Abstract
Background
The effect of statins over time on coronary atherosclerosis in patients with inflammatory joint diseases (IJD) is unknown. Our aim was to evaluate the change in coronary plaque morphology and volume in long-term statin-treated patients with IJD.
Methods
Sixty-eight patients with IJD and carotid artery plaque(s) underwent coronary computed tomography angiography before and after a mean of 4.7 (range 4.0–6.0) years of statin treatment. The treatment target for low density lipoprotein cholesterol (LDL-c) was ≤1.8 mmol/L. Changes in plaque volume (calcified, mixed/soft and total) and coronary artery calcification (CAC) from baseline to follow-up were assessed using the 17-segment American Heart Association-model.
Results
Median (IQR) increase in CAC after statin treatment was 38 (5–236) Agatston units (p<0.001). Calcified and total plaque volume increased with 5.6 (0.0–49.1) and 2.9 (0.0–23.5) mm3, respectively (p<0.001 for both). The median (IQR) change in soft/mixed plaque volume was -10 (-7.1–0.0), p = <0.001. Patients who had obtained the LDL-c treatment target at follow-up, experienced reduced progression of both CAC and total plaque volume compared to patients with LDL-c >1.8mmol/L (21 [2–143] vs. 69 [16–423], p = 0.006 and 0.65 [-1.0–13.9] vs. 13.0 [0.0–60.8] mm3, p = 0.019, respectively).
Conclusions
A progression of total atherosclerotic plaque volume in statin-treated patients with IJD was observed. However, soft/mixed plaque volume was reduced, suggesting an alteration in plaque composition. Patients with recommended LDL-c levels at follow-up had reduced atherosclerotic progression compared to patients with LDL-c levels above the treatment target, suggesting a beneficial effect of treatment to guideline-recommended lipid targets in IJD patients.
Introduction
Patients with inflammatory joint diseases (IJD) have an increased risk of acute coronary syndrome [1]. Lipid-lowering treatment with statins is considered as highly effective prophylaxis for coronary artery disease in the general population due to improvements of both lipid-profiles and clinical outcome [2, 3]. Evidence regarding statin treatment in IJD patients is scarce, but promising results from post hoc analyses in 2 randomized controlled statin trials (TNT and IDEAL) revealed comparable lipid lowering effect and risk reduction for future cardiovascular disease (CVD) in patients with and without IJD [4]. Despite this, inadequate preventive treatment with statins has been reported in patients with IJD [5, 6]. In addition to lowering lipids, statins have been shown to possess anti-inflammatory effects [7]. Other positive plaque-related effects such as cell death in the lipid cores and plaque-stabilization due to micro-calcifications have also been described [8, 9]. Whether these statin effects will occur in patients with IJD is uncertain, due to the underlying systemic inflammation, the lipid increasing effect of anti-rheumatic medications and the polypharmacy these patients have [10]. Inflammation is part of the atherogenesis [11], and elevated inflammation as measured by CRP has been shown be a predictor of increased atherogenesis with clinical outcomes [12]. Assessments of plaque morphology are important and of great interest since non-calcified atherosclerotic plaques are more likely to result in acute coronary syndrome than the more stable calcified plaques [13].
Coronary computed tomography angiography (CCTA) has become an established non-invasive method for detection of coronary artery stenosis [14]. It is also a promising and increasingly used tool for characterization of coronary plaques with good correlation to intravascular ultrasound [15]. Statin-treatment has been shown by CCTA to induce regression of coronary plaques in patients without IJD [16], in addition to a slower progression of coronary plaque volume in patients with low LDL-c level [17]. Increased coronary artery calcifications (CAC) have also been reported after statin treatment in the general population [18]. Taking into consideration that patients with IJD have high systemic inflammation and that disease activity has been shown to have an impact on carotid artery plaque composition [19] further warrants evaluation of the statin effect on atherosclerotic plaques in patients with IJD.
The aims of the present study were to evaluate the progression of coronary atherosclerosis/plaques after long-term statin-treatment in patients with IJD, and the effect on plaque morphology evaluated by CCTA. Furthermore, we assessed possible predictors of plaque progression, including patient characteristics, lipids and inflammatory markers.
Materials and methods
Patients and study design
The RORA-AS study (ROsuvastatin in Rheumatoid Arthritis, Ankylosing Spondylitis and other inflammatory joint diseases) was an open, prospective intervention study, and a complete description of inclusion and exclusion-criteria has previously been reported [20]. In short, IJD patients with ultrasound-verified carotid plaque(s) were treated with rosuvastatin with an LDL-c target of ≤1.8 mmol/L, in accordance with the most recent European guidelines [21]. All patients signed an informed consent and the study was approved by the Norwegian South East Regional Committee for Medical and Health Research Ethics and registered with ClinicalTrials.gov Id: NCT01389388. The European Union Drug Regulating Authorities Clinical Trials (EudraCT) number is 2008-005551-20.
CCTA was performed for study purposes in 68 statin-naïve patients with IJD and carotid artery plaques between 2010 and 2012 with a follow-up CCTA in 2016. The follow-up time was prolonged compared to study protocol, due to lack of time available on the scanner. Patients with reduced kidney function (estimated glomerular filtration rate of <45 ml/minute), arrhythmias, previous coronary artery bypass surgery, stents or pacemaker-implantation were excluded. All patients filled in a questionnaire at baseline and follow-up for assessment of characteristics, symptoms of coronary disease and medications. Changes in lipid-profiles and inflammatory parameters were evaluated by laboratory tests drawn and analyzed at Diakonhjemmet Hospital using a COBAS 6000 and COBAS 8000, Roche Diagnostics Norway AS.
