Summary:
Field sterility is commonly used for skin and minor hand surgery performed in the ambulatory setting. Surgical site infection (SSI) rates are similar for these same procedures when performed in the main operating room (OR). In this paper, we aim to look at both current evidence and common sense logic supporting the use of some of the techniques and apparel designed to prevent SSI. This is a literature review of the evidence behind the ability of gloves, masks, gowns, drapes, head covers, footwear, and ventilation systems to prevent SSIs. We used MEDLINE, EMBASE, and PubMed and included literature from the inception of each database up to March 2019. We could not find substantial evidence to support the use of main OR sterility practices such as head covers, gowns, full patient draping, laminar airflow, and footwear to reduce SSIs in skin and minor hand surgery. Field sterility in ambulatory minor procedure rooms outside the main OR is appropriate for most skin and minor hand surgery procedures. SSIs in these procedures are easily treatable with minimal patient morbidity and do not justify the cost and waste associated with the use of main OR sterility.
INTRODUCTION
Since Louis Pasteur discovered that microorganisms caused infections, the evolution of sterility has had the pattern of the following: “If some sterility is good, more must be better.”1 Applying evidence-based medicine and common sense to sterility will allow us to evolve into the practice of the following: “What level of sterility do we actually need in this particular circumstance?”
The American Hospital Association estimated that two-thirds of all surgeries are performed in the ambulatory setting with field sterility.2 In Canada, typical outpatient procedures include laceration repair, skin lesion excision and reconstruction, and simple hand surgery, such as K wiring hand fractures, carpal tunnel decompression, and trigger finger release. In this paper, we arbitrarily define field sterility to include a mask, sterile gloves, and sterile draping of an area of 40 cm by 40 cm or less around a wound. Full standard sterility used in the main operating room (OR) involves the additional use of head covers, neck to knee sterile surgeon gowns, shoe covers, laminar airflow, and full patient body sterile draping.
The aim of aseptic technique is to prevent surgical site infections (SSIs), which is defined by the Centers for Disease Control and Prevention as a skin or subcutaneous tissue infection around the incision site occurring within 30 days after a procedure.3 There is a growing body of evidence indicating that SSI does not differ significantly between main OR sterility and field sterility for many surgical procedures.4–8,10–15 However, the difference in cost and garbage production is immense (Figs. 1–3).
Fig. 1.
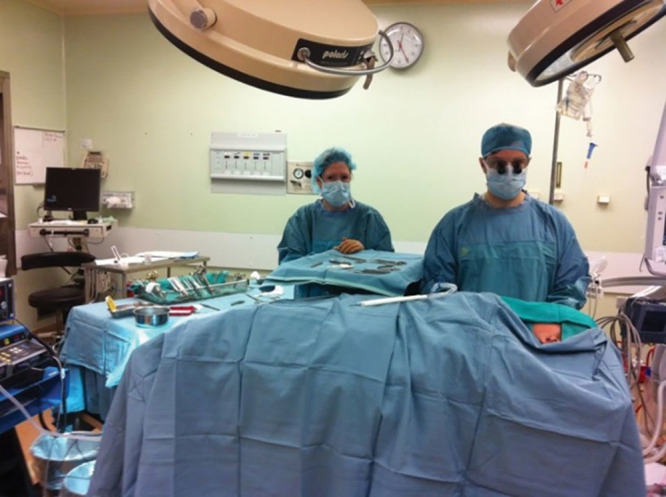
Main OR sterility to remove an accessory auricle. This is a minor skin procedure that could very safely be performed with field sterility.
Fig. 3.
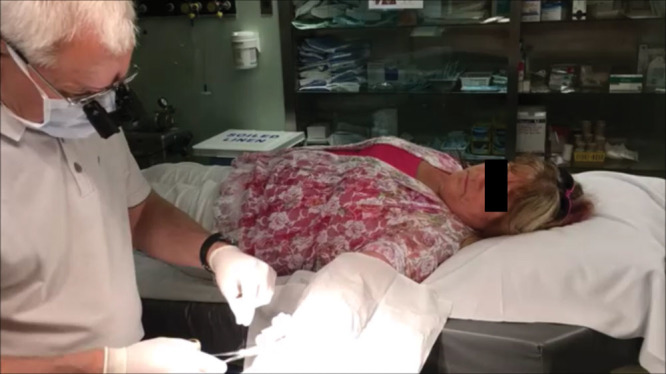
Field sterility for carpal tunnel surgery. More than 90% of Canadian carpal tunnel operations are performed this way in minor procedure rooms with an infection rate of 0.39%.14
Fig. 2.
