Abstract
Transition metal-catalyzed enantioselective Sonogashira-type oxidative C(sp3)—C(sp) coupling of unactivated C(sp3)−H bonds with terminal alkynes has remained a prominent challenge. The difficulties mainly stem from the regiocontrol in unactivated C(sp3)—H bond functionalization and the inhibition of readily occurring Glaser homocoupling of terminal alkynes. Here, we report a copper/chiral cinchona alkaloid-based N,N,P-ligand catalyst for asymmetric oxidative cross-coupling of unactivated C(sp3)—H bonds with terminal alkynes in a highly regio-, chemo-, and enantioselective manner. The use of N-fluoroamide as a mild oxidant is essential to site-selectively generate alkyl radical species while efficiently avoiding Glaser homocoupling. This reaction accommodates a range of (hetero)aryl and alkyl alkynes; (hetero)benzylic and propargylic C(sp3)−H bonds are all applicable. This process allows expedient access to chiral alkynyl amides/aldehydes. More importantly, it also provides a versatile tool for the construction of chiral C(sp3)—C(sp), C(sp3)—C(sp2), and C(sp3)—C(sp3) bonds when allied with follow-up transformations.
Subject terms: Asymmetric catalysis, Asymmetric synthesis, Synthetic chemistry methodology
Enantioselective oxidative cross-coupling of unactivated C(sp3)−H bonds and terminal alkynes is challenging. Here, the authors developed a copper/cinchona alkaloid catalyst for the asymmetric Sonogashira-type alkynylation of C(sp3)-H bonds via radical intermediates.
Introduction
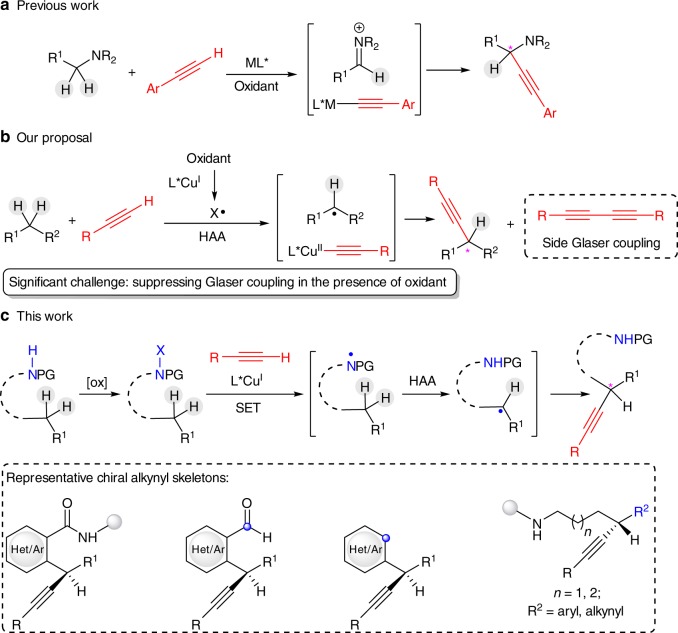
As one of the most fundamental motifs in organic chemistry, chiral alkynes play an essential role in biology, medicinal chemistry, and material science1,2. They also serve as vital synthetic precursors for many functionalities such as alkanes, alkenes, aldehydes, carboxylic acids, and heterocycles on both laboratory and industrial scales1,2. Accordingly, a variety of catalytic methods have been developed to enantioselectively deliver chiral C(sp3)—C(sp) bonds3–13. From the viewpoint of step- and atom-economy, a direct enantioselective alkynylation of a C(sp3)−H bond would be highly appealing14–16. Thus, directed palladium-catalyzed C−H activation has been shown to be an effective strategy for C(sp3)−H alkynylation17–19. However, only a few enantioselective examples have been disclosed with the expensive alkynyl bromide/iodine as the alkynylation reagents16,20–22. In comparison, an enantioselective Sonogashira-type cross-dehydrogenative coupling (CDC) of C(sp3)−H bonds with low-cost terminal alkynes would be more ideal due to the ready availability of both coupling partners. In this respect, Li and others have pioneered in establishing enantioselective alkynylation of C(sp3)−H bonds adjacent to nitrogen with terminal alkynes23–32. The stereochemical control was elegantly implemented via chiral Lewis acid-catalyzed nucleophilic addition to the in situ generated iminium ions (Fig. 1a)23–32. However, the enantioselective oxidative cross-coupling of common unactivated C(sp3)−H bonds poses a significant challenge to this strategy. Two major difficulties have to be overcome: (1) The site-selective generation of carbocation species from such unactivated C(sp3)−H bonds is relatively difficult compared with that adjacent to nitrogen. (2) The chemo- and enantiocontrol of the non-heteroatom-stabilized carbocations are also more challenging due to their inherently high reactivity. Clearly, a conceptually different approach is highly desirable to achieve the enantioselective oxidative cross-coupling of unactivated C(sp3)−H bonds and terminal alkynes.
Fig. 1. Sonogashira-type enantioselective oxidative cross-coupling of C(sp3)−H bonds with terminal alkynes.
a Previous ionic-type dehydrogenative coupling of C(sp3)—H bonds adjacent to nitrogen. b Our proposal: tandem HAA and copper-catalyzed Sonogashira-type coupling. c Amide-directed enantioselective coupling of C(sp3)−H bonds with terminal alkynes. HAA hydrogen atom abstraction, [ox] oxidant, SET single-electron transfer.
Enantioselective C(sp3)−H bond functionalization by merging a site-selective hydrogen atom abstraction (HAA) process with asymmetric copper-catalyzed cross-coupling has received much attention over the past several years33–42. At the same time, we have recently developed a copper/cinchona alkaloid-based N,N-ligand catalyst, which could intimately associate with alkyl radical species for realization of asymmetric alkene difunctionalization43–46. Inspired by these works, we wondered if this catalyst system would be also suitable for tandem site-selective HAA on unactivated C(sp3)−H bonds and enantioselective coupling with terminal alkynes (Fig. 1b). However, common oxidants for HAA readily lead to copper-catalyzed Glaser homocoupling of terminal alkynes47,48. To this end, we have been encouraged by the mild oxidation power of N-halogenated amides employed in recent remote C(sp3)−H functionalization transformations49–59 based on the classic Hofmann–Löffler–Freytag60,61 and Barton62 reactions. More importantly, the corresponding amidyl radicals49–51 commonly exhibit robust HAA reactivity and site-selectivity. Thus, we questioned if a removable directing amide group would be viable for sequential N-oxidation and asymmetric copper-catalyzed Sonogashira-type coupling of unactivated C−H bonds with terminal alkynes.
