Supplemental Digital Content is available in the text.
Abstract
Background:
Mastopexy is one of the most performed cosmetic surgery procedures in the United States. Despite the numerous mastopexy techniques that were published in the past decades, preventing pseudoptosis to ensure longer lasting results remains the principal challenge.
Objectives:
This paper describes a new mastopexy technique developed for moderate to severe ptosis/pseudoptosis associated with upper pole deflation. Considering some of the commonest risk factors generally considered predictive of worse outcomes (massive weight loss, multiple pregnancies, skin quality, smoking, age), we aimed to assess whether this technique could be beneficial in the support of the desired breast shape over time.
Methods:
Twelve patients, all featuring 1 or more of the abovementioned preoperative risk factors, were operated on by the same senior surgeon with the hammock mastopexy technique using dermal flaps as a support for the glandular reshaping (6 bilateral mastopexies and 6 unilateral mastopexies for contralateral symmetrization after breast reconstruction). Patients’ characteristics, such as smoking, weight loss, or multiparity with consequent inelastic skin, age, and lengthy nipple–areola complex lift, were considered as independent risk factors for ptosis recurrence and bottoming out. Patients were divided into 3 subgroups according to the number of their risk factors. Aesthetic results were assessed at 12 months postoperatively. Changes in postoperative were assessed for each patient by breast measurements and a superposition of the standardized breast photographs. Long-term outcomes were compared with a control group of 6 patients who benefited from mastopexy without “hammock technique.”
Results:
Satisfactory maintenance of shape and stable nipple–areola complex position was seen at 12 months regardless of the number of risk factors. However, a statistically significant difference was found in lower pole lengthening between patients with more than 3 risk factors compared to other groups. Aesthetic measurement results were consistent between the patient and surgeon reporting a satisfying cosmetic result, regardless of the number of risk factors. In the control group, we found a significant increase in breast lower pole measurements at 12 months when compared with the hammock group.
Conclusions:
This mastopexy technique improves projection and reinforces the lower pole support with lateral and medial dermal flaps. The technique is safe and reliable and provides easily reproducible results for patients with risk factors for postoperative pseudoptosis.
INTRODUCTION
Breast uplift or mastopexy is the seventh most commonly performed cosmetic procedure in the United Stattes.1 Forty years ago, Regnault defined and classified ptosis using the nipple position with respect to the inframammary fold (IMF) (Table 1).2 This classification is still being used today. All mastopexy techniques will try to overcome the gravitational effect by lifting the breast, tightening the skin, and correcting the upper pole deflation.
TABLE 1.
Ptosis Classification According to the Study by Regnault1
| Type | Degree | Description |
|---|---|---|
| Grade | Mild | Nipple position at the level of the IMF |
| Grade II | Moderate | Nipple position below the IMF but above the lower breast contour |
| Grade III | Severe | Nipple position below the IMF but at the lower breast contour |
| Pseudoptosis | – | Nipple position above the IMF but the breast is below the fold |
An ideal breast shape is characterized by a nipple position above the IMF, a breast abdominal angle of greater than 90 degrees with no breast tissue lying on the abdominal part, and a full upper pole. This ideal shape will be inevitably affected by lifestyle factors such as the number of pregnancies, breast feeding, smoking, weight loss, and age. All of these factors increase ptosis by exacerbating the stretching of the native internal suspensory ligaments and the skin envelope.3 This last attribute is often underestimated. The dermis differs greatly in quality and thickness from 1 patient to another and plays a major role on the extent of lower pole lengthening and the subsequent bottoming out.
Numerous mastopexy techniques have been described, all of which aim to improve breast projection and to increase upper pole fullness by glandular reshaping to give a durable result that does not bottom out over time. “Auto-augmentation” to enhance breast projection refers to the use of autologous (usually glandular) flaps to increase upper pole fullness.3 The most used glandular reshape technique to improve breast contour and lift is the inferior pedicle-based mastopexy, popularized by Botti.4 This technique recalls the publication by Ribeiro et al on glandular remodeling in breast reductions.5,6
Finally, to overcome the problem of pseudoptosis, which compromises long-lasting results, many authors proposed a number of support or “hammock” techniques for the lower pole. Some used autologous dermal or glandular support,7,8 whereas others described the use of synthetic matrices to support the lower pole.9,10 Graf et al also described the pectoralis muscle sling to support the parenchyma moved into the upper pole to avoid bottoming out.11
This article describes a new mastopexy technique that is developed for ptosis associated with upper pole deflation, especially in those patients with risks of recurrent ptosis. The based dermal support is designed to act as a “hammock” for the lower pole of the breast. When associated with the auto-augmentation, this technique aims to reinforce the lower pole support with lateral and medial dermal slings to prevent bottoming out over time. Most importantly, it reshapes the inferior base and lateral profile of the breast, thus tightening its base.
