Supplemental Digital Content is available in the text.
Background:
Free flaps have evolved from musculocutaneous flaps to perforator-based cutaneous flaps. The subdermal plexus is now thought to play a significant role in skin paddle perfusion. We propose a new concept, the “intradermal plexus,” allowing survival of dermis flaps, according to our study.
Methods:
A dermis flap was used in 6 cases to reconstruct small defects. The superficial branch of the superficial circumflex iliac artery was traced distally using an ultrasound device with a 70-MHz linear array transducer until the artery’s branch entered the dermis. The location of the dermis entry site was marked and the vessels running inside the dermis were observed and video-recorded. A flap was elevated above the superficial fascia, and the adipose tissue was removed using scissors after confirmation of the vessels’ dermis entry point.
Results:
The use of 70-MHz ultrasonography permitted observation in all patients of small arteries entering the dermis layer. The artery was observed to give off branches after entering the dermis, in effect constituting an “intradermal plexus.” Small veins entering the dermis were similarly visualized using 70 MHz ultrasonography. All flaps survived completely.
Conclusions:
Small arteries and veins entering and running inside the dermis were visualized for the first time with 70 MHz real-time ultrasonography. Knowledge of the existence of this “intradermal plexus” made it possible to discard nearly all subdermal adipose tissue quickly and safely, without resorting to the elaborate measures described in previous reports.
INTRODUCTION
In the quest for flaps with lower donor-site morbidity, flaps have evolved from musculocutaneous flaps to perforator flaps.1,2 In 1989, Koshima and Soeda3 reported the first use of the deep inferior epigastric artery perforator flap, proving for the first time that the rectus abdominis was not necessary for survival of the skin paddle. In 2015, Laungani et al4 reported the importance of the subdermal plexus.
In this study, we propose a new concept, the “intradermal plexus,” to substantiate the feasibility of dermis flaps. Recent advancements in technology have brought about an ultrasound system with ultra-high resolution (resolution capacity of 30 μm), which emits ultrasound with frequencies as high as 70 MHz. It was first developed for use in small animals, but recent applications in the human body have led to innovations in supermicrosurgery.5–7 Using ultra-high-frequency ultrasound, we present visualization of the “intradermal” arteries and veins, entering the dermis from the subdermal plexus. This provided a basis for our new concept. Based on these ultrasound findings, and to corroborate the feasibility of the intradermal plexus concept, a dermis free flap transfer was performed for reconstruction in 6 cases under the University of Tokyo Hospital ethics committee-approved protocol.
MATERIALS AND METHODS
Visualization of Intradermal Plexus Using Ultra-high-resolution Ultrasonography
A dermis flap was used for reconstruction of small defects in 6 cases under the University of Tokyo Hospital ethical committee-approved protocol. Patients’ demographic data are given in Table 1. The superficial branch of the superficial circumflex iliac artery (SCIA) was identified first using conventional ultrasonography preoperatively; it was then traced distally until it penetrated the superficial fascia. Conventional ultrasonography was performed with Noblus (Hitachi Medical Corporation, Tokyo, Japan) with a linear EUP-L65 probe in color Doppler mode at a frequency of 15 MHz. The superficial branch of the SCIA was then traced distally until it entered the dermis using a Vevo MD ultrasound device (Fujifilm Visual Sonics, Amsterdam, the Netherlands) with a 70-MHz linear array transducer. The location of the dermis-entering site was marked and the vessels running inside the dermis were observed and video-recorded.
Table 1.
Patient Data and Reconstructive Summary
Surgical Technique
The skin paddle’s design placed the artery’s dermis entry point in the center. The superficial branch of the SCIA was identified first as described in our previous report; the flap was then elevated above the superficial fascia.8 The flap was elevated and turned upside-down, with its pedicle still connected to the femoral artery. The terminal branch of the superficial branch of the SCIA was then exposed, “unflooring” the pedicle (not “unroofing” because the flap is now turned upside-down). The terminal branch of the SCIA was traced down to the dermis-entering site via direct visualization under surgical microscope. After performing the same procedure with the superficial vein, the remaining adipose tissues were removed until the dermis was exposed using a pair of scissors, as shown in the video. Since this was a flap transfer entailing arterial and venous anastomoses, no compression was applied to the flap postoperatively (See Video 1 [online], which demonstrates removal of subdermal adipose tissue using scissors. After confirmation of the vessels’ dermis entry points, the adipose layer was removable without requiring special precautions).
Video 1. This video demonstrates removal of subdermal adipose tissue using scissors. After confirmation of the vessels’ dermis entry points, the adipose layer was removable without requiring special precautions.
Intravenous vasodilator was administered postoperatively: 40 mcg of lipo-prostaglandin E1 (Prostandin; Ono, Osaka, Japan) with 100 mL of saline was infused over 2 hours twice a day, in the morning and evening, for 5 days. The flap perfusion was checked via color monitoring and pinprick test using a 26-gauze needle every 3 hours for 3 days. This report was published with the consent and permission of the patients involved.