Medications
After baseline CCTA, all patients received rosuvastatin, with dose titration to achieve an LDL-c goal of ≤1.8mmol/L. The lipids were frequently monitored for the first 18 months. Due to national regulations the lipid lowering medication was switched to atorvastatin after the first 18 months unless there was a specific reason to continue rosuvastatin treatment, such as side effects or inadequate lipid lowering effect with other statins. After 18 months the patient was followed by the primary care physician who had received a discharge report including specification of diagnosis, present medication use, LDL-c goal and follow-up recommendations.
Imaging technique
All baseline and follow-up CCTA examinations were performed on a Philips Brilliance 64-slice CT scanner (Philips Healthcare, Cleveland, Ohio, USA) with protocols as previously described [22]. Initially, a non-contrast scan was conducted for evaluation of CAC. If tolerated, intravenous beta blockage (5–20 mg Seloken®, Astra Zeneca) was used to reduce the heart rhythm and Nitroglycerin 0.4mg (Nitrolingual®, Pohl-Boskamp, Hohenlockstedt, Germany) was administered for the vasodilating effect sublingually 1–3 minutes prior to the contrast-enhanced scan. Prospective ECG-gating was used when achieving a heart rate ≤ 65 beats/min (bpm), while retrospective ECG-gating was required for higher heartrates. The contrast media OmnipaqueTM 350 mg/ml (GE Healthcare, Princeton, New Jersey) was used in both the baseline and follow-up examinations.
Image analysis
The image analyses were performed on a Philips Workstation (Intellispace v5, Philips Healthcare) with dedicated software (Plaque Analysis, Comprehensive Cardiac, Philips Healthcare) [23]. The inter-observer variability was calculated on a per-segment level after two independent readers blinded to patient characteristics measured the plaque volume in left ascending artery in 30% of the patients, with an interclass correlation coefficient 0.92. The same segments were evaluated twice by one reader with an intra-observer variability of 0.93. The analyses were assessed using the 17-segment model of the American Heart Association [24]. All segments with sufficient image quality and a diameter >1.5 mm were included in the analyses.
CAC was calculated by the Agatston method [25]. The morphology of the plaques was defined according to plaque density, measured with Hounsfield Units (HU). Plaques were defined as calcified if ≥90% of the total volume had a density ≥130 HU, and soft when ≥10% had a density of ≥130HU. Mixed plaques were all in between [23]. Coronary artery disease (CAD) was defined as “presence of any plaque.” Segment involvement score (SIS) and segment stenosis score was used to assess extent and severity of the CAD with previously described definitions [22].
Statistical analysis
Descriptive data are presented with number (%) for dichotomized variables, mean±standard deviation (SD) for normally distributed characteristics or median with interquartile range (IQR) if not normally distributed. Analysis of variance and X2 were used to compare variables between groups. The paired samples t-test was applied in assessment of changes in variables from baseline to follow-up. The Wilcoxon signed rank test was used for non-normally distributed variables.
Independent samples t-test was used to test the difference in atherosclerotic change between patients with obtained and non-obtained LDL-c goal at follow-up. Non-normally distributed variables were log-transformed before these analyses were conducted.
Linear regression models were constructed with a stepwise backwards approach to assess predictors of change in plaque volumes and CAC. Pearsons correlation coefficient was used to test correlation to variables (patient characteristics and CVD risk factors), but due to a correlation less than 0.2, none of the variables tested in the univariate analyses were included in the multivariate models. Variables considered to be of clinical relevance (lipids, inflammatory markers and sDMARDS/dDMARDS) were therefore the only variables included in the models.
For further evaluation of atherosclerotic progression, we arbitrary divided the change in total plaque volume into percentiles (25%, 50% and 75%). Differences were evaluated with analysis of variance. Multiple logistic regression was used to identify predictors for <25th percentile and >75th percentile. All analyses were performed using IBM SPSS version 21.
Results
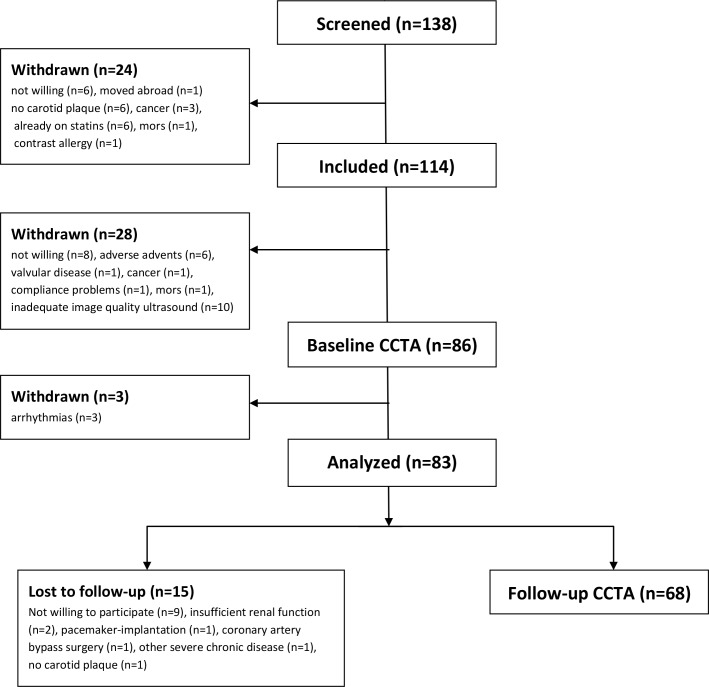
Of the 83 patients initially included at baseline, 15 patients were lost to follow up; 2 due to insufficient renal function, 1 due to pacemaker-implantation, 1 had a coronary artery bypass surgery, 1 due to severe chronic disease, 1 because of screening failure (no presence of carotid artery plaque at baseline) and 9 did not want to participate (Fig 1). Evaluations from the remaining 68 patients are included in the analyses. Mean follow-up time was 4.7 (range 4.0–6.0) years.
Fig 1. Study flow chart.
Out of 83 patients analyzed at baseline CCTA, 68 patients were included at follow-up CCTA.
