Abstract
Corneal transplantation is the only option to cure corneal opacities. However, there is an imbalance between supply and demand of corneal tissues in the world. To solve the problem of corneal shortage, corneal xenotransplantation studies have been implemented in the past years using porcine corneas. The corneal xenografts could come from 1) wild type pigs, 2) genetically-engineered pigs, 3) decellularized porcine corneas, and 4) decellularized porcine corneas that are recellularized with human corneal cells, eventually with patients’ own cells, as in all type of xenografts. All approaches except, the former would reduce or mitigate recipient immune responses. Although several techniques in decellularization have been reported, there is still no standardized protocol for the complete decellularization of corneal tissue. Herein, we reviewed different decellularization methods for porcine corneas based on the mechanism of action, decellularization efficacy, biocompatibility, and the undesirable effects on corneal ultrastructure. We compared 9 decellularization methods including: (i) sodium dodecyl sulfate, (ii) triton x-100, (iii) hypertonic saline, (iv) human serum with electrophoresis, (v) high hydrostatic pressure, (vi) freeze-thaw, (vii) nitrogen gas, (viii) phospholipase A2, and (ix) glycerol with chemical crosslinking methods. It appears that combined methods could be more useful to perform efficient corneal decellularization.
Keywords: Cornea transplantation, Decellularization, Porcine xenograft, Tissue engineering
1. INTRODUCTION
According to data from the Global Vision Database, 36 million people worldwide are blind.1 The World Health Organization indicates that corneal opacities account for 5% of all causes of blindness.2 When corneal transparency becomes defective, corneal transplantation is the only curative option.3 Currently, deceased human donors are the only source of corneal grafts. Despite the existence of countries that export corneas, e.g., the United States (that accounts for 85% of the world’s corneal ‘exports’), Sri Lanka (9%), and Italy (3%), there is a major imbalance between supply and demand.4,5 In the last decade, tissue engineering has become a more prominent method of potentially solving the problem of corneal shortage.
Tissue-engineered corneal scaffolds should have complex features that are essential to corneal function. These features, which include (i) light transparency, (ii) resistance to ultraviolet light, (iii) insensitivity to photons, and (iv) specific tensile strength, make the replication of a corneal scaffold from biomaterials much more difficult.6,7 To combat this issue, decellularized corneas have been successfully tested in pre-clinical animal studies.4,8–10 The most significant advantage of a decellularization process is conservation of the extracellular matrix (ECM) proteins and mechanical properties of the cornea.8,11 For these reasons, a decellularized corneal scaffold could be a good option for creating tissue-engineered corneal xenografts.
Normally, the cornea is an immune-privileged site, but in some pathological conditions, especially infections and after transplantation, this privilege can be diminished.12,13 In addition, decellularized porcine corneal xenografts (DPCXs) offer another important advantage in that they exhibit a decreased risk of an inflammatory reaction compared to the fresh porcine corneal xenografts.14,15 Therefore, DPCXs may provide a basis for successful corneal transplantation.8
Decellularization of a three-dimensional matrix scaffold (i.e. organ or tissue, cornea) enables the removal of cellular and immunogenic components from an organ/tissue while preserving ECM with its native micro- and macroscopic anatomy.16 Many different protocols for decellularizing human and porcine corneal tissues have been reported using biological, physical and chemical compounds.17–20 However, there is still no standardized protocol. An ideal decellularization method should maximize the elimination of cellular components and nuclear materials, while limiting the possible detrimental effect on histo-architecture and mechanical properties of the ECM. In addition, being able to repopulate host cells (e.g., keratocytes, epithelial cells) should be an important factor after decellularization.
In the present review, we will assess favorable, promising, and new decellularization protocols and agents for developing DPCXs.
2. DECELLULARIZATION METHODS
To date, there has been no documented method that can achieve complete decellularization while maintaining the principal properties of the cornea (Table 1). Moreover, the term “complete decellularization” is currently not well-defined. Crapo et al.21 suggested that it is impossible to remove 100% of cellular material by decellularization techniques and defined the term of “sufficient decellularization” by conditioning minimal criteria those are following (i) ECM should contain <50ng dsDNA per mg dry weight, (ii) DNA fragments should be shorter than 200 bp, and (iii) there should be no visible nuclear component within the ECM. Besides decellularization, reduced antigenicity in the collagen tissues of cornea is also essential if a recipient immune response is to be prevented. Additional conditions must be implemented to reduce edema, maintain or achieve transparency, and increase mechanical strength. Albeit, there are numerous procedures and agents to reverse undesirable effects. However, the balance between decellularization and damage must be established well in advance in order to achieve effective decellularization.
