Supplemental Digital Content is available in the text.
Background:
Liposuction is the treatment of choice for solid predominant extremity lymphedema. The classic lymphedema liposuction technique does not remove skin excess created following bulk removal. The skin excess is presumed to resolve with spontaneous skin contracture. We investigated the technique of simultaneously performing liposuction with immediate skin excision in patients with solid predominant lymphedema and compared the outcome with that from the classic technique.
Methods:
Modified liposuction with skin excision (mLIPO) and standard liposuction without skin excision (sLIPO) were offered to patients with solid predominant extremity lymphedema. Skin traction of 4 cm and undulating skin mobility constituted positive “flying squirrel” sign. Patients with negative “flying squirrel” sign were excluded. mLIPO patients underwent skin excision. Surgical outcomes and postoperative complications were compared.
Results:
The study enrolled 15 and 26 patients into the sLIPO and mLIPO groups, respectively. mLIPO patients demonstrated statistically significant decrease in seroma/hematoma, contour irregularity, and skin necrosis, while experiencing increased procedural satisfaction.
Conclusions:
Skin excision following liposuction for solid predominant lymphedema is safe. It decreases postoperative complication and improves surgical outcome.
BACKGROUND
Modern advancements in reconstructive microsurgery and supermicrosurgery turn lymphedema from an untreatable condition to one that can now be surgically managed.1–8 With this comes further development in disease diagnosis,9–11 severity staging,12–15 and disease classification.9,12–17 Although initially thought of as a condition characterized by pathologic edema, the presence of lymphedema-derived lipodystrophy and subcutaneous fibrosis, or the solid disease component, is now widely recognized.10,18,19 Only subtly present in early disease, the solid disease component becomes increasingly prominent as the pathology progresses. In the intermediate to late stages, it causes serious lymphedema-related morbidities.20 The solid disease component does not resolve with physiologic reconstruction such as vascularized lymph node transfer and lymphaticovenular anastomosis (LVA).21 Direct surgical debulking remains the only effective treatment.22 Commonly performed debulking techniques include liposuction,23 serial excision,24 and the Charles procedure.25 Liposuction is particularly appealing because of its technical simplicity and minimally invasive nature. Lymphedema liposuction is classically performed without concomitant skin excision.21,26 The skin redundancy created following bulk reduction is presumed to resolve with spontaneous skin contracture.21,26 We investigated the technique of simultaneously performing liposuction and skin excision in lymphedema patients and compared the outcome with that from the standard technique.
METHODS
Patients
With approval from the institutional review board of the University of Iowa Hospitals and Clinics and with written informed patient consent, lymphedema liposuction was offered to 43 consecutive patients with solid predominant limb lymphedema. The diagnosis of lymphedema was confirmed with indocyanine green lymphography in all patients. All patients showed extensive “stardust” and/or “diffuse” lymphographic patterns consistent with advanced lymphatic injury (reference).13 The criteria for solid predominant disease include (1) irreversible limb bulkiness, (2) limb bulkiness out of proportion to the volume suggested by quantitative bioimpedance spectroscopy (Figs. 1 and 3) magnetic resonance imaging demonstrating abundant lipodystrophy (Fig. 2). All patients had International Society of Lymphology stage II and III disease. All International Society of Lymphology stage III patients had only mild cutaneous fibrosis (Fig. 3). All patients lacked signs/symptoms characteristically associated with lipedema, a condition that could mimic solid predominant lymphedema, including painful lipodystrophy, easy bruising, distal parts (hand, feet) preservation, negative Stemmer sign, and only mild or normal lymphatic drainage seen on lymphatic imaging.27
Fig. 1.
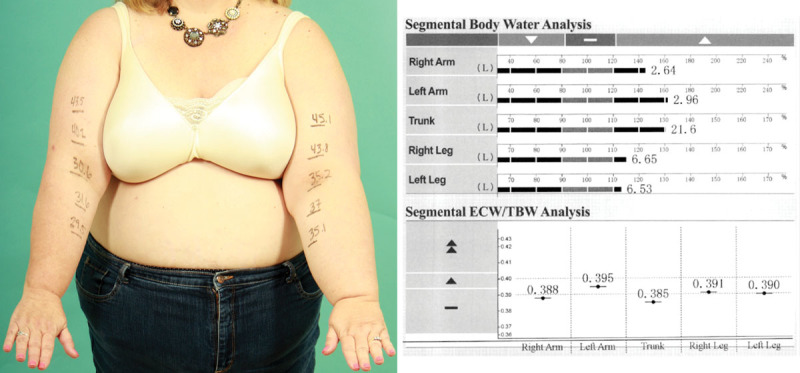
Bioimpedance spectroscopy in this patient with solid predominant left arm lymphedema showed an interarm body water discrepancy of only 0.32 L (2.96–2.64 L). This amount of discrepancy does not sufficiently explain the observed left arm bulk.