Table 1 presents the patient characteristics at baseline. Two-thirds of the patients had RA (66%), and the majority of these patients were females (64%). Mean age was 60.5±8.6 years. Only a few patients had diabetes mellitus (6%) or previous CVD (10%), but other risk factors of CVD were prevalent; hypertension (47%), hyperlipidemia (64%) and smoking (22%).
Table 1. Patient characteristics at baseline.
| IJD n = 68 (100) |
RA n = 45 (66.1) |
AS n = 15 (22.1) |
PsA n = 8 (11.8) |
|
|---|---|---|---|---|
| Age (years), mean±SD | 60.5±8.6 | 61.2±8.8 | 60.3±8.2 | 57.4±8.6 |
| Women, n(%) | 44 (63.8) | 34 (73.9) | 7 (46.7) | 3 (37.5) |
| Disease duration (years), median (IQR) | 17.1±11.9 | 15.8±1.7 | 22.7±2.7 | 13.6±5.0 |
| BMI (kg/m2), mean±SD | 25.1±3.0 | 25.1±3.1 | 24.5±2.2 | 25.8±3.6 |
| Waist circumference (cm), mean±SD | 91±11 | 91±11 | 90±9.0 | 94±11 |
| Systolic BP (mmHg) mean±SD | 142±20 | 141±21 | 144±13 | 146±28 |
| Diastolic BP(mmHg), mean±SD | 83±9 | 83±9 | 83±7 | 86±12 |
| HT,n(%) | 32 (47.1) | 21 (46.7) | 8 (27.6) | 3 (37.5) |
| Diabetes mellitus, n(%) | 4 (5.8) | 3 (6.5) | 1 (6.7) | 0 (0.0) |
| Smoking, n(%) | 15 (21.7) | 11 (23.9) | 2 (13.3) | 2 (25.0) |
| Family history of CVD, n(%) | 11 (15.9) | 7 (15.6) | 1 (6.7) | 3 (37.5) |
| Previous CVD, n(%) | 7 (10.3) | 5 (11.1) | 2 (13.3) | 0 (0.0) |
| Angina, n(%) | 12 (17.4) | 10 (21.7) | 2 (13.3) | 0 (0.0) |
| Hyperlipidemia, n(%) | 44 (63.8) | 27 (58.7) | 4 (73.3) | 6 (75.0) |
| Medications | ||||
| Synthetic DMARDs, n(%) | 40 (62.5) | 28 (65.1) | 4 (30.8) | 8 (100.0) |
| Biologic DMARDs, n(%) | 22 (34.4) | 13 (32.5) | 5 (38.5) | 4 (50.0) |
| NSAIDs, n(%) | 19 (32.8) | 13 (32.5) | 4 (30.8) | 2 (10.5) |
| Anti-hypertensives, n(%) | 10 (14.7) | 6 (15.4) | 3 (33.3) | 1 (16.7) |
| Inflammatory markers | ||||
| ESR (mm/hour), mean±SD | 11.8±9.3 | 13.0±10.5 | 8.4±4.8 | 9.3±3.6 |
| CRP (mg/L), mean±SD | 3.6±4.7 | 4.0±5.1 | 2.6±3.6 | 2.8±2.9 |
IJD: inflammatory joint disease, RA: rheumatoid arthritis, AS: ankylosing spondylitis, PsA: psoriatric arthritis, BMI: body mass index, BP: blood pressure, HT: hypertension, CVD: cardiovascular disease, DMARDS: disease modifying anti-rheumatic drug, NSAIDs: Non-steroidal Anti-Inflammatory Drugs, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein.
Hyperlipidemia: total cholesterol ≥6.0mmol/l.
Hypertension: systolic BP >140 mmHg and diastolic BP >90 mmHg.
CAD was detected in 42 (62%) patients at baseline, compared to 51 (75%) at follow-up. In total, atherosclerotic plaques were present in 133 of 913 (14.6%) segments at baseline compared to 203 of 874 (23.2%) at follow-up. Forty-six (34.6%) of the plaques were defined as mixed or soft at baseline compared to 16 (7.9%) at follow-up.
The atherosclerotic progression is shown in Table 2.
Table 2. CCTA findings, lipids and inflammatory markers at baseline and follow-up (per-patient-level).
| Baseline (n = 68) | Follow-up (n = 68) | Change (n = 68) |
p-value | |
|---|---|---|---|---|
| CCTA findings | ||||
| CAC, Agatston units, median(IQR) | 15(0–221) | 73(6–514) | 38 (5–236) | <0.001a |
| Total plaque volume, mm3, median(IQR) | 5.1(0.0–36.7) | 8.0(0.5–77.2) | 2.9 (0.0–23.5) | <0.001a |
| Calcified plaque volume, mm3, median(IQR) | 0.2(0.0–15.5) | 9.5(6.0–77.2) | 5.6 (0.0–49.1) | <0.001a |
| Mixed/soft plaque volume, mm3, median(IQR) | 0(0–8) | 0(0–0) | -10 (-7.1–0.0) | 0.001a |
| Segment Involvement Score | 2.0±2.5 | 3.1±2.9 | 1.1±1.4 | <0.001b |
| Segment Stenosis Score | 2.9±4.0 | 5.7±6.3 | 2.8±3.1 | <0.001 b |
| Lipids | ||||
| Total cholesterol, mmol/L | 6.44±1.09 | 4.34±0.85 | -2.09±1.14 | <0.001b |
| HDL cholesterol, mmol/L | 1.75±0.55 | 1.81±0.61 | 0.07±0.31 | 0.059b |
| LDL cholesterol, mmol/L | 4.02±1.02 | 1.97±0.70 | -2.06±1.09 | <0.001b |
| Triglycerides, mmol/L | 1.52±0.98 | 1.27±0.80 | -0.24±0.82 | 0.019b |
| Inflammation-markers | ||||
| ESR, mm/hour | 13.71±9.17 | 11.88±11.93 | -1.83±12.55 | 0.24b |
| CRP, mg/L | 3.71±3.86 | 3.70±5.76 | -0.01±6.58 | 0.99b |
Values are presented as the mean ± SD unless otherwise stated.
