Summary:
Breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) is a rare lymphoma that has been associated with textured breast implants. Most cases present as a delayed (>1 year) seroma, which can be aspirated for diagnosis. Fewer patients present with masses or skin signs. Surgical resection is the cornerstone of treatment for this form of lymphoma. For advanced disease, treatment is multidisciplinary and incorporates adjuvant chemotherapy, radiation therapy, and potentially, the immunotherapeutic agent brentuximab vedotin, an anti-CD30 antibody-drug conjugate. However, relapse rates are high among patients with peripheral ALCL. We present the case of a 39-year-old woman who developed BIA-ALCL 13 years after augmentation with silicone, textured implants and had a complete pathologic response to neoadjuvant cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine (Oncovin), etoposide, prednisolone (CHOEP) at time of bilateral removal of implants and capsules. CHOEP is a long-standing regimen for treatment of peripheral ALCL and is a suggested regimen for treatment of BIA-ALCL. This case report is the first to demonstrate the use of neoadjuvant chemotherapy in the treatment of BIA-ALCL and suggests a role for its use in advanced disease.
Breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) is a rare disease associated with textured breast implants with incidence as high as 1/2,832 among implants made with Allergan BioCell (Dublin, Ireland) texturing by the salt loss technique.1,2 The National Comprehensive Cancer Network 2019 consensus guidelines emphasize the critical role of complete surgical excision, which results in remission when disease is limited to the fibrous implant capsule.3,4 Patients with advanced disease may have worse prognosis; Miranda et al5 found that only 72% of patients with tumor masses achieved remission, as opposed to 93% when disease was confined to the fibrous capsule.6 There may be a role for neoadjuvant chemotherapy in the advanced patient.
We present a woman with stage IV BIA-ALCL with lymph node metastasis who achieved complete pathologic response after neoadjuvant chemotherapy.
CASE REPORT
A 39-year-old, otherwise healthy, woman with a history of bilateral breast augmentation with silicone, EuroSilicone (Dublin, Ireland) textured implants 13 years before presentation developed a 5 cm left axillary mass. Examination revealed edematous and hyperpigmented skin changes and 2 masses: a firm, fixed left axillary mass 5 cm in largest dimension and a left lateral breast mass, 2 cm in largest dimension. There was neither cervical nor clavicular adenopathy. Diagnostic mammogram revealed multiple left-sided masses, largest measuring 5 cm in the left axilla. Magnetic resonance imaging (MRI) again revealed the large left-sided masses with regional adenopathy and additionally revealed a small left intracapsular fluid collection with slight thickening and mild enhancement of the capsule but no implant rupture (Fig. 1). MRI also showed right implant rupture with “linguine sign” and focal nodularity of the capsule in the lower inner portion which were positron emission tomography (PET) avid and concerning for contralateral disease.
Fig. 1.
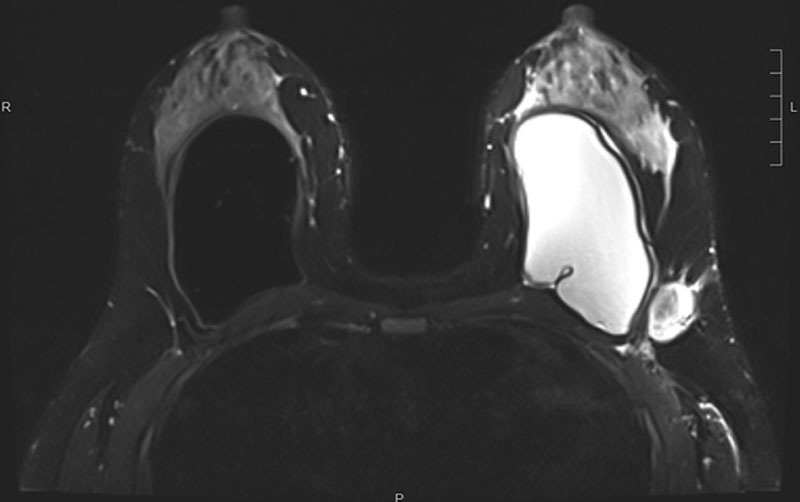
MRI of left-sided axillary masses before neoadjuvant chemotherapy. MRI indicates magnetic resonance imaging.
Biopsy of the left axillary mass revealed CD30+, CD4+, ALK-1 negative T-cell neoplasm suggestive of BIA-ALCL. Cells were positive for BCL2 and Ki-67 index was high. Cells were negative for keratin markers, including E-cadherin, ER, Her2, S100, CD8, CD5, CD23, CD20, kappa, and lambda. Biopsy of the left breast mass was benign. Further lymphoma work-up included a bone marrow biopsy which was negative for lymphoma.