Table 1:
Advantages and disadvantages of decellularization methods and bioprinting for replacing the cornea
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
|
Sodium Dodecyl Sulfate |
Sufficiently removes cellular and nuclear components. |
Harms ECM, lamellar structure. Causes collagen fibril disorganization. Reduces transparency. |
17, 22, 23, 24, 26 |
| Triton X-100 | Can be useful as an additional treatment with another main decellularization agent. |
Cannot remove cellular and nuclear components sufficiently. Damages glycosaminoglycans. Reduces transparency. |
17, 30, 32, 35 |
| Hypertonic Saline | Maintains ECM. Removes cellular components sufficiently. Used in a clinical trial. |
Mixed reports on transparency. Causes edema. |
20, 21, 29, 37, 38, 39 |
|
Human Serum with Electrophoresis |
Maintains ECM and transparency. |
Cannot remove cellular components sufficiently and homogenously. |
43, 44 |
|
High Hydrostatic Pressure |
Maintains lamellar and collagenous structure. Short time-frame. Used in a clinical trial. |
Expensive. Disorganizes collagen fibrils and causes edema. Reduces transparency. |
45, 46, 62 |
| Freeze-Thaw | Removes cellular components sufficiently. |
Harms ECM. Reduces transparency. Reduces mechanical strength. |
48, 49, 50 |
| N2 Gas | Maintains transparency. | Causes edema. Studies needed on mechanical strength. |
20, 51 |
| Phospholipase A2 | Maintains ECM, transparency, and collagenous structure. Biocompatible. |
Additional treatment needed to achieve sufficient decellularization. |
9 |
| Glycerol with Chemical Crosslinking | Maintains corneal transparency and mechanical properties. |
Small amount of residual nucleic acids. | 54 |
Legend: ECM = Extracellular matrix
2.1. Sodium Dodecyl Sulfate (SDS)
SDS is a strong ionic detergent and is the most widely used agent in corneal decellularization practices (either alone or in combination with other agents) due to its extreme efficiency in removing cells and solubilizing cellular membranes.22–30 Numerous studies have reported ‘complete’ or ‘sufficient’ decellularization at different concentrations and exposure times.19,22–24 However, Sasaki et al. was not able to replicate the findings despite using higher concentrations and the same or prolonged time-points.30 These results indicate that other conditions, besides exposure time and concentration, may be important to reach sufficient corneal decellularization. In this regard, studies have suggested different optimal conditions. For instance, Zhou et al. recommended 0.1% (wt/vol) SDS for 7 hours at 37°C24, Gonzalez-Andrades et al. recommended 0.1% (vol/vol) SDS for 48 hours at room temperature with 300 rpm continuous shaking17, and Du and Wu recommended 0.5% (wt/vol) SDS for 24 hours at 4°C with protease inhibition and continuous shaking22.
Despite its effectiveness in decellularization, SDS has undesirable effects on the cornea (especially in high concentrations) including matrix edema, disruption of collagen layers, disordered fibrils, and reduced transparency. Nonetheless, these effects can be managed by decreasing the concentration and/or exposure time. There is a delicate balance between decellularization and damage. Therefore, determining the best conditions with the least possible damage to the cornea is of upmost importance.24 To restore transparency (the effect of edema, and/or disrupted collagen conformation), glycerol and dextran can be utilized.20,31,32 In some animal studies, reduced transparency, edema, and disrupted histo-architecture gradually returned to normality after corneal transplantation.22–24,26
2.2. Triton X-100 (TX)
TX is a non-ionic detergent and the second most commonly-used detergent in corneal decellularization experiments.17–19 Its decellularization activity is based on the disruption of lipid-to-lipid and lipid-to-protein interactions, but not protein-to-protein interactions.33 The efficacy of decellularization agents greatly fluctuates with the tissue being treated. For instance, while TX is able to decellularize cartilage, corneal decellularization studies have shown great variability.34 Du et al. found that decellularization of pig corneas using TX was unachievable, regardless of high concentrations (5% w/v) and prolonged time (96h).17 Others have also failed to obtain satisfactory decellularization on pig and human corneas.17,30,32
These studies suggest that TX alone is not a strong enough detergent to sufficiently decellularize corneas. On the other hand, since TX is not able to break protein-to-protein interactions, disruption of the histo-architecture is not as complete as with SDS, and there remains reduced transparency and edema after use of TX.30,35