aWilcoxon signed rank test
bPaired samples t-test
* coefficient of variation: 4.3%
CCTA: coronary computed tomography angiography, CAC: coronary artery calcification, SD: standard deviation, HDL: high density lipoprotein, LDL: low density lipoprotein, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein
Median (IQR) increase in CAC increase was 38(5–236) Agatston units (p<0.001). Calcified and total plaque volume increased with 5.6 (0.0–49.1) and 2.9(0.0–23.5) mm3, respectively (p<0.001 for both). The median (IQR) change in soft/mixed plaque volume was -10 (-7.1–0.0), p = <0.001. Regarding lipids, all levels were reduced except for high density lipoprotein cholesterol (HDL-c), as expected. The inflammatory markers were comparable at baseline and follow-up. Both segment involvement score and segment stenosis score increased (p<0.001 for both).
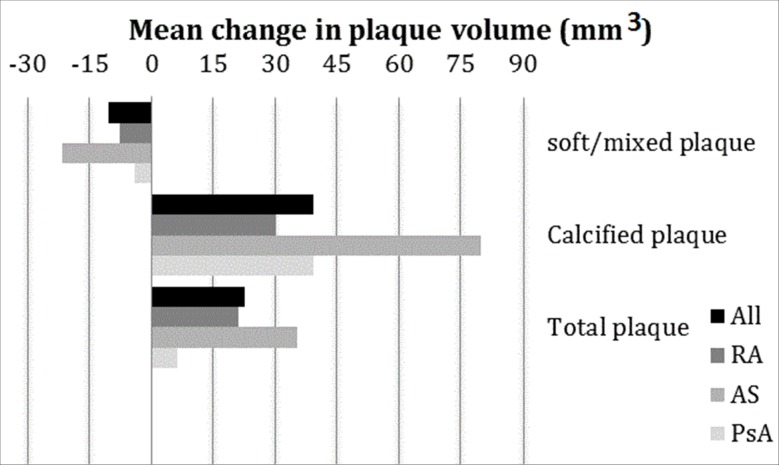
Fig 2 shows the mean change in plaque volume in the 3 IJD groups. The ankylosing spondylitis (AS) group had a larger reduction in soft/mixed plaque volume, and more extensive increase in calcified and total plaque volume than RA and psoriatic arthritis (PsA) patients.
Fig 2. Mean change in plaque volume in the 3 IJD groups.
Data shown as mean change in soft/mixed, calcified and total plaque volume (mm3). The soft/mixed plaque was over-all reduced, and calcified and total plaque volume increased in all groups. The plaque alterations are highest in the AS-group. RA:rheumatoid arthritis, AS:ankylosing spondylitis, PsA:psoriatic arthritis.
At follow-up, 34 (50%) of the patients had an LDL-c level below study target (≤1.8mmol/L). Table 3 shows the difference in the CCTA-measurements between patients with an LDL-c level above or below 1.8mmol/l at follow-up. The change in CAC, calcified plaque volume and total plaque volume was reduced in the group with an LDL-c ≤1.8 mmol/L. The reduction in soft/mixed plaque volume was numerically larger in the group with LDL-c-level above treatment target, although this difference was not statistically significant (p = 0.71).
Table 3. Lipid status and CAD-progression in patients with and not with LDL-c ≤ 1.8mmol/l.
| LDL ≤1.8mmol/l n = 34 |
LDL >1.8mmol/l n = 34 |
p-value | |
|---|---|---|---|
|
LDL-c level Baseline, mmol/L, mean±SD |
3.7±0.9 | 4.4±1.0 | <0.001 |
| LDL-c level Follow-up, mmol/L, mean±SD | 1.5±0.2 | 2.4±0.7 | <0.001 |
|
Change LDL-c level, mmol/L, mean±SD |
-2.2±0.9 | -1.9±1.3 | 0.38 |
|
Change CAC, median (IQR) |
21 (2–143) | 69 (16–423) | <0.001a |
|
Change Soft/Mixed plaque, mm3, median (IQR) |
0 (-3.5–0.0) | 0 (-15.7–0.0) | 0.71a |
| Change calcified plaque, mm3, median (IQR) | 1.7 (0.0–17.3) | 13.4 (1.5–107.6) | <0.019a |
|
Change Total Plaque, mm3, median (IQR) |
0.65 (-1.0–13.9) | 13.0 (0.0–60.8) | <0.001a |
aindependent samples t-test using log-transformed variables.
CAD: coronary artery disease, LDL-c: low density lipoprotein cholesterol, SD: standard deviation, CAC: coronary artery calcifications, IQR: interquartile range
An LDL-c level >1.8mmol/l was associated with change in CAC (model A) and change in total plaque volume (model B), after adjusting for age and sex, but was not significantly associated with change in soft/mixed plaque volume (model C) (Table 4).
