Abstract
5-Aminolevulinate (ALA) synthase (ALAS), a homodimeric pyridoxal-5’-phosphate (PLP)-dependent enzyme, catalyzes the first step of heme biosynthesis in metazoa, fungi and α-proteobacteria. In this review, we focus on the advances made in unraveling the mechanism of the ALAS-catalyzed reaction during the past decade. The interplay between the PLP cofactor and the protein moiety determines and modulates the multi-intermediate reaction cycle of ALAS, which involves the decarboxylative condensation of two substrates, glycine and succinyl-CoA. Substrate binding and catalysis are rapid, and product (ALA) release dominates the overall ALAS kinetic mechanism. Interconversion between a catalytically incompetent, open conformation and a catalytically competent, closed conformation is linked to ALAS catalysis. Reversion to the open conformation, coincident with ALA dissociation, defines the slowest step of the reaction cycle. These findings were further substantiated by introducing seven mutations in the16-amino acid loop that gates the active site, yielding an ALAS variant with a greatly increased rate of catalytic turnover and heightened specificity constants for both substrates. Recently, molecular dynamics (MD) simulation analysis of various dimeric ALAS forms revealed that the seven active site loop mutations caused the proteins to adopt different conformations. In particular, the emergence of a β-strand in the mutated loop, which interacted with two preexisting β-strands to form an anti-parallel three-stranded β-sheet, conferred the murine heptavariant with a more stable open conformation and prompted faster product release than wild-type mALAS2. Moreover, the dynamics of the mALAS2 active site loop anti-correlated with that of the 35 amino acid C-terminal sequence. This led us to propose that this C-terminal extension, which is absent in prokaryotic ALASs, finely tunes mammalian ALAS activity. Based on the above results, we extend our previous proposal to include that discovery of a ligand inducing the mammalian C-terminal extension to fold offers a good prospect for the development of a new drug for X-linked protoporphyria and/or other porphyrias associated with enhanced ALAS activity and/or porphyrin accumulation.
Keywords: 5-aminolevulinate synthase, heme, porphyria, porphyrin, anemia, pyridoxal 5’-phosphate
1. Introduction
In humans approximately 2×1011 red blood cells (RBCs), also named erythrocytes, are produced per day to sustain homeostatic oxygen delivery to tissues. A steady supply of heme is critically important for maintenance of erythropoiesis, the differentiation process of bone marrow stem cells into mature erythrocytes. With each RBC containing 280 million oxygen-carrying hemoglobin molecules and each of the polypeptide chains of the hemoglobin tetramer binding one heme, over 2 × 1020 heme molecules must be synthesized per day just to meet the requirements of RBCs. 5-Aminolevulinate synthase (ALAS; EC 2.3.1.37) catalyzes the first reaction step in mammalian heme biosynthesis, the condensation of glycine and succinyl-CoA to form 5-aminolevulinate (ALA), CoA, and CO2 [1, 2]. Activity of the erythroid-specific ALAS isoform (ALAS2) along with erythroid iron uptake have determining regulatory roles in heme biosynthesis [1, 3]. It is, in fact, the coordination between iron acquisition, heme biosynthesis and globin synthesis that makes erythropoiesis possible [3].
Two genes, ALAS1 and ALAS2, encode the housekeeping and erythroid-specific ALAS isoforms, respectively [4, 5]. The ALAS1 gene is expressed in every single cell and its transcription is strongly influenced by the intracellular concentration of heme. There are two known mechanisms through which the intracellular pool of heme influences the expression of the ALAS1 gene. In the first mechanism, down-regulation of the ALAS1 gene is accomplished through a heme-mediated up-regulation of the early growth response 1 (Egr-1) transcription factor, whose assembly with its major co-repressors, NAB1/NAB2 onto heme responsive elements located in the proximal region of ALAS1 gene suppresses its expression [6]. The second mechanism entails heme-dependent regulation of the nuclear receptor Rev-erbα [7], which in turn down-regulates the expression of the ALAS1 gene through transcriptional repression of the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α). Post-transcriptionally, heme also regulates the physiological concentration of ALAS1 available for heme biosynthesis by inhibiting the translocation of the precursor ALAS1 protein into the mitochondrial matrix [8] and by adversely affecting the stability of the mature protein against proteolytic degradation by Lon peptidase 1 [9].
