Abstract
Biological neural networks produce information backgrounds of multi-scale spontaneous activity that become more complex in brain states displaying higher capacities for cognition, for instance, attentive awake versus asleep or anesthetized states. Here, we review brain state-dependent mechanisms spanning ion channel currents (microscale) to the dynamics of brain-wide, distributed, transient functional assemblies (macroscale). Not unlike how microscopic interactions between molecules underlie structures formed in macroscopic states of matter, using statistical physics, the dynamics of microscopic neural phenomena can be linked to macroscopic brain dynamics through mesoscopic scales. Beyond spontaneous dynamics, it is observed that stimuli evoke collapses of complexity, most remarkable over high dimensional, asynchronous, irregular background dynamics during consciousness. In contrast, complexity may not be further collapsed beyond synchrony and regularity characteristic of unconscious spontaneous activity. We propose that increased dimensionality of spontaneous dynamics during conscious states supports responsiveness, enhancing neural networks' emergent capacity to robustly encode information over multiple scales.
Keywords: computational neuroscience, neural network models, mean-field models, membrane biophysics, low-dimensional manifold, cerebral cortex, coupling, desynchronized
Introduction
Brain activity transitions between healthy states, including stages of sleep, restful and aroused waking, as well as pathological states such as epilepsy, coma, and unresponsive wakefulness syndrome. From such a diversity of brain states, phenomenological categories encompassing similar spatio-temporal activity patterns can roughly, but usefully, be defined: unconscious (e.g., sleep and anesthesia) and conscious (e.g., waking and dreaming) brain states. At the macroscopic, global scale, unconscious brain states are dominated by high voltage, low frequency oscillatory brain activity related to the microscopic alternation of synchronous neuronal spiking and near silence (Steriade et al., 1993; Brown et al., 2010). Conversely, conscious states are macroscopically characterized by low voltage, high frequency, complex “disorganized” dynamics resulting from more asynchronous irregular (AI) microscopic network activity (Tsodyks and Sejnowski, 1995; Van Vreeswijk and Sompolinsky, 1996; Brunel, 2000), thought to be important for neural coding (Skarda and Freeman, 1987; Van Vreeswijk and Sompolinsky, 1996; Tononi and Edelman, 1998; Zerlaut and Destexhe, 2017).
Much as different states of matter like solids, liquids, and gases emerge from interactions between populations of molecules, different brain states may emerge from the interactions between populations of neurons. Statistical physics provides a mathematical framework to uncover structures of microscopic interactions underlying macroscopic properties. In this sense, macroscopically observed high synchrony, low complexity brain signals recorded from unconscious states may be accounted for by an increased coupling in the system's components, behaving more like a solid (Peyrache et al., 2012; Le Van Quyen et al., 2016; Olcese et al., 2016; Nghiem et al., 2018a). In contrast, conscious brain states may be described as higher complexity (Sitt et al., 2014; Engemann et al., 2018; Nghiem et al., 2018a), perhaps liquid-like.
Though quantitative expressions directly linking order and complexity are not straightforward, various definitions and metrics of complexity have been described to vary between brain states. Reports of enhanced complexity in conscious compared to unconscious states may be understood as increased dimensionality (El Boustani and Destexhe, 2010), namely the number of degrees of freedom needed to capture a system's dynamics. Intuitively, dimensionality relates, though is not reducible to, algorithmic complexity which quantifies the length of a deterministic algorithm required to reproduce an exact signal. For a random signal resulting from purely stochastic dynamics (similar to neural activity observed during conscious states), the length of the algorithm would be as long as the signal itself. In contrast, a purely oscillatory signal (reminiscent of unconscious brain dynamics) can be recapitulated by a shorter algorithm, easily described by a periodic trajectory in few dimensions.
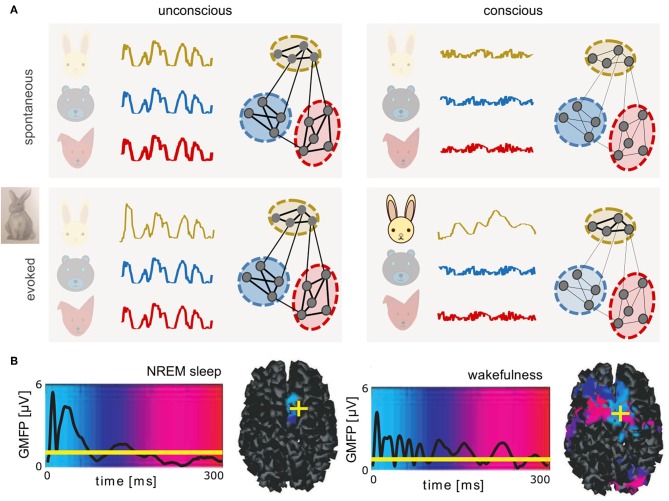
Here, we aim to connect spatial scales from microscopic (nanometers to micrometers—molecules to whole neurons) to macroscopic brain activity (centimeters to meters—brain areas to individual subjects' brains), describing both spontaneous and evoked dynamics. Toward linking interpretations of studies between scales, mesoscopic data (micrometers to millimeters—populations of thousands to tens of thousands of neurons) have been useful to inform models of neuronal assemblies. The perspective concludes by discussing a hypothesis best tested with a multi-scale understanding of brain function: the global complexity of neural activity increases in conscious brain states so as to enhance responsiveness to stimuli. We suggest responsiveness may depend on the capacity of neural networks to transiently collapse the dimensionality of collective dynamics—in particular neural assemblies sensitive to stimulus features—into evoked low-dimensional trajectories supporting neural codes (Figure 1A).
Figure 1.