2.3. Hypertonic Saline (HS)
HS has been used to decellularize human, porcine, rabbit, and ostrich corneas.3,8,35,36 HS is an effective decellularization agent due to its ability to disrupt DNA-to-protein interactions and to induce osmotic lysis.21 HS is able to ‘completely’ decellularize corneal tissue on its own or in combination with TX, though its effects on natural ultrastructure are variable.20,29,37 For instance, Lee et al. obtained decellularized opaque corneas by using 1.5 mol/L HS for 24 hours, but Luo et al. obtained decellularized transparent corneas by using 2 mol/L for 30 min.20,38 HS protocol treated porcine corneas resulted in well-maintained collagen fibrils after the transplantation into the rabbit corneal bed.37
Choi et al. transplanted HS-treated and fresh porcine corneas to nonhuman primates. The decellularized corneas showed encouraging results over the fresh corneas with less rejection rate and longer graft survival.14 On the other hand, TX-assisted HS is one of the methods that has been used in a clinical trial.39 A different approach to decellularize corneas was implemented in which corneas were first soaked in ultrapure water to induce swelling, after which 2 mol/L HS was used to decellularize, followed by a wash with 0.2% TX. Dehydration and sterilization has been achieved by using glycerol and cobalt-60 irradiation, respectively.39
2.4. Human Serum with Electrophoresis
Galactose-α−1,3-galactose (α-Gal) is the major carbohydrate antigen to porcine cells.40 Cohen et al. found that α-Gal expression exists in the limbal and stromal areas of porcine corneal tissue but not in the endothelium and epithelium.41 However, Lee et al. demonstrated increased α-Gal expression in all layers of cornea after xenotransplantation.42 The protocol (originally reported by Shao et al.43) is based on the cytotoxic effect of human serum to α-Gal antigenic wild-type porcine cells through binding of anti-Gal IgM and IgG antibodies, which induce complement-mediated cell destruction.43 This method equates with the effect of pig corneas in which α-Gal gene-knockout has been achieved by genetic engineering. Anterior and posterior lamellas have been used separately after decellularization, and the results showed a decreasing number of cells in the anterior, but not posterior, stroma.43 These findings indicate heterogeneity of corneal stromal structure and keratocytes.
The aim of using electrophoresis after exposure to human serum is to enhance the removal of dead cells. Evaluation of the biomechanical properties and collagen fibril interactions of DPCX demonstrated ultrastructural similarity to native corneas, with remarkable transparency.44
2.5. High Hydrostatic Pressure (HHP)
The main decellularization mechanism of the HHP method is the disruption of cellular membranes. In this method, a pressure of 980 MPa or 10.000 kPa is applied to the cornea for 10 min at 10°C or 30°C. In order to achieve sufficient decellularization and maintain corneal histoarchitecture, corneas must then be washed with PBS or EGM-2 medium containing dextran and DNase I.10,18,30 Applying the HHP method at 10°C instead of 30°C maintained collagen fibrils and interactions to a greater extent.45 When compared to TX and SDS, HHP preserved more glycosaminoglycans, removed more DNA content, and decellularization was achieved in a shorter time-frame.45,46 Hashimoto et al. have performed anterior lamellar and intra-lamellar keratoplasties in animals, and showed that corneal transparency was preserved without any immune reaction for 6 months.10,18 HHP has many advantages, especially maintaining the native histo-architecture of the cornea, but the high expense of this technology is seen as a disadvantage.
2.6. Freeze-Thaw
Freezing and thawing lyses and removes the cells to produce a decellularized graft. Freezing can be carried out at −80°C (freezer) or −196°C (liquid nitrogen), and thawing should be carried out at a biological temperature of 37°C.47 Li et al. achieved sufficient decellularization through a freeze-thaw method by using different adjuvants, including DNase, RNase, and Tris hydrochloride, resulting in corneas that were transparent.48 Subsequent transplantation in rabbits showed consistent transparency, with no immune reaction for 3 months. However, neovascularization and inflammation was observed in amniotic membrane transplanted group during the same period.48 Lin et al. applied the freeze-thaw method in a different way, and were unable to obtain transparent corneas.49 However, after transplantation into rabbits, the corneas gradually regained transparency and the angiogenesis that developed immediately after transplantation started to regress after the first two weeks.49 Van den Bogerd et al. have successfully used the freeze-thaw method on anterior capsule of the lens to make a useful scaffold for corneal endothelial cells (CECs).50