In contrast to the ubiquitous expression of ALAS1, expression of the ALAS2 gene is restricted to developing erythrocytes, and its transcription appears to be regulated by factors that are essential for proper erythropoiesis, such as GATA-1, the erythroid Kruppel-like factor (EKLF)/Sp1, NF-E2, and the hypoxia inducible factor-1 (HIF-1) [10]. Among these factors, HIF-1 has an important role in the up-regulation of ALAS2 under hypoxic conditions [11]. On a post-transcriptional level, the translation of the ALAS2 mRNA is strongly influenced by the intracellular concentration of iron [12]. Specifically, when the intracellular concentration of iron is low, the binding of iron regulatory proteins (IRP) to iron responsive elements (IRE) located on the 5’ untranslated region of the ALAS2 mRNA sequence inhibits its translation [12–14]. This mechanism is essential in preventing the toxic accumulation of highly reactive porphyric intermediates during iron deficiency. Lastly, genetic mutations in human ALAS2 (hALAS2) are associated with two different blood disorders, X-linked sideroblastic anemia (XLSA; MIM# 300751) [15, 16] and X-linked protoporphyria (XLPP; MIM# 300751) [17–19]. ALAS2 loss-of-function mutations, resulting in decreased hALAS2 activity, cause XLSA [20, 21], and ALAS2 gain-of-function mutations lead to XLPP [21, 22]. As a consequence of the reduced hALAS2 activity, impaired heme synthesis and iron overload are typical of XLSA, which is clinically characterized by a mild hypochromic microcytic anemia with bone marrow ring sideroblasts [15]. Contrastingly, increased hALAS2 activity prompts propotorphyrin IX and zinc-propotorphyrin IX accumulation in erythrocytes of XLPP patients [23]. Upon light irradiation, the accumulated porphyrins give rise to reactive oxygen species and, ultimately, cellular damage, which is the basis for the photosensitivity symptoms of XLPP patients [21, 23]. The available treatment options for XLSA and XLPP remain solely supportive [15, 21]. While in recent years several comprehensive reviews have been published that deal with the regulatory aspects of the rate-limiting step in heme biosynthesis and its underlying genetic abnormalities [1, 17, 21, 24–27], here we focus on experimental results obtained with selected recombinant variants of ALAS2 to highlight the mechanism of the ALAS-catalyzed reaction. We refer to the advances in defining the ALAS dimeric structure, in particular the microenvironment of the pyridoxal 5’-phosphate (PLP) cofactor, as they pertain to the interpretation of the enzymatic mechanism.
2. How does ALAS work? Or the interplay between the protein moiety and the cofactor to achieve enzyme function
Well before ALAS was isolated and purified to homogeneity, the research groups of Shemin [28] and Neuberger [29] described the enzymatic synthesis of ALA based on studies using radiolabeled substrates and cellular extracts. These investigators demonstrated that glycine and succinyl-CoA reacted in the presence of PLP to produce ALA and CO2, and they postulated that succinyl-CoA condensed with a stabilized carbanion formed from glycine and PLP. Two possible mechanistic routes leading to the formation of this carbanion were suggested, involving either decarboxylation or deprotonation of the PLP-glycine external aldimine (Fig. 1). In the latter mechanism, the stabilized carbanion accepts the succinyl group of succinyl-CoA to yield the Schiff base of PLP with 2-amino-3-ketoadipate. This intermediate then decarboxylates to give ALA. At the time, it could not be determined if the decarboxylation step was enzymatic or if the 2-amino-3-ketoadipate intermediate was released free into solution and spontaneously decarboxylated (Fig.1 - 2.1 vs. 2.2 route). Subsequently, Akhtar and co-workers [28, 30] established that ALAS catalyzes the stereospecific removal of the pro-R proton of glycine and that the pro-S proton of glycine resided entirely in the pro-S position of C-5 of ALA. These studies supported both the mechanism involving deprotonation of the PLP-glycine external aldimine and the enzyme requirement for the decarboxylation of the 2-amino-3-ketoadipate intermediate (Fig.1 - 2.1 route).