Table 4. Associations between progression of CAC (A), total plaque volume (B), soft/mixed plaque volume (C) with lipids, inflammatory markers and sDMARDS/bDMARDS.
| Univariate | Multivariated | ||||
|---|---|---|---|---|---|
| β (95%CI) | p-value | β (95%CI) | p-value | ||
| Aa | Age | 5.62 (-2.51-13-75) | 0.17 | 8.80 (1.14–16.45) | 0.025 |
| Male | 57.21 (-86.39- -200.80) | 0.43 | 79.25 (-52.69–211.19) | 0.23 | |
| LDL-c >1.8mmol/l | 199.63 (69.09–330.16) | <0.001 | 225.79 (95.48–356.09) | 0.001 | |
| HDL-c | 49.90 (-164.97–264.75) | 0.64 | |||
| Triglycerides | 47.11 (-38.40–132.62) | 0.28 | |||
| CRP follow-up | -2.85 (-15.03–9.34) | 0.64 | |||
| ESR follow-up | 0.67 (-5.48–6.83) | 0.83 | |||
| Non-bDMARDs user | 70.39 (82.99–223.77) | 0.36 | |||
| Non-sDMARDs user | 122.13 (26.22–270.47) | 0.11 | |||
| Bb | Age | 0.57 (-0.97–2.12) | 0.46 | 0.97 (-0.53–2.47) | 0.20 |
| Male | 21.87(-5.03–48.76) | 0.11 | 23.5 (-2.68–49.80) | 0.078 | |
| LDLc >1.8mmol/L | 30.42 (5.06–55.79) | 0.019 | 33.8 (8.2–59.4) | 0.010 | |
| Cc | Age | -0.37 (-1.15–0.41) | 0.35 | -0.48 (-1.24–0.27) | 0.21 |
| Male | -18.06 (-31.26- -4.85) | <0.001 | -18.05 (-31.25- -4.84) | 0.008 | |
| LDL-c >1.8mmol/L | -9.15 (-22.39–4.09) | 0.17 | -11.52 (-24.41–1.37) | 0.079 | |
Linear regression Aa: change in CAC as dependent variable, Bb: change in total plaque volume, Cc: change in soft/mixed plaque volume
dAdjusted for number of months between baseline and follow-up.
CAC: coronary artery calcification, CI: confidence interval, LDL: low-density lipoprotein, sDMARDs: synthetic disease modifying anti-rheumatic drugs, bMARDS: biologic disease modifying anti-rheumatic drugs, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate.
S1 Fig shows a near linear relationship between change in total mixed/soft plaque volume per patient and baseline mixed/soft plaque volume (R = 0.898).
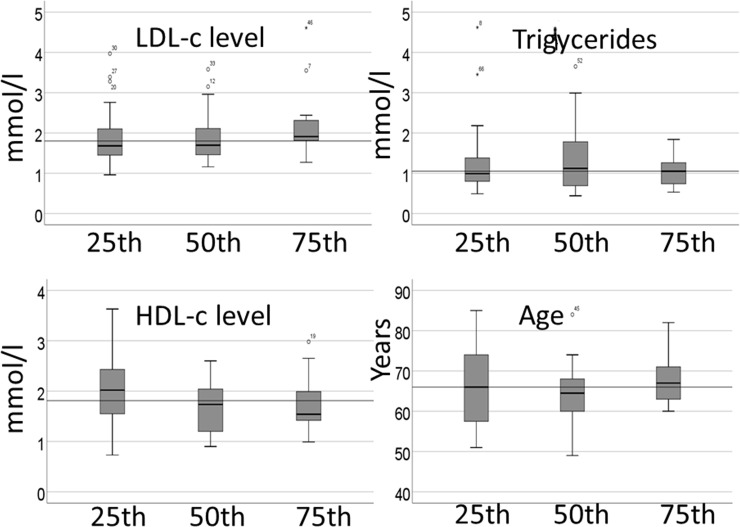
Fig 3 presents the difference in HDL-c, LDL-c, triglycerides and age between the percentiles of change in total plaque volume, with no significant difference between the groups. However, in the multiple logistic regression analysis, the HDL-c level at follow up was associated with <25th percentile (<2.9 mm3) increase in total plaque volume, OR (95%CI): 3.36 (1.16–9.74), p = 0.029, after adjusting for age and sex. In addition, LDL-c (OR: (95%CI): 1.3 (1.2–11.0), p = 0.022) was associated with the >75th percentile (>23.5mm3) of change in total plaque volume after adjusting for sex and age. All patients with ≥400 CAC increase had an LDL-c-level at follow-up above the treatment target.
Fig 3. Difference in lipids and age between percentiles of increase in total plaque volume (mm3).
The reference line is set to median in all variables. LDL-c; low density lipoprotein-cholesterol, HDL-c; high density lipoprotein-cholesterol.
The correlation between biologic DMARD-use and change in CAC, total plaque volume, soft/mixed plaque volume and calcified volume were: r = -0.14 (p = 0.28), r = 0.12 (p = 0.36), r = 0.03 (p = 0.81) and r = -0.02 (p = 0.88), respectively.
Discussion
In this study, we have shown that a progression of coronary atherosclerosis in statin-treated patients with IJD occurs after nearly 5 years of statin treatment. However, an increase in calcified plaque volume and a decrease in soft/mixed plaque volume suggested a conversion in plaque-composition. We also revealed that LDL-c-levels were associated with atherosclerotic progression in the sense that the patients who obtained LDL-c treatment target experienced a more moderate progression of atherosclerotic plaque volume compared to those with LDL-c-levels above the LDL-c treatment target of 1.8 mmol/L. To our knowledge, this is the first study to assess the effects of statin-treatment on coronary plaques in patients with IJD.
The CAC increased significantly from baseline to follow-up. CAC has a well-documented prognostic value for future cardiac events, and a linear relationship between CAC and CVD risk has been established [26–28]. CAC has been shown to be a greater determinant of atherosclerotic progression than traditional risk-factors, sex or age in asymptomatic individuals [29]. However, the relationship of CAC progression and events has not been fully elucidated in statin users [30]. Puri et al. described that an increase in CAC induced by statins had a positive plaque-stabilizing effect due to induction of micro-calcifications [8]. Shaw et al. suggested that CAC may loose its predictive value after initiation of plaque-altering therapies such as statins [30]. From the MESA-study [31] it was reported an inverse association between plaque density and risk of CVD events, suggesting that denser plaques may be protective for CVD events. Whether the increased CAC in our study was a marker for healing of plaques (induced by statins) or for progression of disease, is difficult to interpret. However, the volume measurements add valuable information to this evaluation, as the total plaque volume also increased significantly in our study. If the increased CAC was solely due to statin-treatment, the volume may not increase significantly and thus, one may argue that the CAC increase in our study is, most likely, an effect caused by both plaque-stabilizing and disease progression.