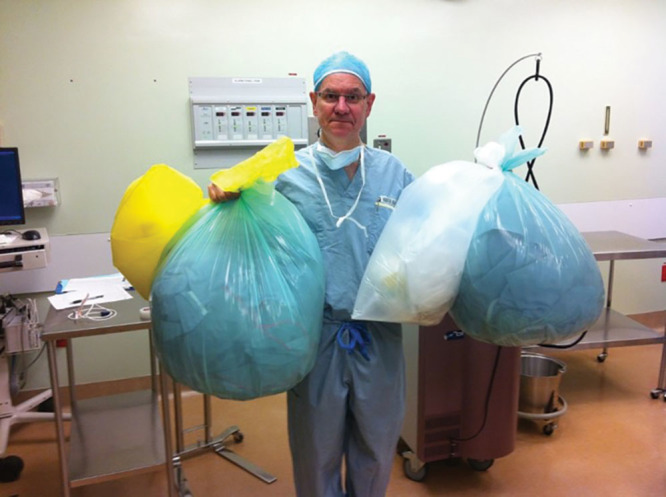
The waste produced from main OR sterility to remove an accessory auricle. Main OR carpal tunnel surgery produces similar waste.
The authors hypothesize that there is insufficient scientific evidence to support the costs and garbage production of main OR sterility practices such as head covers, sterile gowns, full patient draping, laminar airflow, and footwear for skin and minor hand surgery procedures. In this paper, we therefore look at both current evidence and common sense behind the use of techniques and apparel designed to prevent SSI. We will focus on the evidence behind the ability of gloves, masks, gowns, drapes, head covers, footwear, and ventilation systems to prevent SSIs. We will also introduce the concept that SSIs are not equal in their impact to a patient’s well-being and cost to the health-care system.
MATERIALS AND METHODS
A review of the literature was conducted using 3 medical databases (Ovid MEDLINE, EMBASE, and PubMed). Literature from the inception of each database up to March 2019 was searched. Keywords and MeSH terms related to “surgical wound infection,” “drapes,” “garments,” “protective clothing,” “gloves,” “shoes,” “surgical attire,” “ventilation system,” “HEPA,” “operating room,” “head covering,” and “surgical cap” were used. Animal studies and non-English articles were excluded.
RESULTS AND DISCUSSION
Gloves
The use of surgical gloves has become standard practice to protect health-care providers and prevent SSI.16–20 Much of the recent relevant research has focused upon the efficacy of sterile gloves versus clean boxed gloves. Boxed gloves are almost 8 times less expensive than sterile gloves and generate less sterile packing waste.21
Glove studies examining SSIs have yielded conflicting results. Recent work has failed to clearly demonstrate the benefit of sterile gloves over clean gloves.22 Two observational studies found no statistical differences in rates of SSI using sterile gloves versus clean boxed gloves in Mohs micrographic surgery.23,24 One study found a higher rate of infection in more complicated flap procedures performed with nonsterile clean boxed gloves but infection control precautions varied with the procedures.20 A more recent systematic review and meta-analysis of 14 studies, 8 of which were randomized controlled trials (RCTs), found no difference in the rate of postoperative SSI in outpatient cutaneous surgical procedures performed with sterile versus clean boxed gloves.25 It may be that boxed gloves are appropriate for minor cutaneous skin procedures.
It should also be noted most of the above studies look at facial surgery where infection rates may to be lower than other anatomical areas. The authors speculate that hand infection rates may be like facial infections because of high blood flow rates in both sites.
