Abstract
Background:
We have achieved greater than a 6-month survival of a life-supporting kidney co-transplanted with a vascularized thymic graft into non-human primates (NHP). Although we have achieved pig-specific unresponsiveness in vitro, immunosuppression was not able to be fully weaned. Studies in mice and humanized mice suggest that a hybrid pig thymus (Hyb-thy) containing host thymic epithelial cells (TECs) can optimize intra-thymic selection, achieving xenograft tolerance with improved reconstitution of T cell function.
Methods:
We have tested the feasibility of preparation of a Hyb-thy that contains NHP TECs in the donor thymic grafts. We first prepared the Hyb-thy in the donor pigs 2–3 weeks before xeno-Tx. We performed 6 cases of Hyb-thy preparation in six juvenile miniature swine. Two pigs received non-manipulated cynomolgus monkey thymic cells that were isolated from an excised atrophic thymus via injection into their thymic lobes (Group 1). The remaining four received thymic cells that were isolated from non-atrophic thymic glands (Groups 2 and 3). Pigs in Group 2 received un-manipulated thymic cells in one thymic lobe, as well as CD2 positive cell-depleted TEC-enriched cells in the contralateral lobe. Pigs in Group 3 received TEC-enriched cells alone.
Results:
All thymus-injected pigs received tacrolimus and rapamycin until endpoint (POD16). We detected cynomolgus monkey TEC networks in pig thymus from Groups 1 and 3, while pigs in Group 2 rejected the thymic cells. We demonstrated preparation of Hyb-thy in pigs using tacrolimus plus rapamycin therapy.
Conclusion:
Our results suggest that enrichment of TEC from the excised NHP thymus facilitated NHP TEC engraftment in pig thymus.
Keywords: Vascularized thymus, Hybrid Thymus, Pig, Non-human primate, Xenotransplantation
Introduction
Recent technological advancements in genomic manipulation have markedly improved the efficiency of multiple gene manipulations in porcine xeno-transplantation (xeno-Tx) donors 1, 2. Utilizing this technology, xeno-Tx from multi-transgenic alpha-1,3-galactosyltransferase knockout (GalT-KO) pigs has demonstrated marked prolongation of renal xenograft survival ranging from days to greater than six months in a life-supporting model 3–5 , >12 months in islets in induced diabetes in NHPs 6–10 , and >2 years of a heterotopic non-life-supporting cardiac xenograft in pig-to-baboon models 10. More recently, six months’ survival of baboons bearing life-supporting porcine cardiac grafts in baboons has been reported 11. These results have since allowed xeno-Tx to become a much more realistic strategy in regards to solving the organ shortage crisis. However, in order to prevent rejection, continuous administration of multiple immunosuppressive drugs is still required. Even though these current immunosuppressive regimens seem to be controlling the T cell responses 3–5, 10, 12, it appears that low levels of T cell-dependent antibodies 13, 14 and the activation of innate immune responses 15 still led to xenograft rejection. Shin et al. reported that >500 days engrafted pig islets in NHPs were fully rejected by activated immune cells, particularly CD4+ and CD8+ T cells, when immunosuppressive maintenance drugs were discontinued 16. Therefore, approaches utilizing specific tolerance induction must be included to avoid persistent immune reactivity.
Thymic Tx has proven to be a powerful strategy to induce T cell tolerance across both allogeneic and xenogeneic barriers in pig-to-pig, pig-to-mouse and pig-to-humanized mouse models 17,18,19,20. We have developed two techniques for transplanting a vascularized thymic graft which allow the donor thymus to function immediately after revascularization. One technique is “composite thymo-kidney (TK)” 21 and the other is “vascularized thymic lobe graft (VTL)” 22. Using these two techniques, we have demonstrated that vascularized thymic tissue can successfully induce tolerance and support thymopoiesis across fully allogeneic barriers in MGH miniature swine 23,24,25. By extending this strategy to pig-to-baboon models, we have recently achieved survival of a life-supporting TK for over 6 months 26. Notably, multiple recipients bearing a functional TK for over 3 months developed donor specific unresponsiveness at the T and B cell levels 26,27. Although new thymic emigrants (CD4+/CD31+/CD45+) were developed (Yamada K et al. manuscript in preparation) beyond 2 months following transplantation, we have not yet been able to fully stop immunosuppression, although we have been successful with tapering it markedly 26. Therefore, additional strategies are required for the rapid induction of tolerance in the pig-to- NHP model.
While robust tolerance is induced by xenogeneic thymic Tx 17,18, sixty percent of grafted nude mice and 10% of grafted thymectomized B6 mice developed a clinical illness resembling chronic graft-versus-host disease in T cell-depleted, thymectomized mice that received fetal pig thymus grafts. This is thought to be due to the incomplete negative selection of host tissue-specific antigens and insufficient development of T regulatory cells (Tregs) with those specificities 28. It was subsequently found that this can be overcome by injecting recipient TECs into the porcine thymus graft 29. More recent data have demonstrated that a hybrid pig thymus containing human hematopoietic stem cell donor TECs (Hyb-thy) optimizes intra-thymic selection of human T cells which are tolerant of both the pig and human in humanized mouse models (Maharlooei MK. Data presented in the IXA 2017). Likewise, we hypothesize that Tx of a vascularized hybrid pig VTL (Hyb-VTL) graft, which contains the recipient baboon’s TECs, into the recipient baboon will permit rapid and stable induction of tolerance with rapid development of thymopoiesis as well as thymic selection. However, our technique for transplanting a vascularized thymic graft is specifically designed for large animals as we use pigs as donors, and it would not be possible to perform this procedure in a pig-to-humanized mouse model. Therefore, we first examined whether preparation of Hyb-thy is both technically and immunologically feasible in pigs for transplant as a Hyb-VTL graft. In this study, we determined the optimal conditions as well as an immunosuppressive regimen to prepare Hyb-VTL which is specifically designed for pig-to-NHP Tx as well as possible future clinical trials. Results in this study demonstrate that NHP thymic TECs successfully engraft in miniature swine cervical thymic lobes, indicating the success of Hyb-VTL graft preparation.
Materials and Methods.
Animals
Recipients:
CLAWN miniature swine (n=6) between the ages of 5 and 6 months old and weighing between 12.0 to 20.9 kg were obtained from the Miniature Swine Research Institute, Isa, Kagoshima, Japan 30.
Donors:
Cynomolgus monkeys were used as a source of NHP thymic cells. Male cynomolgus monkeys (n=3) between the ages of 3 to 4 years old, weighing between 3.7 kg and 4.9 kg, were purchased from the Shin Nippon Biomedical Laboratories, Ltd., Kagoshima, Japan. The study protocol was approved by the Ethical Committee of the Faculty of Medicine at Kagoshima University in Japan, and all animal care and procedures were performed in accordance with the guidelines of the National Society for Medical Research and the ‘‘Guide for the Care and Use of Laboratory Animals’’ prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health.
Experimental follow up period following NHP thymic cell implantation:
Since our protocol for transplanting pig kidneys with VTL xeno-Tx (K+VTL xeno-Tx) includes a host thymectomy 2–3 weeks prior to K+VTL xeno-Tx 27,31, we used this same 2–3 week period in this experiment to prepare the Hyb-VTL in the donor swine. In order to determine whether this period is feasible for preparation of a Hyb-VTL, we set the end points of this study at 16 days after cynomolgus monkey thymic cell implantation into the thymic lobes of the miniature swine.