Complex dynamics associated with conscious brain states provide a potential substrate for neural coding. (A) Schematics of spontaneous (top) and evoked (bottom) dynamics in connected neuronal assemblies encoding different related concepts (different colors) in unconscious (left) and conscious (right) brain states. In unconscious brain states, slow, synchronous, large amplitude oscillations are observed. Stimuli delivered during unconscious states evoke large amplitude, transient responses similar to spontaneous activity. In contrast, during conscious states, asynchronous, irregular firing of neurons results in macroscopically desynchronized, low amplitude signals. Only networks recruited by the perturbation (here, a rabbit) produce lower-dimensional patterns that propagate relatively further in time and space. (B) Global mean-field power (GMFP) recorded with EEG in response to transcranial magnetic stimulation, during deep, non-rapid eye movement (NREM) sleep versus wakefulness. Mean EEG signal is represented by black traces. Background colors represent temporal latency (light blue, 0 ms; red, 300 ms) of maximum current sources, also shown in cortical space on the right, where yellow crosses represent the location of stimulation (right dorsolateral premotor cortex). Reprinted with permission from AAAS (Massimini et al., 2005). If brain dynamics between states may be described in analogy to states of matter, perturbing unconscious brains results in large, brief signals perhaps akin to a small perturbation of a solid, which can displace the solid briefly, but will not modify its internal structure. In contrast, the same perturbation delivered during conscious, liquid-like brain states results in smaller but more complex patterns that propagate further in time and space. Under this interpretation, in coding networks, responses evoked during conscious states could represent a form of transient “crystallization,” consistent with neural trajectories lying on low-dimensional manifolds.
Macroscopic Signals Vary Robustly Between Brain States
Both spontaneous and evoked (Figures 1A,B) neural signals vary macroscopically across brain states, as demonstrated in electroencephalography (EEG), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI). In unconscious states, neural activity is dominated by low-frequency, high-amplitude signals (Niedermeyer and Lopes da Silva, 2005). Accordingly, analyses of entropy (Sitt et al., 2014; Engemann et al., 2018), complexity (Tononi and Edelman, 1998), and dimensionality (El Boustani and Destexhe, 2010) during unconscious states indicate a relative simplicity of signals compared to conscious states. In unconscious states, synchronous activity slowly sweeps across the cortex (Massimini et al., 2004) along paths formed by cortical tracts (Capone et al., 2017). In both conscious resting and unconscious states, the default mode network (Raichle et al., 2001; Boly et al., 2008) establishes a pattern of synchronization between brain areas, producing correlations in ultra-slow (< 1 Hz) dynamics (Brookes et al., 2011). Sustained, slow oscillations were initially reported in the thalamocortical system (Steriade, 2003), but are also observed experimentally in isolated cortex, without thalamus (Sanchez-Vives and McCormick, 2000; Timofeev et al., 2000). Thalamocortical connections shape slow wave dynamics (Destexhe et al., 2007; Poulet et al., 2012; David et al., 2013; Crunelli et al., 2015; Zucca et al., 2019) although slow oscillations appear to be the default state of cortical networks (Sanchez-Vives and McCormick, 2000; Sanchez-Vives et al., 2017).
Patterns of neocortical regions activated in resting state networks have been successfully retrieved using eigenmodes of the structural connectivity matrix, i.e., the possible oscillatory patterns at frequencies allowed by white matter tract lengths (Atasoy et al., 2016). In active states, the executive control network replaces the default mode (Fox et al., 2005), and the co-activation of different cortical regions is more strongly controlled by correlations in external stimuli than by white matter structural connectivity (Gilson et al., 2018), with patterns of activity propagating recurrently between low-level, sensory areas and high-level, associative areas.
During conscious states, on the background of globally disorganized neural activity, transient patterns emerge (Duncan-Johnson and Donchin, 1982; Goodin and Aminoff, 1984; Sur and Sinha, 2009; Uhlhaas et al., 2009; Luck and Kappenman, 2011; Churchland et al., 2012; Sato et al., 2012; Singer, 2013; Chemla et al., 2019). Under an interpretation of brain states in analogy to states of matter, microscopic changes in the interactions between neurons could permit the emergence of larger-scale structures in brain activity.
Microscopic Mechanisms; Biophysics of Brain States
Experiments have demonstrated that during unconscious brain states, the membrane potential (Vm) of single cells slowly oscillates between hyperpolarized and depolarized potentials associated with alternating periods of silence (Down states, also termed “OFF periods”) and AI-like firing (Up states, also termed “ON periods”) (Steriade et al., 1993) (Figure 2A). During conscious brain states, neurons show sustained but sparse and irregular AI firing patterns (Vreeswijk and Sompolinsky, 1998; Destexhe et al., 1999; Brunel, 2000; Steriade, 2000; Renart et al., 2010; Dehghani et al., 2016; di Volo and Torcini, 2018). It was found that, during AI states, excitatory (E) and inhibitory (I) synaptic inputs are near-balanced (Dehghani et al., 2016), as predicted theoretically (Van Vreeswijk and Sompolinsky, 1996). In AI states, voltage fluctuations drive neurons over the threshold for firing action potentials, resulting in irregular spiking dynamics, also known as fluctuation-driven regimes (Kuhn et al., 2004; Destexhe, 2007; Destexhe and Rudolph-Lilith, 2012). To understand mechanisms at work during fluctuation-driven dynamics, computational models have further shown that three parameters are important to capture neuronal responses in this regime, the average membrane voltage Vm, the amplitude of Vm fluctuations, and the conductance state of the membrane (Reig et al., 2015; Zerlaut et al., 2016).
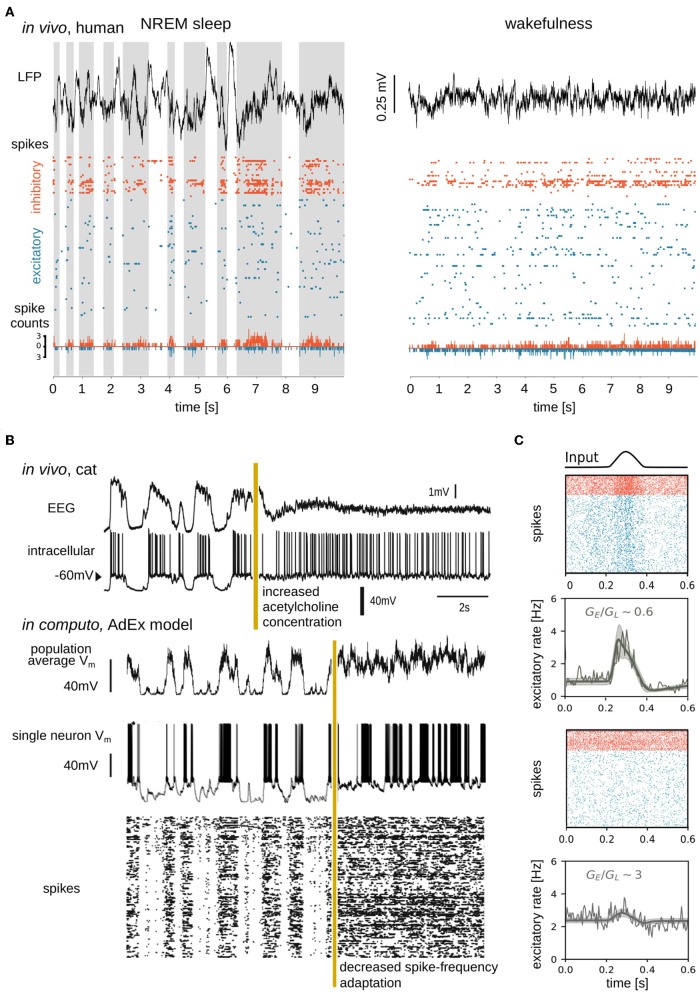
Figure 2.