Another important finding is the reduction in mixed/soft plaques from baseline to follow-up. The presence of soft plaques has been reported to be an independent predictor for acute coronary syndromes [32], and a reduction of soft/mixed plaque is likely to be beneficial for the patient. Previous studies have reported on a difference in plaque morphology between statin users and non-statin users [33]. Further, statins have shown a greater impact on the morphology of non-calcified/partially calcified plaques than on solely calcified plaques [34].
Interestingly, we observed a significantly lower progression of both CAC and plaque volume among the patients who maintained LDL-c-levels of ≤1.8 mmol/l at follow-up. The latter finding is in line with results from a 10-year follow-up study by Goh et al., showing a slower progression of CAC in patients on aggressive statin treatment regimens [35]. Two other studies have found reduced progression of plaque volume additionally to CAC in patients who achieved lower LDL-c-levels [17, 36]. Zeb et. al found a slower progression in non-calcified atheroma after 1 year follow-up in statin-users compared to non-statin-users [37]. A recently published study, described a significant association between individual lipoprotein variability and coronary atheroma progression and also to adverse CVD events [38]. We did not manage to detect a significant difference in regression/progression in soft/mixed plaque volume in those with an LDL-c level above vs. below the LDL-c treatment target. However, there was a near linear relationship between the regression of the volume of mixed/soft plaque and mixed/soft plaque at baseline (S1 Fig). Thus, the group with the largest burden of soft/mixed plaques at baseline experienced most regression/alteration (i.e. those with LDL-c >1.8mmol/l). This finding might be influenced by the “regression towards the mean-“phenomenon. However; the number of soft/mixed plaques was also significantly reduced. Fig 2 shows more plaque alterations/regression of soft/mixed plaque in the AS-group compared to the RA and PsA groups. The AS-group consisted of more males in comparison with the RA and PsA groups, which may explain the higher presence of more soft/mixed plaque at baseline.
In our study, LDL-c and HDL-c-levels in addition to age turned out as important predictors of atherosclerotic progression. The significant association between LDL-c level and progression of both CAC and total plaque volume was maintained after adjusting for sex and age in multivariate analyses. Along the same lines, the LDL-c level was predictive of the patient ending up with a total plaque volume above the 75th percentile, suggesting that the LDL-c level also plays an important role in plaque progression in patients with IJD. Moreover, a higher HDL-c-level was a predictor for having a small increase in total plaque volume (<25th percentile). This finding is consistent with previous reports on the protective effect of HDL-c on atherosclerosis [39].
Atherosclerosis is a multifactorial and complex disease in which inflammation has been shown to play an important role. The pleiotropic effect of statins has shown to also reduce the inflammation markers [7], which may be beneficial in patients with systemic inflammation. In our study, both ESR and CRP were not significantly reduced from baseline to follow-up, and neither was related to plaque progression/regression during the follow up period of 4.7 years. Furthermore, we did not find an association between markers of inflammatory disease activity at baseline and progression of CAD. The latter is probably due to the fact that the patient cohort was well treated with anti-inflammatory drugs when entering the study (mostly in remission or with low disease activity). The lack of association between CAD and inflammatory markers in our study may therefor suffer from a type II error, as we may not have sufficient variations in these variables to detect statistically significant associations. We cannot exclude a type II error also in the negative associations to biologic DMARDS in present study. Only 13 patients were on biologic DMARDS which may have resulted in lack of power.
A clear limitation to our study is the absence of a placebo controlled arm of non-statin users, which would have been helpful in identification of plaque progression/regression caused by statins, especially the reported statin-effect on CAC progression. Furthermore, the loss of 15 patients to follow-up may have influenced our results, as the progression of atherosclerosis in these patients is unknown.
A recently published systematic review implies that CCTA has a potential role in assessment on the response of statin therapy on plaque volume and composition [40]. Such serial plaque assessments demand usage of the same software [41, 42]. In our study, plaque assessments were performed with a software previously shown to have a high degree of inter-observer variability on calcified and mixed lesions [23]. However; overestimation of calcified plaques due to blooming artifacts is a known limitation in CCTA [43]. Therefore we also evaluated CAC-score and number of plaques, with comparable results as with the volume-measurements. CAC is an established method with a high degree of reproducibility [44]. Importantly, the observer variability in our study was shown to be smaller than the actual change in plaque burden when comparing serial CT examinations [45].
After the 18 months follow-up in the study, the patients’ cardiovascular preventive care was transferred to the primary care physician, who was responsible for further management of the statin-treatment. A lack of control of the medicine intake and lipid-levels in the period between 1.5 and 4.7 years may have influenced our results as we have not measured sequential LDL-c levels at regular intervals during this period. However; we believe it is of clinical importance to evaluate the development of plaque progression and lipid profiles in a real-life, clinical setting. Interestingly, 50% of the patients maintained the LDL-c treatment target of ≤ 1.8 mmol/L during the follow up time, which is higher than reported from the general population [46].
In conclusion, we revealed a progression of atherosclerotic plaque volume in statin-treated patients with IJD. However, after long-term statin treatment the number of soft, unstable plaques was reduced, and the calcified plaques were more abundant. An explanation for this may be that statin treatment induced an alteration in plaque composition from mixed/soft plaques into calcified plaques in patients with IJD. Patients with recommended LDL-c levels below 1.8 mmol/L after nearly 5 years of statin-treatment, experienced a reduced atherosclerotic progression compared to patients with LDL-c levels above this treatment target. Our results support the importance of treatment to guideline recommended lipid targets in IJD patients. Longitudinal studies for assessment of the effect of statins and plaque morphology on CVD events in IJD patients are warranted.