The article also aims to evaluate the outcomes of our technique by measuring changes in key parameters of the breast shape over time. These parameters were compared with previous patients who were also operated on by the same surgeon, but did not benefit from the hammock modification. Outcomes were specifically related to the presence of risk factors generally associated with worse outcomes (weight loss, multiparity, bad skin quality, smoking, age, and nipple–areola complex (NAC) lift >8 cm),12,13 to evaluate whether this technique could be particularly indicated in such preoperative conditions.
METHODS
From January 2014 to January 2016, mastopexy procedures performed at our institution by the senior author using the present technique were included in a prospectively maintained database. A retrospective analysis of the prospectively maintained database was performed. Medical records from the operative reports, discharge notes, and anesthesia charts were used to complete missing data. Smoking, weight loss, multiparity, poor skin quality, age > 50 years, and NAC lift > 8 cm were considered as independent risk factors for ptosis recurrence and bottoming and used for statistical analysis. A matched control group of 6 patients who benefitted from mastopexy procedures (performed by the same senior author, from January to December 2013, using the same mastopexy glandular remodeling technique, but without the described modification) was used as controls.
All patients presented ptosis with grades II–III according to Regnault and a deflated upper pole with lower pole bottoming out. Mastopexy augmentation with implant procedures, mastopexy without glandular remodeling, and mastopexy/reduction procedures were excluded from the analysis.
Informed consent was obtained from all patients, including approval for photographic/video documentation. The study was conducted in accordance with the Declaration of Helsinki on human research (1964).
Surgical Technique
Drawings were made on patients in the standing position the day before surgery. The IMF, sternal midline, and breast midlines were marked. The intended new nipple position was decided by the projection of the IMF (Pitanguy’s point).14 The extent of NAC lift was limited to a maximum of 12 cm to avoid any potential compromise in its vascularity. An inverted T pattern skin resection was drawn from the base of the NAC to IMF, generally between 4.5 and 6 cm (with an intentional overcorrection, expecting a physiological lower pole stretch over time).
Patients were positioned in supine position with arms abducted and secured on armrests. The superior NAC pedicle and the lower pole were both de-epithelialized, carefully preserving the inferomedial and infero-lateral dermal triangles. The breast was dissected in the subcutaneous plane with curved mayo scissors to the level of the IMF caudally and to the prepectoral fascia at the medial and lateral breast boundaries. The superiorly based glandular flap was then dissected off the prepectoral fascia from caudal to cranial up to the level of the NAC to allow sufficient cranial mobilization of the breast parenchyma, which was entirely preserved and redistributed. Once released from its inferior attachments, the gland was sufficiently mobile to be folded under the NAC and sutured cranially and medially to the prepectoral fascia with 2.0 Vicryl sutures (Vicryl; Ethicon, J&J) up to the level of the second intercostal space to improve upper breast contour. An overcorrection of the upper pole was generally desired. Once the gland was repositioned, the medial and lateral dermal triangles were de-epithelialized with a blade or a mayo scissors. Care was taken to preserve dermis, ensuring a robust blood supply. The caudal edges of the lower de-epithelialized triangles were then incised. With the patient in a semiupright position, the lateral triangular dermal flap was later sutured to the medial aspect of the pectoralis muscle fascia. This was important to prevent lateral glandular displacement into the axilla, redefine the lateral curvature of the breast, and to narrow the breast base thus further maintaining projection. This was followed by suturing the medial triangular dermal flap to the chest wall at the same level as the lateral triangle to complete the dermal suspensory hammock (Figs. 1 and 2). Occasionally, the dermis at the lateral edge of the flaps may be slightly released (especially on the lateral flap) to allow a better medialization and marking of the lateral breast shape and IMF. Blake suction drains were placed in the lower part of the breast exiting laterally and kept for 24 hours. The vertical pillars were then repositioned, and the skin closure performed with 3.0 and 4.0 monocryl (Monocryl; Ethicon, J&J). The wounds were dressed with simple water-resistant dressings, and a foam elastic bandage (Microfoam Surgical Tape, 3M, Flemington, NJ, USA) applied. Patients stayed in hospital overnight and were discharged at day 1 postoperatively after drains removal. They were advised to wear a surgical support bra for 6 weeks postoperatively (See Video [online], which displays the surgical steps to perform the hammock technique).
Fig. 1.
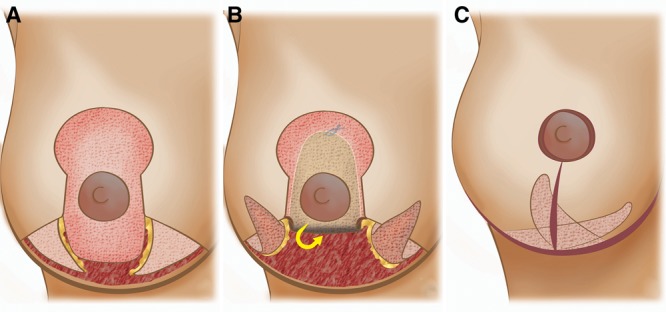
Illustration summarizing the different surgical steps. A, Superiorly based pedicled glandular flap dissected and both dermal flaps de-epidermized. B, Cranial rotation to increase upper pole fullness. C, Final result after medial fixation of the lateral triangular flap.