Herein, we describe our efforts toward the development of radical asymmetric oxidative cross-coupling of unactivated C(sp3)−H bonds with terminal alkynes enabled by copper(I)/cinchona alkaloid-based N,N,P-ligand catalysis. Notably, this protocol not only provides a range of chiral alkynyl amides and alkynyl aldehydes (Fig. 1c) but also, together with further transformations, offers a general way for chiral C(sp3)–C(sp), C(sp3)–C(sp2), and C(sp3)–C(sp3) bond construction.
Results
Reaction optimization
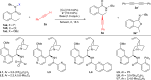
To verify our hypothesis, N-halo-amides 1aa and 1ab as well as O-acylhydroxylamide 1ac were prepared, which all can generate the amidyl radical via single-electron reduction by a Cu(I) catalyst54,56. Initial treatment of N-fluoroamide 1aa with phenylacetylene 2a and Cs2CO3 in the presence of CuI and our designed bidentate quinine-derived sulfonamide ligands43 L1 and L2 did not provide any products (entries 1 and 2, Table 1). We tentatively ascribed the results to the insufficient reducing capability of Cu(I) catalysts. Thus, we speculated that an additional coordinative amine or phosphine moieties on the ligands might help increase the electron-density on copper and enhance its reducing power. As such, cinchona alkaloid-derived Nakamura’s tridentate N,N,N-ligand63 L3 and Dixon’s N,N,P-ligand64 L5 as well as their analogs L4 and L6, respectively, were examined. And most of them led to the desired product 3a with promising enantioselectivity (entries 3–6, Table 1). In contrast, N-chloroamide 1ab and O-acylhydroxylamide 1ac only afforded Glaser homocoupling product 3a′ (entries 7 and 8, Table 1) under such reaction conditions, indicating the importance of the chosen amidyl radical precursor. Further screening of Cu(I) catalysts and solvents (entries 9–15, Table 1) proved that CuI in Tetrahydrofuran (THF) was the best (88% yield and 94% ee; entry 12, Table 1). An evaluation of base indicated significant impacts on the efficiency and selectivity (entries 16–18, Table 1) and Cs2CO3 was found to be particularly effective to inhibit the formation of side product 3a″. Lowering the catalyst loading affected the yield but not the enantioselectivity (entries 19 and 20, Table 1). Control experiments showed that both the N,N,P-ligand and the alkyne were necessary for efficient amide consumption (entries 21–23, Table 1). Thus, both of them are required for the copper center to efficiently reduce the mild N–F oxidant.
Table 1.
Screening of reaction conditionsa.
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | 1 | [Cu] | Base | L* | Solvent | Yield (%, 3a, 3a′, 3a″)b | Ee (%)c |
| 1 | 1aa | CuI | Cs2CO3 | L1 | Dichloromethane (DCM) | –d, 0, 0 | – |
| 2 | 1aa | CuI | Cs2CO3 | L2 | DCM | –d, 0, 0 | – |
| 3 | 1aa | CuI | Cs2CO3 | L3 | DCM | <5, 0, 0 | 6 |
| 4 | 1aa | CuI | Cs2CO3 | L4 | DCM | 8, 0, 0 | 74 |
| 5 | 1aa | CuI | Cs2CO3 | L5 | DCM | 53, 0, –d | 68 |
| 6 | 1aa | CuI | Cs2CO3 | L6 | DCM | 15, 0, 0 | 51 |
| 7 | 1ab | CuI | Cs2CO3 | L5 | DCM | –d, 48, 0 | – |
| 8 | 1ac | CuI | Cs2CO3 | L5 | DCM | 0, 0, –d | – |
| 9 | 1aa | CuI | Cs2CO3 | L5 | 1,2-Dichloroethane | 50, 0, –d | 76 |
| 10 | 1aa | CuI | Cs2CO3 | L5 | Benzene | 77, 0, 5 | 64 |
| 11 | 1aa | CuI | Cs2CO3 | L5 | EtOAc | 92, 0, 0 | 91 |
| 12 | 1aa | CuI | Cs2CO3 | L5 | THF | 88, 0, 0 | 94 |
| 13 | 1aa | CuBr | Cs2CO3 | L5 | THF | 64, 0, 0 | 93 |
| 14 | 1aa | CuTc | Cs2CO3 | L5 | THF | 91, 0, 0 | 92 |
| 15 | 1aa | CuOAc | Cs2CO3 | L5 | THF | 80, 0, 0, | 94 |
| 16 | 1aa | CuI | Na2CO3 | L5 | THF | <5, 0, 81 | 85 |
| 17 | 1aa | CuI | K2CO3 | L5 | THF | 27, 0, 62 | 93 |
| 18 | 1aa | CuI | KOtBu | L5 | THF | 71, 0, 26 | 93 |
| 19e | 1aa | CuI | Cs2CO3 | L5 | THF | 66, 0, 0 | 94 |
| 20f | 1aa | CuI | Cs2CO3 | L5 | THF | 31, 0, 0 | 92 |
| 21 | 1aa | CuI | Cs2CO3 | – | THF | 0, 0, 0 | – |
| 22g | 1aa | CuI | Cs2CO3 | – | THF | 0, 0, 0 | – |
| 23g | 1aa | CuI | Cs2CO3 | L5 | THF | 0, 0, 0 | – |
aReaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), [Cu] (10 mol%), L* (10 mol%), and base (0.1 mmol) in dry solvent (1.2 mL) at room temperature (rt) for 16 h
bYield based on 1H NMR analysis of the crude product using CH2Br2 as an internal standard
cEe values based on HPLC analysis