Simple, high-amplitude signals in unconscious brain states are associated with synchronous regular neuronal firing, whereas complex, low-amplitude signals in conscious brain states emerge from asynchronous irregular firing. (A) Data sample from Peyrache et al. (2012), Dehghani et al. (2016), Le Van Quyen et al. (2016), Teleńczuk et al. (2017), and Nghiem et al. (2018b), containing local field potential (LFP; top), spike times (action potentials; middle), and spike counts (bottom) recorded from a human subject during NREM sleep (left) and wakefulness (right). Spikes from inhibitory (orange) and excitatory (blue) neurons were separated and spike counts were calculated in bins of 5ms. Up states shaded in the left panel. (B) Transition between slow-wave (unconscious) and activated (conscious) state dynamics in vivo (top) and in silico (bottom). Experimentally the transition is generated by electrical stimulation of acetylcholine neurons in the pedunculopontine tegmentum (PPT) of anesthetized cat (Volgushev et al., 2011), triggering awake-like, desynchronized dynamics in cortex (Rudolph et al., 2005). A prominent consequence of enhancing cholinergic signaling in cortex is a reduction of spike-frequency adaptation (McCormick, 1992). In silico, a similarly desynchronizing effect can be generated by reducing the parameter responsible for spike-frequency adaptation. Simulated traces shown in the bottom were modified from Destexhe (2009), which used a network of adaptive exponential integrate-and-fire neurons. The average Vm of the network, the Vm of a randomly chosen neuron, and the raster plot of the network are shown. Reproduced with permission from Destexhe (2009). (C) State dependence of network responsiveness. The responsiveness of two spiking networks to a Gaussian pulse is shown. Raster plots display spike times of excitatory (blue) and inhibitory (orange) neurons connected by conductance-based synapses. Population activity (spike counts, thin line), as well as mean-field model (thick lines), and standard deviation (shaded area) of population firing rate generated by a mean-field model developed in di Volo et al. (2019). Responsiveness is found to vary between different network states, obtained by changing the ratio of the time-averaged global excitatory conductance (GE) (Destexhe et al., 2003) to membrane leakage conductance (GL).
Neuromodulators, including acetylcholine, play important biological roles in modulating the membrane properties of neurons (McCormick, 1992) and thus transitions between AI and slow oscillatory dynamics through the regulation of membrane currents (Hill and Tononi, 2005). Neuromodulators are present at higher concentrations during conscious states (McCormick, 1992; Jones, 2003) and, most generally, inhibit potassium (activity-dependent and leak K+) channels, which leads to depolarization of cells and suppression of spike-frequency adaptation. At low neuromodulatory concentrations, during unconscious states, K+ leak channels are constitutively open and activity-dependent K+ channels open when neurons spike, allowing K+ ions to exit cells thus hyperpolarizing the membrane. Accumulating self-inhibition in the form of spike-frequency adaptation during Up periods results in the transition to Down states. Conversely, spike-frequency adaptation wears off during Down states, allowing noise fluctuations (present ubiquitously; Destexhe and Rudolph-Lilith, 2012) to trigger transitions to Up states (Destexhe, 2009; Jercog et al., 2017; Nghiem T.-A. E. et al., 2018; di Volo et al., 2019) (Figure 2B). Computationally speaking, for high values of spike-frequency adaptation, bistability can be observed, with solutions at firing rate zero (Down state) and non-zero (Up state) values (Holcman and Tsodyks, 2006; di Volo et al., 2019). The more chaotic dynamics of AI states associated with consciousness allows for more reliable stimulus encoding (D'Andola et al., 2017), more reliable propagation (Zerlaut and Destexhe, 2017), and more sustained responses (Nghiem T.-A. E. et al., 2018) to stimuli over time. In contrast, during unconscious states, neuronal responses are more unreliable and vary greatly depending on the stimulus amplitude and whether cells receive inputs in Up or Down periods (Rosanova and Timofeev, 2005; Reig et al., 2015).
The Ising model for spin glasses (Jaynes, 1982) fitted to neural data (Schneidman et al., 2006) has revealed divergent types of emergent neuronal dynamics in conscious and unconscious states. While neuronal interactions are pairwise in wakefulness (Nghiem et al., 2017), coupling becomes population-wide in deep sleep (Tavoni et al., 2017; Nghiem et al., 2018b). In particular, inhibitory neurons organize synchronous activity across populations (Nghiem et al., 2018b; Zanoci et al., 2019), especially during deep sleep (Peyrache et al., 2012; Le Van Quyen et al., 2016; Olcese et al., 2016) where inhibitory neurons regulate rhythms of slow wave dynamics (Compte et al., 2008; Funk et al., 2017; Zucca et al., 2017, 2019).
To summarize, between unconscious and conscious brain states, microscopic data appear intuitively related to macroscopic data: synchronous microscopic Up and Down states resulting from constitutive and activity-dependent, hyperpolarizing currents due to reduced neuromodulation correspond to relatively simple, high-amplitude macroscopic dynamics observed in unconscious states. Active, disorganized, desynchronized, AI, low adaptation, high neuromodulation conditions correspond to low amplitude, complex, conscious brain signals. On backgrounds of differing spontaneous dynamics, generalizable patterns of activity (a.k.a. neural graphoelements) are observed. Cash et al. have elegantly shown that K-complexes (graphoelements characteristic of sleep stage 2) are complementarily observed both at microscopic and macroscopic scales (Cash et al., 2009). Other identifiable patterns also begin to emerge in empirical and theoretical data, including phase cones (Freeman and Barrie, 2000) and interacting traveling waves (Sato et al., 2012; Chemla et al., 2019). Since statistical physics has successfully described neuronal interactions for different brain states, we ask next whether mesoscale methods from statistical physics can help represent spontaneous and evoked dynamics of neuronal populations, thus formally linking knowledge between micro- and macroscopic scales.