Supporting information
(PDF)
(PDF)
(PDF)
A linear relationship between baseline soft/mixed plaque volume and change in soft/mixed plaque volume was detected (R = 0.898, p<0.001).
(TIF)
Data Availability
Due to ethical restrictions by the Norwegian Regional Committees for Medical and Health Research Ethics South East, data is only available upon request. An ethically compliant dataset will be provided by the authors who may be contacted at monasvanteson@gmail.com.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Han C, Robinson DW Jr., Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. The Journal of rheumatology. 2006;33:2167–72. [PubMed] [Google Scholar]
- 2.Hirayama A, Saito S, Ueda Y, Takayama T, Honye J, Komatsu S, et al. Qualitative and quantitative changes in coronary plaque associated with atorvastatin therapy. Circulation journal: official journal of the Japanese Circulation Society. 2009;73:718–25. [DOI] [PubMed] [Google Scholar]
- 3.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet (London, England). 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 4.Semb AG, Kvien TK, DeMicco DA, Fayyad R, Wun CC, LaRosa JC, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis and rheumatism. 2012;64:2836–46. 10.1002/art.34524 [DOI] [PubMed] [Google Scholar]
- 5.Toms TE, Panoulas VF, Douglas KM, Griffiths H, Sattar N, Smith JP, et al. Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: evidence for substantial undertreatment of lipid-associated cardiovascular risk? Annals of the rheumatic diseases. 2010;69:683–8. 10.1136/ard.2009.115717 [DOI] [PubMed] [Google Scholar]
- 6.Ikdahl E, Wibetoe G, Rollefstad S, Salberg A, Bergsmark K, Kvien TK, et al. Guideline recommended treatment to targets of cardiovascular risk is inadequate in patients with inflammatory joint diseases. International journal of cardiology. 2019;274:311–8. 10.1016/j.ijcard.2018.06.111 [DOI] [PubMed] [Google Scholar]
- 7.Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Current cardiology reviews. 2017;13:209–16. 10.2174/1573403X13666170426104611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. Journal of the American College of Cardiology. 2015;65:1273–82. 10.1016/j.jacc.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 9.Zheng G, Li Y, Huang H, Wang J, Hirayama A, Lin J. The Effect of Statin Therapy on Coronary Plaque Composition Using Virtual Histology Intravascular Ultrasound: A Meta-Analysis. PloS one. 2015;10:e0133433 10.1371/journal.pone.0133433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford, England). 2014;53:2143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross R. Atherosclerosis—an inflammatory disease. The New England journal of medicine. 1999;340:115–26. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. 10.1161/01.cir.0000053730.47739.3c [DOI] [PubMed] [Google Scholar]
- 13.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. Journal of the American College of Cardiology. 2009;54:49–57. 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 14.van Velzen JE, Schuijf JD, de Graaf FR, Boersma E, Pundziute G, Spano F, et al. Diagnostic performance of non-invasive multidetector computed tomography coronary angiography to detect coronary artery disease using different endpoints: detection of significant stenosis vs. detection of atherosclerosis. European heart journal. 2011;32:637–45. 10.1093/eurheartj/ehq395 [DOI] [PubMed] [Google Scholar]
- 15.de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, Lelieveldt BP, et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. The international journal of cardiovascular imaging. 2013;29:1177–90. 10.1007/s10554-013-0194-x [DOI] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama. 2007;297:499–508. 10.1001/jama.297.5.499 [DOI] [PubMed] [Google Scholar]
- 17.Shin S, Park HB, Chang HJ, Arsanjani R, Min JK, Kim YJ, et al. Impact of Intensive LDL Cholesterol Lowering on Coronary Artery Atherosclerosis Progression: A Serial CT Angiography Study. JACC Cardiovascular imaging. 2017;10:437–46. 10.1016/j.jcmg.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 18.Henein M, Granasen G, Wiklund U, Schmermund A, Guerci A, Erbel R, et al. High dose and long-term statin therapy accelerate coronary artery calcification. International journal of cardiology. 2015;184:581–6. 10.1016/j.ijcard.2015.02.072 [DOI] [PubMed] [Google Scholar]
- 19.Semb AG, Rollefstad S, Provan SA, Kvien TK, Stranden E, Olsen IC, et al. Carotid plaque characteristics and disease activity in rheumatoid arthritis. The Journal of rheumatology. 2013;40:359–68. 10.3899/jrheum.120621 [DOI] [PubMed] [Google Scholar]
- 20.Rollefstad S, Ikdahl E, Hisdal J, Olsen IC, Holme I, Hammer HB, et al. Rosuvastatin-Induced Carotid Plaque Regression in Patients With Inflammatory Joint Diseases: The Rosuvastatin in Rheumatoid Arthritis, Ankylosing Spondylitis and Other Inflammatory Joint Diseases Study. Arthritis & rheumatology (Hoboken, NJ). 2015;67:1718–28. [DOI] [PubMed] [Google Scholar]
- 21.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European journal of preventive cardiology. 2016;23:Np1–np96. 10.1177/2047487316653709 [DOI] [PubMed] [Google Scholar]
- 22.Svanteson M, Rollefstad S, Klow NE, Hisdal J, Ikdahl E, Semb AG, et al. Associations between coronary and carotid artery atherosclerosis in patients with inflammatory joint diseases. RMD open. 2017;3:e000544 10.1136/rmdopen-2017-000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klass O, Kleinhans S, Walker MJ, Olszewski M, Feuerlein S, Juchems M, et al. Coronary plaque imaging with 256-slice multidetector computed tomography: interobserver variability of volumetric lesion parameters with semiautomatic plaque analysis software. The international journal of cardiovascular imaging. 2010;26:711–20. 10.1007/s10554-010-9614-3 [DOI] [PubMed] [Google Scholar]
- 24.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. 10.1161/01.cir.51.4.5 [DOI] [PubMed] [Google Scholar]