Experimental Groups:
Miniature swine recipients were divided into 3 groups depending on the proportion of injected NHP thymic tissues (Table 1). Animals in Group 1 (n=2) received digested NHP thymic cells injected into their cervical thymic lobes using a 24-gauge needle (details in Surgical Procedures). Animals in Group 2 (n=2) received injections of (1) NHP CD2-depleted thymic cells into the right cervical thymic lobes and (2) NHP non-depleted thymic cells into the left cervical thymic lobes to determine the effects of thymocyte depletion from injected populations. CD2 sorting of thymic cells was performed using negative selection with anti-human CD2 Ab (abcam, ab185791, MA) 32 and MACS (Milteny Biotech, CA). Animals in Group 3 (n=2) received an injection of CD2-negative cells at all three sites in the recipient’s right thymic lobes. The number of cells injected into each site is shown in Table 1.
Table 1:
Experimental groups
| Group | Animal ID age |
Thymic Donors IDs Age |
Thymic atrophy | CD2 depletion | Number of cells to inject |
|---|---|---|---|---|---|
| Group 1 | #28022 (5.5m) |
M28405 4y1m |
+ | − | Digested cells but contained only a small number of thymic cells (6.4×106 cells) |
| #28023 (5.5m) |
Digested cells but contained only a small number of thymic cells (6.4×106 cells) | ||||
| Group 2 | 28090 (6m) |
M28406 4y11m |
− | Left lobe
− Right lobe + |
Left lobe: Digested cells with a large
number of thymic cells (50×106 cells) Right lobe: CD2-cells (1.6×106 cells) |
| 28091 (6m) |
Left lobe
− Right lobe + |
Left lobe: Digested cells with a large
number of thymic cells (50×106 cells) Right lobe: CD2-cells (1.6×106 cells) |
|||
| Group 3 | 29003 (5m) |
M29401 (3y7m) |
− | + | CD2-cells (1.8×106 cells) |
| 29004 w(5m) |
CD2-cells (1.8×106 cells) | ||||
Immunosuppressive regimen:
Since we found that pig anti-cynomolgus monkey T cell responses are similar to swine anti-swine allogeneic responses and that pigs also have preformed IgM against cynomolgus monkeys (data in Results section), we started rapamycin and tacrolimus therapy prior to injection of the thymic cells into the pig recipients (Fig. 1). All miniature swine received rapamycin at 1.0 mg/kg/day through a G-tube and 0.1–0.15 mg/kg/day of tacrolimus via continuous infusion through a central line to adjust levels between 30 and 35 ng/ml, starting 13 days before the cynomolgus monkey thymic injection and continuing until post-operative day (POD) 16 (Fig. 1). This target level was determined based on our previous study on the induction of tolerance of fully mismatched kidneys in miniature swine 33.
Fig 1:
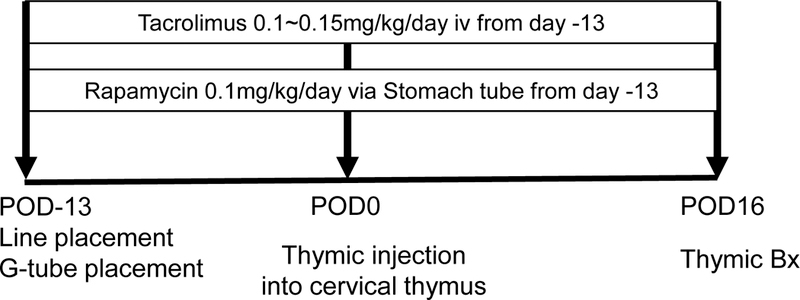
Immunosuppressive protocol. All recipients received bilateral central line insertions and G-tube placement on Day −13 and 0.1 mg/kg of rapamycin was given via G-tube every day. Tacrolimus was also administered at 0.1 ~ 0.15 mg/kg/day to maintain target blood levels between 30 to 35 ng/ml until the end of the experimental period. Thymic injection was done on Day 0, and thymic biopsies were performed on POD 16.
Preparation of digested NHP thymic cells to administer into thymic lobes of recipient swine
A schematic diagram of the preparation of thymic tissue is shown in Fig. 2. Resected cynomolgus monkey thymic tissue was cut into small pieces (2 square mm) with sterile scissors. Thymic pieces were transferred to a 50ml conical with 10 ml of digestion buffer which contained 1U/ml liberase with 0.1% DNAse I in RPMI by dissolving 5mg liberase (26 U) in 26ml RPMI and 0.26ml DNase 1mg/ml (10% DNase). The pieces were then incubated for 60 minutes at 37 °C. The digested thymic tissues were filtered with a mesh to remove remaining fibrotic or fatty tissue. Eluted thymic cells were centrifuged at 1600 rpm/5 min and washed with HBSS (Invitrogen, Grand Island, NY). Non-sorted thymic cells were injected into the cervical thymic lobes of pigs in Group 1 and Group 2. All porcine thymocytes express CD2 34. Therefore, in order to determine the effects of thymocytes on engraftment in Groups 2 and 3, CD2 negative selection was performed to prepare a TEC-enriched population. Anti-human CD2 monoclonal antibody (Abcam, ab185791, MA) 32 was used to collect the CD2 negative population using MACS separation methods (Milteny Biotech, CA) 35,36. >97% of cells were CD2 negative in the eluted population (Fig. 2).
Fig 2:
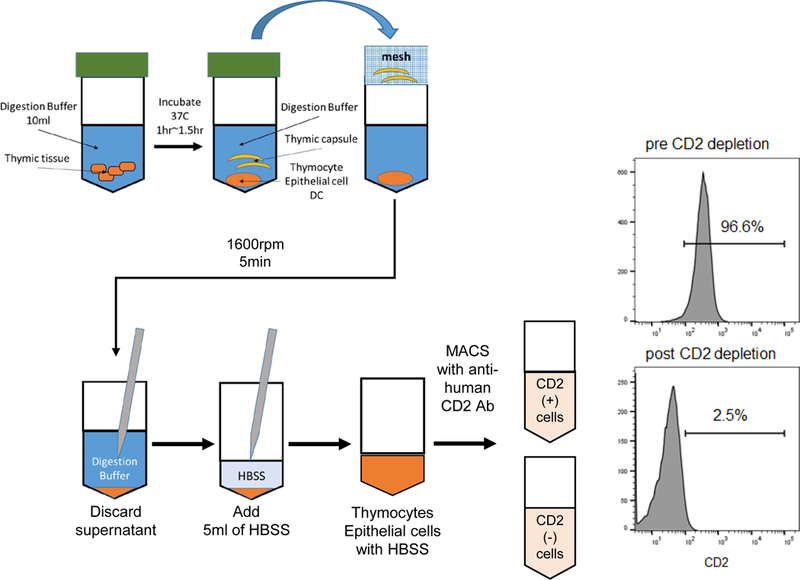
Preparation of injected thymic cells from resected cynomolgus monkey’s thymus. FCM profile of CD2 staining of non-atrophic cynomolgus thymus; pre CD2 depletion (right top panel) and post CD2 depletion (right bottom panel).