dA trace amount of product
eCuI (5 mol%), L* (5 mol%) for 24 h
fCuI (2 mol%), L* (2 mol%) for 36 h
gWithout 2a
Substrate scope
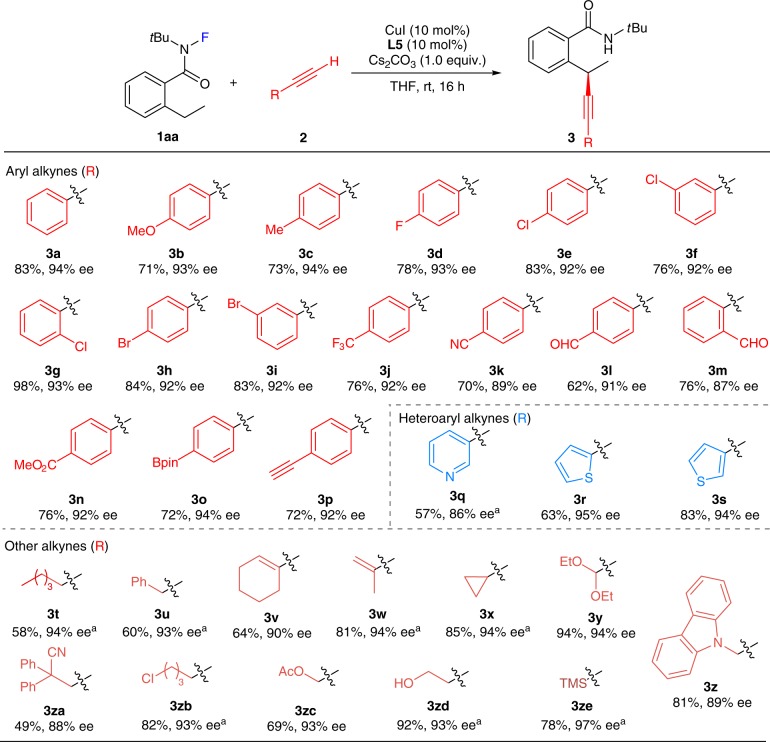
With the optimized conditions in hand, the scope of alkynes was next explored (Fig. 2). A series of aryl alkynes, including those having electron-donating or -withdrawing groups at different positions (ortho, meta, or para) of phenyl rings, reacted smoothly to afford 3a–3p in 62–98% yield with 87–94% ee. Many functional groups, such as methoxyl (3b), halo (3d–3i), trifluoromethyl (3j), cyano (3k), formyl (3l and 3m), methoxylcarbonyl (3n), pinacolborato (3o), and terminal alkynyl (3p), were all compatible with the reaction conditions. Furthermore, a range of heteroaryl alkynes, such as 3-pyridinyl, 2-thiophenyl, and 3-thiophenyl, all worked well to deliver 3q–3s in good yields with excellent enantioselectivity. We were especially pleased to find that alkyl alkynes were also competent coupling partners. For example, the aliphatic alkynes underwent the reaction to give 3t and 3u with good results in the presence of 2 equivalents of Cs2CO3. A wide range of functional groups, such as conjugating alkenyl (3v and 3w), cyclopropanyl (3x), acetal (3y), carbazole (3z), cyano (3za), primary chloride (3zb), ester (3zc), and even hydroxy (3zd) groups, at different distances away from the reacting alkynes were well tolerated, therefore giving products in excellent enantioselectivity. In addition, a silyl alkyne was also applicable to provide 3ze in 78% yield with 97% ee.
Fig. 2. Substrate scope of alkynes.
Standard conditions: 1aa (0.2 mmol), alkyne (0.4 mmol), CuI (10 mol%), L5 (10 mol%), and Cs2CO3 (1.0 equiv.) in THF (2.4 mL) at rt for 16 h. Isolated yield based on 1aa is given. Ee values are based on HPLC analysis. aCuTc (15 mol%), L5 (10 mol%), and Cs2CO3 (2.0 equiv.) in THF at rt for 24 h. Bpin pinacolborato, TMS trimethylsilyl.
The scope of N-fluorocarboxamides bearing secondary benzylic C(sp3)−H bonds was next evaluated (Fig. 3a). Simple alkyl-substituted substrates worked well to give alkynylation products 3zf–3zh with excellent regio- and enantioselectivity. Noteworthy is that a terminal alkenyl group was tolerated under the reaction conditions to provide 3zi in 78% yield with 96% ee. A range of electron-donating or -withdrawing substituents at different positions of the backbone phenyl rings were well accommodated to deliver 3zj–3zn in 55–85% yield with 90–93% ee. In addition, direct alkynylation of the α-C−H bond of a thiophene heterocycle also proceeded smoothly to give 3zo in 69% yield with 88% ee. The N-tBu group could be replaced with another tertiary alkyl group without any interferences on either yield or enantioselectivity (3zp). The above method could also be extended to more remote benzylic C−H bonds via a 1,6-HAA56,65–67 process to give alkynylated amide 3zq in 69% yield with 89% ee.
Fig. 3. Substrate scope of N-fluorocarboxamides.
a The reaction is compatible with a variety of (hetero)aryl-fused N-fluorocarboxamide substrates. b Linear N-fluorocarboxamides are also applicable under slightly modified conditions. aStandard conditions: 1 (0.2 mmol), alkyne (0.4 mmol), CuI (10 mol%), L5 (10 mol%), and Cs2CO3 (1.0 equiv.) in THF (2.4 mL) at rt for 16 h. bIsolated yield based on 1. cEe values based on HPLC analysis. dL6 (10 mol%) was used in DCM at rt for 24 h. eL7 (Table 1, 10 mol%) was used in CHCl3 at rt for 24 h.
To further test the compatibility of the reaction in more structurally diverse contexts, an N-benzoylated amine with a tethered phenyl ring was evaluated and the desired product 3zr was obtained in 72% yield and 91% ee (Fig. 3b). Noteworthy is that the reaction is not restricted to benzylic or heterobenzylic C(sp3)–H bond functionalization. For example, various propargylic C(sp3)–H bonds were amenable to this transformation, therefore providing 3zs−3zu in moderate to high enantioselectivity. Interestingly, a substrate containing only simple alkyl δ-C−H bonds also underwent the reaction to deliver 3zv in excellent regioselectivity, albeit with no enantioselectivity. The reaction is currently under further optimization for potential enantiocontrol in our laboratory.
