Abstract
Monoamine neurotransmitters play essential roles in the regulation of arousal and sleep. Impaired metabolism of monoamine neurotransmitters could result in the accumulation of neurotoxic aldehyde metabolites and, hence, neuronal degeneration. Aldehyde dehydrogenases play an important role in the metabolism of the neurotoxic aldehyde metabolites, including the aldehyde metabolites of dopamine, serotonin, and noradrenaline. Deficient aldehyde dehydrogenase 2 (ALDH2) has been suggested to result in the accumulation of these biogenic aldehydes. An ALDH2 single nucleotide polymorphism (SNP), rs671 (A), results in significantly reduced ALDH2 enzyme activity. A total of 83 Parkinson’s disease (PD) patients were recruited in this study. In addition to the genotypes of rs671, the patients were assessed with the PD sleep scale-2nd version (PDSS-2) and the Epworth sleepiness scale (ESS) for symptoms of daytime and nocturnal sleep disturbances. The patients carrying rs671 (A) had more frequent dozing while lying down to rest in the afternoon (ESS item5) (F = 7.308, p = 0.008) than the rs671 (GG) patients. The patients with rs671 (A) reported a trend toward more frequent difficulty staying asleep than the patients with rs671 (GG). (F = 3.278, p = 0.074). The results indicate that patients carrying allele rs671 (A) are more likely to experience impairment in the regulation of arousal and sleep. The results also support the hypothesis that the accumulation of neurotoxic monoamine neurotransmitter aldehyde metabolites secondary to reduced ALDH2 enzyme activity may cause more severe monoaminergic neuronal loss and, hence, more severe symptoms in the regulation of wakefulness and sleep.
Subject terms: Behavioural genetics, Neurodegeneration, Parkinson's disease
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder. A meta-analysis of the worldwide data showed increasing prevalence with age, and the prevalence was suggested to be 1,087 and 1,903 per 100,000 persons among those between 70 to 79 years of age and those above 80 years of age, respectively1. Although Parkinson’s disease is traditionally characterized by the motor symptoms, non-motor symptoms were shown to cause more significant impairment in the quality of life (QoL), with symptoms of sleep and fatigue highly prevalent and strongly predictive of QoL change among patients with PD2. Around two-thirds of patients with PD report insomnia or are classified as poor sleepers, and around 30% experience excessive daytime sleepiness (EDS)3,4.
Although the pathological change in substantia nigra pars compacta (SNpc) is the hallmark of motor symptoms in PD, the pathological change responsible for the sleep-related disorders in patients with PD remains mostly unclear. Some studies suggested the pathological changes responsible for the sleep disorders may precede and are unrelated to the changes in substantia nigra5. Supporting evidence includes the research demonstrating that rapid eye movement sleep behavior disorder (RBD) may precede the motor symptoms of PD by decades6, suggesting that the pathological changes develop earlier than nigral dopaminergic neuronal degeneration. Furthermore, another study showed that the locus coeruleus-subcoeruleus complex, the arousal-modulating neuron groups, is affected earlier than the SNpc7. In recent years, it has been shown that monoaminergic neurotransmitters are involved in the regulation of the sleep-wake system, and monoaminergic neuronal degeneration causes sleep disorders8. Oxidative deamination of monoamine neurotransmitters results in the production of a number of neurotoxic aldehydes, which may accumulate in the central nervous system if not metabolized adequately by enzymes within the group of aldehyde dehydrogenases9. Among the aldehyde dehydrogenases, aldehyde dehydrogenase 2 (ALDH2) is of critical importance. It is well known that ALDH2 deficiency causes significant impairment in the metabolism of acetaldehyde, the accumulation of which causes alcohol-flush syndrome10. ALDH2 deficiency also causes impairment in the metabolism of biogenic aldehydes. The biogenic aldehyde products of dopamine, noradrenaline, and serotonin through the metabolism of monoamine oxidase (MAO) are 3,4-dihydroxyphenylacetaldehyde (DOPAL), 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL) and 5-hydroxyindole-3-acetaldehyde (5-HIAL), respectively. These monoaminergic aldehydes may accumulate under the circumstances of ALDH2 deficiency11,12. An Asian-specific ALDH2 single nucleotide polymorphism (SNP), rs671 (A), causes an amino acid substitution from glutamic acid to lysine, and results in the greatly reduced enzyme activity of the protein product of ALDH2 gene13. Almost half of the population in south Han Chinese and Japanese carries the impaired enzyme phenotype14.
Results obtained from both in vitro and in vivo experiments suggest that the accumulation of these monoamine-degraded aldehydes can lead to neurotoxicity12,15–17. Compatible with the findings suggestive of the neurotoxicity due to accumulation of monoamine neurotransmitter metabolites, our previous study showed that patients with PD carrying rs671 (A) allele showed a lower score in the mini-mental state examination (MMSE), higher frequency in cognitive impairments and deterioration in hobbies, and more severe disorganization and hypersexuality than patients without the (A) allele18.