Surgical Procedures:
The following surgical procedures were performed in the operating room under general anesthesia. All swine received central venous lines inserted into bilateral external jugular veins 14 days prior to thymic injection. One line was used specifically for administration of tacrolimus via 24-hour continuous infusion pumps 37, and the other was used for blood collection. Gastric tubes (G-tube) were placed into the recipient’s stomach via the abdominal wall for administration of rapamycin (Pfizer Inc., Philadelphia). NHP donor thymic tissues were obtained via thymectomy on the day of thymic injection. Details of preparation of thymic tissues and enrichment of TECs are described in a previous section. Thymic injections into cervical lobes of miniature swine were performed via cervical incision in the recipients on the same day as thymectomy of the cynomolgus monkey donors (POD 0). Thymic tissue was injected into cervical thymic lobes using a 24-g angio-catheter. Thymic capsules of the injection sites were closed with 7–0 prolene to prevent leakage of injected cells as well as to mark the injection sites. The cervical incision was closed with 3–0 Vicryl sutures. The right column in Table 1 indicates the sites and numbers of NHP thymic cells injected into miniature swine recipients. Thymic biopsies were performed via a 2–3-inch cervical incision at POD 4 or 7 and POD 16 after thymic tissue injection.
Laboratory assessment:
To assess the condition of the animals, complete blood count (CBC) and blood chemistry were assessed twice a week until termination (POD 16). Immunologic assessments were performed by carboxyfluorescein diacetate succinimidyl ester (CFSE) - mixed lymphocyte reaction (MLR) assays as well as anti-donor antibody development in sera of recipients (pre and POD 16). Histological examination to elucidate the chimeric formation of cynomolgus monkey donor thymic cells in pig thymus was performed by hematoxylin and eosin (H&E) as well as immunostaining (described below).
Preparation of PBMC in swine and NHP:
PBMCs were prepared from freshly collected, heparinized whole blood. Mononuclear cells were obtained by gradient centrifugation using HISTOPAQUE (Sigma, St Louis, MO). The cells were washed once with HBSS (Invitrogen, Grand Island, NY). Contaminating red cells were lysed with ACK buffer (B&B Research Laboratory, Fiskeville, RI) for swine PBMC, and with 1.26 Mm CaCl2 containing FACS™ lysing Solution (BD Biosciences, San Jose) for cynomolgus monkey PBMC, and the cells were washed again with HBSS and re-suspended in tissue culture medium. All cell suspensions were stored at 4 °C.
Assessment of immuno-responses by CFSE-MLR
MLR cultures were performed as previously reported 38. CFSE MLR assays were performed by plating 2×106 CFSE-labeled pig PBMC as responder cells, in triplicate in 24-well flat-bottom plates (Costar, Corning, NY). Cells were stimulated with 2×106 stimulator cells (irradiated with 3000 cGy). Three-color flow cytometry measurements (FCM) were carried out on a FACSVerse (Becton Dickinson, Mountain View, CA) using standard Cell Quest acquisition and analysis software. Stimulation index (SI) as a measure of the allo-specific reactivity of responder cells were determined by CFSE fluorescence intensities as previously described 39, 40.
Assessment of anti-donor antibodies
Anti-NHP IgM and IgG antibodies (Ab) in the serum samples were assessed by indirect FACS as previously reported 23.
Histologic assessment of engraftment of NHP donor thymic epithelial cells (TECs) in swine thymic lobes
All miniature swine recipients underwent thymic biopsies on POD 16. Samples were prepared by 10% formalin fixation and paraffin embedding, followed by H&E. To detect TECs, immunohistochemistry for cytokeratin (CK) was performed using monoclonal mouse anti-human cytokeratin Ab (AE1/AE3, DAKO). In addition, to assess the network construction of NHP TECs and porcine TECs in pig thymic lobes, double immunostaining with anti-cytokeratin Ab (AE1/AE3 or WSS) and anti-HLA class I (anti-HLA ABC Ab, W6/32, GeneTex, Irvine, CA) was performed. To determine thymopoiesis around NHP TECs, double immunostaining for pig CD1 Ab (76–7-4)) and CK Ab (AE1/AE3) was performed, and accumulation of pig CD1 cells in and around CK+ TECs with linear structures was analyzed. All biopsy samples were assessed by a blinded pathologist using light microscopy.
Results
Pigs have anti-cynomolgus monkey cellular responses as well as preformed antibodies
SLA types of CLAWN miniature swine were either SLA C1, C2 or mixed SLA 38. We tested C1-type and C2 type CLAWN miniature swine anti-cynomolgus monkey, anti-allogeneic CLAWN or anti-human MLR responses. As shown in Fig 3A and B, both naïve C1 and C2 CLAWN miniature swine had anti-cynomolgus monkey MLR responses similar to allo CLAWN or human MLR responses. We also tested anti-cynomolgus monkey preformed IgM and IgG Ab using the actual recipients’ sera that were collected prior to starting immunosuppression. Although no CLAWN pigs (n=6) had anti-cynomolgus monkey preformed IgG, all had anti-cynomolgus monkey IgM preformed natural antibodies (nAb).
Fig 3:
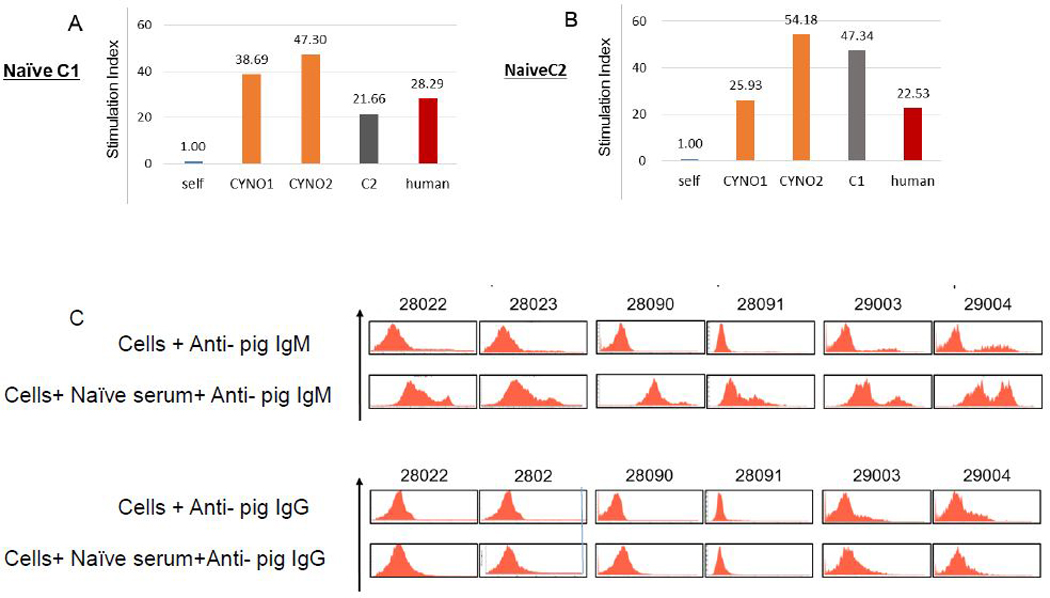
Immuno-responses of naive C1 and C2 CLAWN miniature swine. A, B) MLR responses of C1 and C2 CLAWN miniature swine against the Ag of self, allogeneic swine, cynomolgus monkey, and human as a third party. C) Anti-cynomolgus monkey preformed nAb of IgM (upper panel) and IgG (lower panel) in CLAWN miniature swine.
Tacrolimus levels were not different between the three groups
All miniature swine survived throughout the experimental period without any side effects from immunosuppression and surgery. Blood levels of tacrolimus averaged at 34.8 ± 9.4 ng/ml in Group 1, 31.8±2.1 ng/ml in Group 2, and 33.8±2.4 ng/ml in Group 3. More specifically, blood levels of tacrolimus during the period before NHP thymic injections (from day −14 to Day 0) were an average at 36.4±10.3 ng/ml in Group 1, 35.4±2.7 ng/ml in Group 2, and 32.2±4.0 ng/ml in Group 3. Blood levels of tacrolimus after thymic injection (Day 0 to POD 16) were 32.5±7.9 ng/ml, 28.4±1.7 ng/ml, and 32.0±1.0 ng/ml respectively. Assays for blood concentration of rapamycin were not available in our laboratory.