Fig. 3.
Despite having ISL stage III disease, this patient demonstrated relatively mild cutaneous fibrosis. In authors’ experience, the degree of cutaneous fibrosis correlates well with the severity of subcutaneous fibrosis. Liposuction remains an effective debulking method for this patient. ISL, International Society of Lymphology.
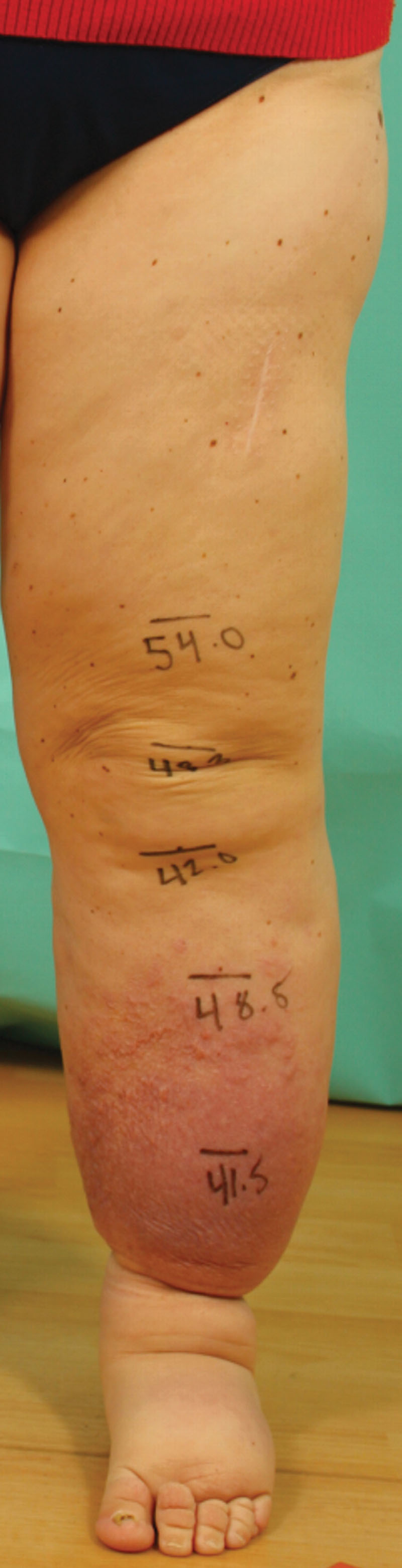
Fig. 2.
An abundance of adiposity can be seen in this patient with solid predominant leg lymphedema. Although there is also significant subcutaneous edema (grey area), it contributes relatively less to the overall bulk of the leg.
After explaining both surgical approaches, the patients elected to undergo either the standard liposuction without skin excision (sLIPO) or the modified liposuction with skin excision (mLIPO).
Surgical Technique
See Video 1 [online], which displays the “flying squirrel” lymphedema liposuction technique. Following tumescent infiltration, liposuction was performed with power assistance to increase surgical efficiency. Liposuction reached endpoint when achieving physiologic thickness of subcutaneous fat. The skin excess was assessed with pinch test and double ellipse technique and removed in tailor-tack fashion to avoid over-resection.
Video 1. This video demonstrates the “Flying Squirrel“ lymphedema liposuction technique. Following tumescent infiltration, liposuction was performed with power-assistance to increase surgical efficiency. Liposuction reached endpoint when achieving physiologic thickness of subcutaneous fat. The skin excess was assessed with pinch test and double ellipse technique, and removed in tailor-tack fashion to avoid over resection.