There is currently a lack of evidence supporting the use of sterile gloves for simple skin surgery. As the theoretical possibility of bacterial contamination of boxed unsterile gloves does exist, common sense still guides most of us to use sterile gloves until stronger evidence is produced, especially in less vascularized spaces such as the carpal tunnel and trigger finger.
Masks
Surgical face masks were originally developed to contain bacteria-filled droplets expelled from the nose and mouth of health-care providers to protect the surgical field from contamination. A study by Meleney and Stevens suggested that mask use may reduce the incidence of postoperative hemolytic streptococcus wound infections to 5%.26 The practice of wearing masks during surgery subsequently became more widespread. Interestingly, the same authors refuted their initial findings 9 years later and reported infection rates with consistent mask use to be much higher than had been anticipated in their initial study.27
Recent studies comparing outcomes with or without surgical face masks have found little to no difference in SSIs.28–31 Cochrane reviews have found insufficient evidence for the benefit of surgical face masks in clean surgical procedures on reducing wound infection rates.32 Furthermore, large variations exist in the filtering ability of surgical masks, even from the same manufacturer. There is currently no standard method of measuring filtering capability of masks, and it is not known what effect the use of surgical masks from different manufacturers would have on SSIs.
We have not been able to find good evidence that surgical masks reduce the incidence of SSIs in skin and minor hand surgery. However, all of us have seen droplets of saliva or nasal mucous emanating in speech or sneezing. Spraying an open wound with these bacteria-laden droplets is a difficult problem to disregard. Therefore, until stronger evidence is available, common sense still guides us to use a mask with field sterility in these procedures.
Surgical Gowns and Drapes
Sterile gowns are thought to prevent the surgeon’s skin or clothing bacteria from shedding onto the patient either from direct contact or via the air. Gowns are fabricated from either reusable or single-use materials. Reusable surgical gowns are often made from tightly woven polyester sheeting or a combination of fabrics with a film coating. Single-use surgical gowns consist of nonwoven materials such as wood pulp and polyester fibers with plastic films for liquid protection. Due to the wide variety of gowns used, interpretation of the literature regarding their efficacy is difficult.
Eisen reviewed prospective studies comparing woven cotton gown material with nonwoven ones and found conflicting evidence.22 Three prospective crossover studies showed a benefit of disposable gowns over reusable gowns whereas 2 RCTs and 1 prospective crossover study showed no difference. Notably, the only level I evidence paper showed no difference in gown material on SSI rates.33 In a separate study, the release of airborne bacteria from dedicated main OR scrub suits compared with personal clothing worn outside the hospital was not significantly different.34
Most surgeons wear unsterile scrubs or regular clothing when performing field sterility procedures outside of the main OR. We could not find any studies that compared the rate of SSI when surgeons used sterile surgical gowns with when surgeons did not use sterile surgical gowns.
The rationale behind using main OR sterile drapes to cover the entire patient far beyond the surgical field is to prevent contact between sterile and unsterile surfaces and the patient’s wound. The World Health Organization (WHO) evaluated the most optimal draping material to prevent SSI in the main OR environment. They recommend either1 sterile, disposable, nonwoven drapes, or2 sterile, reusable, woven drapes, based on a meta-analysis which found no difference between these draping materials.35
Regarding the use of adhesives, a recent Cochrane review (level I evidence) based on 5 trials found that the use of adhesive drapes was associated with an increased SSI risk compared with no drapes.36 This review also found that iodine-impregnated adhesive drapes likely made no difference to SSI risk compared with nonadhesive drapes.37 They concluded that adhesives may increase SSI risk by attracting and promoting the colonization of bacteria.38
We could not find any evidence to support that surgeons’ gowns or full patient draping decreases SSI in skin and minor hand surgery procedures over field sterility in skin and minor hand surgery. Common sense would suggest that, if a difference exists, it would be extremely small and not worth the cost and garbage of gowns and drapes given the low field sterility rates of infection and minimal patient morbidity in these procedures.10,11,13,14
Head Covers
Summers et al stated that 46% of medical and nursing personnel carried pathogenic organisms (most commonly Staphylococcus aureus) in hair and nasal passages.39 Because bacterial shedding could theoretically occur through the air from the scalp hair, the authors recommended that health-care workers completely cover hair during surgical procedures. This type of infection theory paper spawned the widespread practice of using head covers over the scalp and facial hair in the main OR. However, Humphreys et al found that the use of disposable head coverings in an experimental setting was not associated with reduced bacterial air counts.40
There are currently no studies associating the use of head covers with a reduction in SSI in skin and simple hand surgery. Multiple literature reviews have concluded that there is insufficient evidence to suggest that the use of head coverings reduces rates of SSI.22,41–43 Furthermore, no studies have proven superiority of any specific type of surgical headwear over another, in any clinical capacity.44 Despite the lack of definitive evidence, most organizations recommend that head coverings be used in the main OR and perioperative setting given the theoretical benefit.45 The fact that different organizations have so many different head dress policies is likely reflective of the fact that there is no good evidence that 1 policy is better than the other. Neither common sense nor the evidence supports the use of head cover for skin and minor hand surgery.