Engraftment of NHP TECs in miniature swine thymi in Groups 1 and 3 but not in Group 2
Excised porcine thymic lobes with thymic injection sites in Group 1 animals showed successful engraftment of NHP TECs (Fig. 4). Although the age of the cynomolgus monkey used in Group 1 was similar to the ages of the animals in Groups 2 and 3, the thymus of the cynomolgus monkey in Group 1 was grossly atrophic. Only 12.8×106 thymic cells were isolated from the atrophic cynomolgus monkey thymus, as opposed to >100×106 thymic cells from each of the thymi from Groups 2 and 3, which were grossly non-atrophic (Table 1). Fig 4 panels show the recipient thymic samples from pig #28023 which are similar to the other pig (#28022) in Group 1. No injection sites in the pig thymus (Fig. 4A and B) had well identified lobular structures. Many cytokeratin positive (CK+) native pig cells were differentiated in the round-shaped medullar zones in the center of each lobule (Fig 4B). The thymic injection sites, which were marked with a 7–0 prolene suture at the time of injection, showed loss of lobular structure (Fig. 4C) as well as many CK+ cells with linear structures (Fig. 4D, E and F). With a high-power view (Fig. 4F), morphologically Hassall’s body as well as a well-maintained round structure of medullary area were found beside the linear structure. Post-injection findings of the other pig (#28022) were similar.
Fig 4:
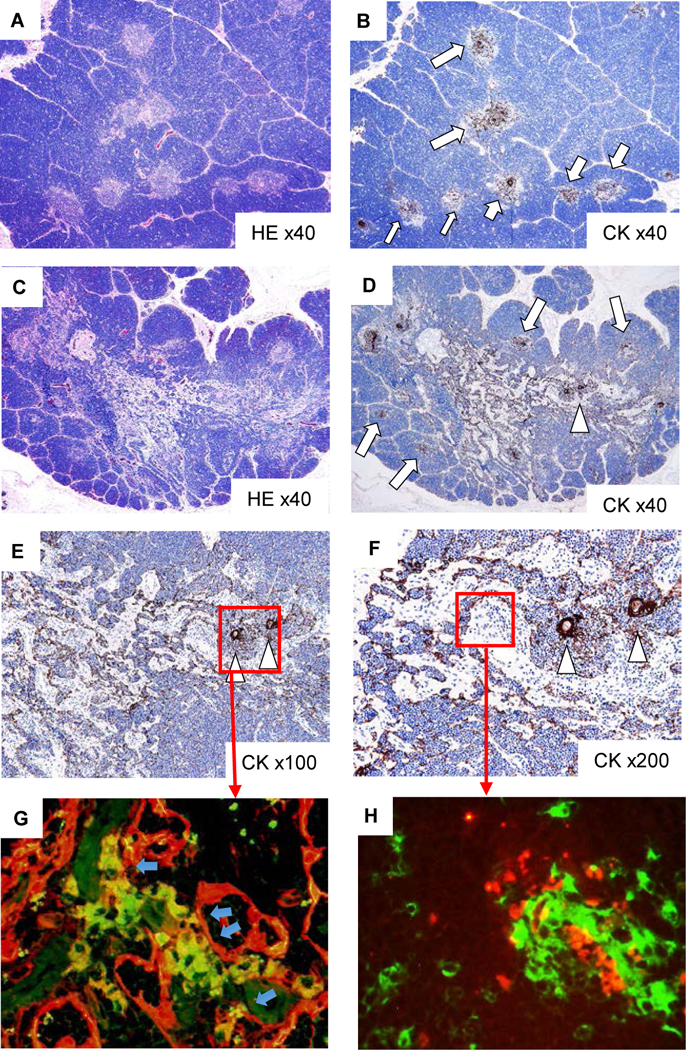
Histologic findings of injected sites of cynomolgus monkey thymic cells in porcine cervical thymic lobes at POD 16 in Group 1 (#28022), demonstrating successful engraftment of injected cynomolgus monkey thymic cells. In non-injection sites of porcine thymus (A: HE staining, B: CK staining), well preserved cortex and medullary structure (A) was noted in thymic lobes with CK+ thymic epithelial cells (TECs) (arrows in B) in medulla. In the injection sites of porcine thymus (C: HE staining, D: CK staining), loss of lobular structure where many CK+ TECs with linear structures (arrowhead in D) were observed. Thymic medulla with CK+ TECs also remained around these areas (E, F: CK staining) and linear structures of CK+ TECs were connected to CK+ TECs in thymic medulla. In double staining with CK and HLA class I (G), the network of injected cynomolgus monkey-TECs (yellow) and porcine TECs (red) were developed. In double staining with CK (red) and pig CD1 (green) (H), the porcine CD1+ cells accumulated around CK+ TECs with linear structure, suggesting thymopoiesis around cynomolgus monkey-TECs.
To confirm whether these linear structures were injected cynomolgus monkey TECs, we stained the injection site of porcine thymic tissue with CK (red) and human class I HLA-ABC (green) (Fig. 4G). We detected HLA-ABC positive CK+ cells (yellow), indicating that injected cynomolgus monkey CK cells were located between HLA-ABC negative CK cells (porcine CK. red). Moreover, we found pig CD1 positive cells (green) (Fig. 4H) in the area of the CK network of linear structures (red square in Fig. 4F), suggesting that early porcine thymopoiesis developed in the NHP TEC network of the porcine Hyb-thy.
In contrast to the Hyb-thy in Group 1, the thymic injection sites of the excised porcine thymic lobes in Group 2 (that received both CD2 positive thymic cells and CD2 negative thymic cells) showed evidence of hemorrhagic changes (Fig. 5). One (#28090) had obvious hemorrhagic changes around hypo-cellular lesions in the thymic injection sites of the CD2 non-depleted thymic population (Fig. 5A), while focal hemorrhagic lesions were seen in the sites of the CD2 depleted population (Fig. 5B). Hypo-cellular lesions consisting of a small number of CK+ cells were discerned in both CD2 non-depleted as well as CD2 depleted sites. (Fig 5 C and D). The excised thymic tissue from the other pig (#28091. Fig. 5 E and F) showed slightly less obvious sites of hemorrhage, especially in the injection area of CD2-depleted thymic cells (Fig. 5F) when compared to those found in #28090. A few scattered CK+ cells were also observed. (Fig. 5 G and H)
Fig 5:
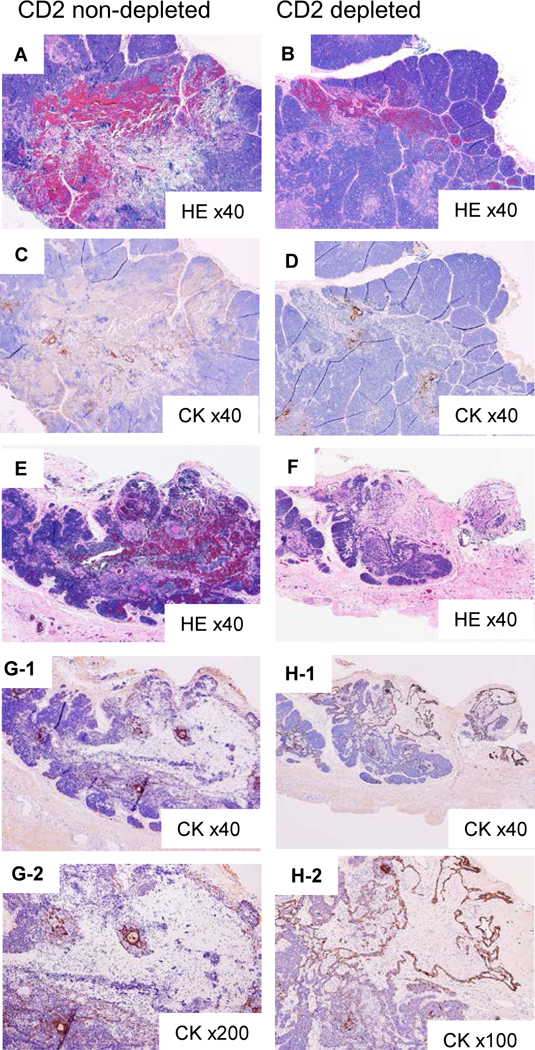
Histologic findings of injected sites of cynomolgus monkeythymic cells in porcine cervical thymic lobes at POD 16 in Group 2 (#28090), demonstrating rejected cynomolgus monkey TEC in the porcine thymic lobe. A, B) HE staining of CD2 non depleted and CD2 depleted thymic cells in #28090 (x40). C, D) CK staining of the same fields of A and B (x40). E, F) HE staining of CD2 non depleted and CD2 depleted thymic cells in #28091 (x40). G-1 shows CK staining of the same fields of E (x40), and G-2 shows a high-power view (x200). H-1 shows CK staining of the same fields of F (x40), and H-2 shows a high-power view (x200).