Fig. 2.
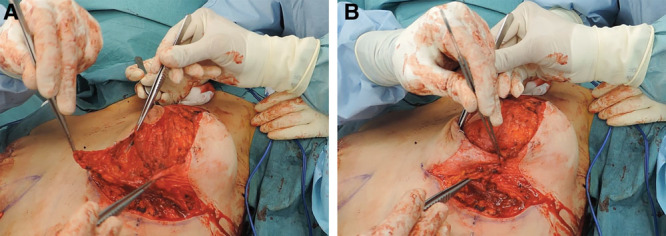
Intraoperative pictures illustrating the dermal flaps. A, Both dermal flaps are de-epidermized and glandular flap is cranially rotated. B, Repositioning of dermal flaps that will act as support.
Video. This video summarizes the surgical steps to perform the Hammock technique.
Postoperative Assessment
All patients were examined at 2 weeks, 3 months, 6 months, and 1 year postoperatively, and data were inserted in a prospectively maintained database as common department practice. Standardized photographs were taken at each appointment. Clinical photography consisted of patient standing comfortably upright with arms at sides, and 5 views were performed (frontal, oblique, and lateral). Framing was standardized with the position of clavicles at the top of image and camera to patient distance at 1 m.
Breast measurements were made with a simple metric tape. All measurements were recorded for the distance between the inframammary crease and the inferior border of the NAC (segment III), the distance from the sternal notch to the nipple (SN-N), the NAC average diameter, and the breast base width.
After identifying the main risk factors for recurrent ptosis (smoking, weight loss, multiparity, poor skin quality, age over 50 years, and a NAC lift distance of more than 8 cm),12 we divided patients into 3 subgroups according to the number of their risk factors. Patients with 1 risk factor were assigned to group A, patients with 2–3 risk factors were assigned to group B, and the remaining patients with more than 3 risk factors were assigned to group C.
Changes in postoperative breast measurements were assessed for each patient by a superposition of the standardized breast photographs taken at consecutive clinic appointments, and as a ratio of measurements at different intervals. Percentage variations in measurements from the initial postoperative results were recorded. A visual analog scale (VAS) was used to assess aesthetic results, using a scale of 1 (very poor) to 10 (excellent). The grading questionnaire was submitted to a third party (attending surgeon blinded to the study) for evaluating preoperative and postoperative photographs at 1 year postoperatively. Patients were also asked to evaluate their postoperative result at 1 year, using the same scale. Complication rates and reoperation rates for ptosis recurrence were accurately recorded.
Statistical Analysis
All breast parameters were statistically analyzed (average, range, standard error of the mean) and graphs building was performed with GraphPad Prism 6.00 (GraphPad Software, La Jolla, CA, USA). Statistical significance was determined as *P < 0.05, **P < 0.01.
RESULTS
Among the initially identified patients, 20 underwent bilateral aesthetic mastopexy and 65 patients had contralateral symmetrization mastopexy after autologous (n = 44) or prosthetic (n = 21) breast reconstruction. Of the 20 aesthetic cases, 6 patients met the study inclusion criteria. The rest of the 14 cases which involved single-stage augmentation mastopexy, minor ptosis not requiring glandular remodeling, and patients with no risk factor for ptosis recurrence, were excluded from the study. Regarding symmetrization mastopexy, reconstruction rarely needed contralateral upper pole fullness, especially after autologous flaps. Six cases (29%) required symmetrization with marked improvement to upper pole matching our inclusion criteria. Considering the 6 retained aesthetic bilateral procedures and the 6 retained symmetrizations, a total of 18 breasts were operated on with the hammock technique. Considering the control group, following the application of exclusion criteria, we could retrieve from our database 6 patients (5 aesthetics, 1 symmetrizing procedure, total of 11 operated breasts) who presented similar preoperative conditions and risk factors for ptosis and lower pole lengthening recurrence, but did not receive the hammock technical modification for lower pole support. All procedures were performed by the senior author.
In the hammock group, the average age was 44 years (range, 23–71 years). The mean follow-up was 13 months (range, 12–18 months). Operative time for bilateral cases was 150 ± 30 minutes (average ± SEM). Unilateral cases lasted for 100 ± 30 minutes (average ± SEM). Patient’s characteristics such as smoking, age, weight loss, parity status, and skin quality are reported in Table 2. Three women (25 %) had grade II ptosis, 5 (41.7 %) had grade III ptosis, and 4 (33.3 %) had pseudoptosis.
Table 2.