Mesoscale Bridges; Populations of Neurons
Brain dynamics at mesoscopic scales, describing thousands of neurons, are investigated empirically by electrophysiology and more recently, voltage-sensitive dyes (Arieli et al., 1996; Chemla and Chavane, 2010). At mesoscales, brain activity follows the trend of increasing complexity of spontaneous activity with consciousness (Figure 2A). Studying the effects of inputs at the mesoscale, studies have shown that perturbations during deep sleep states induce slow waves, but, during waking states, perturbations can result in chains of phase-locked activity (Pigorini et al., 2015) leading to causal global interactions (Rosanova et al., 2018).
Mean-field models offer a formalism for scaling up microscopic detail to collective macroscopic dynamics using few equations, offering a computational advantage for simulations. In describing states of matter, mean-field models simplify the probabilistic behavior of molecules to the relatively more predictable behavior of macroscopic states (Kadanoff, 2009). A rich literature has begun to develop mean-field models of neuronal populations, showing that global variables describing population activity can be usefully derived from the biophysics of neurons and their interactions (Ohira and Cowan, 1993; Ginzburg and Sompolinsky, 1994; El Boustani and Destexhe, 2009; Buice et al., 2010; Dahmen et al., 2016). Mean-field models have qualitatively reproduced temporal features of spontaneous dynamics including AI (El Boustani and Destexhe, 2009), Up and Down dynamics (Compte et al., 2003; Jercog et al., 2017; Tartaglia and Brunel, 2017; di Volo et al., 2019), and transitions between these states (di Volo et al., 2019; Tort-Colet et al., 2019). In addition, connecting mean-fields provides a tool for simulating the propagation of patterns through time and space, across mesoscale structures. For example, recent work deriving mean-field models of networks with conductance-based synapses has reproduced the suppressive interaction between traveling waves observed in visual cortex during conscious states, a biological phenomenon that could not be captured by current-based networks (Chemla et al., 2019).
Mean-field models have highlighted that, while complicated to apply mathematically in the framework of conductance-based models (di Volo et al., 2019), voltage-dependent interactions constitute a significant non-linearity in the membrane evolution equations. Voltage-dependent interactions appear to be important for explaining non-trivial responses of biological neurons, through the mean and fluctuations of the cells' membrane voltage (Reig et al., 2015). In fact, while these results do not imply that differences in responsiveness are due only to conductances, they show that voltage dependent synapses play a role in the nonlinear state-dependent response of a neural network. As shown in Figure 2C, various levels of membrane conductance, regulated by voltage-dependent synapses, are shown to differently shape population responses.
Finally, renormalization group theory, a method of coarse-graining microscopic detail to obtain macroscopic laws helping to understand how order can emerge from apparent disorder (Wilson, 1979; Cardy, 1996; Goldenfeld, 2018) has recently begun to be applied to neural assemblies (Meshulam et al., 2019), laying further foundation for the formal connection of our understanding of brain function across scales.
Discussion
In this paper, we briefly reviewed work on the measurement and modeling of brain states at different scales, from single neurons to cell assemblies and global brain activity, considering both spontaneous and evoked dynamics. In particular, we highlighted that increased complexity in the dynamics of conscious brain states relates to changes in single-neuron biophysics, tuned by neuromodulation. In unconscious states, reduced neuromodulation promotes activity-dependent self-inhibition of excitatory neurons as they spike, leading to alternating, synchronous transients of silence and firing, that produce high-amplitude, low-complexity, synchronous signals, on resonant frequencies of the structural connectome. During conscious states, neuronal discharges are asynchronous, irregular and fluctuation-driven, resulting from sustained membrane depolarization in cortical neurons, promoting effective neural communication.
Beyond conscious and unconscious categories proposed here for the sake of brevity, important differences exist within categories of unconscious and conscious states (Brown et al., 2010; El Boustani and Destexhe, 2010; Nghiem et al., 2018a). Unlike healthy wakefulness and sleep, epileptic networks display both excessively high conductance and strongly synchronized, regular dynamics (El Boustani and Destexhe, 2010). Further, brain signals in coma are both low-amplitude and low-complexity, in contrast to high-amplitude signals observed in other unconscious states, but also to complex signals observed in conscious states (El Boustani and Destexhe, 2010). Such anomalous deviations from the overall trend of coordinated changes in complexity and amplitude may illuminate mechanisms underlying disease-causing deviations from healthy brain states (Mackey and Glass, 1977).
To characterize brain states, it has been useful to consider not only spontaneous dynamics but also patterns evoked by perturbations. It was found that macroscopic responsiveness highly depends on brain state and different patterns of responses are evoked in conscious versus unconscious states (Massimini et al., 2005). Such state-dependent responsiveness can also be seen at the level of local networks in vivo and in silico, for example in the different reliability of responses to perturbations given during Up and Down periods of slow waves (Reig et al., 2015; Zerlaut and Destexhe, 2017). In simulations, different responsiveness could be accounted for by three parameters: membrane voltage, voltage fluctuation amplitude, and membrane conductance (Reig et al., 2015). These parameters could be well described by mean-field models (di Volo et al., 2019), able to capture fundamental properties of spontaneous dynamics and also state-dependent responses at mesoscales. As such, the data-driven coupling of such mean-field models may serve as natural candidates for modeling the emergence of mesoscopic and macroscopic-scale patterns.
Transient collapses of dimensionality found in encoding networks were also discussed as substrates potentially supporting neural codes. Such collapses in complexity have been observed in active ensembles at scales spanning microscopic (Churchland et al., 2010; Fairhall, 2019) to macroscopic (Quiroga et al., 2001; Zang et al., 2004) activity. This echoes recent work studying recordings of neural populations which highlighted that neural representations of stimuli may lie on low-dimensional manifolds (Churchland et al., 2012; Sadtler et al., 2014; Gallego et al., 2017; Zhao and Park, 2017; Golub et al., 2018; Chaudhuri et al., 2019; Recanatesi et al., 2019; Stringer et al., 2019). Indeed neurons do not fire independently, which would yield dynamics of dimensionality as high as the number of neurons, but instead follow constrained trajectories of activity that can be captured by descriptions of much lower dimensionality that depend on spontaneous and evoked dynamics. For example, a neural population firing in synchrony could be fully described by a periodic orbit trajectory constrained to a low-dimensional space (Churchland et al., 2012). Since spontaneous global network activity increases in dimensionality during conscious states, we ask whether the transient collapse of complexity in specific networks, translating the emergence of simpler dynamical structures from disorder, may be associated to neural codes.