In this study, we investigated whether ALDH2 deficiency secondary to rs671 (A) can cause sleep disturbances in patients with PD to gain a deeper understanding of the impact of monoamine neurotransmitters in the sleep-awake system regulation and the effect of rs671 (A) on the non-motor symptoms of PD patients.
Results
A total of 41 patients with the genotype of rs671 (GG) and 42 patients with the genotype of rs671 (AG) or (AA) were included in the study. The allele frequency of rs671 (A) was 31.3% (52 out of 166). The genotype frequencies of the patients were in accordance with Hardy-Weinberg equilibrium (χ2 = 0.896, p = 0.344). There were no significant differences in age, gender, education years, age at onset19,20, disease duration, levodopa equivalent daily dose (LED), levodopa equivalent dose attributable to dopamine agonist, disease severity (Hoehn and Yahr stage, H&Y stage) and severity of motor symptoms (the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale, MDS-UPDRS part I, II, III) between the two groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Study Groups.
| rs671 (GG) (n = 41) |
rs671 (AG) + (AA) (n = 42) |
Statistic | p Value | |||
|---|---|---|---|---|---|---|
| mean | SD | Mean | SD | |||
| Age (years) | 64.937 | 8.5353 | 64.870 | 8.8799 | t = 0.035 | 0.972 |
| Gender (female/male) | 15/26 | — | 20/22 | — | χ2 = 1.036 | 0.309 |
| Education (years) | 12.146 | 3.9215 | 10.690 | 4.2512 | U = 695.000 | 0.121 |
| Age at Onset (years) | 58.683 | 9.4113 | 58.500 | 9.3313 | U = 848.000 | 0.906 |
| Disease duration (months) | 72.439 | 43.8532 | 76.286 | 38.0002 | t = −0.427 | 0.670 |
| Levodopa equivalent dose | 731.427 | 417.3687 | 736.226 | 375.0122 | t = −0.055 | 0.956 |
| Levodopa equivalent dose attributable to dopamine agonist | 99.573 | 98.1356 | 119.345 | 109.0187 | U = 760.000 | 0.354 |
| MMSE | 28.244 | 1.5456 | 28.071 | 1.7305 | U = 824.000 | 0.730 |
| Hoehn and Yahr stage | 2.073 | 0.6852 | 2.381 | 0.7949 | U = 672.500 | 0.059 |
| I | 17.1% | — | 11.9% | — | — | — |
| II | 61.0% | — | 45.2% | — | ||
| III | 19.5% | — | 35.7% | — | ||
| IV | 2.4% | — | 7.1% | — | ||
| sum | 100.0% | — | 100.0% | — | ||
| MDS-UPDRS part I (mentality) | 7.805 | 5.4278 | 6.595 | 5.0609 | t = 1.050 | 0.297 |
| MDS-UPDRS part II (daily activities) | 9.854 | 7.5682 | 9.381 | 6.7714 | U = 854.000 | 0.949 |
| MDS-UPDRS part III (motor) | 25.854 | 12.1708 | 25.786 | 12.3022 | U = 845.500 | 0.888 |
| RBD with/without | 20/21 | — | 16/26 | — | χ2 = 0.964 | 0.326 |
| Age at onset, divided into three groups, proposed by Mehanna et al.19 | ||||||
| AAO ≦ 49 | 7 | — | 7 | — | χ2 = 0.479 | 0.787 |
| 50 ≦ AAO ≦ 69 | 27 | — | 30 | — | ||
| AAO ≧ 70 | 7 | — | 5 | — | ||
| Age at onset, divided into two groups, proposed by Wickremaratchi et al.20 | ||||||
| AAO < 49 | 6 | — | 5 | — | χ2 = 0.002 | 0.966 |
| AAO ≧ 49 | 35 | — | 37 | — | ||
Abbreviations: SD, standard deviation; AAO, age at onset; MMSE, Mini-mental state examination, MDS-UPDRS, Movement Disorder Society-Unified Parkinson’s disease rating scale.
In terms of daytime sleepiness, which was evaluated with Epworth sleepiness scale (ESS) scale, there was a significant difference in the ESS item 5 (Table 2). The patients carrying rs671 (A) had more frequent dozing while lying down to rest in the afternoon (ESS item5) (F = 7.308, p = 0.008) than the rs671 (GG) patients (Fig. 1). The percentage of patients with the sum of ESS ≧ 10 in the group carrying rs671 (A) is 28.6% (12 out of 42), and the percentage in the group carrying only rs671 (G) is 19.5% (8 out of 41). Although the percentage of patients with excessive daytime sleepiness (the sum of ESS ≧ 10) is higher in the group carrying rs671 (A), there was no statistical significance between the two study groups (χ2 = 0.502, p = 0.479).
Table 2.