Under general anesthesia, circumferential, power-assisted liposuction was performed under tourniquet control for both upper and lower extremities after infiltrating the limb with tumescent solution which consisted of 1 mL of 1:1,000 epinephrine mixed with 1 L of normal saline. The tumescent infiltration was performed until achieving strong tissue turgor. After waiting for 10 minutes, the limb was exsanguinated and a tourniquet was deployed. Liposuction was performed using 4- and 5-mm cannulae via 4–6 stab incisions. Liposuction was performed to achieve a uniform subcutaneous tissue thickness of 0.5–1 cm, depending on the anatomic region. For example, aggressive liposuction was performed at pretibial region to achieve 0.5-cm thickness, whereas in thigh region 1-cm thickness was used as treatment endpoint. Liposuction was performed in a deep to superficial plane sequence to preserve dermal plexus. When the limb segment distal to the tourniquet was fully treated, compression bandage was applied, and the tourniquet was released to allow treatment of the proximal segment previously covered by the tourniquet. For the upper extremity, the entire limb was treated in a single procedure. For lower extremity, the leg and thigh were sequentially treated in separate procedures due to high removal volumes and for ease of recovery. After liposuction, the degree of skin excess was assessed. The ability to traction the skin away from the deep fascia by 4 cm and the undulating mobility of the skin excess constituted positive “flying squirrel” sign (FSS). (See Video 2 [online], which displays a positive FSS following lymphedema liposuction. Undulating skin mobility due to skin excess could be seen in the arm and leg. Leaving these skin excess behind would cause increased risks of seroma/hematoma, contour deformity, difficulty with postoperative compression, and skin necrosis. Removing them would improve the limb contour and decrease the risks of all the abovementioned complications.)
Video 2. This video demonstrates a positive “Flying Squirrel” sign following lymphedema liposuction. Undulating skin mobility due to skin excess could be seen in arm and leg. Leaving these skin excess behind would cause increased risks of seroma/hematoma, contour deformity, difficulty with postoperative compression, and skin necrosis. Removing them would improve the limb contour and decrease risks of all above-mentioned complications.
The use of 4-cm threshold as indication for skin excision was empirically derived. We nicknamed this indication “flying squirrel” due to the postliposuction appearance of the limb with skin excess resembling this animal in flight. mLIPO patients with positive FSS received skin excision. The skin redundancy was assessed with the “double ellipse” technique28 and removed with tailor-tack approach. For upper extremity, the long axis of the excised area was positioned on the posterior aspect of the upper arm. For lower extremity, it was placed on the medial aspect of the leg and thigh. The excision included the full length of the limb segment. For example, upper arm skin excision extended from elbow to axilla and leg excision extended from medial malleolus to the knee. No drain was used. Compression with short stretch bandage was immediately applied following the procedure.
Postoperative Care and Outcome Tracking
All patients were admitted for 1–3 days for pain management. Therapy session started on postoperative day 1 during which the therapists instructed proper bandaging technique. Patients were instructed to keep the treated limb elevated with light use only for 4 weeks. Bandage compression continued for 4 postoperative weeks. On the 5th postoperative week, all started custom-ordered 30–40-mm Hg compression garment. The use of compression garment was continued indefinitely. Patients were seen at 1 week, 4 weeks, 3 months, 6 months, and 12 months following the surgery, then annually. Outcomes tracked were contour irregularity, seroma/hematoma, wound dehiscence, skin necrosis, infection, and patient satisfaction. The patient satisfaction assessment was evaluated with a simple, nonvalidated, 1–10 scale. Data were analyzed using descriptive statistics, chi-square test, and Student’s t test where indicated. Demographic and surgical outcome variables were analyzed via chi-square tests. Patient satisfaction scores were analyzed with 2-sample t test assuming unequal variance (heteroscedastic). Statistical analysis was performed with Stata 15.1 (StataCorp, College Station, TX, USA). Unless specified, all statistical testing assumes 2-tailed test with P < 0.05 considered as statistically significant.
RESULTS
Fifteen patients self-selected into the sLIPO group and 28 into the mLIPO group. All patients in the sLIPO group demonstrated positive FSS. Two patients in the mLIPO group had negative FSS and therefore were excluded from the study. All remaining mLIPO patients had positive FSS and underwent skin excision. The patient demographics, disease etiology, distribution, and severity are listed in Table 1. The average liposuction volumes for arm, leg, and thigh were 1,612, 1,717, and 3,609 mL, respectively, for sLIPO, and 1,448, 1,872, and 3,964 mL, respectively, for mLIPO. Skin resection in the mLIPO group increased operative time by an average of 48 minutes. The average follow-up was 20 months (range 13–26 months). The sLIPO group developed postoperative seroma/hematoma requiring percutaneous drainage in 5 cases (33%), contour irregularity in 8 cases (53%), and skin necrosis in 3 cases (20%) (Fig. 4). In the mLIPO group, none experienced seroma/hematoma or skin necrosis (Figs. 5 and 6), and contour irregularity was identified in 5 cases (19%). One case (3.8%) in the mLIPO group developed minor wound dehiscence which healed secondarily. Due to not having skin excision performed, none in the sLIPO group had wound dehiscence. None in the study developed postoperative infection. Patient satisfaction scores were 7.1 and 9.3 for sLIPO and mLIPO, respectively (Table 2).