2.7. Nitrogen (N2) Gas
The decellularization mechanism of N2 gas is by inducing apoptosis through the creation of a hypoxic environment. Amano et al. reported decellularization with complete transparency after treatment of porcine corneas with N2 gas for 7 days at room temperature. Furthermore, after transplantation in rabbits, N2 gas-treated corneas maintained transparency for 6 months.51 The maintenance of transparency could be a major advantage of the N2 gas method. However, future studies need to demonstrate the effects of decellularization on the mechanical strength and ultrastructure of N2 gas-treated corneal stroma. Lee et al. compared modified and classic N2 gas methods on porcine corneas, including methods using 2800 centi-gray irradiation and 4% TX. The modified methods were associated with ‘complete’ decellularization only when N2 gas treatment was combined with TX treatment.20
2.8. Phospholipase A2 (PLA)
PLA is a specific type of esterase which breaks down the ester bonds between the second fatty acid and the glycerol molecule of membrane glycerophospholipids. PLA activation requires a significant Ca2+ concentration.52 Wu et al. accomplished the sufficient decellularization of porcine corneas using PLA and sodium deoxycholate. Staining with hematoxylin and eosin demonstrated no cells or cellular components, but a small amount of nucleonic acid debris was detected on transmission electron microscopy. No significant change in mechanical properties and ultrastructure was observed. Lamellar keratoplasty performed in rabbits showed maintained transparency and significant biocompatibility, such that no immunological reaction occurred in the 12 months after transplantation.9
2.9. Glycerol with Chemical Crosslinking
Chemical crosslinking is defined as the intermolecular or intramolecular joining of two or more molecules by a covalent bond.53 The general purpose of using crosslinkers is to modify the mechanical, biological, and degradation properties of hydrogels.54 Lin et al. used glycerol to decellularize porcine corneas by creating a high osmotic pressure.55 Thereafter, decellularized corneas were treated with a crosslinker (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysulfosuccinimide) to improve biomechanical and biocompatibility properties, while also maintaining transparency. Additionally, this group used γ-irradiation to reduce immunogenicity. Following completion of these procedures, transparent and mechanically-stable corneas were obtained. Lamellar keratoplasty was performed in rabbits to treat fungal keratitis, and demonstrated that corneal grafts effectively controlled the infection while also maintaining transparency.55
2.10. Other Decellularization Methods
Sodium deoxycholate is an ionic detergent that can sufficiently decellularize corneal tissue, but only if treated for an extended period of time. This protocol has negative effects which result in the disruption of corneal histo-architecture, including Descement’s membrane and Bowman’s layer.19
‘Complete’ decellularization was not achieved by 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate (CHAPS, a zwitterionic detergent). In certain conditions, it resulted in the destruction of the corneal histo-architecture.18 Benzalkonium chloride is a cationic surfactant that was unable to ‘completely’ decellularize corneal tissue. Undesired effects occurred, including collagen damage and alteration of the fibrillary pattern.17 Octylphenoxypolyethoxyethanol (Igepal) is a non-ionic detergent that has been previously used for heart valve, pericardial, and vein decellularization.17 Although Igepal preserved the proteoglycans and fibrils very well, it was unable to achieve a significant percentage of decellularization.17
Trypsin is a protease that hydrolyses peptide bonds, and ethylenediaminetetraacetic acid (EDTA) is a chelating agent; the trypsin-EDTA combination has only been used in human corneal tissue engineering once, and was demonstrated to be a more effective way to decellularize corneas than SDS and TX. Furthermore, the trypsin-EDTA method preserved natural corneal features to a greater extent than did SDS and TX.23