The Chinese version of the Epworth sleeping scale in the 2 study groups.
| rs671 (GG) (n = 41) |
rs671 (AG) and (AA) (n = 42) | Mann-Whitney U | p-Value | F of Qaude’s test | P-value of Quade’s test | |||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||||
| ESS1 | 0.683 | 0.9338 | 0.738 | 1.0136 | U = 857.000 | 0.967 | 0.006 | 0.939 |
| ESS2 | 0.976 | 1.0365 | 1.262 | 1.1275 | U = 739.500 | 0.247 | 1.387 | 0.242 |
| ESS3 | 0.732 | 0.9493 | 0.571 | 0.8874 | U = 765.500 | 0.327 | 1.072 | 0.304 |
| ESS4 | 1.049 | 1.1391 | 1.000 | 1.2494 | U = 810.000 | 0.616 | 0.316 | 0.576 |
| ESS5 | 0.707 | 1.0781 | 1.405 | 1.2309 | U = 600.000 | 0.010 | 7.308 | 0.008** |
| ESS6 | 0.341 | 0.6561 | 0.333 | 0.5703 | U = 848.000 | 0.880 | 0.020 | 0.889 |
| ESS7 | 0.927 | 1.0814 | 1.119 | 1.1729 | U = 795.500 | 0.526 | 0.391 | 0.534 |
| ESS8 | 0.146 | 0.3578 | 0.190 | 0.4547 | U = 840.500 | 0.767 | 0.109 | 0.742 |
| ESS sum | 5.561 | 4.9348 | 6.619 | 5.3827 | U = 776.000 | 0.436 | 0.567 | 0.453 |
| EDS (with/without) | 8/33 | 12/30 | χ2 = 0.502 | 0.479 | — | — | ||
Figure 1.
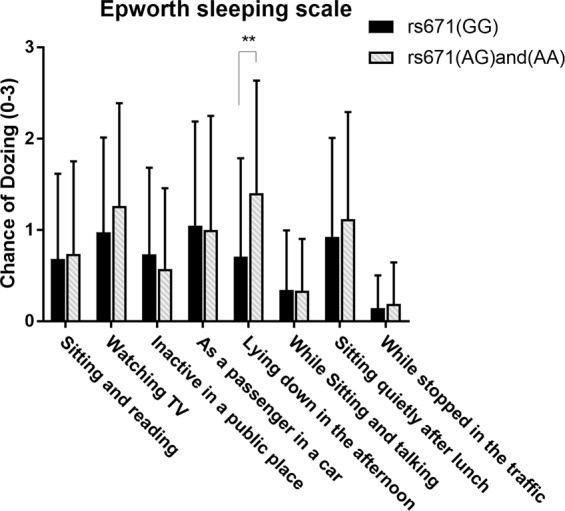
The means and standard deviations of each item on the Epworth sleeping scale in the 2 study groups. Patients with rs671 (AG) and (AA) showed significantly more frequent chance of dozing while “lying down in the afternoon when circumstances permit” (F = 7.308, p = 0.008). **Indicate p < 0.01.
With regard to nocturnal disturbances, there was a trend toward statistical significance in the PD sleep scale-2nd version (PDSS-2) item 3, “difficulty staying asleep” (Table 3). The results indicate that patients carrying rs671 (A) tend to more frequently report difficulty staying asleep than the patients with the genotype of rs671 (GG) (F = 3.278, p = 0.074) (Fig. 2). There was no significant difference in the proportion of patients diagnosed with RBD between the two groups with different genotypes (Table 1).
Table 3.
Parkinson’s disease sleep scale-2nd version in the 2 study groups.
| rs671 (GG) (n = 41) |
rs671 (AG) and (AA) (n = 42) | Statistic | p- Value | F of Qaude’s test | P-value of Quade’s test | |||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||||
| PDSS1 | 1.195 | 1.4003 | 1.357 | 1.3761 | U = 806.500 | 0.599 | 0.301 | 0.585 |
| PDSS2 | 0.854 | 1.2954 | 0.929 | 1.2375 | U = 805.500 | 0.578 | 0.262 | 0.610 |
| PDSS3 | 0.634 | 1.1348 | 1.095 | 1.3217 | U = 686.000 | 0.073 | 3.278 | 0.074 |
| PDSS4 | 0.366 | 0.7667 | 0.310 | 0.6803 | U = 811.000 | 0.535 | 0.427 | 0.515 |
| PDSS5 | 0.244 | 0.5823 | 0.238 | 0.7262 | U = 836.500 | 0.724 | 0.082 | 0.775 |
| PDSS6 | 0.951 | 1.2031 | 1.190 | 1.1943 | U = 752.500 | 0.293 | 1.063 | 0.306 |
| PDSS7 | 0.415 | 1.0482 | 0.262 | 0.7005 | U = 831.500 | 0.671 | 0.245 | 0.622 |
| PDSS8 | 3.293 | 1.1010 | 3.214 | 1.2003 | U = 832.000 | 0.758 | 0.151 | 0.698 |
| PDSS9 | 1.098 | 1.6249 | 0.976 | 1.5848 | U = 805.000 | 0.554 | 0.522 | 0.472 |
| PDSS10 | 0.268 | 0.6334 | 0.571 | 1.2325 | U = 807.000 | 0.494 | 0.458 | 0.500 |
| PDSS11 | 0.439 | 0.7433 | 0.595 | 0.8851 | U = 772.500 | 0.346 | 0.845 | 0.361 |
| PDSS12 | 0.293 | 0.6798 | 0.357 | 0.7594 | U = 839.000 | 0.776 | 0.079 | 0.779 |
| PDSS13 | 0.366 | 0.9684 | 0.381 | 1.0110 | U = 858.500 | 0.972 | 0.000 | 0.988 |
| PDSS14 | 0.927 | 1.1703 | 0.857 | 1.2212 | U = 810.500 | 0.615 | 0.198 | 0.658 |
| PDSS15 | 0.171 | 0.4417 | 0.167 | 0.5372 | U = 824.000 | 0.551 | 0.305 | 0.583 |
Figure 2.