Table 1.
Summary of Patient Demographics
| sLIPO (n = 15) | mLIPO (n = 26) | ||
|---|---|---|---|
| Male | 1 | 4 | |
| Female | 14 | 22 | |
| Age | 53.6 | 58.1 | |
| BMI | 32.9 | 34.1 | |
| Primary disease | 7.3 | 9 | P = 0.52 |
| Secondary disease | 12 | 18 | |
| ISL stage II | 14 | 23 | P = 0.61 |
| ISL stage III | 1 | 3 | |
| Arm cases | 6 | 13 | P = 0.34 |
| Leg cases | 6 | 5 | |
| Thigh cases | 3 | 8 | |
| Arm volume removed | 1,612 mL | 1,448 mL | |
| Leg volume removed | 1,717 mL | 1,872 mL | |
| Thigh volume removed | 3,609 mL | 3,964 mL | |
| Area of skin removed | 0 | 292.9 cm2 | |
| Operative time | 101 min | 149 min |
BMI, body mass index; ISL, International Society of Lymphology.
Fig. 4.
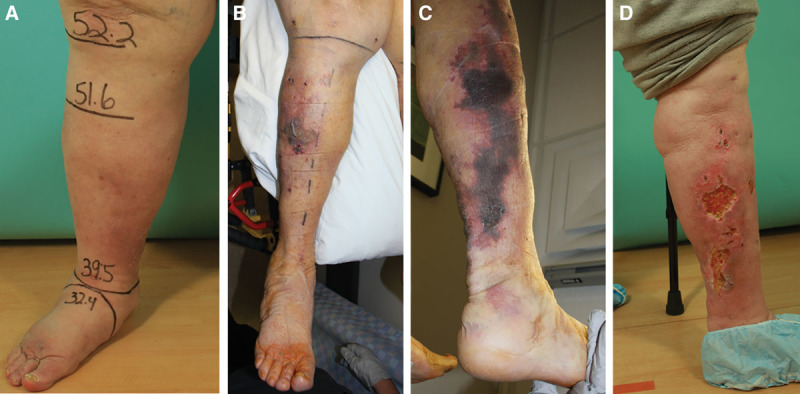
Contour irregularity and skin necrosis seen in an sLIPO patient. A, This patient had bioimpedance spectroscopy and MRI suggestive of solid predominant disease. B, On postoperative day 3 following sLIPO, multiple areas of skin ischemia were observed. C, Skin necrosis and contour irregularity seen at a month postoperatively. D, Despite the complications, the patient was happy with the surgical outcome. MRI, magnetic resonance imaging.
Fig. 5.
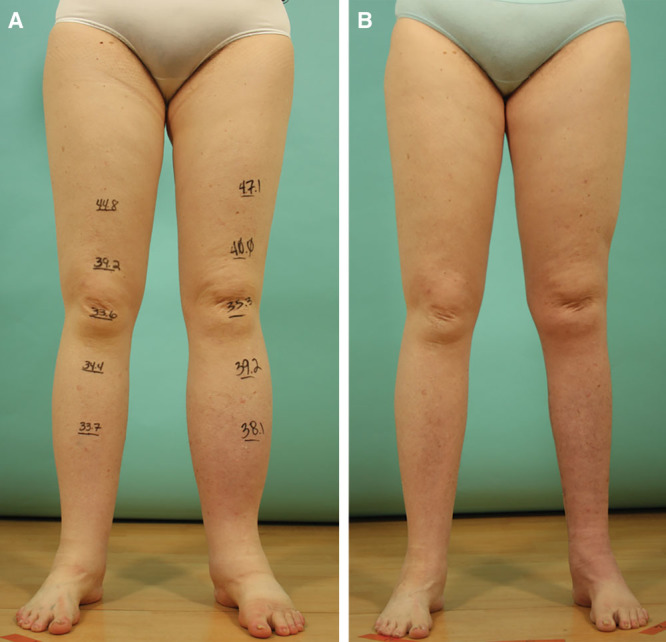
An mLIPO patient with left leg lymphedema preoperatively (A) and at a month postoperatively (B). Her postoperative course was uneventful. The skin excess was excised from the medial aspect of the leg. No contour irregularity was observed. She was happy with the surgical outcome.