Straightforward transformation
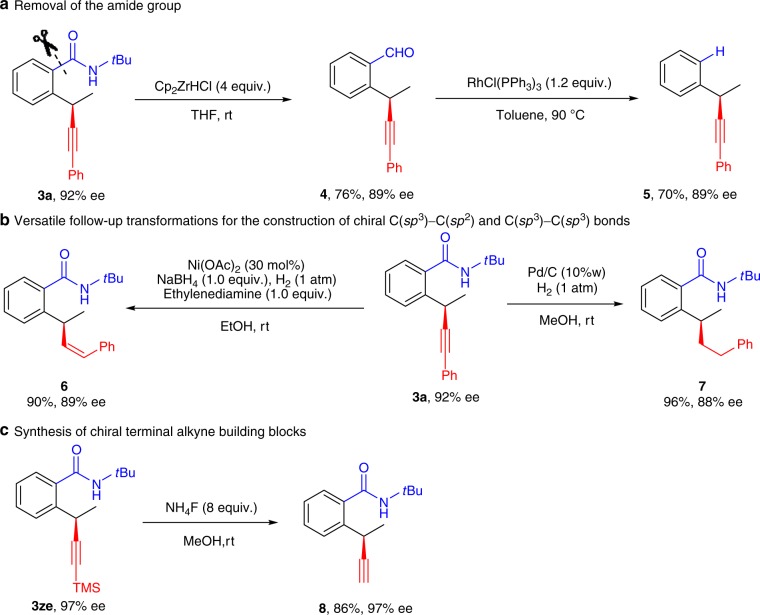
One feature of this strategy is the requirement for an amide directing group. In fact, the amide group in product 3a was readily removed by sequential treatment with the Schwartz’s reagent and the Wilkinson’s catalyst in two steps (Fig. 4a). In this sense, this protocol provides an indirect approach for enantioselective Sonogashira-type oxidative coupling. The absolute configuration of 5 was determined to be R by comparing its HPLC spectrum and optical rotation with those reported in literature68,69. Another feature of this protocol is the reactive alkyne moieties in products, which could be readily converted to a Z-alkene group in 6 and a saturated alkyl group in 7 upon hydrogenation to different extents (Fig. 4b), respectively. Therefore, it provides a versatile and complementary tool to other direct methods for the construction of chiral C(sp3)–C(sp2) and C(sp3)–C(sp3) bonds. In addition, the TMS group in 3ze could be straightforwardly unmasked to provide the chiral terminal alkyne 8 in 86% yield without any loss of enantioselectivity (Fig. 4c). Such enantioenriched terminal alkynes are valuable chiral building blocks for a range of transformations.
Fig. 4. Straightforward transformation.
a The directing amide group was readily removed by sequential amide reduction to aldehyde and decarbonylation. b The essential alkyne moiety in the product was straightforwardly transformed into Z-alkene and alkane featuring chiral C(sp3)–C(sp2) and C(sp3)–C(sp3) bonds. c Silyl alkyne was easily converted to terminal alkyne, thus providing valuable chiral building blocks.
Mechanism investigation
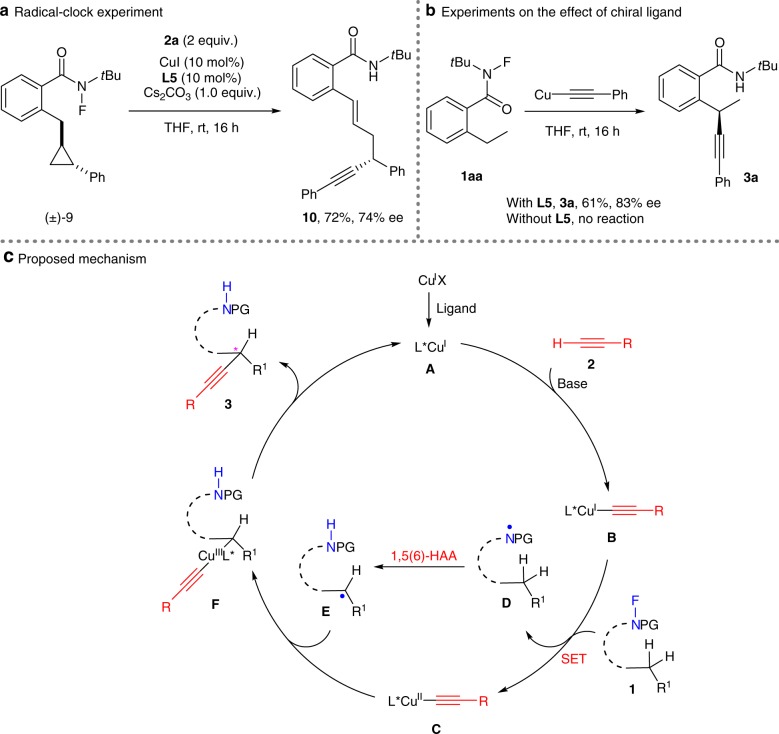
To gain some insight into the reaction mechanism, control experiments with radical inhibitors 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and butylated hydroxytoluene (BHT) were conducted, respectively, indicating reaction inhibition (Supplementary Fig. 1a). Moreover, a radical clock experiment showed that substrate 9 underwent a tandem cyclopropane ring-opening/alkyne trapping process to provide 10 in 72% yield with 74% ee (Fig. 5a). These observations support the possible formation of alkyl radical species from in situ-generated amidyl radical species via 1,5(6)-HAA processes. Deuterium-labeling experiments indicated an intramolecular kinetic isotope effect (KIE) value of 1.94 and an intermolecular KIE value of 1.16. Thus, the 1,5-HAA process might be not involved in the rate-determining step(s) (Supplementary Fig. 1b and 1c)70. In addition, only in the presence of L5 could copper phenylacetylide initiate the reaction (Fig. 5b). This result, together with the aforementioned effects of ligand and phenylacetylene for efficient amide consumption (entries 21–23, Table 1), demonstrates that the complex of copper phenylacetylide with the chiral ligand might be the initial complex to start this reaction.