Hammock Group Patients’ Demographics, Surgical Outcomes, and Complications
| Side | Age (y) | Identified Risk Factors* | NAC Lift (cm) | Complications | VAS | ||
|---|---|---|---|---|---|---|---|
| Patient | Surgeon | ||||||
| 1 | B | 23 | NAC, weight loss, multiparity, skin | 12 | – | 9.5 | 9 |
| 2 | L | 51 | Age, tobacco | 4 | Liponecrosis | 9 | 8 |
| 3 | L | 61 | Age | 4 | – | 8.5 | 8 |
| 4 | B | 31 | Tobacco, skin | 5 | – | 9.5 | 8 |
| 5 | B | 71 | NAC, age | 8 | – | 8 | 9 |
| 6 | R | 37 | Weight loss | 3 | – | 9 | 9 |
| 7 | B | 24 | Skin quality | 7 | – | 8.5 | 9.5 |
| 8 | L | 45 | Tobacco, multi | 3 | Hypertrophic scar | 9 | 9 |
| 9 | B | 40 | NAC, weight loss, tobacco | 10 | Liponecrosis | 8.5 | 8 |
| 10 | L | 52 | Age, skin, multiparity, recurrence | 2 | – | 8 | 7 |
| 11 | R | 41 | Tobacco | 1 | – | 8.5 | 8 |
| 12 | B | 54 | Age | 6 | – | 9 | 8 |
| Average | 44.2 | 5.4 | 8.8 | 8.4 | |||
*Identified risk factors were NAC lift greater than 12 cm, history of weight loss, multiparity, smoking, and age greater than 50 y.
B, bilateral; L, left; R, right.
SN-N distance was 18.7 ± 0.5 cm (average ± SEM) immediately postoperatively. This length did not vary significantly at 12 months with an average increase of 5.7% [19.7 ± 0.6 cm (average ± SEM)], representing roughly 1 cm. No statistical significant difference was found in NAC position among groups at 12 months, when compared to immediate postoperative values (1 risk factor: 2.3% ± 1.5; 2–3 risk factors: 8.2% ± 1.9%; >3 risk factors 6.25% ± 1.25%, all expressed as average % increase of SN-N length ± SEM) (Fig. 3). Indeed, the 3 largest increases in SN-N distance corresponded to the patients who needed the greatest lift of NAC in postoperative (more than 8 cm).
Fig. 3.
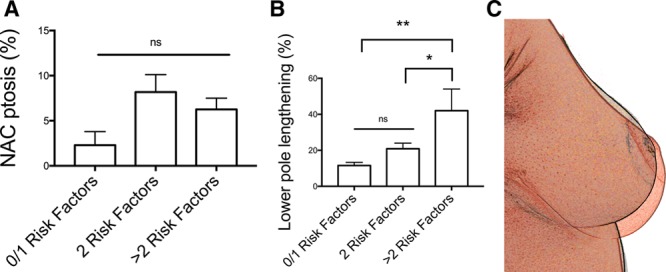
Relationship between number of risk factors and breast dimensions. A, NAC ptosis percentage measured at 12 months according to the number of identified risk factors in the hammock group. Values are expressed as average ± SEM. B, Lower pole lengthening percentage measured at 12 months according to the number of identified risk factors in the hammock group. Values are expressed as average ± SEM. (*P < 0.1; **P < 0.01). C, Superposition of breast standardized images in a patient with more than 3 risk factors after hammock mastopexy with progressive breast shape modifications. NS, no statistical significance.
Lower pole arc length (distance from lower areolar border to IMF) was measured intraoperatively at 5.4 ± 0.2 cm (average ± SEM). At 12 months, the lower pole arc length was 6.5 ± 0.3 cm (average ± SEM), representing an average increase of 21.4% ± 4 % (average ± SEM). We found a statistically significant difference in lower pole lengthening between patients with more than 3 risk factors compared to other groups (**1 risk factor: 11.5% ± 1.8; *2–3 risk factors: 20.8% ± 3.1; >3 risk factors 42% ± 12, all expressed as average % increase of lower pole length ± SEM) (Fig. 3).
On a scale of 1 (very poor) to 10 (excellent), postoperative ptosis correction at 1 year was rated as 8.8 by the patients, which concurred with the grade of 8.4 by a blinded attending surgeon. Patient satisfaction according to the VAS assessment is reported in Table 2. Risk factors did not influence aesthetic outcomes, with no statistically significant differences in VAS score among risk factor groups.