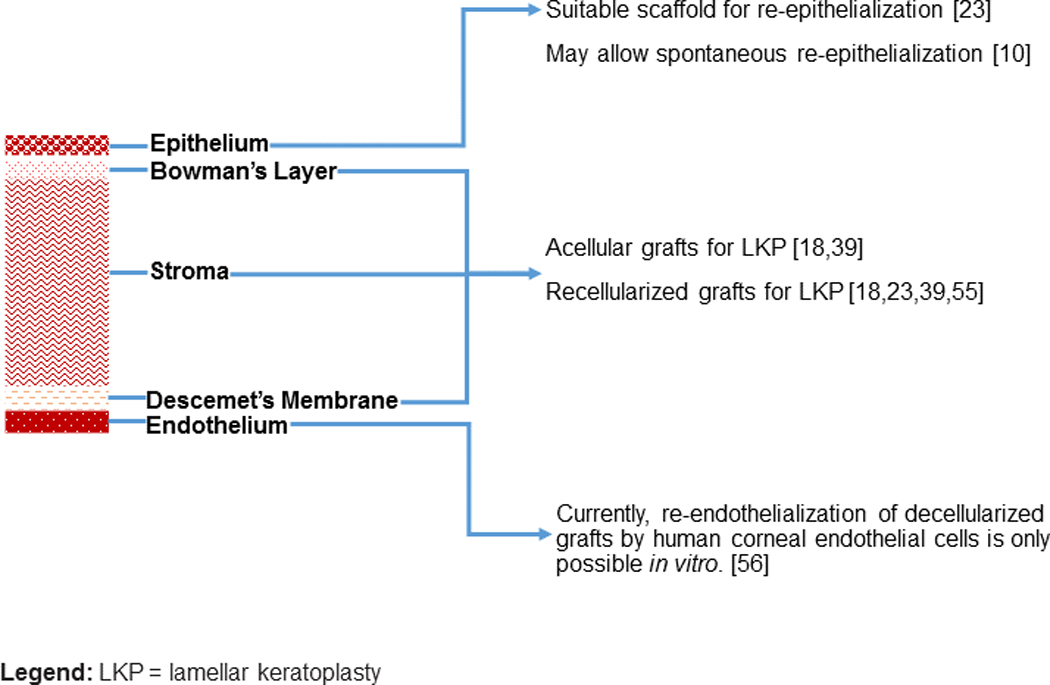
3. RECELLULARIZATION
Ideally, decellularized porcine corneas should have suitable stroma and surface features that allow for repopulation by multiple human corneal cell lines, including endothelial cells, keratocytes, and epithelial cells. However, creating an endothelial sheet (ES) might be the most important challenge to overcome. Unlike murine, rabbit, and bovine cells, human CECs have limited proliferative capability in vivo.56 Although decellularized and layered human corneal lamellae have shown biocompatibility with human CECs, making endothelialized human lamellae a feasible option for endothelial keratoplasty in the future. Currently, re-endothelialization by human CECs is only possible in vitro.57,58 This is a limitation of decellularized corneas that cannot be directly used in many corneal diseases with endothelial cell damage.
A comparative study found that the acellular human lens capsule is a better scaffold to create ES when compared with human Descemet’s membranes and human amniotic membranes.50 Lee et al. demonstrated a porcine model of corneal endothelialization, and successfully obtained an ES on DPCXs with porcine CECs.20 Recellularization of the posterior portion of the DPCXs with human CECs is necessary in order to perform endothelium included keratoplasties. Yoeruek et al. completed an DPCX endothelialization with hCECs by using a modified SDS method with EDTA and aprotinin for decellularization. However, in vitro studies are needed to investigate the biocompatibility of other decellularization methods.23
In animal studies, freeze-thaw, N2 gas, HHP, and SDS-treated DPCXs displayed repopulation of recipient keratocytes in graft stroma after lamellar keratoplasty.10,22,48,51 However, the recellularization activity of PLA and glycerol combined with chemical crosslinking seems to be a preferred alternative.9,57 On the other hand, SDS- and HS-treated DPCXs successfully repopulated with human keratocytes in vitro.23 HS has been found to be a convenient protocol for recellularization through its establishment of a more ideal environment for human keratocytes.23,28 Decellularized human corneas, by using PLA, have also been shown to have sufficient biocompatibility with human keratocytes.59 Human adipose-derived stem cells have also been used to recellularize decellularized human corneas, and differentiate into functional keratocytes in the stroma.60 This same technique exhibited functional biocompatibility with thin human corneal stromal laminas (created by femtosecond laser) which were successfully and safely transplanted into patients in a clinical trial.61 As an alternative to injections and cell cultures, bioreactor platforms can be a useful technique for the recellularization of stroma.
Epithelial repopulation of the cornea is a simpler process than repopulation of the other layers. Adequate graft epithelization has been detected on DPCXs obtained through different decellularization methods, including PLA, HHP, and freeze-thaw.9,10,48,49 In animal studies, Hashimoto et al. demonstrated the complete epithelialization of DPCXs within 3 months after lamellar keratoplasty.10 Likewise, the seeding of human limbal corneal epithelial cells onto the anterior surface of DPCXs resulted in successful epithelialization in 5–8 days.23
4. FUTURE PERSPECTIVES
In order to reach ‘sufficient or complete’ decellularization with less damage to the cornea, combinations of a strong and a weak decellularization agent might be useful. For instance, in 2015, Zhang et al. reported the results of the first known clinical trial by using DPCXs. Hypertonic saline was used to prepare the DPCXs with Triton-X 100. Lamellar keratoplasty was performed on 47 patients who had fungal keratitis, and progress was documented over three years.39 This study showed the safety and efficiency of using DPCXs to treat fungal keratitis, and encouraged more applications with new indications.39 In a recent clinical trial, Shi et al. reached the sufficient decellularization mainly using high hydrostatic pressure but they also utilized distilled water, glycerol, endonucleases and a protective medium. Lamellar keratoplasty was performed on 23 patients who had different type of corneal ulcers. This study also showed the safety of DPCXs for corneal ulcers with 22/23 graft survival for one year.62 Therefore, we suggest that combined methodologies should be used for ‘sufficient’ corneal decellularization.