Fig. 5. Mechanistic investigations.
a The radical-clock substrate (±)-9 underwent ring opening before the C–C bond formation, thus indicating the initial generation of a benzylic radical. b The reaction of 1aa with copper acetylide did not occur in the absence of L5, indicating that both chiral ligand and terminal alkyne are indispensable for reaction initiation. c The reaction was proposed to proceed through sequential single-electron reduction of substrate 1, 1,5(6)-HAA, and copper-catalyzed C(sp3)–C(sp) coupling.
On the basis of abovementioned observations and previous reports38–46,52–59, a plausible mechanism was tentatively proposed, as shown in Fig. 5c. Initially, CuIX reacts with chiral ligand and terminal alkyne in the presence of base, giving chiral Cu(I) acetylide complex B. Subsequent single-electron transfer (SET) of B with N-fluorocarboxamide 1 results in the formation of Cu(II) acetylide complex C and the amidyl radical D. The amidyl radical then undergoes intramolecular 1,5(6)-HAA to generate alkyl radical species E. Next, C(sp3)–C(sp) coupling via reductive elimination of a Cu(III) intermediate F33–42 gives rise to enantioenriched product 3 and chiral Cu(I) complex A.
Discussion
We have discovered copper/cinchona alkaloid-based N,N,P-ligand catalysts to accomplish a radical asymmetric oxidative C(sp3)–C(sp) cross-coupling of unactivated C(sp3)−H bonds and terminal alkynes. The utilization of a removable amide group to direct the site-selective formation of alkyl radical species via a HAA process is crucial to the success of the transformation. In addition, the use of N-fluorocarboxamides as mild amidyl radical precursors is critical for inhibiting the Glaser homocoupling. Further, the strategic utilization of chiral cinchona alkaloid-derived N,N,P-ligands proved to be essential for eliciting the initial SET between copper and the mild N-fluorocarboxamides oxidant while imparting excellent enantiodiscrimination in the final C–C coupling step. This strategy allows for the facile assembly of chiral alkynyl amides/aldehydes and also provides a generally robust tool for the construction of chiral C(sp3)–C(sp), C(sp3)–C(sp2), and C(sp3)–C(sp3) bonds. Further studies toward the development of direct enantioselective oxidative Sonogashira-type coupling of unactivated C(sp3)−H bonds with terminal alkynes are ongoing in our laboratory.
Methods
General procedure A
This procedure applies to compounds 3a–p, 3r, 3s, 3v, 3y–za, 3zc, 3zf–zq. Under argon atmosphere, an oven-dried resealable Schlenk tube equipped with a magnetic stir bar was charged with CuI (3.8 mg, 0.020 mmol, 10 mol%), L5 (12.2 mg, 0.020 mmol, 10 mol%), Cs2CO3 (65.2 mg, 0.20 mmol, 1.0 equiv.), and anhydrous THF (2.4 mL). Then, N-fluorocarboxamide (0.20 mmol, 1.0 equiv.) and alkyne (0.40 mmol, 2.0 equiv.) were sequentially added into the mixture and the reaction mixture was stirred at (rt) for 16 h. Upon completion (monitored by thin-layer chromatography (TLC)), the precipitate was filtered off and washed by DCM. The filtrate was evaporated and the residue was purified by column chromatography on silica gel to afford the desired product.
General procedure B
This procedure applies to compounds 3q, 3t, 3u, 3w, 3x, 3zb, 3zd, 3ze. Under argon atmosphere, an oven-dried resealable Schlenk tube equipped with a magnetic stir bar was charged with CuTc (5.7 mg, 0.030 mmol, 15 mol%), L5 (12.2 mg, 0.020 mmol, 10 mol%), Cs2CO3 (130.4 mg, 0.40 mmol, 2.0 equiv.), and anhydrous THF (2.4 mL). Then, N-fluorocarboxamide (0.20 mmol, 1.0 equiv.) and alkyne (0.40 mmol, 2.0 equiv.) were sequentially added into the mixture and the reaction mixture was stirred at rt for 24 h. Upon completion (monitored by TLC), the precipitate was filtered off and washed by DCM. The filtrate was evaporated and the residue was purified by column chromatography on silica gel to afford the desired product.
General procedure C
This procedure applies to compounds 3zr, 3zs, 3zu, 3v. Under argon atmosphere, an oven-dried resealable Schlenk tube equipped with a magnetic stir bar was charged with CuI (3.8 mg, 0.020 mmol, 10 mol%), L6 (16.7 mg, 0.020 mmol, 10 mol%), Cs2CO3 (65.2 mg, 0.20 mmol, 1.0 equiv.), and anhydrous DCM (2.4 mL). Then, N-fluorocarboxamide (0.20 mmol, 1.0 equiv.) and alkyne (0.40 mmol, 2.0 equiv.) were sequentially added into the mixture and the reaction mixture was stirred at rt for 24 h. Upon completion (monitored by TLC), the precipitate was filtered off and washed by DCM. The filtrate was evaporated and the residue was purified by column chromatography on silica gel to afford the desired product.
General procedure D
This procedure applies to compound 3zt. Under argon atmosphere, an oven-dried resealable Schlenk tube equipped with a magnetic stir bar was charged with CuI (3.8 mg, 0.020 mmol, 10 mol%), L7 (13.9 mg, 0.020 mmol, 10 mol%), Cs2CO3 (65.2 mg, 0.20 mmol, 1.0 equiv.), and anhydrous chloroform (2.4 mL). Then, N-fluorocarboxamide (0.20 mmol, 1.0 equiv.) and alkyne (0.40 mmol, 2.0 equiv.) were sequentially added into the mixture and the reaction mixture was stirred at rt for 24 h. Upon completion (monitored by TLC), the precipitate was filtered off and washed by DCM. The filtrate was evaporated and the residue was purified by column chromatography on silica gel to afford the desired product.
For nuclear magnetic resonance and high-performance liquid chromatography spectra, see Supplementary Figures.
Supplementary information
Acknowledgements
Financial support from the National Natural Science Foundation of China (Nos. 21722203, 21831002, 21801116, and 21804066), Shenzhen special funds for the development of biomedicine, internet, new energy, and new material industries (No. JCYJ 20180302174416591), Shenzhen Nobel Prize Scientists Laboratory Project (No. C17783101), and Shandong Provincial Natural Science Foundation (No. ZR2017BB065) is appreciated.