Footwear
Amirfeyz et al compared bacterial contamination of designated theatre-only shoes with street footwear.45 He found more pathogenic bacteria on all studied shoes at the end of the day, with street shoes being significantly more contaminated than theatre-only shoes at both the beginning and end of the day. Nagai et al demonstrated increased rates of bacterial floor contamination with proximity to areas where footwear is changed.46 Copp and Copp found increased rates of bacterial transfer to the OR floor from street shoes than from designated theatre-only shoes or shoes with covers.47 In these studies, the relationship to SSIs was not studied. A recent systematic review study confirmed a lack of research exploring the effect of shoe surfaces as a potential cause of increased infection rates.48
We could not find any evidence that either high bacterial counts on the floor or increased concentrations of bacteria on shoes increase infection rates in skin or minor hand surgery. The fact that policies vary so widely from center to center reflects this lack of evidence. Unless there is gross contamination such as large amounts of pus on the floor, common sense would suggest that footwear or floor washing between cases makes little to no difference in minor skin and hand surgery procedures that occur way above the floor at the surgeons’ elbow height.
Ventilation Systems
Most procedure rooms outside the main OR have no special ventilation. Most main ORs use laminar airflow filtration (LAF) and high-efficiency particulate air (HEPA) filters to achieve ultraclean air. Most of the literature on the effect of ventilation systems relates to major orthopedic procedures where infection creates great morbidity for patients.
LAF systems provide unidirectional airflow. In theory, they decrease infection rates by preventing airborne bacteria from landing and infecting the wound. A landmark RCT in the early 1980s showed that LAF reduced deep prosthetic joint infections from 1.5% to 0.6%, spurring increased use of ultraclean air systems especially in orthopedic surgery.49
However, multiple recent studies failed to reproduce these results or expand these conclusions to other procedures. A recent systematic review found no difference in overall SSI between laminar airflow and conventional ventilation in abdominal and vascular surgeries.50 The same study also found a possible increased risk of deep SSI for hip and knee arthroplasties with LAF.50 Possible explanations for this discrepancy include changes in the modern-day OR environment with the use of positive pressure rooms, flaws with the original RCT such as lack of random allocation and heterogenic use of antibiotic prophylaxis, and a possible harmful effect of ultraclean air systems if they are not used or maintained properly.51
High-efficiency particulate filters can remove 99.97% of particles >0.3 µm, whereas conventional filters remove 95% of particles >5 µm. However, HEPA filters are significantly more expensive to purchase and maintain.52 A retrospective cohort study showed that HEPA with laminar or turbulent ventilation reduced SSI rates in knee arthroscopy compared with no artificial ventilation but failed to show this benefit in hip arthroscopy.53 Another retrospective comparative study found that HEPA made no difference in SSI rates in total joint arthroplasty surgeries using forced air warmers.54
Given the inconclusive evidence of laminar airflow systems and HEPA filters in high infection risk surgeries with large artificial joint replacements, it seems unlikely that either ventilation system would impact infection for most skin and minor hand surgery procedures. This is especially relevant when evidence has shown the low rate of infections with skin and minor hand surgery in procedure rooms outside the main OR where there is no laminar airflow or HEPA filters10,11,13,14
The Evidence Supporting Field Sterility for Skin and Minor Hand Surgery
K wire pinning of hand fractures without sterile gowns and with no full patient draping has been shown to produce very low infection rates.10,11 Unlike infection after internal plate fixation or prosthetic joint replacement, the cost of infection after hand fracture K wire pinning to a patient’s quality of life or to the health-care system is very low. Most infections after K wire pinning respond with minimal patient morbidity to removing the K wire and oral antibiotics. In Canada, field sterility for K wire insertion procedure rooms outside the main OR is a well-accepted long-standing practice in many hospital centers. It has produced very few cases of infection that end up requiring intravenous antibiotics or producing osteomyelitis. Infection after K wire insertion seems more related to what the patient does after the surgery than the field versus man OR sterility with which we insert them.
