janssen, grant support, advisory board fees, and travel support from AbbVie and Celgene, grant support from Gilead Sciences, Takeda Pharmaceutical, and Acerta Pharma, advisory board fees from AstraZeneca, Astellas Pharma, and Novartis, fees for serving on a data and safety monitoring board and travel support from BeiGene, and fees for patent litigation and travel support from Genentech; Dr. Barr, receiving consulting fees from Pharmacyclics, AbbVie, Gilead Sciences, Merck, Genentech, Seattle Genetics, Verastem Oncology, TG Therapeutics, and Celgene; Dr. Cashen, receiving fees for serving on a speakers’ bureau from Celgene, Seattle Genetics, and Novartis; Mr. Mato, receiving grant support, consulting fees, fees for serving on a data and safety monitoring board, and advisory board fees from TG Therapeutics, grant support, consulting fees, and advisory board fees from Pharmacyclics, Johnson & Johnson, AbbVie, and AstraZeneca, grant support from Regeneron, fees for serving on a data and safety monitoring board and advisory board fees from Celgene, grant support and advisory board fees from Sunesis Pharmaceuticals and Loxo Oncology, and lecture fees, fees for continuing medical education events, and other events from prIME Oncology; Dr. Erba, receiving consulting fees and lecture fees from Agios, Incyte, Jazz Pharmaceuticals, MacroGenics, and Novartis, consulting fees from Amgen, Astellas Pharma, ImmunoGen, Pfizer, and Seattle Genetics, consulting fees, lecture fees, fees for serving as chair of a steering committee from Celgene, grant support and consulting fees from Daiichi Sankyo, grant support, consulting fees, and fees for serving as chair of a data and safety monitoring board from GlycoMimetics, and fees for serving as chair on an independent review board from Covance; Dr. Stone, receiving consulting fees, and research support, paid to his institution, from AbbVie, Agios, Arog Pharmaceuticals, and Novartis, advisory board fees from Actinium Pharmaceuticals, Amgen, and Astellas Pharma, fees for serving on a data and safety monitoring board from Argenx and Takeda Pharmaceutical, consulting fees from AstraZeneca, Celator Pharmaceuticals, Cornerstone Pharmaceuticals, Fujifilm, Jazz Pharmaceuticals, MacroGenics, Ono Pharmaceutical-Theradex Oncology, Orsenix, Otsuka-Astex Pharmaceuticals, Pfizer, Roche, Stemline Therapeutics, and Daiichi Sankyo, and consulting fees, fees for serving on a steering committee, and fees for serving on a data and safety monitoring board from Celgene; and Dr. Litzow, receiving grant support and consulting fees from Amgen, grant support from Astellas Pharma, AbbVie, Actinium Pharmaceuticals, Novartis, and Pluristem Therapeutics, and consulting fees from Sanofi and NewLink Genetics. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the participating patients and their families, who made this trial possible; Drs. Jennifer Woyach and John Byrd for collaboration in the conception and design of the trial; Drs. Peter J. O’Dwyer and Mitchell D. Schnall (co-chairs of Eastern Cooperative Oncology Group-American College of Radiology Imaging Network [ECOG-ACRIN] Cancer Research Group) for assistance in trial coordination; and the ECOG-ACRIN data managers and protocol specialists for their work in the trial.
Footnotes
The views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH), nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–74. [DOI] [PubMed] [Google Scholar]
- 2.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–10. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17: 928–42. [DOI] [PubMed] [Google Scholar]
- 4.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 2014; 123: 3247–54. [DOI] [PubMed] [Google Scholar]
- 5.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 2016; 127: 208–15. [DOI] [PubMed] [Google Scholar]
- 6.Jain P, Nogueras González GM, Kanagal-Shamanna R, et al. The absolute percent deviation of IGHV mutation rather than a 98% cut-off predicts survival of chronic lymphocytic leukaemia patients treated with fludarabine, cyclophosphamide and rituximab. Br J Haematol 2018; 180: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016; 127: 303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma 2015; 56: 1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer G, Montserrat E. Critical molecular pathways in CLL therapy. Mol Med 2018; 24: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011; 117: 6287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-κB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014; 123: 3286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiestner A The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015; 100: 1495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371: 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373: 2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelmann J, Gribben JG. Managing patients with TP53-deficient chronic lymphocytic leukemia. J Oncol Pract 2017; 13: 371–7. [DOI] [PubMed] [Google Scholar]
- 18.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2015; 33: 3199–212. [DOI] [PubMed] [Google Scholar]
- 19.Hallek M, Cheson BD, Catovsky D, et al. iwCLL Guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018; 131: 2745–60. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol 2012; 30: 2820–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549–56. [PubMed] [Google Scholar]
- 22.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–70. [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972; 34: 187–220. [Google Scholar]
- 24.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018; 379: 2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica 2017; 102: 1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes A, Sehn LH, Johnson P, et al. A global, randomized, placebo-controlled, phase 3 study of ibrutinib plus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) in patients with previously untreated non-germinal center B-cell-like (GCB) diffuse large B-cell lymphoma (DLBCL). Blood 2018; 132: 784 abstract. [Google Scholar]
- 27.Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood 2019; 133: 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanafelt TD, Borah BJ, Finnes HD, et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract 2015; 11: 252–8. [DOI] [PubMed] [Google Scholar]
- 29.Landau DA, Sun C, Rosebrock D, et al. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat Commun 2017; 8: 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrio S, Shanafelt TD, Ojha J, et al. Genomic characterization of high-count MBL cases indicates that early detection of driver mutations and subclonal expansion are predictors of adverse clinical out-come. Leukemia 2017; 31: 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.