As an analogy, windmills facing all in one direction display low complexity, but can only be synchronously active or inactive. Windmills facing in random directions, in contrast, are a higher complexity configuration able to represent wind from any direction through the activity of a subset. The activity of an ensemble of windmills tuned to a particular direction of wind could represent a collapse of complexity and the generation of information by that subset (in this case, about the direction of wind). Similarly, enhanced dimensionality associated with conscious states could subserve neural information through the collapse of complexity in neural assemblies tuned to encode particular representations.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Simone Blanco Malerba, Cristiano Capone, Stephen E. Clarke, Damien Depannemaecker, Anton Filipchuk, Enrique Hansen, J. N. K. Jaynes, Vicente Medel, Archibald Parsons, Mattias Peuvrier, Wolf Singer, Kat Uesat, Bahar Hazal Yalçnkaya, and Yann Zerlaut for useful discussion of the manuscript.
Footnotes
Funding. This research was supported by the Centre National de la Recherche Scientifique (CNRS), the European Community (Human Brain Project, H2020-785907), and by École des Neurosciences de Paris (ENP).
References
- Arieli A., Sterkin A., Grinvald A., Aertsen A. (1996). Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273, 1868–1871. 10.1126/science.273.5283.1868 [DOI] [PubMed] [Google Scholar]
- Atasoy S., Donnelly I., Pearson J. (2016). Human brain networks function in connectome-specific harmonic waves. Nat. Commun. 7:10340. 10.1038/ncomms10340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Phillips C., Tshibanda L., Vanhaudenhuyse A., Schabus M., Dang-Vu T. T., et al. (2008). Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann. N. Y. Acad. Sci. 1129:119 10.1196/annals.1417.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes M. J., Woolrich M., Luckhoo H., Price D., Hale J. R., Stephenson M. C., et al. (2011). Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl. Acad. Sci. U.S.A. 108, 16783–16788. 10.1073/pnas.1112685108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. N., Lydic R., Schiff N. D. (2010). General anesthesia, sleep, and coma. New Engl. J. Med. 363, 2638–2650. 10.1056/NEJMra0808281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 8, 183–208. 10.1023/A:1008925309027 [DOI] [PubMed] [Google Scholar]
- Buice M. A., Cowan J. D., Chow C. C. (2010). Systematic fluctuation expansion for neural network activity equations. Neural Comput. 22, 377–426. 10.1162/neco.2009.02-09-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone C., Rebollo B., Muñoz A., Illa X., Del Giudice P., Sanchez-Vives M. V., et al. (2017). Slow waves in cortical slices: how spontaneous activity is shaped by laminar structure. Cereb. Cortex 29, 319–335. 10.1093/cercor/bhx326 [DOI] [PubMed] [Google Scholar]
- Cardy J. (1996). Scaling and Renormalization in Statistical Physics. Vol. 5. Cambridge: Cambridge University Press. [Google Scholar]
- Cash S. S., Halgren E., Dehghani N., Rossetti A. O., Thesen T., Wang C., et al. (2009). The human k-complex represents an isolated cortical down-state. Science 324, 1084–1087. 10.1126/science.1169626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R., Gerçek B., Pandey B., Peyrache A., Fiete I. (2019). The intrinsic attractor manifold and population dynamics of a canonical cognitive circuit across waking and sleep. Nat. Neurosci. 22, 1512–1520. 10.1038/s41593-019-0460-x [DOI] [PubMed] [Google Scholar]
- Chemla S., Chavane F. (2010). Voltage-sensitive dye imaging: technique review and models. J. Physiol. 104, 40–50. 10.1016/j.jphysparis.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Chemla S., Reynaud A., di Volo M., Zerlaut Y., Perrinet L., Destexhe A., et al. (2019). Suppressive traveling waves shape representations of illusory motion in primary visual cortex of awake primate. J. Neurosci. 39, 4282–4298. 10.1523/JNEUROSCI.2792-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland M. M., Byron M. Y., Cunningham J. P., Sugrue L. P., Cohen M. R., Corrado G. S., et al. (2010). Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat. Neurosci. 13, 369–378. 10.1038/nn.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland M. M., Cunningham J. P., Kaufman M. T., Foster J. D., Nuyujukian P., Ryu S. I., et al. (2012). Neural population dynamics during reaching. Nature 487, 51–56. 10.1038/nature11129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A., Reig R., Descalzo V. F., Harvey M. A., Puccini G. D., Sanchez-Vives M. V. (2008). Spontaneous high-frequency (10–80 hz) oscillations during up states in the cerebral cortex in vitro. J. Neurosci. 28, 13828–13844. 10.1523/JNEUROSCI.2684-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A., Sanchez-Vives M. V., McCormick D. A., Wang X.-J. (2003). Cellular and network mechanisms of slow oscillatory activity (<1 HZ) and wave propagations in a cortical network model. J. Neurophysiol. 89, 2707–2725. 10.1152/jn.00845.2002 [DOI] [PubMed] [Google Scholar]
- Crunelli V., David F., Lőrincz M. L., Hughes S. W. (2015). The thalamocortical network as a single slow wave-generating unit. Curr. Opin. Neurobiol. 31, 72–80. 10.1016/j.conb.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Dahmen D., Bos H., Helias M. (2016). Correlated fluctuations in strongly coupled binary networks beyond equilibrium. Phys. Rev. X 6:031024 10.1103/PhysRevX.6.031024 [DOI] [Google Scholar]
- D'Andola M., Rebollo B., Casali A. G., Weinert J. F., Pigorini A., Villa R., et al. (2017). Bistability, causality, and complexity in cortical networks: an in vitro perturbational study. Cereb. Cortex 28, 2233–2242. 10.1093/cercor/bhx122 [DOI] [PubMed] [Google Scholar]