Besides immune rejection and infection recurrence, xenozoonosis is also one of the most important concerns post-operatively. In the all potential microorganisms, porcine endogenous retrovirus (PERV) is the most significant vector for cornea.63,64 To date, no evidence has yet shown that PERV can be transmitted to hosts in preclinical and clinical trials.39,62
In conclusion, currently DPCXs seem to be the most viable option to use in corneal transplantation with limited indications (Figure 1). However, a Korean group is currently planning to initiate a clinical trial of corneal xenotransplantation using fresh porcine corneas65, thus bringing corneal xenotransplantation as a clinical therapy one step closer.
Figure 1:
What can decellularized porcine corneal xenografts (DPCXs) offer to corneal xenotransplantation?
ACKNOWLEDGEMENTS
This research was made possible by Indiana University Health and the Indiana Clinical and Translational Sciences Institute (CTSI), funded in part by grant # UL1TR001108 from NIH, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and the Advances in Medicine (AIM) grant from Cook Medical. Research reported in this publication was also supported by the Office of the Director, NIH under the award number S10OD023595. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Part of this research was also funded by IU Health Values Fund for Research Award. Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959, and by a grant from United Therapeutics.
ABBREVIATIONS
- CECs
Corneal endothelial cells
- DPCX
Decellularized porcine corneal xenograft
- ECM
Extracellular matrix
- ES
Endothelial sheet
- HHP
High hydrostatic pressure
- HS
Hypertonic saline
- LKP
Lamellar keratoplasty
- PA
Peptide amphiphiles
- PERV
Porcine endogenous retrovirus
- PLA
Phospholipase A2
- SDS
Sodium dodecyl sulfate
- TX
Triton X-100
- α-Gal
Galactose-α1,3-galactose
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.World Health Organization. Global Initiative for the Elimination of Avoidable Blindness: action plan 2006–2011. 2007. Available from URL: https://www.who.int/blindness/Vision2020_report.pdf. Accessed Sep 9, 2019.
- 2.Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Bikkuzin T, Song Z, et al. Comprehensive evaluation of decellularized porcine corneal after clinical transplantation. Xenotransplantation. 2017;24(6). [DOI] [PubMed] [Google Scholar]
- 4.Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016;134(2):167–173. [DOI] [PubMed] [Google Scholar]
- 5.Lamm V, Hara H, Mammen A, Dhaliwal D, Cooper DK. Corneal blindness and xenotransplantation. Xenotransplantation. 2014;21(2):99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinberg AW. Engineered tissue grafts: opportunities and challenges in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2012;4(2):207–220. [DOI] [PubMed] [Google Scholar]
- 7.De Stefano VS, Dupps WJ Jr., Biomechanical Diagnostics of the Cornea. Int Ophthalmol Clin. 2017;57(3):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Wang X, Wan P, et al. Tectonic lamellar keratoplasty with acellular corneal stroma in high-risk corneal transplantation. Mol Vis. 2011;17:1909–1917. [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Zhou Y, Li N, et al. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials. 2009;30(21):3513–3522. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Funamoto S, Sasaki S, et al. Corneal Regeneration by Deep Anterior Lamellar Keratoplasty (DALK) Using Decellularized Corneal Matrix. PLoS One. 2015;10(7):e0131989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JS, Williams JK, Greven M, et al. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31(26):6738–6745. [DOI] [PubMed] [Google Scholar]
- 12.Niederkorn JY. Corneal transplantation and immune privilege. Int Rev Immunol. 2013;32(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Front Immunol. 2012;3:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HJ, Kim MK, Lee HJ, et al. Efficacy of Pig-to-Rhesus Lamellar Corneal Xenotransplantation. Investigative Ophthalmology & Visual Science. 2011;52(9):6643–6650. [DOI] [PubMed] [Google Scholar]
- 15.Choi HJ, Lee JJ, Kim MK, et al. Cross-reactivity between decellularized porcine corneal lamellae for corneal xenobridging and subsequent corneal allotransplants. Xenotransplantation. 2014;21(2):115–123. [DOI] [PubMed] [Google Scholar]