- 25.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte JM, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–32. 10.1016/0735-1097(90)90282-t [DOI] [PubMed] [Google Scholar]
- 26.Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N, et al. Long-Term Prognosis After Coronary Artery Calcification Testing in Asymptomatic Patients: A Cohort Study. Annals of internal medicine. 2015;163:14–21. 10.7326/M14-0612 [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Granillo GA, Carrascosa P, Bruining N. Progression of coronary artery calcification at the crossroads: sign of progression or stabilization of coronary atherosclerosis? Cardiovascular diagnosis and therapy. 2016;6:250–8. 10.21037/cdt.2016.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology. 2013;61:1231–9. 10.1016/j.jacc.2012.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon HC, Emerick AM, Hill JA, Gjertson DW, Goldin JG. Calcium begets calcium: progression of coronary artery calcification in asymptomatic subjects. Radiology. 2002;224:236–41. 10.1148/radiol.2241011191 [DOI] [PubMed] [Google Scholar]
- 30.Shaw LJ, Narula J, Chandrashekhar Y. The never-ending story on coronary calcium: is it predictive, punitive, or protective? Journal of the American College of Cardiology. 2015;65:1283–5. 10.1016/j.jacc.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 31.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. Jama. 2014;311:271–8. 10.1001/jama.2013.282535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation. 2006;113:427–37. 10.1161/CIRCULATIONAHA.105.568147 [DOI] [PubMed] [Google Scholar]
- 33.Nakazato R, Gransar H, Berman DS, Cheng VY, Lin FY, Achenbach S, et al. Statins use and coronary artery plaque composition: results from the International Multicenter CONFIRM Registry. Atherosclerosis. 2012;225:148–53. 10.1016/j.atherosclerosis.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM, et al. Coronary Plaque Morphology and the Anti-Inflammatory Impact of Atorvastatin: A Multicenter 18F-Fluorodeoxyglucose Positron Emission Tomographic/Computed Tomographic Study. Circulation Cardiovascular imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goh VK, Lau CP, Mohlenkamp S, Rumberger JA, Achenbach S, Budoff MJ. Outcome of coronary plaque burden: a 10-year follow-up of aggressive medical management. Cardiovascular ultrasound. 2010;8:5 10.1186/1476-7120-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achenbach S, Ropers D, Pohle K, Leber A, Thilo C, Knez A, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002;106:1077–82. 10.1161/01.cir.0000027567.49283.ff [DOI] [PubMed] [Google Scholar]
- 37.Zeb I, Li D, Nasir K, Malpeso J, Batool A, Flores F, et al. Effect of statin treatment on coronary plaque progression–A serial coronary CT angiography study. Atherosclerosis. 2013;231:198–204. 10.1016/j.atherosclerosis.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 38.Clark D 3rd, Nicholls SJ, St John J, Elshazly MB, Kapadia SR, Tuzcu EM, et al. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. European heart journal. 2018. [DOI] [PubMed] [Google Scholar]
- 39.Kontush A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovascular research. 2014;103:341–9. 10.1093/cvr/cvu147 [DOI] [PubMed] [Google Scholar]
- 40.Andelius L, Mortensen MB, Norgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. European heart journal cardiovascular Imaging. 2018;19:850–8. 10.1093/ehjci/jey012 [DOI] [PubMed] [Google Scholar]
- 41.Oberoi S, Meinel FG, Schoepf UJ, Nance JW, De Cecco CN, Gebregziabher M, et al. Reproducibility of noncalcified coronary artery plaque burden quantification from coronary CT angiography across different image analysis platforms. AJR American journal of roentgenology. 2014;202:W43–9. 10.2214/AJR.13.11225 [DOI] [PubMed] [Google Scholar]
- 42.Symons R, Morris JZ, Wu CO, Pourmorteza A, Ahlman MA, Lima JA, et al. Coronary CT Angiography: Variability of CT Scanners and Readers in Measurement of Plaque Volume. Radiology. 2016;281:737–48. 10.1148/radiol.2016161670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dettmer M, Glaser-Gallion N, Stolzmann P, Glaser-Gallion F, Fornaro J, Feuchtner G, et al. Quantification of coronary artery stenosis with high-resolution CT in comparison with histopathology in an ex vivo study. European journal of radiology. 2013;82:264–9. 10.1016/j.ejrad.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 44.Sabour S, Rutten A, van der Schouw YT, Atsma F, Grobbee DE, Mali WP, et al. Inter-scan reproducibility of coronary calcium measurement using Multi Detector-Row Computed Tomography (MDCT). European journal of epidemiology. 2007;22:235–43. 10.1007/s10654-007-9123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papadopoulou SL, Garcia-Garcia HM, Rossi A, Girasis C, Dharampal AS, Kitslaar PH, et al. Reproducibility of computed tomography angiography data analysis using semiautomated plaque quantification software: implications for the design of longitudinal studies. The international journal of cardiovascular imaging. 2013;29:1095–104. 10.1007/s10554-012-0167-5 [DOI] [PubMed] [Google Scholar]
- 46.Kotseva K, De Bacquer D, De Backer G, Ryden L, Jennings C, Gyberg V, et al. Lifestyle and risk factor management in people at high risk of cardiovascular disease. A report from the European Society of Cardiology European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) IV cross-sectional survey in 14 European regions. European journal of preventive cardiology. 2016;23:2007–18. 10.1177/2047487316667784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
A linear relationship between baseline soft/mixed plaque volume and change in soft/mixed plaque volume was detected (R = 0.898, p<0.001).
(TIF)
Data Availability Statement
Due to ethical restrictions by the Norwegian Regional Committees for Medical and Health Research Ethics South East, data is only available upon request. An ethically compliant dataset will be provided by the authors who may be contacted at monasvanteson@gmail.com.