Author contributions
Z.-H.Z., X.-Y.Do, and X.-Y.Du. performed the experiments. Q.-S.G. and Z.-L.L. helped with characterizing some new compounds. X.-Y.L. conceived and directed the project and wrote the paper. All authors discussed the results and commented on the manuscript.
Data availability
Experimental procedure and characterization data of new compounds are available within Supplementary Information. Any further relevant data are available from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Andrew Parsons and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhen-Hua Zhang, Xiao-Yang Dong, Xuan-Yi Du.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13705-1.
References
- 1.Diederich, F., Stang, P. J. & Tykwinski, R. R. (eds) Acetylene Chemistry: Chemistry, Biology, and Material Science (Wiley-VCH, Weinheim, 2005).
- 2.Trost, B. M. & Li, C.-J. (eds) Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations (Wiley-VCH, 2015).
- 3.Chen Q, et al. Copper/guanidine-catalyzed asymmetric alkynylation of isatins. Angew. Chem. Int. Ed. 2016;55:5286–5289. doi: 10.1002/anie.201600711. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z-X, Bai X-Y, Li B-J. Recent progress of transition-metal-catalyzed enantioselective hydroalkynylation of alkenes. Synlett. 2017;28:509–514. doi: 10.1055/s-0036-1588392. [DOI] [Google Scholar]
- 5.Vaillant, F. L. & Waser, J. Alkynylation of radicals: spotlight on the “third way” to transfer triple bonds. Chem. Sci. 10, 8909–8923 (2019). [DOI] [PMC free article] [PubMed]
- 6.Dabrowski JA, Gao F, Hoveyda AH. Enantioselective synthesis of alkyne-substituted quaternary carbon stereogenic centers through NHC−Cu-catalyzed allylic substitution reactions with (i-Bu)2(alkynyl)aluminum reagents. J. Am. Chem. Soc. 2011;133:4778–4781. doi: 10.1021/ja2010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada A, Makida Y, Sato T, Ohmiya H, Sawamura M. Copper-catalyzed enantioselective allylic alkylation of terminal alkyne pronucleophiles. J. Am. Chem. Soc. 2014;136:13932–13939. doi: 10.1021/ja5084333. [DOI] [PubMed] [Google Scholar]
- 8.Cui X-Y, et al. (Guanidine)copper complex-catalyzed enantioselective dynamic kinetic allylic alkynylation under biphasic condition. J. Am. Chem. Soc. 2018;140:8448–8455. doi: 10.1021/jacs.7b12806. [DOI] [PubMed] [Google Scholar]
- 9.Fu L, Zhou S, Wan X, Chen P, Liu G. Enantioselective trifluoromethylalkynylation of alkenes via copper-catalyzed radical relay. J. Am. Chem. Soc. 2018;140:10965–10969. doi: 10.1021/jacs.8b07436. [DOI] [PubMed] [Google Scholar]
- 10.Bai X-Y, Wang Z-X, Li B-J. Iridium-catalyzed enantioselective hydroalkynylation of enamides for the synthesis of homopropargyl amides. Angew. Chem. Int. Ed. 2016;55:9007–9011. doi: 10.1002/anie.201601792. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z-X, Bai X-Y, Yao H-C, Li B-J. Synthesis of amides with remote stereocenters by catalytic asymmetric γ-alkynylation of α,β-unsaturated amides. J. Am. Chem. Soc. 2016;138:14872–14875. doi: 10.1021/jacs.6b10415. [DOI] [PubMed] [Google Scholar]
- 12.Bai X-Y, Zhang W-W, Li Q, Li B-J. Highly enantioselective synthesis of propargyl amides through Rh-catalyzed asymmetric hydroalkynylation of enamides: scope, mechanism, and origin of selectivity. J. Am. Chem. Soc. 2018;140:506–514. doi: 10.1021/jacs.7b12054. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z-X, Li B-J. Construction of acyclic quaternary carbon stereocenters by catalytic asymmetric hydroalkynylation of unactivated alkenes. J. Am. Chem. Soc. 2019;141:9312–9320. doi: 10.1021/jacs.9b03027. [DOI] [PubMed] [Google Scholar]
- 14.Davies HML, Beckwith REJ. Catalytic enantioselective C–H activation by means of metal–carbenoid-induced C–H insertion. Chem. Rev. 2003;103:2861–2903. doi: 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]
- 15.Newton CG, Wang S-G, Oliveira CC, Cramer N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev. 2017;117:8908–8976. doi: 10.1021/acs.chemrev.6b00692. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Denis TG, Zhu R-Y, Chen G, Wu Q-F, Yu J-Q. Enantioselective C(sp3)−H bond activation by chiral transition metal catalysts. Science. 2018;359:eaao4798. doi: 10.1126/science.aao4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ano Y, Tobisu M, Chatani N. Palladium-catalyzed direct ethynylation of C(sp3)−H bonds in aliphatic carboxylic acid derivatives. J. Am. Chem. Soc. 2011;133:12984–12986. doi: 10.1021/ja206002m. [DOI] [PubMed] [Google Scholar]
- 18.He J, Wasa M, Chan KSL, Yu J-Q. Palladium(0)-catalyzed alkynylation of C(sp3)−H bonds. J. Am. Chem. Soc. 2013;135:3387–3390. doi: 10.1021/ja400648w. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, et al. Palladium-catalyzed stereoretentive olefination of unactivated C(sp3)−H bonds with vinyl iodides at room temperature: synthesis of β-vinyl α-amino acids. Org. Lett. 2014;16:6260–6263. doi: 10.1021/ol503248f. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q-F, et al. Formation of α-chiral centers by asymmetric β-C(sp3)−H arylation, alkenylation, and alkynylation. Science. 2017;355:499–503. doi: 10.1126/science.aal5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerhaoui S, Djukic J-P, Wencel-Delord J, Colobert F. Asymmetric, nearly barrierless C(sp3)−H activation promoted by easily-accessible N-protected aminosulfoxides as new chiral ligands. ACS Catal. 2019;9:2532–2542. doi: 10.1021/acscatal.8b04946. [DOI] [Google Scholar]