FIGURE 1. Elucidation of the ALAS mechanism:
Investigators worked from the late 1950s [28, 29] to the late 1970s [30–32] to unravel the reaction mechanism from the three originally proposed mechanisms [28]. The deprotonation and decarboxylation steps in question are indicated in red and green, respectively. [Internal aldimine refers to the Schiff base linkage between the PLP cofactor and an active site lysine, while external aldimine refers to the Schiff base linkage between the PLP cofactor and the glycine substrate.]
According to the reaction mechanism 2.1 in Figure 1, two bonds to the glycine substrate α-carbon are cleaved in the ALAS reaction, as the enzyme catalyzes both a deprotonation and a decarboxylation. Cleavage of two bonds is atypical for most PLP-dependent enzymes [33, 34], and how ALAS accomplishes this undertaking and yet adheres to the stereoelectronic control hypothesis (also designated as “Dunathan’s hypothesis”) was an intriguing question. Dunathan [35] proposed that the extraordinary reaction specificity of PLP-dependent enzymes was attained through stereoelectronic control exerted by the PLP cofactor, such that the amino acid α-carbon bond to be cleaved had to be oriented perpendicular to the plane of the PLP ring. Essentially, by binding the external aldimine complex, PLP-dependent enzymes orient the labile bond to the α-carbon perpendicular to the plane of the cofactor, thus exposing this single bond to the electron withdrawing property of the cofactor, while simultaneously preventing side reactions. Hunter et al. [36] resolved the conundrum of how ALAS catalyzes the sequential cleavage of two bonds to the glycine α-carbon without requiring some intermediate torsion about the aldimine linkage and yet obeying the Dunathan hypothesis. These authors determined that deprotonation, corresponding to abstraction of the pro-R proton of PLP-bound glycine by the active site lysine to yield a quinonoid intermediate (Fig. 2), occurs through the PLP cofactor ring, but decarboxylation of the 2-amino-3-ketoadipate intermediate (Figs. 1 and 2) uses the β-keto group, instead of the cofactor ring, as the alternate electron sink [36].
FIGURE 2. Proposed chemical mechanism for the ALAS-catalyzed reaction.
In the absence of substrates, PLP binds covalently to an active site lysine as an internal aldimine (I) [PLP-K313 internal aldimine in mALAS2]. Binding of glycine in the active site proceeds with the formation of an external aldimine (II). K313-catalyzed abstraction of the glycine pro-R proton yields a first quinonoid intermediate (III), which acts as the nucleophile in the Claisen condensation with succinyl-CoA to give an unstable tetrahedral intermediate (IV). This intermediate converts into an external aldimine with 2-amino-3-ketoadipate (V) accompanied with the release of CoA. When the resulting 2-amino-3-ketoadipate intermediate (V) is decarboxylated (H207-assisted decarboxylation in the mALAS2 reaction), an enol intermediate is formed (VI) which is in rapid equilibrium with a second quinonoid intermediate (VII). Upon protonation of the enol intermediate (VI), the ALA-external aldimine (VIII) is formed. Inset shows positioning of the glycine external aldimine in the active site of ALAS and the primary catalytic residues. R. capsulatus ALAS amino acids H142 and K248 (in black font) correspond to mALAS2 H207 and K313 (in blue font), respectively. The image was created using the PDB 2BWP coordinates and PyMOL. Amino acid numbering for mALAS2 as is in [36, 38, 42].