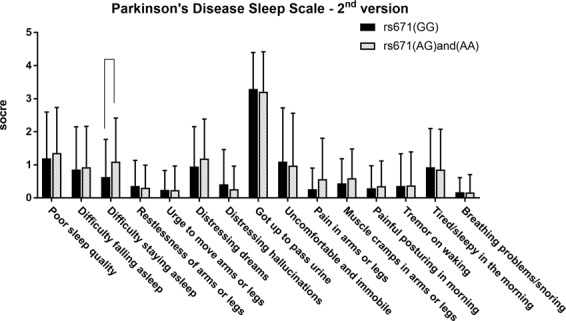
The means and standard deviations of each item on the Parkinson’s disease sleep scale-2nd version in the 2 study groups. Patients with rs671 (AG) and (AA) showed a trend toward more frequent “difficulty staying asleep” (F = 3.278, p = 0.074).
Discussion
To the best of our knowledge, this is the first study investigating the effect of ALDH2 on sleep disturbances in patients with PD, including EDS and nocturnal disturbances. In general, our results indicate that the patients carrying rs671 (A) allele are more likely to develop excessive daytime sleepiness and also tend to have difficulty maintaining asleep than those carrying only rs671 (G) allele.
Previous studies reported the prevalence of EDS among patients with PD to be 16–74%, and usually around 33%3. In this study, the EDS rate was higher in patients carrying rs671 (A) (28.6%) than patients with rs671 (GG) (19.5%), although there was no significant difference (χ2 = 0.502, p = 0.479). The EDS was proposed to be associated with LED, and dopamine agonist was suggested to have an impact on sleep attack21. The LED, as well as the LED attributable to dopamine agonists, were comparable between the two groups in this study. As described in our previous study, evaluating daytime sleepiness with ESS might underestimate the prevalence of sleep disturbances in Taiwan4, so it is possible that the prevalence of EDS may also be underestimated in this study.
In the PDSS-2 questionnaire, patients carrying rs671 (A) allele tend to more frequently report difficulty maintaining asleep. Nonetheless, despite the potential difference in maintaining asleep at night, other items on PDSS-2 failed to reveal a significant difference between the two groups. The result suggests that ALDH2 deficiency contributes specifically to the difficulty in sleep maintenance, which can be due to a perturbed circadian rhythm or an unstable sleep-awake transition. In addition, the item “get up to pass urine” had the highest score in both groups, and the finding is compatible with previous results indicating frequent need to pass urine at night as one of the leading causes of sleep disturbances among patients with PD3.
In the present study, we utilized Quade’s test for controlling the impact attributable to confounding variables (age, age at onset, and LED). The analysis made the result of ESS item 5 more significant after controlling the confounders.
The A allele of ALDH2 rs671 SNP causes an amino acid substitution from glutamic acid to lysine, resulting in the significantly reduced enzyme activity. ALDH2 is a tetrameric enzyme, and one study reported that one inactive subunit encoded by the SNP rs671 (A) results in the inactivation of the tetramer enzyme. Hence, the percentage of ALDH2 enzyme with four active subunits in heterozygous individuals through the random association of the active and inactive subunits would be 6%22. One more recent study showed that the tissue ALDH2 activity in individuals with rs671(AG) was 17% that of individuals with rs671 (GG), while the activity in individuals with rs671(AA) was too low to be determined precisely23. The reduced enzyme activity results in the accumulation of aldehydes, and is responsible for the well-known Asian flush and can even cause alcohol-related cancers as a result of accumulation of acetaldehyde12,24,25.
ALDH2 also plays a major role in the metabolism of monoamine neurotransmitters. The oxidative deamination of dopamine, norepinephrine, and serotonin by MAO results in the production of DOPAL, DOPEGAL, and 5-HIAL, respectively. These aldehydes have been shown to be neurotoxic with both in vitro and in vivo experiments, and the crucial role of ALDH2 in the metabolism of these aldehydes into non-toxic metabolites has been proposed26–28. At the concentration close to the physiological levels reported in healthy human postmortem specimens of the substantia nigra29, DOPAL caused reduced viability of PC12 cells, a cell line with dopaminergic properties, by around 30%30. DOPAL has also been shown to be toxic to cell lines of neuron-like cells, SH-SY5Y31 and SK-N-SH cells32, and neurons from fetal rat mesencephalic tissues and neostriatal synaptosomal preparations33. Stereotaxic injection of DOPAL in the substantia nigra caused selective loss of dopaminergic neurons in the substantia and triggered a behavioral phenotype consistent with other PD animal models34. The neurotoxicity of DOPEGAL has also been demonstrated across different studies, in which DOPEGAL was shown to cause a concentration- and time-dependent toxicity to PC-12 cells at concentrations as low as 5.9 μM35. Injection of DOPEGAL to rostral ventrolateral medulla (RVLM) in amounts as low as 50 ng was shown to cause dose- and time-dependent loss of adrenergic neurons36. Furthermore, the aldehyde metabolite of serotonin, 5-hydroxyindole-3-acetaldehyde (5-HIAL), accumulates in the presence of daidzin, an ALDH2 inhibitor37, and has been shown to cause oligomerization of alpha-synuclein like DOPAL17.