- 22.Han Y-Q, et al. Pd(II)-catalyzed enantioselective alkynylation of unbiased methylene C(sp3)−H bonds using 3,3′-fluorinated-BINOL as a chiral ligand. J. Am. Chem. Soc. 2019;141:4558–4563. doi: 10.1021/jacs.9b01124. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Bohle DS, Li C-J. Cu-catalyzed cross-dehydrogenative coupling: a versatile strategy for C–C bond formations via the oxidative activation of sp3 C–H bonds. Proc. Natl Acad. Sci. USA. 2006;103:8928–8933. doi: 10.1073/pnas.0601687103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C-J. Cross-dehydrogenative coupling (CDC): exploring C–C bond formations beyond functional group transformations. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 25.Girard SA, Knauber T, Li C-J. The cross-dehydrogenative coupling of Csp³–H bonds: a versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Li C-J. CuBr-catalyzed efficient alkynylation of sp3 C–H bonds adjacent to a nitrogen atom. J. Am. Chem. Soc. 2004;126:11810–11811. doi: 10.1021/ja0460763. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Li C-J. Catalytic enantioselective alkynylation of prochiral sp3 C–H bonds adjacent to a nitrogen atom. Org. Lett. 2004;6:4997–4999. doi: 10.1021/ol047814v. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Macleod PD, Li C-J. Studies on Cu-catalyzed asymmetric alkynylation of tetrahydroisoquinoline derivatives. Tetrahedron Asymmetry. 2006;17:590–597. doi: 10.1016/j.tetasy.2006.02.007. [DOI] [Google Scholar]
- 29.Sun S, Li C, Floreancig PE, Lou H, Liu L. Highly enantioselective catalytic cross-dehydrogenative coupling of N-carbamoyl tetrahydroisoquinolines and terminal alkynes. Org. Lett. 2015;17:1684–1687. doi: 10.1021/acs.orglett.5b00447. [DOI] [PubMed] [Google Scholar]
- 30.Perepichka I, Kundu S, Hearne Z, Li C-J. Efficient merging of copper and photoredox catalysis for the asymmetric cross-dehydrogenative-coupling of alkynes and tetrahydroisoquinolines. Org. Biomol. Chem. 2015;13:447–451. doi: 10.1039/C4OB02138J. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z, Liu X, Liu L. Copper-catalyzed aerobic enantioselective cross-dehydrogenative coupling of N-aryl glycine esters with terminal alkynes. Org. Lett. 2016;18:2982–2985. doi: 10.1021/acs.orglett.6b01328. [DOI] [PubMed] [Google Scholar]
- 32.Huang T, et al. Asymmetric aerobic oxidative cross-coupling of tetrahydroisoquinolines with alkynes. ACS Catal. 2017;7:5654–5660. doi: 10.1021/acscatal.7b01912. [DOI] [Google Scholar]
- 33.Choi J, Fu GC. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science. 2017;356:eaaf7230. doi: 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu GC. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 2017;3:692–700. doi: 10.1021/acscentsci.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Q, Glorius F. Radical enantioselective C(sp3)−H functionalization. Angew. Chem. Int. Ed. 2017;56:49–51. doi: 10.1002/anie.201609105. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Chen P, Liu G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 2018;51:2036–2046. doi: 10.1021/acs.accounts.8b00265. [DOI] [PubMed] [Google Scholar]
- 37.Loup J, Dhawa U, Pesciaioli F, Wencel-Delord J, Ackermann L. Enantioselective C–H activation with earth-abundant 3d transition metals. Angew. Chem. Int. Ed. 2019;58:12803–12818. doi: 10.1002/anie.201904214. [DOI] [PubMed] [Google Scholar]
- 38.Kharasch MS, Sosnovsky G. The reactions of t-butyl perbenzoate and olefins—a stereospecific reaction. J. Am. Chem. Soc. 1958;80:756–756. doi: 10.1021/ja01536a062. [DOI] [Google Scholar]
- 39.Eames J, Watkinson M. Catalytic allylic oxidation of alkenes using an asymmetric Kharasch–Sosnovsky reaction. Angew. Chem. Int. Ed. 2001;40:3567–3571. doi: 10.1002/1521-3773(20011001)40:19<3567::AID-ANIE3567>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science. 2016;353:1014–1018. doi: 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Wu L, Chen P, Liu G. Enantioselective arylation of benzylic C–H bonds by copper-catalyzed radical relay. Angew. Chem. Int. Ed. 2019;58:6425–6429. doi: 10.1002/anie.201902191. [DOI] [PubMed] [Google Scholar]
- 42.Yu P, et al. Enantioselective C–H bond functionalization triggered by radical trifluoromethylation of unactivated alkene. Angew. Chem. Int. Ed. 2014;53:11890–11894. doi: 10.1002/anie.201405401. [DOI] [PubMed] [Google Scholar]
- 43.Li X-T, Gu Q-S, Dong X-Y, Meng X, Liu X-Y. A copper catalyst with a cinchona-alkaloid-based sulfonamide ligand for asymmetric radical oxytrifluoromethylation of alkenyl oximes. Angew. Chem. Int. Ed. 2018;57:7668–7672. doi: 10.1002/anie.201804315. [DOI] [PubMed] [Google Scholar]
- 44.Lin J-S, et al. A dual-catalytic strategy to direct asymmetric radical aminotrifluoromethylation of alkenes. J. Am. Chem. Soc. 2016;138:9357–9360. doi: 10.1021/jacs.6b04077. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Y-F, Dong X-Y, Gu Q-S, Yu Z-L, Liu X-Y. Achiral pyridine ligand-enabled enantioselective radical oxytrifluoromethylation of alkenes with alcohols. Angew. Chem. Int. Ed. 2017;56:8883–8886. doi: 10.1002/anie.201702925. [DOI] [PubMed] [Google Scholar]