Kinetic and structural data have led to the postulated chemical reaction mechanism of ALAS outlined in Figure 2. The first catalytic step involves displacement of the Schiff base linkage between a conserved, active site lysine (Lys313 in murine ALAS2 (mALAS2) [37]; Table 1) and the PLP cofactor (Fig. 2, I) by the incoming glycine substrate to form an external aldimine (Fig. 2, II). Abstraction of the glycine pro-R proton is carried out by the ε-amino group of the active site lysine that was liberated from the internal aldimine with PLP (Lys313 or K313 in mALAS2; Fig. 2, II), yielding a carbanionic intermediate, commonly designated quinonoid intermediate (Fig. 2, III). The active site lysine (Lys313 in mALAS2) acts as a general base to labilize the C-α to pro-R proton bond of glycine [38] (Fig. 2, II). In fact, the ALAS crystallographic structure indicates that the positioning of the active site lysine is optimal for its catalytic function, with the glycine Cα-H bond to be broken perpendicular to the plane of the PLP ring (Fig. 2, II). Similar to aspartate aminotransferase [39], the cofactor pyridinium N in ALAS needs to be protonated for catalysis to occur, as enhancement of the electron withdrawing inductive effect of the PLP cofactor stabilizes protonation of C4′ and increases reactivity of Cα. Although the contribution of the pyridine N protonation in the carbanionic intermediate to tuning the reactivity of Cα has yet to be calculated, the modulatory role of the ALAS active site environment in stabilizing the protonation state of the PLP cofactor is well recognized. Crystallographic structures of Rhodobacter capsulatus ALAS [40] and, more recently, Saccharomyces cerevisiae ALAS [41] showed that a carboxylate group of an active site aspartate (Asp279 or D279 in mALAS2) is within the distance necessary for a strong ionic interaction with the PLP ring nitrogen, thus ensuring the proper protonation state for catalysis even at elevated pH values. These structural data are supported by studies of a mutated mALAS2 enzyme in which the carboxylate group is absent, as in the D279N variant of mALAS2 [42]. No activity could be detected for the mutant enzyme nor could detectable activity be restored by the addition of N-methyl-PLP. Nevertheless, the presence of N-methyl-PLP did permit the spectroscopic detection (Abs at 510 nm) of a quinonoid intermediate and the monitoring of its accumulation in the reverse reaction [42].
Table 1.
Numbering of catalytically important active site residues
| Thr | Asn | His | Lys | Asp | Lys | Gln | Arg | |
|---|---|---|---|---|---|---|---|---|
| R. capsulatus ALAS | 83 | 85 | 142 | 156 | 214 | 248 | 359 | 374 |
| Mus musculus ALAS2 | 148 | 150 | 207 | 221 | 279 | 313 | 424 | 439 |
The initial carbanion or quinonoid intermediate (Fig. 2, III) is so short-lived, that it can only be detected spectroscopically when glycine is replaced by O-methyl glycine [43]. Actually, transient kinetic studies involving stopped-flow and absorption spectroscopy indicated that the reaction from species II to V occurs over ~ 10 milliseconds at 30°C [36]. The generated quinonoid intermediate or carbanion (Fig. 2, III) acts as a nucleophile in the Claisen condensation with succinyl-CoA to form an unstable tetrahedral intermediate (Fig. 2, IV), which rapidly converts to give an external aldimine with 2-amino-3-ketoadipate (Fig. 2, V). Decarboxylation of this intermediate produces a stable quinonoid intermediate (Fig. 2, VII) [36,44]. However, this reaction does not use the PLP cofactor as an electron sink, but instead it proceeds via an enol (Fig. 2, VI) formed by decarboxylation of the β-keto acid. The reaction has an apparent pKa of 7.7 ± 0.1 and is catalyzed by a histidine (H207 in mALAS2), which resides above the cofactor (Fig. 3). Therefore this active site histidine is optimally located to act as a general acid catalyst by donating a proton to the β-keto group during decarboxylation (Fig. 2, V), and then abstracting it during formation of the resonance stabilized quinonoid intermediate [36] (Fig. 2, VII). Further, analysis of the ALAS crystallographic structures revealed that the succinyl arm of the 2-amino-3-ketoadipate intermediate is expected to be oriented orthogonally to the plane of the ring (Fig. 2, V), and thus in a compatible orientation with the Dunathan hypothesis.