Fig. 6.
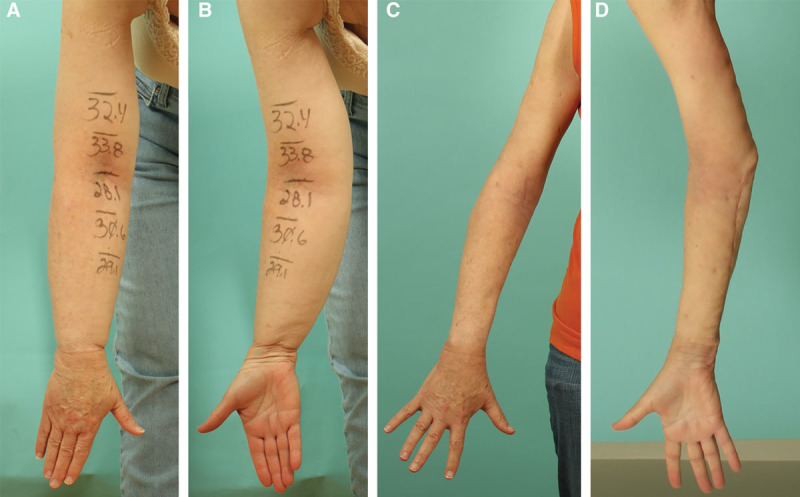
An mLIPO patient with right arm lymphedema preoperatively (A, B) and at 3-month postoperatively (C, D). Skin excision was necessary both from the forearm and the upper arm due to demonstrating positive FSS at both segments. She healed uneventfully. Mild contour irregularity was seen at the elbow and the wrist, secondary to residual skin excess. She was happy with the surgical outcome.
Table 2.
Treatment Data
| sLIPO (n = 15) | mLIPO (n = 26) | P | |
|---|---|---|---|
| Contour irregularity | 8 (53.3%) | 5 (19.2%) | 0.023 |
| Hematoma/seroma | 5 (33.3%) | 0 | 0.002 |
| Wound dehiscence | 0 | 1 (3.8%) | NA |
| Skin necrosis | 3 (20%) | 0 | 0.017 |
| Infection | 0 | 0 | NA |
| Patient satisfaction score | 7.1 | 9.3 | 0.00001 |
DISCUSSION
Recent studies on surgical management of lymphedema mainly focused on microsurgical and supermicrosurgical reconstruction techniques,29–32 leaving liposuction, a time-tested treatment for lymphedema, underinvestigated. Many questions related to lymphedema liposuction remain unanswered, such as the management of skin excess following liposuction, long-term outcome, disease recurrence, and its effect on lymphatic drainage. Our study aimed to address the first question.
The classic lymphedema liposuction technique pioneered by Brorson et al26 does not include skin excision. The skin excess is expected to resolve with spontaneous skin contracture. Despite favorable outcomes reported,26,33,34 our sLIPO patients experienced significantly higher incidences of irregular contour, seroma/hematoma, and skin necrosis. This was likely related to insufficient postoperative skin contracture. With an abundance of skin excess and the inherent dead space, pooling of body fluid and compression of plicated/folded skin (Fig. 7) were likely to occur, predisposing the patients to observed complications. When the skin was removed, the dead space was eliminated, skin folding was less unlikely, and the overall complication decreased. The differential outcomes observed with and without skin excision suggested that spontaneous skin contracture alone could not reliably resolve the skin excess created from bulk removal. Why would even we expect it to? If the skin contracture alone was effective, why there was a need for skin-reducing brachioplasty, thighplasty, and abdominoplasty after massive weight loss?35
Fig. 7.