- 16.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Andrades M, Carriel V, Rivera-Izquierdo M, et al. Effects of Detergent-Based Protocols on Decellularization of Corneas With Sclerocorneal Limbus. Evaluation of Regional Differences. Translational Vision Science & Technology. 2015;4(2):13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Hattori S, Sasaki S, et al. Ultrastructural analysis of the decellularized cornea after interlamellar keratoplasty and microkeratome-assisted anterior lamellar keratoplasty in a rabbit model. Sci Rep. 2016;6:27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du L, Wu X, Pang K, Yang Y. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br J Ophthalmol. 2011;95(3):410–414. [DOI] [PubMed] [Google Scholar]
- 20.Lee W, Miyagawa Y, Long C, Cooper DK, Hara H. A comparison of three methods of decellularization of pig corneas to reduce immunogenicity. Int J Ophthalmol. 2014;7(4):587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du L, Wu X. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif Organs. 2011;35(7):691–705. [DOI] [PubMed] [Google Scholar]
- 23.Yoeruek E, Bayyoud T, Maurus C, et al. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012;90(2):e125–131. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Wu Z, Ge J, et al. Development and characterization of acellular porcine corneal matrix using sodium dodecylsulfate. Cornea. 2011;30(1):73–82. [DOI] [PubMed] [Google Scholar]
- 25.Ponce Marquez S, Martinez VS, McIntosh Ambrose W, et al. Decellularization of bovine corneas for tissue engineering applications. Acta Biomater. 2009;5(6):1839–1847. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Qu M, Wang Y, Wang Z, Shi W. Xenogeneic acellular conjunctiva matrix as a scaffold of tissue-engineered corneal epithelium. PLoS One. 2014;9(11):e111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh M-I, Lee K-P, Kim J, Yi S, Park B-U, Kim Kyun H. Generation of Femtosecond Laser-Cut Decellularized Corneal Lenticule Using Hypotonic Trypsin-EDTA Solution for Corneal Tissue Engineering. Vol 20182018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslan B, Guler S, Tevlek A, Aydin HM. Evaluation of collagen foam, poly(l-lactic acid) nanofiber mesh, and decellularized matrices for corneal regeneration. 2018;106(6):2157–2168. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Andrades M, de la Cruz Cardona J, Ionescu AM, Campos A, Del Mar Perez M, Alaminos M. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest Ophthalmol Vis Sci. 2011;52(1):215–222. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki S, Funamoto S, Hashimoto Y, et al. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol Vis. 2009;15:2022–2028. [PMC free article] [PubMed] [Google Scholar]
- 31.Murab S, Ghosh S. Impact of osmoregulatory agents on the recovery of collagen conformation in decellularized corneas. Biomed Mater. 2016;11(6):065005. [DOI] [PubMed] [Google Scholar]
- 32.Lynch AP, Wilson SL, Ahearne M. Dextran Preserves Native Corneal Structure During Decellularization. Tissue Eng Part C Methods. 2016;22(6):561–572. [DOI] [PubMed] [Google Scholar]
- 33.Yam GH-F, Yusoff NZBM, Goh T-W, et al. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Scientific Reports. 2016;6:26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu K, Kuntz LA, Foehr P, et al. Efficient decellularization for tissue engineering of the tendon-bone interface with preservation of biomechanics. PLoS One. 2017;12(2):e0171577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SL, Sidney LE, Dunphy SE, Dua HS, Hopkinson A. Corneal Decellularization: A Method of Recycling Unsuitable Donor Tissue for Clinical Translation? Curr Eye Res. 2016;41(6):769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XN, Zhu XP, Wu J, et al. Acellular ostrich corneal stroma used as scaffold for construction of tissue-engineered cornea. Int J Ophthalmol. 2016;9(3):325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh JY, Kim MK, Lee HJ, Ko JH, Wee WR, Lee JH. Processing porcine cornea for biomedical applications. Tissue Eng Part C Methods. 2009;15(4):635–645. [DOI] [PubMed] [Google Scholar]
- 38.Luo H, Lu Y, Wu T, Zhang M, Zhang Y, Jin Y. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials. 2013;34(28):6748–6759. [DOI] [PubMed] [Google Scholar]
- 39.Zhang MC, Liu X, Jin Y, Jiang DL, Wei XS, Xie HT. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am J Transplant. 2015;15(4):1068–1075. [DOI] [PubMed] [Google Scholar]
- 40.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016;238(2):288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen D, Miyagawa Y, Mehra R, et al. Distribution of non-gal antigens in pig cornea: relevance to corneal xenotransplantation. Cornea. 2014;33(4):390–397. [DOI] [PubMed] [Google Scholar]