FIGURE 3. Active site H207 is positioned to act as a catalytic acid for decarboxylation.
In this mALAS2 active site model for the external aldimine to the 2-amino-3-ketoadipate intermediate, a tetrad of residues forms a hydrogen bonding network that links the electron sinks of the PLP ring nitrogen to the intermediate ketone functional group. These hydrogen bonds are shown in orange. Hydrogen bonds donated to the PLP phenolate are in pink, while those to the carboxylate tail are in light green. At this point in the reaction cycle, the α-carbon carboxyl is lost and then replaced by a proton donated from K313 to form the product bound external aldimine. The misalignment of the α-carbon carboxyl for decarboxylation directly into the ring is resolved by H207 acting as an acid catalyst for decarboxylation through the ketone to form an enol that is in equilibrium with a quinonoid intermediate. [Reproduced from [2] with permission from Elsevier © 2018 Copyright Clearance Center, Inc.]
While the 2-amino-3-ketoadipate intermediate (Fig. 2, V) was predicted to be in the path of the ALAS catalytic mechanism (Figs 2 and 3), the identification of the chemical nature of this intermediate remained elusive for decades due to its chemical instability. Only when the methyl ester of glycine was used as pseudo-substrate in the ALAS-catalyzed reaction could the chemical nature of the amino-ketoadipate intermediate (Fig. 2, V) be confirmed [43]. Upon the mALAS2-catalyzed reaction of O-methylglycine with succinyl-CoA, the resulting methyl ester of the β-ketoacid-aldimine could not undergo enzymatic decarboxylation, and thus accumulated. This allowed its detection and characterization by ultra-high pressure liquid chromatography coupled with tandem mass spectrometry [43]. Similarly, “trapping” of this Claisen condensation intermediate made it possible to detect the first quinonoid intermediate (Fig. 2, III) using stopped-flow absorption spectroscopy [43, 45]. Of significance, in the absence of the succinyl-CoA substrate, however, the external aldimine (Fig. 2, II) predominated over the “glycine quinonoid” intermediate (Fig. 2, III). These results supported the occurrence of the quinonoid intermediate (Fig. 2, III) and 2-amino-3-ketoadipate intermediate (Fig. 2, V) in the pathway of the ALAS-catalyzed reaction and confirmed that the amino-ketoadipate intermediate stems from deprotonation, rather than decarboxylation, of the ALAS-glycine external aldmine (Fig. 2, II).
Upon decarboxylation of the 2-amino-3-ketoadipate intermediate (Fig. 2, V), the generated enol intermediate (Fig. 2, VI) tautomerizes into the quinonoid intermediate (Fig. 2, VII) in a rapid equilibrium [2], and the subsequent protonation of the enol by Lys313 (mALAS2 numbering) yields the ALA-bound external aldimine (Fig. 2, VII). ALA is then released from the enzyme in the slowest step of the ALAS catalytic cycle [46].