PET revealed multiple additional concerning areas: right axillary lymph nodes with standardized uptake value (SUV) 31.3, right cervical level 3 lymph node with an SUV of 11.3, inferior border of the right breast implant with SUV of 9.8, T5 spinous process with SUV avidity of 10.7, inguinal lymph nodes bilaterally with SUV of 3.5 (Fig. 2). MRI of the spine demonstrated edema at the T5 vertebra concerning for metastatic lesion, and decision was made not to pursue biopsy. Excisional biopsy of the right axillary lymph node at time of port placement was benign.
Fig. 2.
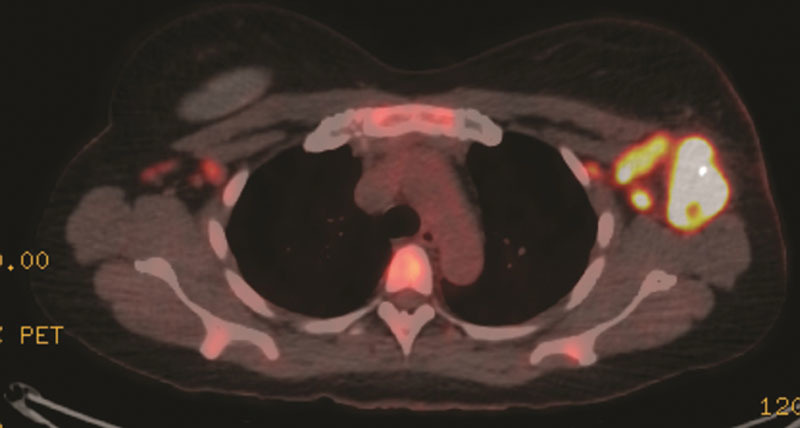
PET scan showing avid left axillary masses before neoadjuvant chemotherapy. PET indicates positron emission tomography.
The patient was designated American Joint Committee on Cancer T4N2M1; Stage IV. Due to her advanced stage and the apparent growth of masses during work-up, the multidisciplinary team elected to proceed with neoadjuvant chemotherapy. Over 4 months, she received 5 of 6 planned cycles of neoadjuvant cyclophosphamide, doxorubicin, vincristine, etoposide, prednisolone (CHOEP). The treatment course was complicated by hospitalization in cycle 1 for vulvar herpes simplex virus cellulitis and in cycle 4 for multiple complications that included symptomatic pulmonary emboli in the right lung, pancytopenia, mild decrease in left ventricular ejection fraction to 40%–45%, neuropathy, and nausea. PET/computed tomography after completion of neoadjuvant chemotherapy revealed resolution of all hypermetabolic areas (Fig. 3).
Fig. 3.
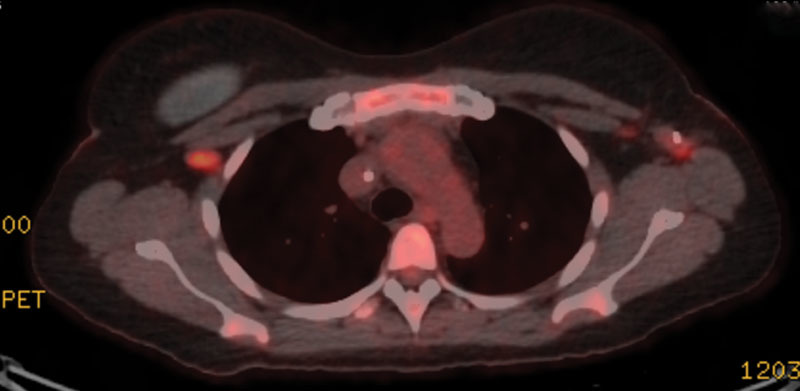
PET scan showing resolution of left axillary avidity after neoadjuvant chemotherapy and biopsy clip remaining. PET indicates positron emission tomography.
Six months after initial presentation, she underwent removal of both implants and their associated capsules plus wire localized excision of the left axillary and lateral breast masses, but no axillary lymph node dissection (Fig. 4). She had a complete pathologic response.
Fig. 4.