Although studies suggest that glutamate and gamma-Aminobutyric acid (GABA) have more direct and immediate impact on the regulation of wakefulness and sleep, the monoaminergic neurotransmitters have been shown in numerous studies to play essential roles in the arousal promoting system and sleep-promoting system8,38. Noradrenergic neurons in the locus coeruleus (LC) have been shown to discharge tonically at highest frequency during wakefulness and to reduce their activity during non-rapid eye movement (NREM) sleep with virtually absent activity during rapid eye movement (REM) sleep39. By applying optogenetic tools in mice, photoinhibition of locus coeruleus neurons was shown to cause a reduction in the duration of wakefulness, while photostimulation of locus coeruleus neurons caused immediate sleep-to-wake transitions40. Besides, photoinhibition of LC neurons during stimulation of Hypocretin (Hcrt) neurons was shown to block Hcrt-mediated sleep-to-wake transitions, while photostimulation of LC neurons with concomitant photostimulation of Hcrt neurons significantly increased the probability of sleep-to-wake transitions compared with stimulation of Hcrt neurons alone41. All the results above indicate the crucial role of noradrenergic neurons in LC as an effector in the regulation of wakefulness.
In addition to noradrenergic neurons, dopaminergic neurons in the ventral tegmental area (VTA) and dorsal raphe nucleus (DRN) have also been found to be involved in the regulation of wakefulness or sleep. VTA dopaminergic neurons were found to be activated during REM sleep, and this activation was similar to the activity measured during the consumption of palatable food42. With fiber photometry to record calcium activity, the activity of VTA dopaminergic neurons was found to be reduced during NREM sleep compared with both wakefulness and REM sleep43. Optogenetic activation of VTA dopaminergic neurons was found to initiate and maintain wakefulness, while chemogenetic inhibition of VTA dopaminergic neurons decreased wakefulness and promoted sleep43. Optogenetic stimulation and chemogenetic inhibition of DRN dopaminergic neurons were shown to promote and reduce wakefulness, respectively44. Furthermore, the importance of dopamine is also evidenced by the results showing that optogenetic activation of nucleus accumbens dopamine D1 receptor (D1R)-expressing neurons induced immediate transitions from NREM sleep to wakefulness, and chemogenetic stimulation prolonged arousal45. These results all indicate the importance of dopamine in the regulation of wakefulness. Moreover, serotonergic DRN neurons were shown to be active during wakefulness and progressively less active during the early, middle and late phases of slow-wave sleep46. Meanwhile, the involvement of serotonin in the regulation of sleep is also evidenced by the finding showing increased REM sleep and decreased serotonin level with DRN microdialysis perfusion of 8-Hydroxy-2-(Di-n-Propylamino)Tetralin (8-OH-DPAT), a selective hydroxytryptamine (5-HT) receptor agonist that inhibits serotonergic neural activity via autoreceptor-mediated inhibition47. At the same time, the sleep-promoting ventral lateral pre-optic nucleus (VLPO) innervates the monoaminergic arousal components (including LC and raphe system) and receives inputs from these areas, reciprocally. Dysfunction of the monoaminergic neurons may hence cause disturbance in the sleep-promoting system as well8,48.
Degeneration of monoaminergic neurons is the histopathological hallmark of PD. Loss of noradrenergic neurons in LC and dopaminergic neurons in VTA has been reported across several studies in patients with PD49–53. Neuronal degeneration in DRN has also been reported in patients with PD, although whether dopaminergic or serotonergic neurons are more severely affected remain unknown52. The monoaminergic neuron degeneration may be the underlying cause responsible for the symptoms of excessive daytime sleepiness and sleep disturbances in patients with PD. Moreover, in the present study, the results demonstrate the differences in the profiles of sleep disorders between the two groups with different rs671 genotypes. The findings support the hypothesis that the accumulation of neurotoxic monoamine neurotransmitter aldehyde metabolites as a result of inadequate ALDH2 enzyme activity can cause more severe monoaminergic neuronal loss and more severe disorders in the regulation of wakefulness and sleep.