3. ALAS kinetic mechanism and the role of active site side chains in determining reaction specificity
3.1. Kinetic mechanism
Stopped-flow and quenched-flow studies have been instrumental in determining the rates associated with the different kinetic steps of ALAS catalysis [36, 44, 46–48]. Hunter et al. concluded from those studies, together with considerations of the ALAS crystal structures, that a conformational change of the protein, and not the ALAS reaction chemistry, limits the overall rate of the ALAS-catalyzed reaction [36, 44, 46]. Substrate binding and the catalytic events leading to the formation of the 2-amino-3-ketoadipate intermediate external aldimine (Fig. 2, I to V) are rapid (no more than five milliseconds), and it is the subsequent steps that dominate the overall kinetics. Specifically, the formation of the ALA-quinonoid intermediate is described by a single kinetic step; it is acid-catalyzed with a pKa of 7.7 ± 0.1 and was ascribed to the decarboxylation of the 2-amino-3-ketoadipate intermediate by His207 (mALAS2 numbering) (Fig. 2, V), while the decay of the quinonoid intermediate occurs in two kinetic steps. The first step is acid-catalyzed with a pKa of 8.1 ± 0.1 and was assigned to protonation of the enol by Lys313 (mALAS2 numbering) to generate the product-bound external aldimine [36]. The second step of ALA-quinonoid intermediate decay is pH-independent, with a rate matching that of the kcat value, and it was assigned to a conformational transition, in particular, the opening of the active site loop to allow ALA dissociation [36].
In 1999, Hunter and Ferreira proposed a model for catalysis by ALAS in which succinyl-CoA triggers interconversion between an open conformation and a closed conformation, which is competent for catalysis, and turnover is limited by reversion to the open conformation, concomitant with release of ALA from the enzyme [46]. This kinetically derived model is consistent with the domain movement (~3.5 Å) inferred from the crystal structure of the R. capsulatus ALAS holoenzyme (i.e., protein moiety and PLP cofactor complex) in relation to that of succinyl-CoA-bound at the active site [40]. Superimposition of the 3D structures of one monomer of holoenzymic and succinyl-CoA-bound R. capsulatus ALAS revealed that a loop seems to move towards the active site cleft upon succinyl-CoA binding [40, 47], supporting an induced-fit type mechanism [2, 47]. In fact, the predominant conformational change within the enzyme is that of this active site loop [40]. Further, mALAS2 variants with one or multiple simultaneous mutations in the active site loop were significantly more active than the wild type enzyme, with at least a ten-fold increase in the catalytic efficiency towards one or both substrates [47]. Of the examined variants, a heptavariant (V423L/Y428R/P432E/R433I/G434N/E435Q/L437K) exhibited not only the largest enhancement in the turnover number (enhancements of 19-, 24-, and 70-fold in the kcat value and specificity constants for glycine and succinyl-CoA, respectively), but its kinetic mechanism was also distinctly different from that of wild-type mALAS2 [47]. Specifically, the opening of the active site loop coincident with ALA release was no longer the rate-limiting step in the heptavariant reaction [47]. These findings supported the proposal that the mobility of the active site loop controls the rate of ALA and porphyrin production in vivo [2, 47].
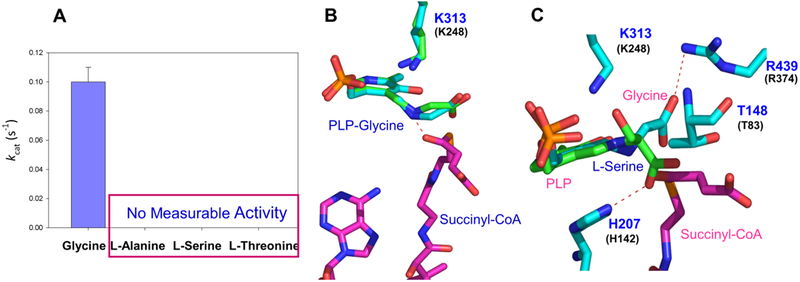