No major complications (eg, ptosis recurrence requiring reoperation or NAC necrosis) were encountered. No hematoma or seroma occurred. Minor complications affected 3 (25%) patients. One patient developed hypertrophic scarring at the level of IMF. Two patients presented clinical localized fat necrosis after 4 weeks and were treated conservatively. Both patients were smokers and did not comply with smoking cessation. No bilateral mastopexy cases had measurable asymmetry at 1 year postoperatively. No infections or wound dehiscence was noted. None of the patients required blood transfusion (Table 2). Patient’s characteristics of the matched control group are reported in Table 3. The mean age was 44 years, 1 patient presented grade III ptosis, 3 patients presented grade II ptosis, and 1 patient presented pseudoptosis. In these patients, SN-N distance was 19.3 ± 0.5 cm (average ± SEM) immediately postoperatively. This length increased an average of 8.6% [21 ± 0.7 cm (average ± SEM)] at 12 months. Lower pole arc length was measured intraoperatively at 5.4 ± 0.2 cm (average ± SEM). At 12 months, the lower pole arc length was 6.7 ± 0.2 cm (average ± SEM), representing an average increase of 25.3% ± 4% (average ± SEM). Because the control group lacked patients with more than 3 risk factors, a comparison was only possible with the hammock group A and B (up to 3 risk factors). This showed a nonstatistical difference in terms of lengthening of the SN-N distance over time (P = 0.17), but a statistically significant reduction in bottoming out and lower pole lengthening (*P < 0.05) (Fig. 4).
TABLE 3.
Control Group Patients’ Demographics, Surgical Outcomes, and Complications
| Side | Age (y) | Identified Risk Factors* | NAC Lift (cm) | Complications | VAS | ||
|---|---|---|---|---|---|---|---|
| Patient | Surgeon | ||||||
| 1 | L | 52 | Age, skin, weight loss | 4 | Pseudoptosis | 8 | 8 |
| 2 | B | 28 | Weight loss | 6 | – | 8 | 8 |
| 3 | B | 36 | Skin | 4 | Hypertrophic scar | 7 | 7 |
| 4 | B | 71 | Age | 8 | – | 8 | 9 |
| 5 | B | 34 | Skin, smoke | 3 | – | 7 | 7 |
| 6 | B | 41 | Age, skin, weight loss | 7 | – | 8 | 7 |
| Average | 44 | 3.7 | 7.7 | 7.7 | |||
*Identified risk factors were NAC lift greater than 12 cm, history of weight loss, multiparity, smoking, and age greater than 50 y.
B, bilateral; L, left; R, right.
Fig. 4.
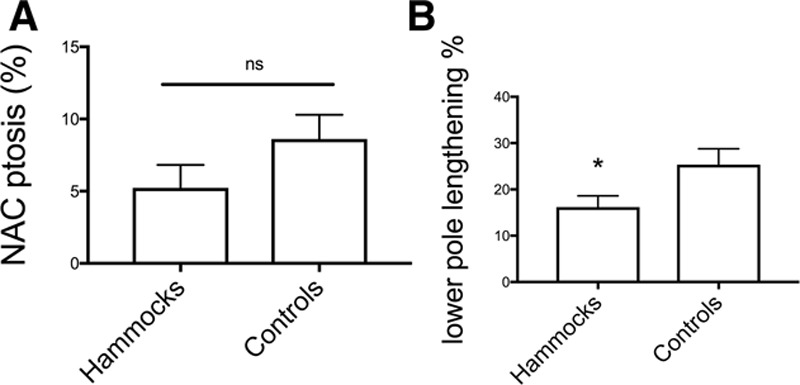
Comparison between Hammock technique and control technique. A, NAC ptosis percentage measured at 12 months in both the hammock and control groups. Values are expressed as average ± SEM. B, Lower pole lengthening percentage measured at 12 months in both the hammock and control groups. Values are expressed as average ± SEM (*P < 0.1). NS, no statistical significance
In the control group, 1 patient required skin-only redraping mastopexy of the lower pole 6 months after surgery. One patient developed hypertrophic scarring that improved after physical therapy without requiring corticoid injections.
DISCUSSION
Numerous techniques for glandular remodeling in mastopexy surgery have been described. These range from a simple dermal manipulation, with or without glandular reshaping, to the use of dermal flaps fixed to the pectoralis fascia, and the use of synthetic mesh or sheets. All techniques have a common objective in trying to position all of the breast tissue above the inframammary crease long term.3 To address the weakened Cooper’s ligaments and cutaneous laxity in retaining the breast weight in an upward direction, we recommended a support or a “hammock” to counteract the lower pole weight. Some authors have even proposed the use of a pectoral muscle sling to maintain durable support.15–17 Recently, Hamdi et al and Khouri et al described the use of an intradermal purse-string suture in an attempt to recreate the IMF and to improve breast projection.18,19
Although previous attempts to support the lower pole have been described,7,8 these were mainly dermoglandular flaps, where the glandular shaping was not dissociated from the dermal support.
In our technique, which aims to achieve a durable result and to reduce lower pole lengthening over time, there are 2 important concepts: repositioning of the breast volume in a more cranial position and dermal support to ensure that it stays there long term. The glandular reshaping allows transposition of a large volume from the lower pole to a more cranial position, thus restoring the depleted upper pole volume. Remodeling the glandular tissue in this fashion augments the upper pole without the need for implants and their inherent complications. This auto-augmentation has previously been shown to reliably ensure upper pole fullness with minimal bottoming out over time.4,20 We used a superiorly based glandular pedicle and folded it under the breast to prevent most of the breast weight from lying in the inferior pole.