In a multicenter prospective study of 1,504 cases, field sterility for carpal tunnel surgery yielded an infection rate of 0.39%.14 More than 90% of Canadian carpal tunnels are performed this way today with the same low infection rate with no gown, no head gear, no protective footwear no full patient draping, and no special airflow systems.12 This SSI rate is comparable with those carpal tunnel surgeries completed in the main OR.55,56
Field sterility is also associated with very low SSI rates in Mohs micrographic surgery. A 2013 publication of 20,821 cases produced an infection rate of 0.37%.13 This is remarkably similar to the multicenter study of the infection rate in carpal tunnel surgery described above. Another Moh’s series also showed very low infection rates of more complex skin surgery such as flaps (1.9%) and in full thickness skin grafts (3.1%).39
Both current evidence and common sense therefore suggest that field sterility is reasonable in minor procedures such as skin surgery, carpal tunnel surgery, and K wiring of hand fractures.
Main OR Sterility Is Expensive
Main OR sterility is costly compared with field sterility.4,6–8,10,11,57 The cost to excise a single skin lesion in the main OR is twice as expensive as with field sterility.15 Carpal tunnel releases are 4 times more costly if performed in the main OR compared with in the minor procedure room, even without anesthesiology.8 Trigger finger surgery is 2–3 times more expensive in the main OR.9
It should also be noted that many of the main OR materials used are for personal protection equipment not sterility. Part of the costs therefore is consumed by health personnel protection.
Main OR Sterility Creates Massive Amounts of Unnecessary Waste
Disposable materials consumed in main OR sterility practices create enormous amounts of waste. Recent Canadian environmental data from 2015 showed that the health-care sector generated 33 million metric tons of carbon dioxide, which is equivalent to 4.6% of Canada’s total greenhouse gas emissions.17 In the United States, hospitals produce 4 billion tons of waste annually with ORs being the highest contributors.58,59 Future studies that evaluate the safety and efficacy of field sterility practices could provide considerable financial savings for the health-care system and environmental benefits for everyone.4–8,57
Not All SSIs Are Equal in Patient Impact and Cost
Infection after carpal tunnel surgery usually responds very well to suture removal or oral antibiotics without significant harm to the patient or cost to the health-care system. Infection after a prosthetic knee insertion can be disastrous to a patient’s life and will generate costly complication management. Both clinical situations are SSIs, but they have very different cost and patient harm implications. Common sense tells us that to use main OR maximal expense “space suit” style sterility is reasonable for knee replacement but not for carpal tunnel surgery. It is important to study not only the rates but perhaps more importantly the patient impact of SSIs in different operations. We should not treat all SSIs as having an equal effect on patients.
The senior author currently uses field sterility outside the main OR environment for closed K wire insertion and simple soft tissue dissection surgery such as carpal tunnel, trigger finger, and ulnar nerve decompression at the elbow. He uses augmented field sterility (gown for the surgeon and a larger drape around the hand) for tendon repair and tendon transfer because infection in these circumstances has more grave consequences than simple closed K wire insertion or carpal tunnel surgery. He prefers traditional full OR sterility for permanent hardware insertion and extensive soft tissue dissection procedures such as forearm tendon transfers.
Main Weakness of the Study
The principal weakness of this study is that there is profound lack of good scientific evidence telling us how much sterility we actually need for different types of procedures. For this reason, the senior author felt compelled to add the common sense of clinical experience to the analysis of the literature.