- David F., Schmiedt J. T., Taylor H. L., Orban G., Di Giovanni G., Uebele V. N., et al. (2013). Essential thalamic contribution to slow waves of natural sleep. J. Neurosci. 33, 19599–19610. 10.1523/JNEUROSCI.3169-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N., Peyrache A., Telenczuk B., Le Van Quyen M., Halgren E., Cash S. S., et al. (2016). Dynamic balance of excitation and inhibition in human and monkey neocortex. Sci. Rep. 6:23176. 10.1038/srep23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A. (2007). High-conductance state. Scholarpedia 2:1341 10.4249/scholarpedia.1341 [DOI] [Google Scholar]
- Destexhe A. (2009). Self-sustained asynchronous irregular states and up–down states in thalamic, cortical and thalamocortical networks of nonlinear integrate-and-fire neurons. J. Comput. Neurosci. 27, 493–506. 10.1007/s10827-009-0164-4 [DOI] [PubMed] [Google Scholar]
- Destexhe A., Contreras D., Steriade M. (1999). Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci. 19, 4595–4608. 10.1523/JNEUROSCI.19-11-04595.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A., Hughes S. W., Rudolph M., Crunelli V. (2007). Are corticothalamic ‘up'states fragments of wakefulness? Trends Neurosci. 30, 334–342. 10.1016/j.tins.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A., Rudolph M., Paré D. (2003). The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 4, 739–751. 10.1038/nrn1198 [DOI] [PubMed] [Google Scholar]
- Destexhe A., Rudolph-Lilith M. (2012). Neuronal Noise. New York, NY: Springer. [Google Scholar]
- di Volo M., Romagnoni A., Capone C., Destexhe A. (2019). Biologically realistic mean-field models of conductance-based networks of spiking neurons with adaptation. Neural Comput. 31, 653–680. 10.1162/neco_a_01173 [DOI] [PubMed] [Google Scholar]
- di Volo M., Torcini A. (2018). Transition from asynchronous to oscillatory dynamics in balanced spiking networks with instantaneous synapses. Phys. Rev. Lett. 121:128301 10.1103/PhysRevLett.121.128301 [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C. C., Donchin E. (1982). The p300 component of the event-related brain potential as an index of information processing. Biol. Psychol. 14, 1–52. 10.1016/0301-0511(82)90016-3 [DOI] [PubMed] [Google Scholar]
- El Boustani S., Destexhe A. (2009). A master equation formalism for macroscopic modeling of asynchronous irregular activity states. Neural Comput. 21, 46–100. 10.1162/neco.2009.02-08-710 [DOI] [PubMed] [Google Scholar]
- El Boustani S., Destexhe A. (2010). Brain dynamics at multiple scales: can one reconcile the apparent low-dimensional chaos of macroscopic variables with the seemingly stochastic behavior of single neurons? Int. J. Bifur. Chaos 20, 1687–1702. 10.1142/S0218127410026769 [DOI] [Google Scholar]
- Engemann D. A., Raimondo F., King J.-R., Rohaut B., Louppe G., Faugeras F., et al. (2018). Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 141, 3179–3192. 10.1093/brain/awy251 [DOI] [PubMed] [Google Scholar]
- Fairhall A. L. (2019). Whither variability? Nat. Neurosci. 22, 329–330. 10.1038/s41593-019-0344-0 [DOI] [PubMed] [Google Scholar]
- Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., Raichle M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102, 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W. J., Barrie J. M. (2000). Analysis of spatial patterns of phase in neocortical gamma EEGs in rabbit. J. Neurophysiol. 84, 1266–1278. 10.1152/jn.2000.84.3.1266 [DOI] [PubMed] [Google Scholar]
- Funk C. M., Peelman K., Bellesi M., Marshall W., Cirelli C., Tononi G. (2017). Role of somatostatin-positive cortical interneurons in the generation of sleep slow waves. J. Neurosci. 37, 9132–9148. 10.1523/JNEUROSCI.1303-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego J. A., Perich M. G., Miller L. E., Solla S. A. (2017). Neural manifolds for the control of movement. Neuron 94, 978–984. 10.1016/j.neuron.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson M., Deco G., Friston K. J., Hagmann P., Mantini D., Betti V., et al. (2018). Effective connectivity inferred from fMRI transition dynamics during movie viewing points to a balanced reconfiguration of cortical interactions. Neuroimage 180, 534–546. 10.1016/j.neuroimage.2017.09.061 [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Sompolinsky H. (1994). Theory of correlations in stochastic neural networks. Phys. Rev. E 50, 3171–3191. 10.1103/PhysRevE.50.3171 [DOI] [PubMed] [Google Scholar]
- Goldenfeld N. (2018). Lectures on Phase Transitions and the Renormalization Group. Boca Raton, FL: CRC Press. [Google Scholar]
- Golub M. D., Sadtler P. T., Oby E. R., Quick K. M., Ryu S. I., Tyler-Kabara E. C., et al. (2018). Learning by neural reassociation. Nat. Neurosci. 21, 607–616. 10.1038/s41593-018-0095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin D. S., Aminoff M. J. (1984). The relationship between the evoked potential and brain events in sensory discrimination and motor response. Brain 107, 241–251. 10.1093/brain/107.1.241 [DOI] [PubMed] [Google Scholar]
- Hill S., Tononi G. (2005). Modeling sleep and wakefulness in the thalamocortical system. J. Neurophysiol. 93, 1671–1698. 10.1152/jn.00915.2004 [DOI] [PubMed] [Google Scholar]
- Holcman D., Tsodyks M. (2006). The emergence of up and down states in cortical networks. PLoS Comput. Biol. 2:e23. 10.1371/journal.pcbi.0020023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes E. T. (1982). On the rationale of maximum-entropy methods. Proc. IEEE 70, 939–952. 10.1109/PROC.1982.12425 [DOI] [Google Scholar]
- Jercog D., Roxin A., Bartho P., Luczak A., Compte A., de la Rocha J. (2017). Up-down cortical dynamics reflect state transitions in a bistable network. Elife 6:e22425. 10.7554/eLife.22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. E. (2003). Arousal systems. Front. Biosci. 8:438–451. 10.2741/1074 [DOI] [PubMed] [Google Scholar]
- Kadanoff L. P. (2009). More is the same; phase transitions and mean field theories. J. Stat. Phys. 137:777 10.1007/s10955-009-9814-1 [DOI] [Google Scholar]