Group 3 received CD2 depleted thymic cells isolated from a cynomolgus monkey thymus that was grossly similar to thymic tissue from Group 2. The isolated CD2 depleted thymic population had less than 2.5% of CD2+ cells. As shown in Figure 6 A–D, the thymic structure of the injected sites showed similar findings to those of Group 1 (Fig. 4 A). Numerous linear structured CK+ cells were seen beside a native pig medulla (Fig. 6D). Double staining with CK (red) and human class I (green) showed that HLA class I+ CK+ cynomolgus monkey CK cells (yellow) made CK networks within HLA-class I negative porcine CK+ cells (red) in the injection sites (Fig. 6E) as well as in the pig medulla (Fig. 6F, G). In addition, pig CD1+ cells (green) accumulated in the area of the HLA-ABC+ cynomolgus monkey thymic epithelial network (Fig. 6H), suggesting that early porcine thymopoiesis developed in the NHP TEC network in the porcine Hyb-thy.
Fig 6:
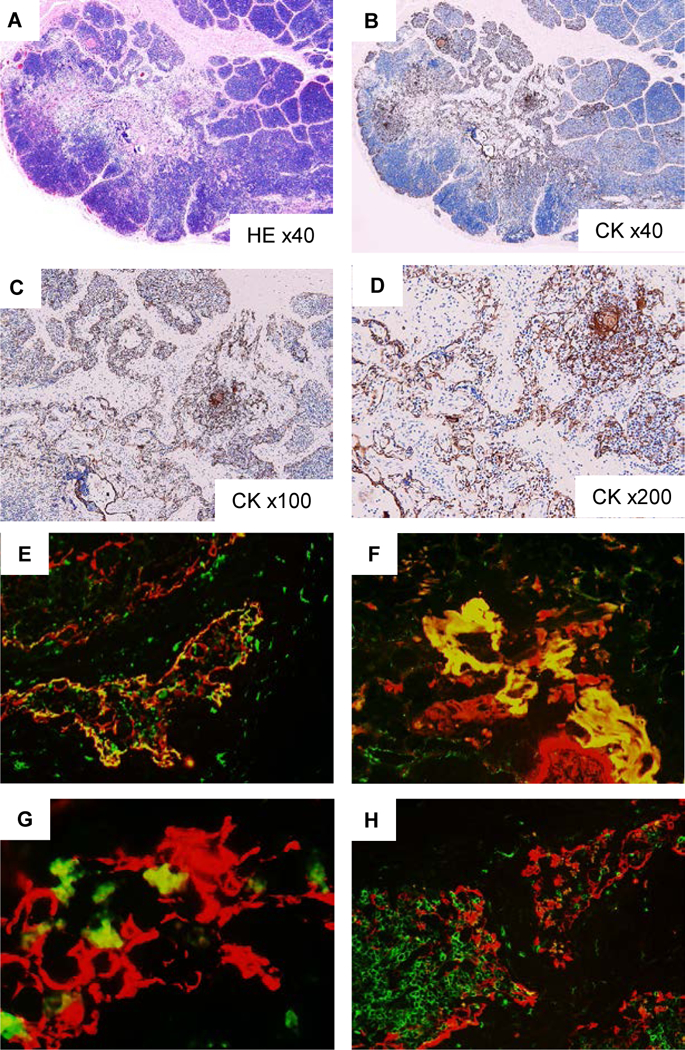
Histologic findings of injected sites of cynomolgus monkey-thymic cells in porcine cervical thymic lobes at POD 16 in Group 3 (#29003), demonstrating cynomolgus monkey-thymic TE network in the porcine thymic lobe. In injection sites in porcine thymus (A: HE staining, B-D: cytokeratin [CK] staining), hypocellular structure was seen where many CK+ TECs with linear structures were observed (A, B). Numerous linear structured CK+ cells were seen beside a native pig medulla (C, D). Double immunostaining with CK (red) and HLA class I (green) (E, F, G) showed the CK network of HLA class I+ CK+ cynomolgus monkey-TECs (yellow) and HLA-class I negative porcine TECs (red) in the injection sites (E) and in the pig medulla (F,G). In double immunostaining with HLA class I (red) and pig CD1 (green) (H), pig CD1+ cells were accumulated in the area of the HLA class I+ network, suggesting thymopoiesis around cynomolgus monkey-TEC network.
Immunologic assessments demonstrating sensitization in Group 2 but not Groups 1 and 3.
- Anti-donor cynomolgus monkey antibody responses
Sera from all recipients at pre (Day −14), Day 0, PODs 7 and 16 were tested for anti-donor pig IgM or IgG binding. As described in the earlier section, all pigs had preformed nAb IgM antibodies (nAb IgM) against donor monkey PBMC (Fig. 7 upper panels), while no IgG was detected by FCM analysis (Fig. 7 lower panels). Both recipients (IDs 28090 and 28091) in Group 2 developed elicited anti-donor IgM abs by POD 7 (+ in the upper panels of Fig. 7) and anti-donor IgG antibodies on POD 16 (+ in the lower panels of Fig. 7), indicating sensitization. Recipient #28090 had a much more marked right shift of anti-donor preformed IgM than the other (#28091). Interestingly, both #29003 and #29004 in Group 3 also had a marked right shift prior to thymic injection which did not cause rejection (Fig. 6). Neither of these pig recipients in Group 3 nor the pigs in Group 1 developed anti-donor elicited antibodies after thymic injection. Although it is not a marked change, #28023 in Group 1, #29003 and #29004 in Group 3 had a slightly left shift of IgM on POD 16 compared to POD 7, suggesting immune modulation after thymic injection.