SUMMARY
We have not been able to find substantial evidence to support many of the main OR sterility practices such as head covers, sterile gowns, full patient draping, laminar airflow, and footwear for skin and minor hand surgery procedures. Field sterility with a mask, sterile gloves, and a small sterile wound drape in minor procedure rooms (outside the main OR) seems appropriate for the majority of these types of operations.
The cost of an infection after implantation of a synthetic knee implant is very high to a patient’s quality of life and to our health-care system. All possible measures of reducing SSIs (theoretical or proven) in this type of procedure seem justified. On the other hand, most infections in skin and minor hand surgery respond to the removal of sutures, K wires, and oral antibiotics. The cost is not massive to a patient’s quality of life or to the health-care system. The costs and garbage production of main OR sterility are not justified with these types of procedures, especially given their low infection rates with field sterility.
PATIENT CONSENT
The patient provided written consent for the use of her image.
Footnotes
Published online 21 November 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Schwartz M. The life and works of Louis Pasteur. J Appl Microbiol. 2001;91:597–601. [DOI] [PubMed] [Google Scholar]
- 2.American Hospital Association. Chapter 3: Utilization and Volume. Trendwatch Chartbook 2016 - trends affecting hospitals and health systems. Available at: https://www.aha.org/system/files/2018-01/2016-chartbook.pdf. Accessed Oct 17, 2019. [Google Scholar]
- 3.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 4.Chatterjee A, McCarthy JE, Montagne SA, et al. A cost, profit, and efficiency analysis of performing carpal tunnel surgery in the operating room versus the clinic setting in the United States. Ann Plast Surg. 2011;66:245–248. [DOI] [PubMed] [Google Scholar]
- 5.Van Demark RE, Jr, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg Am. 2018;43:179–181. [DOI] [PubMed] [Google Scholar]
- 6.Rhee PC, Fischer MM, Rhee LS, et al. Cost savings and patient experiences of a clinic-based, wide-awake hand surgery program at a military medical center: a critical analysis of the first 100 procedures. J Hand Surg Am. 2017;42:e139–e147. [DOI] [PubMed] [Google Scholar]
- 7.Bismil M, Bismil Q, Harding D, et al. Transition to total one-stop wide-awake hand surgery service-audit: a retrospective review. JRSM Short Rep. 2012;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leblanc MR, Lalonde J, Lalonde DH. A detailed cost and efficiency analysis of performing carpal tunnel surgery in the main operating room versus the ambulatory setting in Canada. Hand (N Y). 2007;2:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazmers NH, Stephens AR, Presson AP, et al. Cost implications of varying the surgical setting and anesthesia type for trigger finger release surgery. Plast Reconstr Surg Glob Open. 2019;7:e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dua K, Blevins CJ, O’Hara , et al. The safety and benefits of the semisterile technique for closed reduction and percutaneous pinning of pediatric upper extremity fractures. Hand (N Y). 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garon MT, Massey P, Chen A, et al. Cost and complications of percutaneous fixation of hand fractures in a procedure room versus the operating room. Hand (N Y). 2018;13:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters B, Giuffre JL. Canadian trends in carpal tunnel surgery. J Hand Surg Am. 2018;43:1035.e1–1035.e8. [DOI] [PubMed] [Google Scholar]
- 13.Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378–1385. [DOI] [PubMed] [Google Scholar]
- 14.Leblanc MR, Lalonde DH, Thoma A, et al. Is main operating room sterility really necessary in carpal tunnel surgery? A multicenter prospective study of minor procedure room field sterility surgery. Hand (N Y). 2011;6:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuzzi LC, Greene AK, Meara JG, et al. Surgical site infection after skin excisions in children: is field sterility sufficient? Pediatr Dermatol. 2016;33:136–141. [DOI] [PubMed] [Google Scholar]