The lateral and medial triangular dermal flaps are used as a “hammock” to support the more cranially positioned glandular tissue which will give a more durable result. The de-epithelialized dermal flaps can be adjusted in accordance with the desired breast projection. Tightening this dermal sling allows narrowing of the breast base to improve breast contour and to accentuate the lateral breast curvature. Although not responsible for the breast projection, which largely results from the glandular reshape, the dermal sling played an important role in preserving such a projection. It is important to point out that a limited elongation of the lower pole should be expected and that it does not necessarily denote recurrence of ptosis or bottoming out. The technique showed a significant limitation in bottoming out (21.4%, roughly 1 cm at 12 months, favorably comparing with measurements in the matched control group), and a significant improvement in support, with high patient satisfaction (Figs. 5–8). Noteworthy, the degree of lengthening of the lower pole curvature, even with this technique, increased proportionally to the number of predisposing risk factors (Fig. 3). Indeed, the hammock technique could effectively protect against lower pole lengthening in patients with up to 3 preoperative risk factors. This finding was further strengthened upon comparison with the matched control group, which showed a significant reduction in average lower pole length at 12 months (Fig. 4).
Fig. 5.
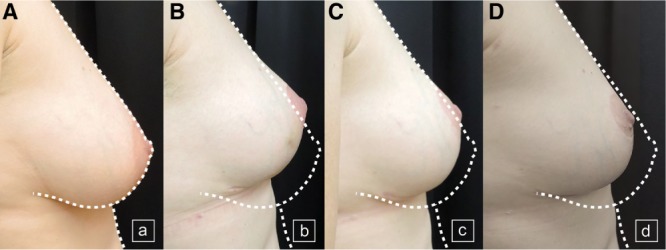
Hammock technique with 1 risk factor. A, Preoperative views of a patient with 1 risk factor requiring symmetrization mastopexy. B, Postoperative result at 3 months after hammock mastopexy. C, Result at 6 months after surgery. D, Result at 12 months after surgery.
Fig. 8.
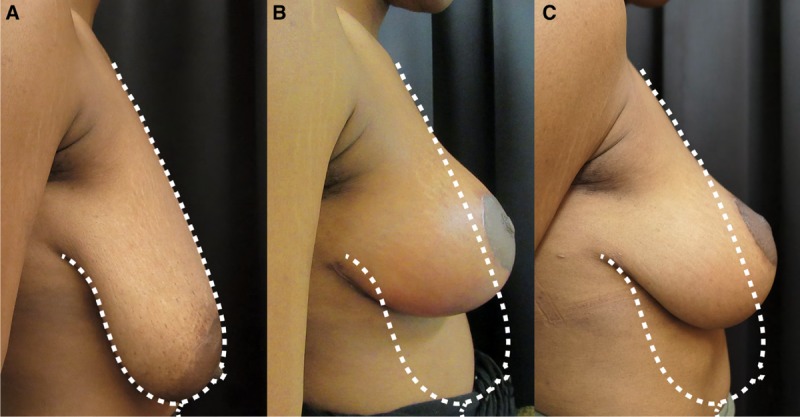
Hammock technique with more than 3 risk factors. A, Preoperative profile view of a patient presenting ptosis with more than 3 risk factors. B, Postoperative result at 6 months after hammock mastopexy. C, Result at 12 months after surgery.
Fig. 6.
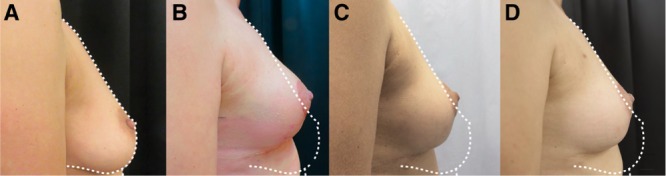
Hammock technique with 2 risk factors. A, Preoperative views of a patient presenting ptosis with 2 risk factors requiring symmetrization mastopexy. B, Postoperative result at 3 months after hammock mastopexy. C, Result at 6 months after surgery. D, Result at 12 months after surgery.
Fig. 7.
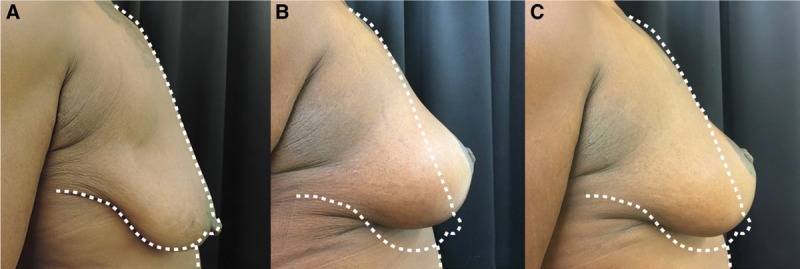
Hammock technique with 3 risk factors. A, Preoperative views of a patient presenting ptosis with 3 risk factors. B, Postoperative result at 6 months after hammock mastopexy. C, Result at 12 months after surgery.