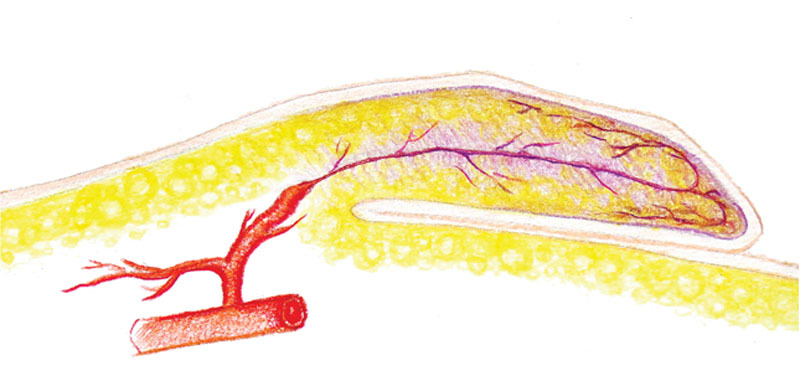
Large amount of skin excess predisposed to skin folding when external compression was applied, resulting in ischemic necrosis.
“Dry” liposuction under tourniquet control has been standardly performed in lymphedema liposuction.21,36 This may be related to the difficulty in injecting tumescent solution into pathologically fibrotic subcutaneous tissue. In authors’ experience, the degree of subcutaneous fibrosis varies significantly, and the injection of tumescent solution is only ineffective in those with severe fibrosis. With our approach of combining tourniquet use and tumescent technique, the observed blood loss was low. None of our patients required blood transfusion, and none showed symptoms of anemia. In patients with severe fibrosis, “fountain sign”35 would be seen even with small-volume injection of tumescent solution. In these patients, driving of the cannula also becomes physically demanding and the tissue removal becomes ineffective. These patients are better served with direct surgical excision.
A common practice in lymphedema liposuction is using the contralateral uninvolved limb as a template to preoperatively prepare compression garment.21 This was not feasible in the majority of our patients who had elevated body mass indexes. Liposuction of the lymphedema-affected limb resulted in the affected limb becoming smaller than the healthy limb (Fig. 8). Garment fitment during the immediate postoperative period was suboptimal due to the inevitable postoperative edema. We found compression bandaging during the immediate postoperative period and transitioning to garment compression at 4 weeks after surgery to be a practical solution.
Fig. 8.
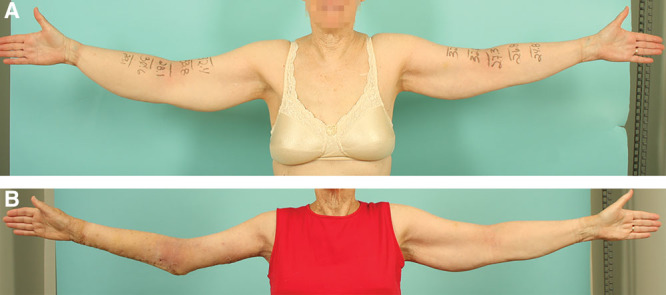
An mLIPO patient with right arm lymphedema preoperative (A) and at 3 months postoperatively (B). As many of our patients had elevated BMIs, the treated limb frequently became smaller than the contralateral healthy limb, making the contralateral limb an inappropriate template for preoperative garment preparation. BMI, body mass index.
Liposuction is presumed to exacerbate edema and weaken skin perfusion, predisposing patients to wound complications.34 Liposuction has been reported as a bridge to future skin excision,37,38 to facilitate skin excision by liposuctioning under the skin to be excised,39 or to provide additional debulking by conservative liposuction adjacent to the skin excision site.40 To our knowledge, circumferential limb liposuction in combination with immediate skin excision has not been reported, in either healthy aesthetic patients or in lymphedema patient. Unexpectedly, when liposuction and skin excision were simultaneously performed, as in the mLIPO group, the incidence of wound-related complications decreased. The complication profile of the mLIPO group compares favorably with the published outcomes for brachioplasty and thighplasty in healthy aesthetic surgery patients in nearly all parameters measured.37,40 In other words, the complication decreased when more invasive surgery was performed in a higher-risk lymphedema patient population. We hypothesized that the favorable outcomes of mLIPO in the lymphedema patients, who theoretically were at higher risks for complications due to their compromised soft tissue quality than those without lymphedema, suggested feasibility of such technique in healthy aesthetic patients also.