The present study has potential limitations. The participants in the study were recruited from tertiary medical centers and may cause selection bias. In addition, to ensure accurate and correct report of the sleep symptoms, PD patients who scored < 24 on MMSE were excluded from the study. On the other hand, patients with more severe sleep disturbances may not be included. Another drawback of this study is the need for combining the results of patients with rs671 (AG) and rs671 (AA) into one group for analysis because the genotype frequency of rs671 (AA) is low and hence the number of patients carrying rs671 (AA) in this study is small. The number of patients carrying rs671 (AA) is much smaller (n = 10) than that of the rs671 (GG) (n = 41) and rs671 (AG) (n = 32). If the three groups are analyzed separately, the possibility of type I error would be too high. The study also did not quantify for doses of sleep-aids. This study also did not apply polysomnography (PSG) to obtain objective parameters for sleep evaluation. Utilizing PSG may provide more information and help differentiate REM sleep from NREM sleep because poor sleep maintenance reported by the patients might reflect an increase in REM sleep bout.
To conclude, the present study is the first study investigating the association between sleep disturbances in patients with PD and ALDH2 SNP rs671. The results indicate that PD patients with allele rs671 (A) are more likely to have excessive daytime sleepiness and may tend to have difficulty maintaining asleep, and provide evidence suggesting that ALDH2 may modulate the accumulation of monoamine neurotransmitters and hence, the non-motor symptoms of patients with PD.
Methods
Participants
This study included 83 patients who were diagnosed with idiopathic PD according to the United Kingdom PD Society Brain Bank clinical diagnostic criteria54. These patients were referred from neurologists from the outpatient departments from three medical centers located in three different cities in Taiwan. We included patients with the following features: mentally able to complete the interview, self-report questionnaires, and neuropsychological tests (scored ≧ 24 in the mini-mental state examination). The neuropsychological tests were completed by the patients themselves and with some assistance from the investigators if needed. We excluded patients with the following features: atypical parkinsonism, illiteracy, history of brain surgery, or any severe systemic disease.
We obtained all of the written informed consent from the participants before enrollment, following the ethical standards outlined in the 1964 Declaration of Helsinki. All study procedures were approved by the ethical research committee of Kaohsiung Medical University Hospital, National Cheng Kung University Hospital, and National Taiwan University Hospital and all methods were performed in accordance with the approved guidelines. The detailed information of demographic, dopamine replacement therapy and motor severity was obtained from the medical record assessed by the neurologists. Levodopa equivalent dose, as well as Levodopa equivalent dose attributable to dopamine agonist of each participant, was calculated according to the method of Tomlinson et al.55. Motor symptoms and severity were assessed according to Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)56 and Hoehn and Yahr staging criteria57, respectively.
Assessment of symptoms
We assessed the 83 PD patients with MMSE, MDS-UPDRS, H&Y staging to investigate the subgroup characteristics of the mental, motor and daily activities. We obtained the history of RBD (Rapid eye movement sleep behavior disorder) from the patient’s sleep companion. We used two sleep-related questionnaires, including the Chinese version of the Epworth sleeping scale (ESS), the Parkinson’s Disease Sleep Scale -2nd version (PDSS-2)-Taiwan form.
ESS is a useful tool to evaluate daytime sleepiness. Each item scored from 0 to 3, with 0 beings “would never doze” and 3 being “ high chance of dozing”. If a total score of the eight items yields ten or more, the patient is categorized as suffering from excessive daytime sleepiness (75% sensitivity and 82.4% specificity for daytime sleep episodes)58. The Chinese version of the ESS is reliable (Cronbach’s α = 0.81, test-retest reliability = 0.74)59.
The English PDSS-2 consists of 15 questions about various sleep and nocturnal disturbances which are to be rated by the patients using one of five categories, from 0 beings “very often” to 4 being “never” in question 1; from 0 being “never” to 4 being “very often” in question 2 to 14. The total score ranges from 0 (no disturbance) to 60 (maximum nocturnal disturbance). The Chinese version had a totally opposite scoring, from 0 beings “never” to 4 beings “very often” in question 1, from 0 beings” very often” to 4 being “never” in question 2 to 14. We replaced the original score (obtained from the patient’s score in Chinese questionnaire) to a final score of “4 minus original score”. PDSS-2 is a test of good reliability (Cronbach’s α = 0.73, test-retest reliability intra-class-coefficient = 0.80)60.
Genetic analysis
We performed blood sampling of the 83 patients’ genomic DNA. We extracted genomic DNA from peripheral blood leukocytes with the Genomic DNA Extraction Kit (Geneaid, New Taipei City, Taiwan). ALDH2 rs671 SNP genotype was determined with TaqMan probes and the StepOnePlusTM system with the StepOne software (Applied Biosystems, Grand Island, NY, USA). The laboratory technicians, who were blinded to the patient’s demographic characteristics, performed the genotyping, and read the results.
Statistical analysis
We examined each item mentioned earlier based on the 83 patient’s ALDH2 rs671 genotype. Proportions were calculated for qualitative variables, and means and standard deviations (SDs) were calculated for quantitative variables. Because there were only ten of the participants carrying the rs671 (AA) genotype, we combined Patients with genotype rs671 (AG) and rs671 (AA) into one group. This combination is valid, based on the study reporting the rs671 (AA)-encoded enzymes is completely inactive and the rs671 (AG)-encoded enzyme is partially inactive (16~17% of activity of that of the rs671 (GG))23. We applied the Kolmogorov-Smirnov test for testing normality. Afterward, we tested quantitative variables with the t-test or Mann-Whitney U test and tested qualitative variables by using chi-squared test. We controlled the impact attributable to confounding variables (age, age at onset and LED) by applying Quade’s test. Statistical significance was predetermined with an alpha level less than 0.05. The statistical analysis was done by a commercially available software program (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.).