- Kuhn A., Aertsen A., Rotter S. (2004). Neuronal integration of synaptic input in the fluctuation-driven regime. J. Neurosci. 24, 2345–2356. 10.1523/JNEUROSCI.3349-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M., Muller L. E., Telenczuk B., Halgren E., Cash S., Hatsopoulos N. G., et al. (2016). High-frequency oscillations in human and monkey neocortex during the wake–sleep cycle. Proc. Natl. Acad. Sci. U.S.A. 113, 9363–9368. 10.1073/pnas.1523583113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. J., Kappenman E. S. (2011). The Oxford Handbook of Event-Related Potential Components. Oxford: Oxford University Press. [Google Scholar]
- Mackey M. C., Glass L. (1977). Oscillation and chaos in physiological control systems. Science 197, 287–289. 10.1126/science.267326 [DOI] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Huber R., Esser S. K., Singh H., Tononi G. (2005). Breakdown of cortical effective connectivity during sleep. Science 309, 2228–2232. 10.1126/science.1117256 [DOI] [PubMed] [Google Scholar]
- Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G. (2004). The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870. 10.1523/JNEUROSCI.1318-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A. (1992). Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 39, 337–388. 10.1016/0301-0082(92)90012-4 [DOI] [PubMed] [Google Scholar]
- Meshulam L., Gauthier J. L., Brody C. D., Tank D. W., Bialek W. (2019). Coarse graining, fixed points, and scaling in a large population of neurons. Phys. Rev. Lett. 123:178103. 10.1103/PhysRevLett.123.178103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem T.-A., Lina J.-M., di Volo M., Capone C., Evans A. C., Destexhe A., et al. (2018a). State equation from the spectral structure of human brain activity. arXiv [preprint]. arXiv:1806.07365. [Google Scholar]
- Nghiem T.-A., Marre O., Destexhe A., Ferrari U. (2017). Pairwise ising model analysis of human cortical neuron recordings, in International Conference on Geometric Science of Information (New York, NY: Springer; ), 257–264. 10.1007/978-3-319-68445-1_30 [DOI] [Google Scholar]
- Nghiem T.-A., Telenczuk B., Marre O., Destexhe A., Ferrari U. (2018b). Maximum-entropy models reveal the excitatory and inhibitory correlation structures in cortical neuronal activity. Phys. Rev. E 98:012402. 10.1103/PhysRevE.98.012402 [DOI] [PubMed] [Google Scholar]
- Nghiem T.-A. E., Tort-Colet N., Gorski T., Ferrari U., Moghimyfiroozabad S., Goldman J. S., et al. (2018). Cholinergic switch between two different types of slow waves in cerebral cortex. bioRxiv [Preprint]. 10.1101/430405 [DOI] [PubMed] [Google Scholar]
- Niedermeyer E., Lopes da Silva F. H. (2005). Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Ohira T., Cowan J. D. (1993). Master-equation approach to stochastic neurodynamics. Phys. Rev. E 48:2259. 10.1103/physreve.48.2259 [DOI] [PubMed] [Google Scholar]
- Olcese U., Bos J. J., Vinck M., Lankelma J. V., van Mourik-Donga L. B., Schlumm F., et al. (2016). Spike-based functional connectivity in cerebral cortex and hippocampus: loss of global connectivity is coupled to preservation of local connectivity during non-REM sleep. J. Neurosci. 36, 7676–7692. 10.1523/JNEUROSCI.4201-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A., Dehghani N., Eskandar E. N., Madsen J. R., Anderson W. S., Donoghue J. A., et al. (2012). Spatiotemporal dynamics of neocortical excitation and inhibition during human sleep. Proc. Natl. Acad. Sci. U.S.A. 109, 1731–1736. 10.1073/pnas.1109895109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigorini A., Sarasso S., Proserpio P., Szymanski C., Arnulfo G., Casarotto S., et al. (2015). Bistability breaks-off deterministic responses to intracortical stimulation during non-rem sleep. Neuroimage 112, 105–113. 10.1016/j.neuroimage.2015.02.056 [DOI] [PubMed] [Google Scholar]
- Poulet J. F., Fernandez L. M., Crochet S., Petersen C. C. (2012). Thalamic control of cortical states. Nat. Neurosci. 15, 370–372. 10.1038/nn.3035 [DOI] [PubMed] [Google Scholar]
- Quiroga R. Q., Rosso O. A., Başar E., Schürmann M. (2001). Wavelet entropy in event-related potentials: a new method shows ordering of EEG oscillations. Biol. Cybern. 84, 291–299. 10.1007/s004220000212 [DOI] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanatesi S., Ocker G. K., Buice M. A., Shea-Brown E. (2019). Dimensionality in recurrent spiking networks: global trends in activity and local origins in connectivity. PLoS Comput. Biol. 15:e1006446. 10.1371/journal.pcbi.1006446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R., Zerlaut Y., Vergara R., Destexhe A., Sanchez-Vives M. V. (2015). Gain modulation of synaptic inputs by network state in auditory cortex in vivo. J. Neurosci. 35, 2689–2702. 10.1523/JNEUROSCI.2004-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A., De La Rocha J., Bartho P., Hollender L., Parga N., Reyes A., et al. (2010). The asynchronous state in cortical circuits. Science 327, 587–590. 10.1126/science.1179850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M., Fecchio M., Casarotto S., Sarasso S., Casali A., Pigorini A., et al. (2018). Sleep-like cortical off-periods disrupt causality and complexity in the brain of unresponsive wakefulness syndrome patients. Nat. Commun. 9:4427. 10.1038/s41467-018-06871-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M., Timofeev I. (2005). Neuronal mechanisms mediating the variability of somatosensory evoked potentials during sleep oscillations in cats. J. Physiol. 562, 569–582. 10.1113/jphysiol.2004.071381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M., Pelletier J.-G., Paré D., Destexhe A. (2005). Characterization of synaptic conductances and integrative properties during electrically-induced EEG-activated states in neocortical neurons in vivo. J. Neurophysiol. 94, 2805–2821. 10.1152/jn.01313.2004 [DOI] [PubMed] [Google Scholar]
- Sadtler P. T., Quick K. M., Golub M. D., Chase S. M., Ryu S. I., Tyler-Kabara E. C., et al. (2014). Neural constraints on learning. Nature 512, 423–426. 10.1038/nature13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives M. V., Massimini M., Mattia M. (2017). Shaping the default activity pattern of the cortical network. Neuron 94, 993–1001. 10.1016/j.neuron.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives M. V., McCormick D. A. (2000). Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 3, 1027–1034. 10.1038/79848 [DOI] [PubMed] [Google Scholar]