Following lymphedema liposuction, all patients became eligible for lymphatic reconstruction with either vascularized lymph vessel transfer41 or LVA.42 This is the so-called hybrid reconstruction. The second stage of the hybrid reconstruction was optional. Surgery was only offered to those who were not confident in lifelong adherence to compression therapy. The choice of vascularized lymph vessel transfer versus LVA depended on the degree of lymphatic regeneration following the liposuction, as observed on postoperative indocyanine green lymphography,41 with LVA only offered to those with the most rigorous regeneration.42
The limitations of this study are the nonrandomized nature of the study, the small sample sizes, and the empirically determined threshold to perform skin excision. These will be addressed in our future study.
CONCLUSIONS
Skin excision following liposuction for solid predominant lymphedema is safe and seems to decrease postoperative complication and improve surgical outcome.
Supplementary Material
Footnotes
Published online 12 November 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265–1275. [DOI] [PubMed] [Google Scholar]
- 2.Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132:1305–1314. [DOI] [PubMed] [Google Scholar]
- 3.Kenworthy EO, Nelson JA, Verma R, et al. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J Surg Oncol. 2018;117:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koshima I, Narushima M, Mihara M, et al. Lymphadiposal flaps and lymphaticovenular anastomoses for severe leg edema: functional reconstruction for lymph drainage system. J Reconstr Microsurg. 2016;32:50–55. [DOI] [PubMed] [Google Scholar]
- 5.Koshima I, Inagawa K, Etoh K, et al. [Supramicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the extremities]. Nihon Geka Gakkai Zasshi. 1999;100:551–556. [PubMed] [Google Scholar]
- 6.Yamamoto T, Yamamoto N, Fuse Y, et al. Optimal sites for supermicrosurgical lymphaticovenular anastomosis: an analysis of lymphatic vessel detection rates on 840 surgical fields in lower extremity lymphedema patients. Plast Reconstr Surg. 2018;142:924e–930e. [DOI] [PubMed] [Google Scholar]
- 7.Masia J, Pons G, Nardulli ML. Combined surgical treatment in breast cancer-related lymphedema. J Reconstr Microsurg. 2016;32:16–27. [DOI] [PubMed] [Google Scholar]
- 8.Masià J. Surgical treatment for lymphedema: state of the art. J Reconstr Microsurg. 2016;32:1. [DOI] [PubMed] [Google Scholar]
- 9.Mihara M, Hayashi Y, Hara H, et al. High-accuracy diagnosis and regional classification of lymphedema using indocyanine green fluorescent lymphography after gynecologic cancer treatment. Ann Plast Surg. 2014;72:204–208. [DOI] [PubMed] [Google Scholar]
- 10.Greene AK, Goss JA. Diagnosis and staging of lymphedema. Semin Plast Surg. 2018;32:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin ES, Bowen MJ, Chen WF. Diagnostic accuracy of bioimpedance spectroscopy in patients with lymphedema: a retrospective cohort analysis. J Plast Reconstr Aesthet Surg. 2018;71:1041–1050. [DOI] [PubMed] [Google Scholar]
- 12.Campisi C. Lymphoedema: modern diagnostic and therapeutic aspects. Int Angiol. 1999;18:14–24. [PubMed] [Google Scholar]
- 13.Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;128:941–947. [DOI] [PubMed] [Google Scholar]
- 14.Cheng MH, Pappalardo M, Lin C, et al. Validity of the novel Taiwan lymphoscintigraphy staging and correlation of Cheng lymphedema grading for unilateral extremity lymphedema. Ann Surg. 2018;268:513–525. [DOI] [PubMed] [Google Scholar]
- 15.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 16.Mihara M, Hara H, Narushima M, et al. Indocyanine green lymphography is superior to lymphoscintigraphy in imaging diagnosis of secondary lymphedema of the lower limbs. J Vasc Surg Venous Lymphat Disord. 2013;1:194–201. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Lu Q, Chen TW, et al. Thickness of soft tissue of lower extremities measured with magnetic resonance imaging as a new indicator for staging unilateral secondary lower extremity lymphedema. Acta Radiol. 2015;56:1016–1024. [DOI] [PubMed] [Google Scholar]
- 18.Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. [DOI] [PubMed] [Google Scholar]