RESULTS
Using 70 MHz ultrasonography, small arteries entering the dermis layer were observed in all patients. The artery was observed to give off branches after entering the dermis, constituting an “intradermal plexus.” Small veins entering the dermis were similarly visualized using 70 MHz ultrasonography. The arteries were distinguished from the veins by applying pressure to the skin: the veins collapsed, whereas the arteries retained their shape, as in conventional ultrasonography (See Video 2 [online], which demonstrates ultrasonography of an artery entering the dermis layer. Note that at the beginning of the video, venae comitantes collapse when additional pressure was applied to the skin. The artery retains its shape. (See Video 3 [online], which demonstrates ultrasonography of the artery entering and then running inside the dermis.
Video 2. This video demonstrates ultrasonography of an artery entering the dermis layer. Note that at the beginning of the video, venae comitantes collapse when additional pressure was applied to the skin. The artery retains its shape.
Video 3. This video demonstrates ultrasonography of the artery entering and then running inside the dermis.
Postoperative courses were uneventful in all patients. All flaps survived completely with no healing complications, and no donor site complications were observed. The average length of hospital stay was 7 days. These findings are summarized in Table 1.
Case Report
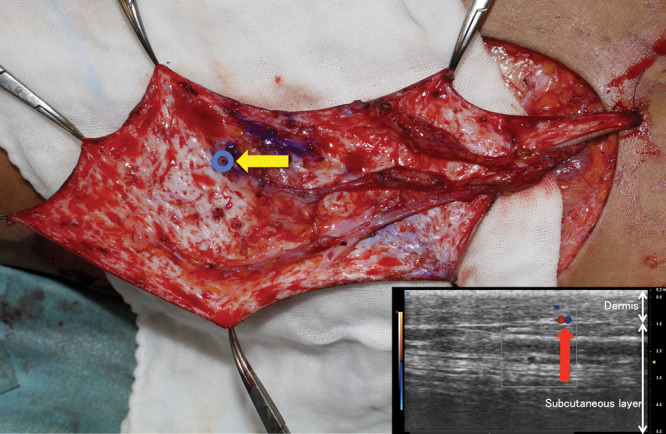
An 81-year-old man had posttraumatic contracture of the palmar skin of his left index finger. Contracture release resulted in an 8 × 3 cm defect at the proximal interphalangeal joint. A 9 × 3 cm dermis flap was designed in the right groin and was elevated based on a small artery and a small vein entering the dermis, branching from the deep branch of the SCIA (Fig. 1). The flap was thinned down to the dermis, resulting in a thickness of 0.9 mm. The SCIA was anastomosed to the proper palmar digital artery in an end-to-side fashion, and the vena comitans and a subcutaneous vein were anastomosed to 2 subcutaneous veins of the finger in an end-to-end fashion, respectively. The flap survived completely, with no immediate postoperative compression, resulting in soft and pliable skin coverage addressing the contracture (Fig. 2).
Fig. 1.
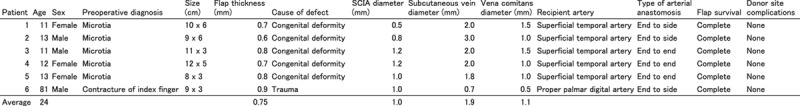
A dermis flap was elevated based on small arteries and veins entering the dermis. Note that almost all adipose tissues were removed, exposing the whitish dermis. The entry point (blue circle) was confirmed preoperatively using ultra-high-resolution ultrasonography (red arrow).
Fig. 2.
A, A 9 × 3 cm dermis flap was transferred after contracture release of the left index finger. B, Postoperative photo taken 9 months after the operation. The flap survived completely, resulting in soft and pliable skin coverage addressing the contracture.
DISCUSSION
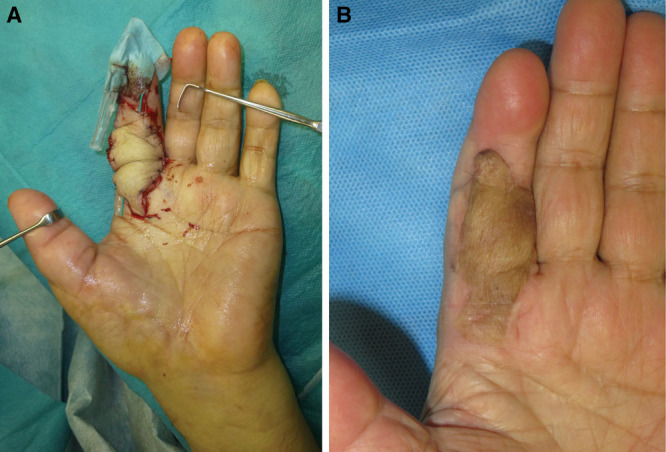
The evolving history of the free flap has led us to postulate that if the “intradermal plexus” exists, a “dermis” flap can be elevated without the subdermal plexus (Fig. 3). Putting the ultrasound findings of the “intradermal plexus” to good use, we found we could remove the adipose tissue to the dermis level with no second-guessing. The thinning procedure was completed in <20 minutes using surgical scissors and necessitated none of the special precautions described in previous reports.9,10 We are confident that the flaps in this series survived as a flap, and not as a full thickness skin graft because of the following 2 reasons: (1) no postoperative compression was applied to the flap; and (2) bleeding was observed from the flap with postoperative pinprick tests.