- 16.Eckelman MJ, Sherman JD, MacNeill AJ. Life cycle environmental emissions and health damages from the Canadian healthcare system: an economic-environmental-epidemiological analysis. PLos Med. 2018;15:e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemsworth S, Selwood K, van Saene R, et al. Does the number of exogenous infections increase in paediatric oncology patients when sterile surgical gloves are not worn for accessing central venous access devices? Eur J Oncol Nurs. 2007;11:442–447. [DOI] [PubMed] [Google Scholar]
- 18.Chiu WK, Cheung LK, Chan HC, et al. A comparison of post-operative complications following wisdom tooth surgery performed with sterile or clean gloves. Int J Oral Maxillofac Surg. 2006;35:174–179. [DOI] [PubMed] [Google Scholar]
- 19.Whyte W, Hambraeus A, Laurell G, et al. The relative importance of the routes and sources of wound contamination during general surgery. II. Airborne. J Hosp Infect. 1992;22:41–54. [DOI] [PubMed] [Google Scholar]
- 20.Rogues AM, Lasheras A, Amici JM, et al. Infection control practices and infectious complications in dermatological surgery. J Hosp Infect. 2007;65:258–263. [DOI] [PubMed] [Google Scholar]
- 21.Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27–31. [DOI] [PubMed] [Google Scholar]
- 22.Eisen DB. Surgeon’s garb and infection control: what’s the evidence? J Am Acad Dermatol. 2011;64:960.e1–960.20. [DOI] [PubMed] [Google Scholar]
- 23.Rhinehart MB, Murphy MM, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170–176. [DOI] [PubMed] [Google Scholar]
- 24.Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234–239. [DOI] [PubMed] [Google Scholar]
- 25.Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008–1014. [DOI] [PubMed] [Google Scholar]
- 26.Meleney FL, Stevens FA. Postoperative haemolytic streptococcus wound infections and their relation to haemolytic streptococcus carriers among the operating personnel. SG&O. 1926;43:338–342. [Google Scholar]
- 27.Meleney F. Infection in clean operative wounds: a nine year study. SG&O. 1935;60264–275. [Google Scholar]
- 28.Beck WC. The surgical mask: another ‘sacred cow’? Aorn J. 1992;55:955–957. [DOI] [PubMed] [Google Scholar]
- 29.Orr N. Why wear a mask in theatre? Natnews. 1982;19:11–12. [PubMed] [Google Scholar]
- 30.Orr NW, Bailey S. Masks in surgery. J Hosp Infect. 1992;20:57. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell NJ, Hunt S. Surgical face masks in modern operating rooms–a costly and unnecessary ritual? J Hosp Infect. 1991;18:239–242. [DOI] [PubMed] [Google Scholar]
- 32.Lipp A, Edwards P. Disposable surgical face masks for preventing surgical wound infection in clean surgery. Cochrane Database Syst Rev. 2016;4:CD002929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garibaldi RA, Maglio S, Lerer T, et al. Comparison of nonwoven and woven gown and drape fabric to prevent intraoperative wound contamination and postoperative infection. Am J Surg. 1986;152:505–509. [DOI] [PubMed] [Google Scholar]
- 34.Bethune DW, Blowers R, Parker M, et al. Dispersal of staphylococcus aureus by patients and surgical staff. Lancet. 1965;1:480–483. [DOI] [PubMed] [Google Scholar]
- 35.Leaper DJ, Edmiston CE. World health organization: global guidelines for the prevention of surgical site infection. J Hosp Infect. 2017;95:135–136. [DOI] [PubMed] [Google Scholar]
- 36.Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2015(4) CD006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Dumville JC, Norman G, et al. Intraoperative interventions for preventing surgical site infection: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2018;2:CD012653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falk-Brynhildsen K, Friberg O, Söderquist B, et al. Bacterial colonization of the skin following aseptic preoperative preparation and impact of the use of plastic adhesive drapes. Biol Res Nurs. 2013;15:242–248. [DOI] [PubMed] [Google Scholar]