This study confirms the importance of patient education and management of expectations especially in the presence of poor skin quality, increased SN-N-NAC distance, multiparity, advanced age, and smoking.
The results demonstrate the maintenance of satisfactory breast shape over time, even if the dermal hammocks cannot support the breast mount entirely when multiple risk factors are present (Fig. 9). However, the NAC position remained stable at 12 months regardless of the number of risk factors (Fig. 3).
Fig. 9.
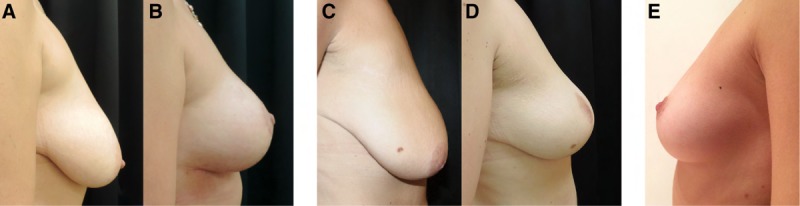
Comparaison between Hammock technique and control technique. A, Deflated upper pole, pseudoptosis, and ptosis in a patient with 2 risk factors. B, Stable maintenance of upper pole fullness and projection, attaining the profile of the “ideal breast” after mastopexy with the hammock technique. C, Preoperative profile image of a patient with 2 risk factors. D, Postoperative image after mastopexy without the hammock technique. E, The ideal breast shape in a 27-year-old model.
Other authors have used synthetic matrices to support the lower pole.10,21,22 We believe that the hammock mimics the role of an acellular dermal matrix for supporting the breast tissue and spares the breast from a foreign body. A recent article which reviewed mastopexy techniques showed that those which included the use of matrixes had a 4-fold increased rate of infection (1.7%), compared to the overall infection rate of mastopexy without matrices (0.4%).23 No patients in the series presented nipple-related complications such as nipple retraction, loss in sensation, asymmetry, and necrosis. This suggests that the breast parenchyma was effectively supported and minimal traction was applied to the NAC. The only reported complication in our series was hypertrophic scarring within the horizontal scar in 1 patient, and liponecrosis in 2 patients who were smokers and did not comply with smoking cessation. All patients were treated conservatively. To reduce potential fat necrosis in smokers, we were less aggressive during fixation of the dermal flaps to limit tissue ischemia. Although this technique required an inverted T scar, the scars were concealed in the IMF and no patients complained of excessive scarring. Consistently, Thoma et al demonstrated that scar pattern in mammoplasty did not really affect the patient in terms of quality of life.24 De-epithelializing, mobilizing and repositioning the triangular skin flaps take a little extraoperative time, but the learning curve is relatively quick with overall operative time much shorter in the second half of this series. The average VAS scores to grade cosmetic appearance were consistent between patient and surgeon as both reported a satisfactory cosmetic result (Table 2). Our study has some limitations. These include the relatively small number of patients and the lack of a prospective control group. However, we believe that the matched retrospective control group may still give some insight into the benefits of the hammock modifications. Indeed, this technique has now become routinely used in our unit for patients presenting risk factors for mastopexy. We hope that we would be able in the coming years to reinvestigate this technique with a larger number of patients and a longer follow-up time. Moreover, the lack of standardization of data analysis in previous literature makes it harder to compare with previous well-established mastopexy techniques. Although we acknowledge that a cosmetic standardized patient outcome questionnaire such as the BREAST-Q25 would have been beneficial, the morphometric measurements and the long-term follow-up results reinforced the VAS results.
CONCLUSIONS
The hammock technique referred to above is safe and reliable and provides easily reproducible results for patients with multiple high-risk factors for postoperative pseudoptosis such as weight loss, multiple pregnancies, smoking, poor skin quality, and advanced age. This mastopexy technique improves projection and reinforces the lower pole support with lateral and medial dermal flaps. It therefore reshapes the breast base and lateral profile while preventing bottoming out over time.
Supplementary Material
Footnotes
Published online 27 November 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com
REFERENCES
- 1.American Society of Plastic Surgeons. 2016 Plastic Surgery Statistics Report. Available at: https://www.plasticsurgery.org/news/plastic-surgery-statistics. Accessed July 13, 2017.