Fig. 3.
An illustration demonstrating the concept of a dermis flap.
In a dermis flap, the extent of the region perfused by a small artery entering the dermis is still unknown. The largest dermis flap in our series was 12 × 5 cm. Because the flap sizes were relatively small in our case series, indocyanine green angiography was not necessary to check flap perfusion; perfusion could be assessed via mere bleeding from the edges. However, in future clinical settings using larger dermis flaps, indocyanine green angiography will play a crucial role in evaluating maximum area of flap perfusion.
In this series, the terminal branch of the SCIA was traced down to its dermis-entering site. Theoretically, this dermis flap concept can be applied to other parts of the body as well. If this is possible, for example, we may be able to cover facial defects with a very large dermis flap elevated based on a deep inferior epigastric artery perforator. Further study is necessary to confirm this hypothesis.
The dermis flap was used mainly for reconstruction of the external ear canal in this series (although the case report describes application to the hand). However, we believe the best indications are for irradiated defects necessitating thin, malleable skin (irradiated dorsum aspect of the foot, for example). For further understanding of its indications, clinical studies with a larger number of patients are warranted.
To the best of our knowledge, this is the first report on real-time visualization of small arteries and veins running inside the dermis. As advances in technology lead to dissemination of the perforator flap concept, this may be the first step into the next dimension: dermis flaps.
CONCLUSIONS
Small arteries and veins entering and running inside the dermis were visualized with 70 MHz ultrasonography in real time for the first time. Existence of the “intradermal plexus” facilitates the discarding of almost all subdermal adipose tissue, without recourse to the painstaking special precautions described in previous reports.
Supplementary Material
Footnotes
Published online 14 November 2019.
Parts of this work was presented at the 2018 Asian Pacific Federation of Societies for Reconstructive Microsurgery, Antalya, Turkey, and at the 2019 Annual Meeting of the American Society of Reconstructive Microsurgery.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Blondeel PN, Van Landuyt KH, Monstrey SJ, et al. The “gent” consensus on perforator flap terminology: preliminary definitions. Plast Reconstr Surg. 2003;112:1378–1383; quiz 1383, 1516; discussion 1384. [DOI] [PubMed] [Google Scholar]
- 2.Hallock GG. The complete nomenclature for combined perforator flaps. Plast Reconstr Surg. 2011;127:1720–1729. [DOI] [PubMed] [Google Scholar]
- 3.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. [DOI] [PubMed] [Google Scholar]
- 4.Laungani AT, Van Alphen N, Christner JA, et al. Three-dimensional CT angiography assessment of the impact of the dermis and the subdermal plexus in DIEP flap perfusion. J Plast Reconstr Aesthet Surg. 2015;68:525–530. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi A, Visconti G, Yamamoto T, et al. Intraoperative imaging of lymphatic vessel using ultra high-frequency ultrasound. J Plast Reconstr Aesthet Surg. 2018;71:778–780. [DOI] [PubMed] [Google Scholar]
- 6.Visconti G, Hayashi A, Yoshimatsu H, et al. Ultra-high frequency ultrasound in planning capillary perforator flaps: preliminary experience J Plast Reconstr Aesthet Surg. 2018;71:1146–1152. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi A, Giacalone G, Yamamoto T, et al. Ultra high-frequency ultrasonographic imaging with 70 MHz scanner for visualization of the lymphatic vessels. Plast Reconstr Surg Glob Open. 2019;7:e2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimatsu H, Yamamoto T, Hayashi A, et al. Proximal-to-distally elevated superficial circumflex iliac artery perforator flap enabling hybrid reconstruction. Plast Reconstr Surg. 2016;138:910–922. [DOI] [PubMed] [Google Scholar]
- 9.Narushima M, Yamasoba T, Iida T, et al. Pure skin perforator flap for microtia and congenital aural atresia using supermicrosurgical techniques. J Plast Reconstr Aesthet Surg. 2011;64:1580–1584. [DOI] [PubMed] [Google Scholar]
- 10.Kimura N, Satoh K. Consideration of a thin flap as an entity and clinical applications of the thin anterolateral thigh flap. Plast Reconstr Surg. 1996;97:985–992. [DOI] [PubMed] [Google Scholar]