- 39.Summers MM, Lynch PF, Black T. Hair as a reservoir of staphylococci. J Clin Pathol. 1965;18:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphreys H, Russell AJ, Marshall RJ, et al. The effect of surgical theatre head-gear on air bacterial counts. J Hosp Infect. 1991;19:175–180. [DOI] [PubMed] [Google Scholar]
- 41.McHugh SM, Corrigan MA, Hill AD, et al. Surgical attire, practices and their perception in the prevention of surgical site infection. Surgeon. 2014;12:47–52. [DOI] [PubMed] [Google Scholar]
- 42.Salassa TE, Swiontkowski MF. Surgical attire and the operating room: role in infection prevention. J Bone Joint Surg Am. 2014;96:1485–1492. [DOI] [PubMed] [Google Scholar]
- 43.Spruce L. Surgical head coverings: a literature review. Aorn J. 2017;106:306.e6–316.e6. [DOI] [PubMed] [Google Scholar]
- 44.Kothari SN, Anderson MJ, Borgert AJ, et al. Bouffant vs skull cap and impact on surgical site infection: does operating room headwear really matter? J Am Coll Surg. 2018;227:198–202. [DOI] [PubMed] [Google Scholar]
- 45.Amirfeyz R, Tasker A, Ali S, et al. Theatre shoes - a link in the common pathway of postoperative wound infection? Ann R Coll Surg Engl. 2007;89:605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagai I, Kadota M, Takechi M, et al. Studies on the mode of bacterial contamination of an operating theatre corridor floor. J Hosp Infect. 1984;5:50–55. [DOI] [PubMed] [Google Scholar]
- 47.Copp G, Copp AJ. Operating room nursing as a scientific discipline: a review of research findings and their implementation. Perioper Nurs Q. 1985;1:22–33. [PubMed] [Google Scholar]
- 48.Rashid T, VonVille HM, Hasan I, et al. Shoe soles as a potential vector for pathogen transmission: a systematic review. J Appl Microbiol. 2016;121:1223–1231. [DOI] [PubMed] [Google Scholar]
- 49.Lidwell OM, Lowbury EJ, Whyte W, et al. Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J (Clin Res Ed). 1982;285:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bischoff P, Kubilay NZ, Allegranzi B, et al. Effect of laminar airflow ventilation on surgical site infections: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:553–561. [DOI] [PubMed] [Google Scholar]
- 51.Thomas AM, Simmons MJ. The effectiveness of ultra-clean air operating theatres in the prevention of deep infection in joint arthroplasty surgery. Bone Joint J. 2018;100-B:1264–1269. [DOI] [PubMed] [Google Scholar]
- 52.Cacciari P, Giannoni R, Marcelli E, et al. [Cost evaluation of a ventilation system for operating theatre: an ultraclean design versus a conventional one]. Ann Ig. 2004;16:803–809. [PubMed] [Google Scholar]
- 53.Song KH, Kim ES, Kim YK, et al. Differences in the risk factors for surgical site infection between total hip arthroplasty and total knee arthroplasty in the Korean nosocomial infections surveillance system (KONIS). Infect Control Hosp Epidemiol. 2012;33:1086–1093. [DOI] [PubMed] [Google Scholar]
- 54.Curtis GL, Faour M, George J, et al. High efficiency particulate air filters do not affect acute infection rates during primary total joint arthroplasty using forced air warmers. J Arthroplasty. 2018;33:1868–1871. [DOI] [PubMed] [Google Scholar]
- 55.Harness NG, Inacio MC, Pfeil FF, et al. Rate of infection after carpal tunnel release surgery and effect of antibiotic prophylaxis. J Hand Surg Am. 2010;35:189–196. [DOI] [PubMed] [Google Scholar]
- 56.Hanssen AD, Amadio PC, DeSilva SP, et al. Deep postoperative wound infection after carpal tunnel release. J Hand Surg Am. 1989;14:869–873. [DOI] [PubMed] [Google Scholar]
- 57.Sieber D, Lacey A, Fletcher J, et al. Cost savings using minimal draping for routine hand procedures. Minn Med. 2014;97:49. [PubMed] [Google Scholar]
- 58.Kwakye G, Brat GA, Makary MA. Green surgical practices for health care. Arch Surg. 2011;146:131–136. [DOI] [PubMed] [Google Scholar]
- 59.Albert MG, Rothkopf DM. Operating room waste reduction in plastic and hand surgery. Plast Surg (Oakv). 2015;23:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]