- Sato T. K., Nauhaus I., Carandini M. (2012). Traveling waves in visual cortex. Neuron 75, 218–229. 10.1016/j.neuron.2012.06.029 [DOI] [PubMed] [Google Scholar]
- Schneidman E., Berry II M. J., Segev R., Bialek W. (2006). Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440, 1007–1012. 10.1038/nature04701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. (2013). Cortical dynamics revisited. Trends Cogn. Sci. 17, 616–626. 10.1016/j.tics.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Sitt J. D., King J.-R., El Karoui I., Rohaut B., Faugeras F., Gramfort A., et al. (2014). Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 137, 2258–2270. 10.1093/brain/awu141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarda C. A., Freeman W. J. (1987). How brains make chaos in order to make sense of the world. Behav. Brain Sci. 10, 161–173. 10.1017/S0140525X00047336 [DOI] [Google Scholar]
- Steriade M. (2000). Corticothalamic resonance, states of vigilance and mentation. Neuroscience 101, 243–276. 10.1016/S0306-4522(00)00353-5 [DOI] [PubMed] [Google Scholar]
- Steriade M. (2003). Neuronal Substrates of Sleep and Epilepsy. Cambridge: Cambridge University Press. [Google Scholar]
- Steriade M., Nunez A., Amzica F. (1993). A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265. 10.1523/JNEUROSCI.13-08-03252.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C., Pachitariu M., Steinmetz N., Carandini M., Harris K. D. (2019). High-dimensional geometry of population responses in visual cortex. Nature 571, 361–365. 10.1038/s41586-019-1346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S., Sinha V. (2009). Event-related potential: an overview. Ind. Psychiatry J. 18, 70–73. 10.4103/0972-6748.57865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia E. M., Brunel N. (2017). Bistability and up/down state alternations in inhibition-dominated randomly connected networks of lif neurons. Sci. Rep. 7:11916 10.1038/s41598-017-12033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoni G., Ferrari U., Battaglia F. P., Cocco S., Monasson R. (2017). Functional coupling networks inferred from prefrontal cortex activity show experience-related effective plasticity. Netw. Neurosci. 1, 275–301. 10.1162/NETN_a_00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleńczuk B., Dehghani N., Le Van Quyen M., Cash S. S., Halgren E., Hatsopoulos N. G., et al. (2017). Local field potentials primarily reflect inhibitory neuron activity in human and monkey cortex. Sci. Rep. 7:40211. 10.1038/srep40211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I., Grenier F., Bazhenov M., Sejnowski T., Steriade M. (2000). Origin of slow cortical oscillations in deafferented cortical slabs. Cereb. Cortex 10, 1185–1199. 10.1093/cercor/10.12.1185 [DOI] [PubMed] [Google Scholar]
- Tononi G., Edelman G. M. (1998). Consciousness and complexity. Science 282, 1846–1851. 10.1126/science.282.5395.1846 [DOI] [PubMed] [Google Scholar]
- Tort-Colet N., Capone C., Sanchez-Vives M. V., Mattia M. (2019). Attractor competition enriches cortical dynamics during awakening from anesthesia. bioRxiv [Preprint]. 10.1101/517102 [DOI] [PubMed] [Google Scholar]
- Tsodyks M. V., Sejnowski T. (1995). Rapid state switching in balanced cortical network models. Network 6, 111–124. 10.1088/0954-898X_6_2_001 [DOI] [Google Scholar]
- Uhlhaas P., Pipa G., Lima B., Melloni L., Neuenschwander S., Nikolić D., et al. (2009). Neural synchrony in cortical networks: history, concept and current status. Front. Integr. Neurosci. 3:17. 10.3389/neuro.07.017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vreeswijk C., Sompolinsky H. (1996). Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274, 1724–1726. 10.1126/science.274.5293.1724 [DOI] [PubMed] [Google Scholar]
- Volgushev M., Chauvette S., Timofeev I. (2011). Long-range correlation of the membrane potential in neocortical neurons during slow oscillation, in Progress in Brain Research, Vol. 193 (Amsterdam: Elsevier; ), 181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeswijk C. V., Sompolinsky H. (1998). Chaotic balanced state in a model of cortical circuits. Neural Comput. 10, 1321–1371. 10.1162/089976698300017214 [DOI] [PubMed] [Google Scholar]
- Wilson K. G. (1979). Problems in physics with many scales of length. Sci. Am. 241, 158–179. 10.1038/scientificamerican0879-158 [DOI] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zanoci C., Dehghani N., Tegmark M. (2019). Ensemble inhibition and excitation in the human cortex: an ising-model analysis with uncertainties. Phys. Rev. E 99:032408. 10.1103/PhysRevE.99.032408 [DOI] [PubMed] [Google Scholar]
- Zerlaut Y., Destexhe A. (2017). Enhanced responsiveness and low-level awareness in stochastic network states. Neuron 94, 1002–1009. 10.1016/j.neuron.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Zerlaut Y., Teleńczuk B., Deleuze C., Bal T., Ouanounou G., Destexhe A. (2016). Heterogeneous firing rate response of mouse layer V pyramidal neurons in the fluctuation-driven regime. J. Physiol. 594, 3791–3808. 10.1113/JP272317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Park I. M. (2017). Variational latent gaussian process for recovering single-trial dynamics from population spike trains. Neural Comput. 29, 1293–1316. 10.1162/NECO_a_00953 [DOI] [PubMed] [Google Scholar]
- Zucca S., D'Urso G., Pasquale V., Vecchia D., Pica G., Bovetti S., et al. (2017). An inhibitory gate for state transition in cortex. Elife 6:e26177. 10.7554/eLife.26177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca S., Pasquale V., de Leon Roig P. L., Panzeri S., Fellin T. (2019). Thalamic drive of cortical parvalbumin-positive interneurons during down states in anesthetized mice. Curr. Biol. 29, 1481–1490. 10.1016/j.cub.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]