- 42.Lee HI, Kim MK, Oh JY, et al. Gal alpha(1–3)Gal expression of the cornea in vitro, in vivo and in xenotransplantation. Xenotransplantation. 2007;14(6):612–618. [DOI] [PubMed] [Google Scholar]
- 43.Shao Y, Yu Y, Pei CG, et al. Evaluation of novel decellularizing corneal stroma for cornea tissue engineering applications. Int J Ophthalmol. 2012;5(4):415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao Y, Tang J, Zhou Y, et al. A novel method in preparation of acellularporcine corneal stroma tissue for lamellar keratoplasty. Am J Transl Res. 2015;7(12):2612–2629. [PMC free article] [PubMed] [Google Scholar]
- 45.Funamoto S, Nam K, Kimura T, et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. 2010;31(13):3590–3595. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto Y, Funamoto S, Sasaki S, et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31(14):3941–3948. [DOI] [PubMed] [Google Scholar]
- 47.Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017;2017:9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q, Wang H, Dai Z, Cao Y, Jin C. Preparation and Biomechanical Properties of an Acellular Porcine Corneal Stroma. Cornea. 2017;36(11):1343–1351. [DOI] [PubMed] [Google Scholar]
- 49.Lin XC, Hui YN, Wang YS, Meng H, Zhang YJ, Jin Y. Lamellar keratoplasty with a graft of lyophilized acellular porcine corneal stroma in the rabbit. Vet Ophthalmol. 2008;11(2):61–66. [DOI] [PubMed] [Google Scholar]
- 50.Van den Bogerd B, Ni Dhubhghaill S, Zakaria N. Characterizing human decellularized crystalline lens capsules as a scaffold for corneal endothelial tissue engineering. J Tissue Eng Regen Med. 2018;12(4):e2020–e2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amano S, Shimomura N, Yokoo S, Araki-Sasaki K, Yamagami S. Decellularizing corneal stroma using N2 gas. Mol Vis. 2008;14:878–882. [PMC free article] [PubMed] [Google Scholar]
- 52.Murakami M, Kudo I. Phospholipase A2. Vol 1312002. [Google Scholar]
- 53.Arora B, Tandon R, Attri P, Bhatia R. Chemical Crosslinking: Role in Protein and Peptide Science. Curr Protein Pept Sci. 2017;18(9):946–955. [DOI] [PubMed] [Google Scholar]
- 54.Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol. 2018;107(Pt A):678–688. [DOI] [PubMed] [Google Scholar]
- 55.Lin Y, Zheng Q, Hua S, Meng Y, Chen W, Wang Y. Cross-linked decellularized porcine corneal graft for treating fungal keratitis. Sci Rep. 2017;7(1):9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Sun H, Hu M, Zhu M, Tighe S, Chen S, Zhang Y, Su C, Cai S, Guo P. Human Corneal Endothelial Cells Expanded In Vitro Are a Powerful Resource for Tissue Engineering. Int. J. Med. Sci. 2017, Vol. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, Niu G, Choi JS, Giegengack M, Atala A, Soker S. Bioengineered multilayered human corneas from discarded human corneal tissue. Biomed Mater. 2015;10(3):035012. [DOI] [PubMed] [Google Scholar]
- 58.He Z, Forest F, Bernard A, et al. Cutting and Decellularization of Multiple Corneal Stromal Lamellae for the Bioengineering of Endothelial Grafts. Invest Ophthalmol Vis Sci. 2016;57(15):6639–6651. [DOI] [PubMed] [Google Scholar]
- 59.Xiao J, Duan H, Liu Z, et al. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials. 2011;32(29):6962–6971. [DOI] [PubMed] [Google Scholar]
- 60.Alio del Barrio JL, Chiesa M, Garagorri N, et al. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res. 2015;132:91–100. [DOI] [PubMed] [Google Scholar]
- 61.Alio Del Barrio JL, El Zarif M, Azaar A, et al. Corneal Stroma Enhancement With Decellularized Stromal Laminas With or Without Stem Cell Recellularization for Advanced Keratoconus. Am J Ophthalmol. 2018;186:47–58. [DOI] [PubMed] [Google Scholar]
- 62.Shi W, Zhou Q, Gao H, Li S, Dong M, Wang T, Jia Y, Dong C, Wang X, Guo Z, Zhao L, Hu X, Xie L. Protectively Decellularized Porcine Cornea versus Human Donor Cornea for Lamellar Transplantation. Adv. Funct. Mater. 2019, 29, 1902491. [Google Scholar]
- 63.Song YW, Pan ZQ. Reducing porcine corneal graft rejection, with an emphasis on porcine endogenous retrovirus transmission safety: a review. Int J Ophthalmol. 2019;12(2):324–33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi HJ, Kim J, Kim JY, et al. Long-term safety from transmission of porcine endogenous retrovirus after pig-to-non-human primate corneal transplantation. Xenotransplantation. 2017;24(4):10.1111/xen.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi HJ, Yoon CH, Hyon JY, et al. Protocol for the first clinical trial to investigate safety and efficacy of corneal xenotransplantation in patients with corneal opacity, corneal perforation, or impending corneal perforation. Xenotransplantation. 2019;26(1):e12446. [DOI] [PubMed] [Google Scholar]