Fig 7:
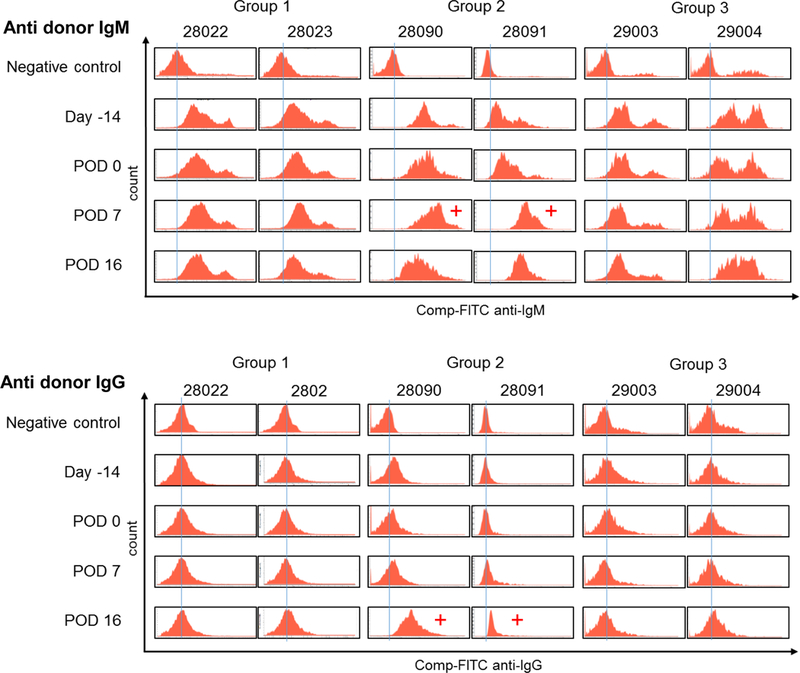
Anti-donor IgM and IgG Ab in pig recipients in Groups 1, 2 and 3 assessed by FCM. Upper panels show all pigs have preformed natural IgM Ab against cynomolgus monkey donors. Anti-donor IgM developed by POD 7 in Group 2, but no elicited ab developed in Groups 1 and 3. Lower panels show no preformed natural IgG Ab was detected in pigs in Groups 1, 2 and 3. However, anti-donor IgG developed at POD 16 in Group 2 while no elicited IgG Ab was found in pigs in Group 1 and Group 3.
- Anti-donor cynomolgus monkey MLR responses
Anti-donor T cell responses were assessed by MLR at Day 0 and at POD 16 following thymic injection. Unfortunately, we did not set up MLR prior to immunosuppression. However, all recipient pigs in this study were CLAWN miniature swine that were partially SLA inbred swine 38. Therefore, as a substitute, we assessed anti-cynomolgus monkey MLR responses as well as anti-allo CLAWN miniature swine and anti-human as described in the earlier section (Fig 3A and B).
Since we found that naive CLAWN miniature swine have not only cellular responses but also preformed nAb (Fig. 3), all miniature swine recipients received immunosuppression beginning at 13 days prior to thymic injection (Day −13. Fig. 1). All recipients had low MLR responses against the donor (orange bars) at Day 0 (Fig. 8A–F) on immunosuppression with tacrolimus and rapamycin that were started at day −13. Recipients in Group 1 and Group 3 maintained general hyporesponsiveness throughout the experimental period (up to POD 16) with the tacrolimus and rapamycin treatment (Fig. 8-A, B, E, F). As a positive control in each MLR assay, we utilized a naive CLAWN pig that was SLA-matched to the recipient against the donor cynomolgus monkey, which showed stimulation index >12 (Fig. 8 yellow bars). Notably, #28090 (Group 2) whose thymus showed marked hemorrhagic changes, restored anti-pig responses markedly at POD 16 (Fig. 8C). The other animal in Group 2 also had higher anti-donor MLR responses compared to the naive SLA-matched pig against the donor at POD 16 (Fig. 8D, orange bar vs yellow bar)
Fig 8:
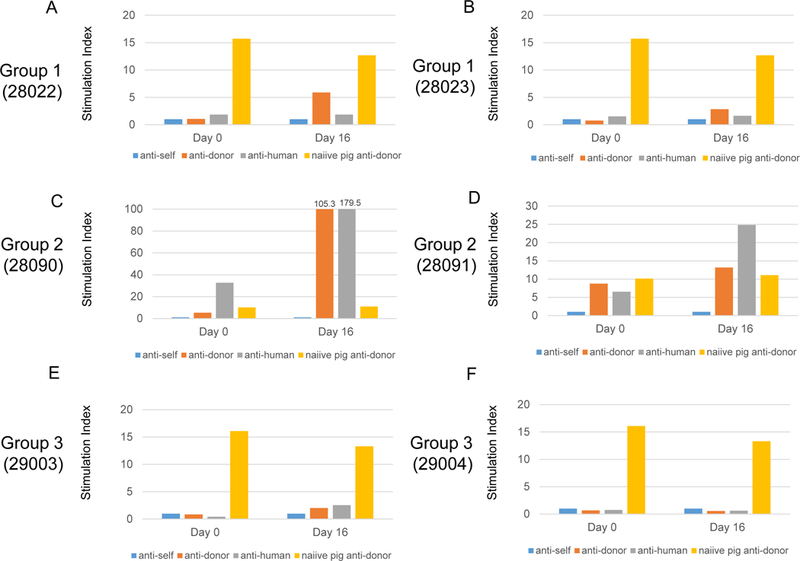
Results of MLR in pigs. All results were indicated as a stimulation index. A, B MLR responses on day 0 (14 days after tacrolimus and rapamycin have started) and POD 16 against self (blue bars), donor cynomolgus monkeys (orange bars) and human (third party, gray bars) in Group 1 (A, B), Group 2 (C, D) and Group 3 (E, F). Responses of naive pig that was SLA-matched to the recipient anti-donor cynomolgus monkey are shown with a yellow bar in each assay.
Discussion
Experiments in a pig-to-mouse model have shown that within swine thymic tissue the T cell pool is positively selected only on pig MHC (SLA), which could result in impaired protective immunity for the host. Positive selection of maturing CD4+ T cells on a xenogeneic thymus graft was mediated exclusively by donor (swine) TECs 41, while negative selection was regulated by elements of both donor and recipient origin (donor APCs and TECs and host APCs) 42,43,44. Cortical TECs (cTECs) are critically important to the development of T cells, while medullary TECs (mTECs) play an important role in the negative selection of tissue antigen (TSA)-specific T cells 45. Hyb-thy tissue that contains both pig and host TECs may optimize both positive selection by cTECs and negative selection by mTECs that express “tissue-specific” antigens under the control of the AIRE transcription factor 45. Since these mTECs and AIRE-driven antigens also positively select Tregs with specificity for them 46–51, the Tregs repertoire specific for the pig donor and the recipient would also be optimal in a hybrid thymus 29. With this hypothesis, Dr. Sykes’s lab was recently able to show that human/pig hybrid thymus achieved immune-tolerance to pig and human antigens in a pig-to-humanized mouse model (Maharlooei MK et al. IXA 2017). However, since we have demonstrated the necessity for donor thymic grafts to be transplanted as a vascularized graft into recipients in order to induce tolerance 23, our procedures of thymic Tx between pig-to-NHP as VTL Tx 25,31,52 are totally different from pig-to-mice as thymic tissue Tx 41. Therefore, the present study is essential in order to determine the optimal conditions and an immunosuppressive regimen to prepare a Hyb-VTL specifically designed for pig-to-NHP.