- 2.Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3:193–203. [PubMed] [Google Scholar]
- 3.Kirwan L. Breast autoaugmentation. Can J Plast Surg. 2007;15:73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botti G. Vertical scar mammaplasty: Stable padding of the superior pole by means of a posteriorly based pedicle autoprosthesis. Aesthetic Surgery Journal. 1999;19:116–123. [Google Scholar]
- 5.Ribeiro L. A new technique for reduction mammaplasty. Plast Reconstr Surg. 1975;55:330–334. [PubMed] [Google Scholar]
- 6.Ribeiro L, Accorsi A, Jr, Buss A, et al. Creation and evolution of 30 years of the inferior pedicle in reduction mammaplasties. Plast Reconstr Surg. 2002;110:960–970. [DOI] [PubMed] [Google Scholar]
- 7.Gümüş N. A versatile modification of dermoglandular hammock flap for mastopexy: extended hammock. J Plast Surg Hand Surg. 2013;47:252–257. [DOI] [PubMed] [Google Scholar]
- 8.Ross GL. One stage mastopexy augmentation in the ptotic patient. The superiorly based dermal flap for autologous reinforcement of the inferior pole. J Plast Reconstr Aesthet Surg. 2015;68:1248–1254. [DOI] [PubMed] [Google Scholar]
- 9.Góes JC. Preventing the “bottoming out and star-gazing” phenomenon in inferior pedicle breast reduction with an acellular dermal matrix internal brassiere. Aesthetic Plast Surg. 2010;34:768. [DOI] [PubMed] [Google Scholar]
- 10.de Bruijn HP, Johannes S. Mastopexy with 3D preshaped mesh for long-term results: development of the internal bra system. Aesthetic Plast Surg. 2008;32:757–765. [DOI] [PubMed] [Google Scholar]
- 11.Graf R, Biggs TM, Steely RL. Breast shape: a technique for better upper pole fullness. Aesthetic Plast Surg. 2000;24:348–352. [DOI] [PubMed] [Google Scholar]
- 12.Rinker B, Veneracion M, Walsh CP. The effect of breastfeeding on breast aesthetics. Aesthet Surg J. 2008;28:534–537. [DOI] [PubMed] [Google Scholar]
- 13.Rinker B, Veneracion M, Walsh CP. Breast ptosis: causes and cure. Ann Plast Surg. 2010;64:579–584. [DOI] [PubMed] [Google Scholar]
- 14.Pitanguy I. Surgical treatment of breast hypertrophy. Br J Plast Surg. 1967;20:78–85. [DOI] [PubMed] [Google Scholar]
- 15.van Deventer PV, Graewe FR, Würinger E. Improving the longevity and results of mastopexy and breast reduction procedures: reconstructing an internal breast support system with biocompatible mesh to replace the supporting function of the ligamentous suspension. Aesthetic Plast Surg. 2012;36:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borovikov A. Use of myofascial flaps in aesthetic breast surgery. Aesthet Surg J. 2004;24:331–341. [DOI] [PubMed] [Google Scholar]
- 17.Ritz M, Silfen R, Southwick G. Fascial suspension mastopexy. Plast Reconstr Surg. 2006;117:86–94. [DOI] [PubMed] [Google Scholar]
- 18.Hamdi M, Anzarut A, Hendrickx B, et al. Percutaneous purse-string suture: an innovative percutaneous technique for inframammary fold creation and improved breast projection in reconstructive surgery. Aesthetic Surgery Journal. 2017sjx190–sjx190. [DOI] [PubMed] [Google Scholar]
- 19.Khouri R. Incisionless & Sutureless Mastopexy. Paper presented at: ISAPS Official Course & Lebanese Plastic Surgery Day “Breast and Breast: The Future” Beirut, Lebanon. [Google Scholar]
- 20.Rubin JP, Gusenoff JA, Coon D. Dermal suspension and parenchymal reshaping mastopexy after massive weight loss: statistical analysis with concomitant procedures from a prospective registry. Plast Reconstr Surg. 2009;123:782–789. [DOI] [PubMed] [Google Scholar]
- 21.Goes JC, Bates D. Periareolar mastopexy with fortaperm. Aesthetic Plast Surg. 2010;34:350–358. [DOI] [PubMed] [Google Scholar]
- 22.Graf R, Biggs TM. In search of better shape in mastopexy and reduction mammoplasty. Plast Reconstr Surg. 2002;110:309–17; discussion 318. [DOI] [PubMed] [Google Scholar]
- 23.di Summa PG, Oranges CM, Wafta W, et al. A comprehensive review of mastopexy techniques: unveiling the breast lift. J Plast Reconstr Aesthet Surg. 2019;72:243–272.30527707 [Google Scholar]
- 24.Thoma A, Ignacy TA, Duku EK, et al. Randomized controlled trial comparing health-related quality of life in patients undergoing vertical scar versus inverted T-shaped reduction mammaplasty. Plast Reconstr Surg. 2013;132:48e–60e. [DOI] [PubMed] [Google Scholar]
- 25.Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: a review of the literature 2009-2015. J Plast Reconstr Aesthet Surg. 2016;69:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