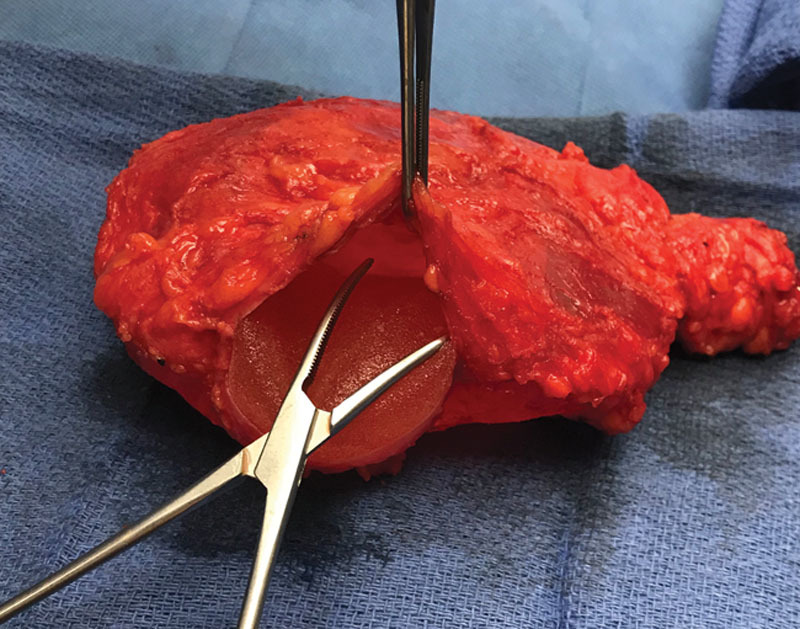
Excised left implant and capsule with adjacent extracapsular mass.
Given her complete pathologic response, multidisciplinary consensus was that adjuvant radiation therapy was unnecessary.
Her response remained durable 8 months postoperatively by PET/computed tomography and clinical examination.
DISCUSSION
Five-year survival of early-stage BIA-ALCL is 89%. However, the rate of adverse events, such as relapse, is significantly greater when the tumor spreads beyond the capsule.7 According to the International Peripheral T-Cell Lymphoma Project and report by Savage et al,8 the 5-year failure-free survival for ALK-negative peripheral ALCL is 36%. In a cohort of 39 patients, Collins et al4 report that patients with positive lymph nodes had significantly higher rates of death (20.8% versus 0%) and lower rates of complete remission (70.8% versus 100%). Notably, no patients in that cohort who died from BIA-ALCL underwent definitive surgical treatment, which supports surgical extirpation of implants, capsules, and lesions as the cornerstone of treatment. In addition, adjuvant chemotherapy and radiation therapy may play a role in advanced disease with high risk of recurrence.3 CHOEP has long been used for treatment of peripheral ALCL, and it should be noted that the U.S. Food and Drug Administration recently, and in light of the ECHELON-2 trial,9 approved brentuximab vedotin, an anti-CD30 antibody-drug conjugate, for first-line treatment of peripheral T-cell lymphomas. While use of brentuximab vedotin was considered for the patient, ultimately, CHOEP was administered.
This case report posits that there may be a role for neoadjuvant chemotherapy in BIA-ALCL to control disease burden before tumor resection, particularly in the advanced widespread disease. Success was demonstrated by complete resolution of hypermetabolic areas concerning for disease and complete pathologic response on examination of surgical specimens. Further examination of the role of neoadjuvant chemotherapy for treatment of advanced BIA-ALCL is necessary as we learn more about this rare disease.
Footnotes
Published online 24 September 2019
Presented at the Mid Atlantic Breast Consortium, March 27, 2019, Washington, D.C.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Statement of Conformity: This case report was written in line with the principles of the Helsinki Declaration.
References
- 1.Van Natta BW. Determining the true incidence of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): the need for accurate data. Aesthet Surg J. 2019;39:NP230. [DOI] [PubMed] [Google Scholar]
- 2.Collett DJ, Rakhorst H, Lennox P, et al. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):30S–40S. [DOI] [PubMed] [Google Scholar]
- 3.Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN consensus guidelines on the diagnosis and treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Suppl 1):S3–S13. 10.1093/asj/sjy331. [DOI] [PubMed] [Google Scholar]
- 4.Collins MS, Miranda RN, Medeiros LJ, et al. Characteristics and treatment of advanced breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):41S–50S. [DOI] [PubMed] [Google Scholar]
- 5.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrufino-Schmidt MC, Medeiros LJ, Liu H, et al. Clinicopathologic features and prognostic impact of lymph node involvement in patients with breast implant-associated anaplastic large cell lymphoma. Am J Surg Pathol. 2018;42:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savage KJ, Harris NL, Vose JM, et al. ; International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the international peripheral T-cell lymphoma project. Blood. 2008;111:5496–5504. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz S, O’Connor OA, Pro B, et al. ; ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]