In this study, we prepared Hyb-VTL which contained histologically proven cynomolgus monkey TEC networks in pig thymic lobes 16 days after the injection of CD2 depleted thymic cells from cynomolgus monkeys with an immunosuppressive regimen comprised of tacrolimus and rapamycin. This 16-day period was set because our protocol of co-Tx of vascularized thymus either as composite TKs or VTL in pig-to-NHP models 27, 31, 53 includes host thymectomy 2–3 weeks prior to xeno-KTx. This period appears to be sufficient to allow for NHP TEC to engraft in miniature swine thymi. The keys to successful preparation of Hyb-thy in pigs most likely consist of (1) Tx of NHP TECs into the intact thymus of pigs to minimize rejection from preformed nAb, and (2) minimization of cell mediated responses in the cynomolgus monkey recipient by pre-treatment of the recipients with tacrolimus and rapamycin as well as depletion of the cynomolgus monkey’s CD2+ thymocytes from the injected thymic cells.
By utilizing our two methods for vascularized thymic Tx, either as a composite TK 23 or a VTL 25, the successful induction of tolerance as well as T-cell development has been achieved in fully allogeneic swine models 23, 25 as well as marked prolongation of survival of xenogeneic kidneys 25, 52. In this study, we chose to inject thymic cells isolated from thymectomized cynomolgus monkeys directly into the pig cervical thymic glands to prepare Hyb-VTL grafts. The major advantage of choosing the Hyb-VTL preparation over composite Hyb-TK is that Hyb-VTL can be co-transplanted with any other organ (hearts, lungs and islets) while composite Hyb-TK applies only for kidney Tx. The hybrid VTL in miniature swine, by using techniques outlined in this study, can be transplanted as a Hyb-VTL graft back into the thymectomized, injected TE host without changing the current preoperative regimen for XTx.
Despite the presence of preformed nAb (predominantly IgM) against cynomolgus monkeys in recipient pigs (Fig 3), the cynomolgus monkey’s TECs successfully engrafted, and we hypothesize that there are three possible reasons for this success. First, the engraftment of injected TEC might be associated with a thymus-blood barrier 54, which could act as a functional and selective barrier separating T-lymphocytes from circulating blood and cortical capillaries. Our colleagues have shown that thymoglobulin (r-ATG) used in vivo in pigs did not deplete thymocytes, and only background staining was observed in the pig thymus by subsequent immunofluorescence (IF). This was despite their demonstration of r-ATG bound to pig lymphocytes in peripheral lymph nodes by IF, as well as FCM analysis on PBMC and isolated thymocytes in vitro (Huang CA, personal communication). These data, when taken together, suggest that vascularized thymus is an immune privileged site. This is of consequence when cynomolgus monkey TECs are mixed with minced pig thymic tissue in a non-vascularized environment at the time of preparation, which may lead to rejection by circulating anti-donor NHP preformed nAb before the hybrid thymic tissue is reconstituted in a pig-to-NHP model 23. The second reason for successful TEC engraftment is the use of rapamycin and tacrolimus 13 days prior to the thymic cell injection. Heidt S et al has reported that rapamycin profoundly inhibits both B-cell proliferation and immunoglobulin production 55. Rapamycin induces B-cell apoptosis and reduces the number of B-cells capable of producing immunoglobulins 55. The third possible reason for engraftment is the use of CD2 depleted TEC-enriched thymic cells. Data from Groups 2 and 3 in this study show that CD2 thymocytes in cells isolated from the cynomolgus monkey thymic glands are immunogenic. Injected NHP thymocytes could be activated in the pig thymus, which would in turn induce an inflammatory environment in the pig thymus. Data from Group 1 suggest atrophic thymus may be useful in this regard even without CD2 depletion. Although pig thymic irradiation can be considered, because we have previously reported that thymic irradiation interfered with the induction of tolerance of allogeneic VTL grafts 56, thymic irradiation is not preferable.
This is the first report of the preparation of a pig-NHP hybrid thymus in miniature swine. In this study, we demonstrated that hybrid thymus can be prepared in a 3-week period. Engrafted NHP TECs in pig thymi of pig donors will not be rejected after the Hyb-VTL graft is transplanted to the NHP as they are autologous TECs. The TECs in the Hyb-VTL should potentially provide not only active positive selection but also negative selection of tissue specific antigens (TSA) 27, 41–43, 45. Although the number of animals used in this study was small, we believe that the study should nevertheless be of value as a proof-of-concept demonstration. Further studies will be carried out to investigate the function of the pig-NHP hybrid thymus, including potential recipient NHP responses to pig as well as TSAs. We also plan to perform experiments to test the effect of Tx of hybrid VTL grafts along with solid organs from GalT-KO pigs into baboons (Fig. 9).
Fig. 9:
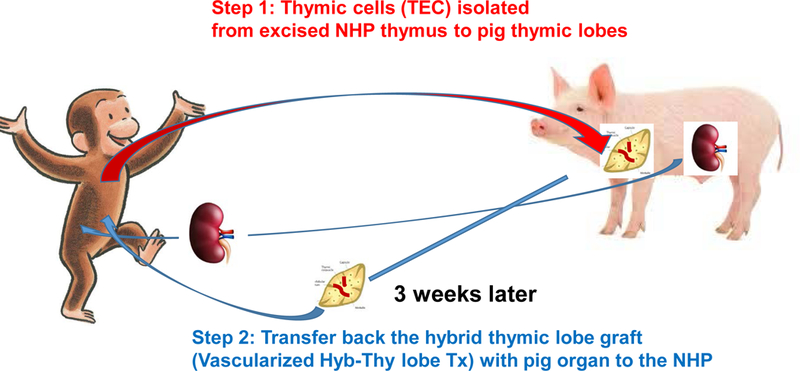
Schematic diagram of preparation of Hyb VT in pig donors (Step 1) and transfer of Hyb VTL to the recipients for Hyb-VTL xeno transplantation. This study is to determine if Step 1 was technically and immunologically feasible.
Acknowledgement
The authors thank Dr. David Sachs for his critical review of the manuscript. We thank Ms. Haruna Shimizu for her editorial assistance. We also thank Kagoshima Miniature Swine Research Center (Isa, Kagoshima, Japan) for providing CLAWN miniature swine, and all the staff members of the Institute of Laboratory Animal Sciences, Natural Science Centre for Research and Education, Kagoshima University for assisting animal care. This study is supported by Kagoshima University Research Awards (SM, SH) as well as NIAID P01AI045897.