- 46.Lin J-S, et al. Cu/Chiral phosphoric acid-catalyzed asymmetric three-component radical-initiated 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 2019;141:1074–1083. doi: 10.1021/jacs.8b11736. [DOI] [PubMed] [Google Scholar]
- 47.Glaser C. Beiträge zur Kenntnifs des acetenylbenzols. Ber. Dtsch. Chem. Ges. 1869;2:422–424. doi: 10.1002/cber.186900201183. [DOI] [Google Scholar]
- 48.Kamata K, Yamaguchi S, Kotani M, Yamaguchi K, Mizuno N. Efficient oxidative alkyne homocoupling catalyzed by a monomeric dicopper-substituted silicotungstate. Angew. Chem. Int. Ed. 2008;47:2407–2410. doi: 10.1002/anie.200705126. [DOI] [PubMed] [Google Scholar]
- 49.Xiong T, Zhang Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 2016;45:3069–3087. doi: 10.1039/C5CS00852B. [DOI] [PubMed] [Google Scholar]
- 50.Kärkäs MD. Photochemical generation of nitrogen-centered amidyl, hydrazonyl, and imidyl radicals: methodology developments and catalytic applications. ACS Catal. 2017;7:4999–5022. doi: 10.1021/acscatal.7b01385. [DOI] [Google Scholar]
- 51.Stateman LM, Nakafuku KM, Nagib DA. Remote C–H functionalization via selective hydrogen atom transfer. Synthesis. 2018;50:1569–1586. doi: 10.1055/s-0036-1591930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H, Studer A. Chemistry with N-centered radicals generated by single-electron transfer-oxidation using photoredox catalysis. CCS Chem. 2019;1:38–49. doi: 10.31635/ccschem.019.20180026. [DOI] [Google Scholar]
- 53.Groendyke BJ, AbuSalim DI, Cook SP. Iron-catalyzed, fluoroamide-directed C–H fluorination. J. Am. Chem. Soc. 2016;138:12771–12774. doi: 10.1021/jacs.6b08171. [DOI] [PubMed] [Google Scholar]
- 54.Li Z, Wang Q, Zhu J. Copper-catalyzed arylation of remote C(sp3)−H bonds in carboxamides and sulfonamides. Angew. Chem. Int. Ed. 2018;57:13288–13292. doi: 10.1002/anie.201807623. [DOI] [PubMed] [Google Scholar]
- 55.Jiang H, Studer A. Amidyl radicals by oxidation of α-amido-oxy acids: transition metal-free amidofluorination of unactivated Alkenes. Angew. Chem. Int. Ed. 2018;57:10707–10711. doi: 10.1002/anie.201804966. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Stateman LM, Nagib DA. δ C–H (Hetero)arylation via Cu-catalyzed radical relay. Chem. Sci. 2019;10:1207–1211. doi: 10.1039/C8SC04366C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friese FW, Mück-Lichtenfeld C, Studer A. Remote C–H functionalization using radical translocating arylating groups. Nat. Commun. 2018;9:2808. doi: 10.1038/s41467-018-05193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z, et al. Copper-catalyzed remote C(sp3)−H trifluoromethylation of carboxamides and sulfonamides. Angew. Chem. Int. Ed. 2019;58:2510–2513. doi: 10.1002/anie.201813425. [DOI] [PubMed] [Google Scholar]
- 59.Jiang, H., Seidler, G. & Studer, A. Carboamination of unactivated alkenes via three-component radical conjugate addition. Angew. Chem. Int. Ed. 58, 16528–16532 (2019). [DOI] [PMC free article] [PubMed]
- 60.Hofmann AW. Ueber die einwirkung des broms in alkalischer lösung auf die amine. Chem. Ber. 1883;16:558–560. doi: 10.1002/cber.188301601120. [DOI] [Google Scholar]
- 61.Löffler K, Freytag C. Über eine neue bildungsweise von N-alkylierten pyrrolidinen. Chem. Ber. 1909;42:3427–3431. doi: 10.1002/cber.19090420377. [DOI] [Google Scholar]
- 62.Barton DHR, Beaton JM, Geller LE, Pechet MM. A new photochemical reaction. J. Am. Chem. Soc. 1961;83:4076–4083. doi: 10.1021/ja01480a030. [DOI] [Google Scholar]
- 63.Hayashi M, Shiomi N, Funahashi Y, Nakamura S. Cinchona alkaloid amides/dialkylzinc catalyzed enantioselective desymmetrization of aziridines with phosphites. J. Am. Chem. Soc. 2012;134:19366–19369. doi: 10.1021/ja309963w. [DOI] [PubMed] [Google Scholar]
- 64.Sladojevich F, Trabocchi A, Guarna A, Dixon DJ. A new family of cinchona-derived amino phosphine precatalysts: application to the highly enantio- and diastereoselective silver-catalyzed isocyanoacetate aldol reaction. J. Am. Chem. Soc. 2011;133:1710–1713. doi: 10.1021/ja110534g. [DOI] [PubMed] [Google Scholar]
- 65.Chen K, Richter JM, Baran PS. 1,3-Diol Synthesis via controlled, radical-mediated C–H functionalization. J. Am. Chem. Soc. 2008;130:7247–7249. doi: 10.1021/ja802491q. [DOI] [PubMed] [Google Scholar]
- 66.Chen K, Baran PS. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 67.Short MA, Blackburn JM, Roizen JL. Sulfamate esters guide selective radical-mediated chlorination of aliphatic C–H bonds. Angew. Chem. Int. Ed. 2018;57:296–299. doi: 10.1002/anie.201710322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guisán-Ceinos M, Martín-Heras V, Tortosa M. Regio- and stereospecific copper-catalyzed substitution reaction of propargylic ammonium salts with aryl Grignard reagents. J. Am. Chem. Soc. 2017;139:8448–8451. doi: 10.1021/jacs.7b05273. [DOI] [PubMed] [Google Scholar]
- 69.Caeiro J, Sestelo JP, Sarandeses LA. Enantioselective nickel-catalyzed cross-coupling reactions of trialkynylindium reagents with racemic secondary benzyl bromides. Chem. Eur. J. 2008;14:741–746. doi: 10.1002/chem.200701035. [DOI] [PubMed] [Google Scholar]
- 70.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental procedure and characterization data of new compounds are available within Supplementary Information. Any further relevant data are available from the authors upon reasonable request.