- 19.Granzow JW. Lymphedema surgery: the current state of the art. Clin Exp Metastasis. 2018;35:553–558. [DOI] [PubMed] [Google Scholar]
- 20.Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 21.Schaverien MV, Munnoch DA, Brorson H. Liposuction treatment of lymphedema. Semin Plast Surg. 2018;32:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brorson H. Adipose tissue in lymphedema: the ignorance of adipose tissue in lymphedema. Lymphology. 2004;37:175–177. [PubMed] [Google Scholar]
- 23.Damstra RJ, Voesten HG, Klinkert P, et al. Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg. 2009;96:859–864. [DOI] [PubMed] [Google Scholar]
- 24.Salgado CJ, Mardini S, Spanio S, et al. Radical reduction of lymphedema with preservation of perforators. Ann Plast Surg. 2007;59:173–179. [DOI] [PubMed] [Google Scholar]
- 25.Dellon AL, Hoopes JE. The Charles procedure for primary lymphedema. Long-term clinical results. Plast Reconstr Surg. 1977;60:589–595. [DOI] [PubMed] [Google Scholar]
- 26.Hoffner M, Ohlin K, Svensson B, et al. Liposuction gives complete reduction of arm lymphedema following breast cancer treatment-a 5-year prospective study in 105 patients without recurrence. Plast Reconstr Surg Glob Open. 2018;6:e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollina U. Lipedema-an update. Dermatol Ther. 2019;32:e12805. [DOI] [PubMed] [Google Scholar]
- 28.Aly A, Pace D, Cram A. Brachioplasty in the patient with massive weight loss. Aesthet Surg J. 2006;26:76–84. [DOI] [PubMed] [Google Scholar]
- 29.Ciudad P, Mouchammed A, Manrique OJ, et al. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center’s approach to the treatment of lymphedema. J Surg Oncol. 2018;117:1346–1347. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Yamamoto N, Hayashi A, et al. Supermicrosurgical deep lymphatic vessel-to-venous anastomosis for a breast cancer-related arm lymphedema with severe sclerosis of superficial lymphatic vessels. Microsurgery. 2017;37:156–159. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: a prospective study of 100 consecutive cases. J Surg Oncol. 2017;115:68–71. [DOI] [PubMed] [Google Scholar]
- 32.Tzou CH, Meng S, Ines T, et al. Surgical anatomy of the vascularized submental lymph node flap: anatomic study of correlation of submental artery perforators and quantity of submental lymph node. J Surg Oncol. 2017;115:54–59. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Perry L, Granzow J. Suction assisted protein lipectomy (SAPL) even for the treatment of chronic fibrotic and scarified lower extremity lymphedema. Lymphology. 2016;49:36–41. [PubMed] [Google Scholar]
- 34.Greene AK, Maclellan RA. Operative treatment of lymphedema using suction-assisted lipectomy. Ann Plast Surg. 2016;77:337–340. [DOI] [PubMed] [Google Scholar]
- 35.Hunstad JP, Aitken ME. Liposuction: techniques and guidelines. Clin Plast Surg. 2006;33:13–25, v. [DOI] [PubMed] [Google Scholar]
- 36.Clayton DN, Clayton JN, Lindley TS, et al. Large volume lipoplasty. Clin Plast Surg. 1989;16:305–312. [PubMed] [Google Scholar]
- 37.Gusenoff JA, Coon D, Nayar H, et al. Medial thigh lift in the massive weight loss population: outcomes and complications. Plast Reconstr Surg. 2015;135:98–106. [DOI] [PubMed] [Google Scholar]
- 38.Symbas JD, Losken A. An outcome analysis of brachioplasty techniques following massive weight loss. Ann Plast Surg. 2010;64:588–591. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen AT, Rohrich RJ. Liposuction-assisted posterior brachioplasty: technical refinements in upper arm contouring. Plast Reconstr Surg. 2010;126:1365–1369. [DOI] [PubMed] [Google Scholar]
- 40.Bossert RP, Dreifuss S, Coon D, et al. Liposuction of the arm concurrent with brachioplasty in the massive weight loss patient: is it safe? Plast Reconstr Surg. 2013;131:357–365. [DOI] [PubMed] [Google Scholar]
- 41.Chen WF, Bowen M, McNurlen M, et al. Vascularized lymph vessel transfer with supermicrosurgical ultra-thin superficial circumflex iliac artery flap for treatment of advanced lymphedema not treatable with supermicrosurgical lymphaticovenular anastomosis. Ann Plast Surg. 2018;80supp 31.29239967 [Google Scholar]
- 42.Chen WF. Is it possible to perform lymphaticovenular after liposuction for lymphedema? J Surg. 2016;12:2. [Google Scholar]