Abbreviations
- TK
thymokidney
- TEC
thymic epithelial cell
- Hyb-thy
hybrid thymus
- NHP
non-human primate
- GalT-KO
alpha-1,3-galactosyltransferase knockout
- Tx
transplantation
- VTL
vascularized thymic lobe
- POD
post-operative day
- PBMC
peripheral blood mononuclear cells
- HBSS
Hanks’ Balanced Salt Solution
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- MLR
mixed lymphocyte reaction
- PBS
phosphate buffered saline
- FBS
fetal bovine serum
- H&E
Hematoxylin and Eosin
- CK
cytokeratin
- HLA
Human leukocyte Antigen
- MHC
Major histocompatibility complex
- SLA
swine leukocyte antigen
- Tregs
T regulatory cells
- nAb
natural antibody
References
- 1.Cowan PJ. The use of CRISPR/Cas associated technologies for cell transplant applications. Curr Opin Organ Transplant 2016, 21:461–6. [DOI] [PubMed] [Google Scholar]
- 2.Hai T, Teng F, Guo R, et al. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res 2014, 24:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015, 22:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 2017, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 2015, 22:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006, 12:301–3. [DOI] [PubMed] [Google Scholar]
- 7.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 2006, 12:304–6. [DOI] [PubMed] [Google Scholar]
- 8.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant 2009, 9:2716–26. [DOI] [PubMed] [Google Scholar]
- 9.Bottino R, Wijkstrom M, van der Windt DJ, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant 2014, 14:2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JS, Kim JM, Kim JS, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant 2015, 15:2837–50. [DOI] [PubMed] [Google Scholar]
- 11.Langin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018. [DOI] [PubMed] [Google Scholar]
- 12.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016, 7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cretin N, Bracy J, Hanson K, et al. The role of T cell help in the production of antibodies specific for Gal alpha 1–3Gal. J Immunol 2002, 168:1479–83. [DOI] [PubMed] [Google Scholar]
- 14.Liang F, Wamala I, Scalea J, et al. Increased levels of anti-non-Gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment. Xenotransplantation 2013, 20:458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation 2010, 17:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin JS, Min BH, Kim JM, et al. Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation 2016, 23:300–9. [DOI] [PubMed] [Google Scholar]
- 17.Lee LA, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci U S A 1994, 91:10864–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Swenson K, Sergio JJ, et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med 1996, 2:1211–6. [DOI] [PubMed] [Google Scholar]
- 19.Nikolic B, Gardner JP, Scadden DT, et al. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol 1999, 162:3402–7. [PubMed] [Google Scholar]
- 20.Kalscheuer H, Onoe T, Dahmani A, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol 2014, 192:3442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada K, Shimizu A, Ierino FL, et al. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney”. Transplantation 1999, 68:1684–92. [DOI] [PubMed] [Google Scholar]
- 22.LaMattina JC, Kumagai N, Barth RN, et al. Vascularized thymic lobe transplantation in miniature swine: I. Vascularized thymic lobe allografts support thymopoiesis. Transplantation 2002, 73:826–31. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol 2000, 164:3079–86. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Vagefi PA, Utsugi R, et al. Thymic transplantation in miniature swine: III. Induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex-mismatched barriers. Transplantation 2003, 76:530–6. [DOI] [PubMed] [Google Scholar]
- 25.Kamano C, Vagefi PA, Kumagai N, et al. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A 2004, 101:3827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivard CJ, Tanabe T, Lanaspa MA, et al. Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study. Transplant international : official journal of the European Society for Organ Transplantation 2018, 31:1164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009, 9:2669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Rodriguez-Barbosa JI, Shimizu A, et al. Despite efficient intrathymic negative selection of host-reactive T cells, autoimmune disease may develop in porcine thymus-grafted athymic mice: evidence for failure of regulatory mechanisms suppressing autoimmunity. Transplantation 2003, 75:1832–40. [DOI] [PubMed] [Google Scholar]
- 29.Fudaba Y, Onoe T, Chittenden M, et al. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. J Immunol 2008, 181:7649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oku M, Okumi M, Shimizu A, et al. Hepatocyte growth factor sustains T regulatory cells and prolongs the survival of kidney allografts in major histocompatibility complex-inbred CLAWN-miniature swine. Transplantation 2012, 93:148–55. [DOI] [PubMed] [Google Scholar]
- 31.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 2005, 11:32–4. [DOI] [PubMed] [Google Scholar]
- 32.Tingstedt JE, Tornehave D, Lind P, et al. Immunohistochemical detection of SWC3, CD2, CD3, CD4 and CD8 antigens in paraformaldehyde fixed and paraffin embedded porcine lymphoid tissue. Vet Immunol Immunopathol 2003, 94:123–32. [DOI] [PubMed] [Google Scholar]
- 33.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation 2001, 71:1368–79. [DOI] [PubMed] [Google Scholar]
- 34.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation 2003, 75:1615–24. [DOI] [PubMed] [Google Scholar]
- 35.Miltenyi S, Muller W, Weichel W, et al. High gradient magnetic cell separation with MACS. Cytometry 1990, 11:231–8. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz J, Petrasch S, van Lunzen J, et al. Optimizing follicular dendritic cell isolation by discontinuous gradient centrifugation and use of the magnetic cell sorter (MACS). Journal of immunological methods 1993, 159:189–96. [DOI] [PubMed] [Google Scholar]
- 37.Utsugi R, Barth RN, Kitamura H, Ambroz J, Sachs DH, Yamada K: Tolerance across a two-haplotype, fully MHC-mismatched barrier induced in miniature swine renal allografts treated with a 12-day course of tacrolimus. Transplantation proceedings 2001, 33:101. [DOI] [PubMed] [Google Scholar]
- 38.Oku M, Okumi M, Sahara H, et al. Porcine CFSE mixed lymphocyte reaction and PKH-26 cell-mediated lympholysis assays. Transpl Immunol 2008, 20:78–82. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y, Ohdan H, Onoe T, et al. Multiparameter flow cytometric approach for simultaneous evaluation of proliferation and cytokine-secreting activity in T cells responding to allo-stimulation. Immunol Invest 2004, 33:309–24. [DOI] [PubMed] [Google Scholar]
- 40.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest 1997, 100:3173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Fishman JA, Sergio JJ, et al. Immune restoration by fetal pig thymus grafts in T cell-depleted, thymectomized mice. J Immunol 1997, 158:1641–9. [PubMed] [Google Scholar]
- 42.Zhao Y, Swenson K, Sergio JJ, et al. Pig MHC mediates positive selection of mouse CD4+ T cells with a mouse MHC-restricted TCR in pig thymus grafts. J Immunol 1998, 161:1320–6. [PubMed] [Google Scholar]
- 43.Zhao Y, Sergio JJ, Swenson K, et al. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J Immunol 1997, 159:2100–7. [PubMed] [Google Scholar]
- 44.Zhao Y, Rodriguez-Barbosa JI, Zhao G, et al. Maturation and function of mouse T-cells with a transgenic TCR positively selected by highly disparate xenogeneic porcine MHC. Cell Mol Biol (Noisy-le-grand) 2001, 47:217–28. [PubMed] [Google Scholar]
- 45.Hamazaki Y, Fujita H, Kobayashi T, et al. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol 2007, 8:304–11. [DOI] [PubMed] [Google Scholar]
- 46.Gray DH, Gavanescu I, Benoist C, et al. Danger-free autoimmune disease in Aire-deficient mice. Proc Natl Acad Sci U S A 2007, 104:18193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aschenbrenner K, D’Cruz LM, Vollmann EH, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 2007, 8:351–8. [DOI] [PubMed] [Google Scholar]
- 48.Guerau-de-Arellano M, Martinic M, Benoist C, et al. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. The Journal of experimental medicine 2009, 206:1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubert FX, Kinkel SA, Davey GM, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 2011, 118:2462–72. [DOI] [PubMed] [Google Scholar]
- 50.Malchow S, Leventhal DS, Nishi S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013, 339:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry JSA, Lio CJ, Kau AL, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 2014, 41:414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanabe T, Watanabe H, Shah JA, et al. Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation. Am J Transplant 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe H, Sahara H, Nomura S, et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplantation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribatti D. The discovery of the blood-thymus barrier. Immunol Lett 2015, 168:325–8. [DOI] [PubMed] [Google Scholar]
- 55.Heidt S, Roelen DL, Eijsink C, et al. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation 2008, 86:1292–300. [DOI] [PubMed] [Google Scholar]
- 56.Nobori S, Samelson-Jones E, Shimizu A, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation 2006, 81:26–35. [DOI] [PubMed] [Google Scholar]