Acknowledgements
The authors are grateful to the participants involved in this study and for the grants from the Ministry of Science and Technology (MOST), Taipei, Taiwan (MOST 107-2628-B-037-002 - and MOST 106-2410-H-006 -039 -MY2), Kaohsiung Medical University (KMU-Q108002) and Kaohsiung Medical University Hospital (KMUH106-6R66).
Author contributions
All authors fulfilled the criteria of authorship, and no one who met the criteria was excluded. C.H. Tan and R.L. Yu had the idea, designed the experiments. C.H. Tan, R.L. Yu and R.M. Wu were involved in sample collection. C.Y. Lin performed literature searches and data analysis and produced the first draft of the manuscript. C.Y. Lin, R.L. Yu, R.M. Wu and C.H. Tan take responsibility for the interpretation of the results. All authors critically reviewed drafts and approved the final version of this article. C.H. Tan accepted full responsibility for the work and controlled the decision to publish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Movement disorders: official journal of the Movement Disorder. Society. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2.Prakash KM, Nadkarni NV, Lye WK, Yong MH, Tan EK. The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: a longitudinal study. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2016;23:854–860. doi: 10.1111/ene.12950. [DOI] [PubMed] [Google Scholar]
- 3.De Cock VC, Vidailhet M, Arnulf I. Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol. 2008;4:254–266. doi: 10.1038/ncpneuro0775. [DOI] [PubMed] [Google Scholar]
- 4.Yu RL, Tan CH, Wu RM. The impact of nocturnal disturbances on daily quality of life in patients with Parkinson’s disease. Neuropsychiatric disease and treatment. 2015;11:2005–2012. doi: 10.2147/ndt.s85483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehgoetz Martens KA, Lewis SJ. Pathology of behavior in PD: What is known and what is not? Journal of the neurological sciences. 2017;374:9–16. doi: 10.1016/j.jns.2016.12.062. [DOI] [PubMed] [Google Scholar]
- 6.Claassen DO, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–499. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erwin VG, Deitrich RA. Brain aldehyde dehydrogenase. Localization, purification, and properties. The Journal of biological chemistry. 1966;241:3533–3539. [PubMed] [Google Scholar]
- 10.Vasiliou V, Pappa A. Polymorphisms of human aldehyde dehydrogenases. Consequences for drug metabolism and disease. Pharmacology. 2000;61:192–198. doi: 10.1159/000028400. [DOI] [PubMed] [Google Scholar]
- 11.Keung WM. Biogenic aldehyde(s) derived from the action of monoamine oxidase may mediate the antidipsotropic effect of daidzin. Chemico-biological interactions. 2001;130-132:919–930. doi: 10.1016/S0009-2797(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 12.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacological reviews. 2007;59:125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet (London, England) 1981;2:982. doi: 10.1016/S0140-6736(81)91172-7. [DOI] [PubMed] [Google Scholar]
- 15.Burke WJ, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/s0161-813x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 16.Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinsmaa Y, Cooney A, Sullivan P, Sharabi Y, Goldstein DS. The serotonin aldehyde, 5-HIAL, oligomerizes alpha-synuclein. Neuroscience letters. 2015;590:134–137. doi: 10.1016/j.neulet.2015.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu RL, Tan CH, Lu YC, Wu RM. Aldehyde dehydrogenase 2 is associated with cognitive functions in patients with Parkinson’s disease. Scientific reports. 2016;6:30424. doi: 10.1038/srep30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC. Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism & related disorders. 2014;20:530–534. doi: 10.1016/j.parkreldis.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Wickremaratchi MM, et al. The motor phenotype of Parkinson’s disease in relation to age at onset. Movement disorders: official journal of the Movement Disorder. Society. 2011;26:457–463. doi: 10.1002/mds.23469. [DOI] [PubMed] [Google Scholar]
- 21.Yeung EYH, Cavanna AE. Sleep Attacks in Patients With Parkinson’s Disease on Dopaminergic Medications: A Systematic Review. Movement disorders clinical practice. 2014;1:307–316. doi: 10.1002/mdc3.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabb DW, Edenberg HJ, Bosron WF, Li TK. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant. The Journal of clinical investigation. 1989;83:314–316. doi: 10.1172/jci113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai CL, et al. Dominance of the inactive Asian variant over activity and protein contents of mitochondrial aldehyde dehydrogenase 2 in human liver. Alcoholism, clinical and experimental research. 2014;38:44–50. doi: 10.1111/acer.12215. [DOI] [PubMed] [Google Scholar]
- 24.Garaycoechea Juan I., Crossan Gerry P., Langevin Frédéric, Mulderrig Lee, Louzada Sandra, Yang Fentang, Guilbaud Guillaume, Park Naomi, Roerink Sophie, Nik-Zainal Serena, Stratton Michael R., Patel Ketan J. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553(7687):171–177. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci. 2017;24:19. doi: 10.1186/s12929-017-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CH, Joshi AU, Mochly-Rosen D. The Role of Mitochondrial Aldehyde Dehydrogenase 2 (ALDH2) in Neuropathology and Neurodegeneration. Acta neurologica Taiwanica. 2016;25(4):111–123. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiological reviews. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu RB, et al. The aldehyde dehydrogenase 2 polymorphisms on neuropsychological performance in bipolar II disorder with or without comorbid anxiety disorder. PloS one. 2018;13:e0192229. doi: 10.1371/journal.pone.0192229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burke WJ, Chung HD, Li SW. Quantitation of 3, 4-dihydroxyphenylacetaldehyde and 3, 4-dihydroxyphenylglycolaldehyde, the monoamine oxidase metabolites of dopamine and noradrenaline, in human tissues by microcolumn high-performance liquid chromatography. Analytical biochemistry. 1999;273:111–116. doi: 10.1006/abio.1999.4196. [DOI] [PubMed] [Google Scholar]
- 30.Kristal BS, et al. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free radical biology & medicine. 2001;30:924–931. doi: 10.1016/S0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 31.Legros H, Dingeval MG, Janin F, Costentin J, Bonnet JJ. Toxicity of a treatment associating dopamine and disulfiram for catecholaminergic neuroblastoma SH-SY5Y cells: relationships with 3,4-dihydroxyphenylacetaldehyde formation. Neurotoxicology. 2004;25:365–375. doi: 10.1016/s0161-813x(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 32.Lamensdorf, I. et al. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. Journal of neuroscience research60, 552–558, doi:10.1002/(sici)1097-4547(20000515)60:4<552::aid-jnr14>3.0.co;2-u (2000). [DOI] [PubMed]
- 33.Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration: a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1995;4:271–281. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 34.Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PloS one. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke WJ, Schmitt CA, Gillespie KN, Li SW. Norepinephrine transmitter metabolite is a selective cell death messenger in differentiated rat pheochromocytoma cells. Brain research. 1996;722:232–235. doi: 10.1016/0006-8993(96)00129-1. [DOI] [PubMed] [Google Scholar]
- 36.Burke WJ, et al. Catecholamine monoamine oxidase a metabolite in adrenergic neurons is cytotoxic in vivo. Brain research. 2001;891:218–227. doi: 10.1016/S0006-8993(00)03199-1. [DOI] [PubMed] [Google Scholar]
- 37.Rooke N, Li DJ, Li J, Keung WM. The mitochondrial monoamine oxidase-aldehyde dehydrogenase pathway: a potential site of action of daidzin. Journal of medicinal chemistry. 2000;43:4169–4179. doi: 10.1021/jm990614i. [DOI] [PubMed] [Google Scholar]
- 38.Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Current opinion in neurobiology. 2017;44:186–192. doi: 10.1016/j.conb.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature neuroscience. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter ME, et al. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2635–2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahan L, et al. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 43.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nature neuroscience. 2016;19:1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho JR, et al. Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron. 2017;94:1205–1219.e1208. doi: 10.1016/j.neuron.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Luo YJ, et al. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nature communications. 2018;9:1576. doi: 10.1038/s41467-018-03889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain research. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 47.Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 49.McRitchie DA, Cartwright HR, Halliday GM. Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson’s disease. Experimental neurology. 1997;144:202–213. doi: 10.1006/exnr.1997.6418. [DOI] [PubMed] [Google Scholar]
- 50.Alberico SL, Cassell MD, Narayanan NS. The Vulnerable Ventral Tegmental Area in Parkinson’s Disease. Basal ganglia. 2015;5:51–55. doi: 10.1016/j.baga.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Tredici K, Braak H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. Journal of neurology, neurosurgery, and psychiatry. 2013;84:774–783. doi: 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- 52.Jellinger KA. Pathology of Parkinson’s disease. Molecular and Chemical Neuropathology. 1991;14:153–197. doi: 10.1007/bf03159935. [DOI] [PubMed] [Google Scholar]
- 53.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Archives of neurology. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 54.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder. Society. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 56.Yu RL, et al. Cross-Cultural Differences of the Non-Motor Symptoms Studied by the Traditional Chinese Version of the International Parkinson and Movement Disorder Society- Unified Parkinson’s Disease Rating Scale. Movement disorders clinical practice. 2017;4:68–77. doi: 10.1002/mdc3.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki K, et al. Excessive daytime sleepiness and sleep episodes in Japanese patients with Parkinson’s disease. Journal of the neurological sciences. 2008;271:47–52. doi: 10.1016/j.jns.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Chen NH, et al. Validation of a Chinese version of the Epworth sleepiness scale. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11:817–821. doi: 10.1023/A:1020818417949. [DOI] [PubMed] [Google Scholar]
- 60.Trenkwalder C, et al. Parkinson’s disease sleep scale–validation of the revised version PDSS-2. Movement disorders: official journal of the Movement Disorder Society. 2011;26:644–652. doi: 10.1002/mds.23476. [DOI] [PubMed] [Google Scholar]