Binding of the succinyl-CoA substrate to ALAS induces a conformational change from open to closed, that enhances the rate of the initial abstraction of the pro-R proton from the glycine substrate by >200,000-fold [44, 46]. Comparison of the R. capsulatus ALAS holoenzymic and succinyl-CoA-bound structures showed that the active site loop closes over the active site and seems to lock succinyl-CoA in the correct position for catalysis in the substrate-bound structure, while it adopts a more open conformation in one subunit of the holoenzyme structure [40]. Moreover, the succinyl-CoA-bound structure indicates that molecular recognition of this substrate includes binding interactions with amino acids from the N-terminal, catalytic and C-terminal domains of one monomer and the catalytic domain from the opposite monomer. While the pantetheine moiety of succinyl-CoA resides in a narrow channel that connects the surface of the active site to the interior, the succinyl moiety is coordinated by amino acids from the glycine-rich stretch and flexible active site loop (142-GAGAGG-147 [49] and 422-Y—R-439 [47]in mALAS2, respectively) (Table 1) [40]. The association between succinyl-CoA and ALAS minimizes the exposure of the active site interior to the solvent, in part due to stabilization of the closed conformation and channel occupancy with the pantetheine moiety. On the other hand, the interaction between the succinyl moiety and the glycine-rich segment and active site loop stabilize the closed conformation of ALAS by reducing the segmental motions of the active site loop. Among the investigated active site amino acids that coordinate the positioning of the succinyl-moiety of succinyl-CoA (e.g., mALAS2 Arg85 [50], Thr148 [43], Thr430 [50], Asn150 [45] and Ile151 [45], here we will focus simply on threonine-148 (T148, murine ALAS2 amino acid numbering; Table 1) to illustrate how the binding of succinyl-CoA not only promotes the transition from the open to the closed conformation of the enzyme, but it also ensures the strict specificity of ALAS towards glycine (Fig 4A).
FIGURE 4. ALAS and amino acid substrate specificity: Succinyl-CoA-coordinating threonine contributes to glycine specificity of ALAS.
A. Specificity of ALAS towards glycine. ALAS shows strict specificity for glycine, as evaluated from ALAS activity with only glycine as amino acid substrate. B. Positioning of the glycine external aldimine in the active site of ALAS. Differences in the positioning of the α-carboxyl group of glycine were detected upon superimposition of the crystal structures for monomers A and E from PDB file 2BWP. The ternary complex formed by the glycine external aldimine and succinyl-CoA was modeled in the active site of ALAS. The succinyl-CoA carboxyl group can strongly interact with the Schiff base nitrogen. The model was built by superimposing the structures with the PDB 2BWP and 2BWO coordinates using PyMOL. Amino acid K248 in R. capsulatus ALAS corresponds to K313 in mALAS2. C. Model depicting the positioning of the (vs. glycine) external aldimine in the active site of ALAS. This structural model depicts the positioning of the L-serine external aldimine in the active site of ALAS. The model was created by superimposing the L-serine external aldimine of Sphingomonas paucimobilis serine palmitoyl transfererase (PDB file 2W8J) with the glycine external aldimine of R. capsulatus ALAS (PDB file 2BWP). L-serine is shown in green and glycine in cyan. Succinyl-CoA was subsequently modeled into the active site by superimposing the generated structure with the structure for the R. capsulatus●succinyl-CoA complex (PDB file 2BWO), using PyMOL. R. capsulatus ALAS amino acids T83, H142, K248 and R374 (in black font) correspond to mALAS2 T148, H207, K313 and R439 (in blue font), respectively (Table 1). [Adapted from [43] originally published in the Journal of Biological Chemistry. Stojanovski, B.M., Hunter, G.A., Jahn, M., Jahn, D. and Ferreira, G.C.). J. Biol. Chem. 2014; 289: 22915–22925. © the American Society for Biochemistry and Molecular Biology]
3.2. ALAS and amino acid substrate specificity: Succinyl-CoA-coordinating threonine
The many interactions between the PLP cofactor and ALAS ensure that the carbonyl group of the succinyl-moiety and the glycine α-carbon are optimally positioned for the condensation to occur between the two substrates (Fig. 4B). In the absence of succinyl-CoA, the catalytic removal of the pro-R proton of glycine to give the quinonoid intermediate is considerably retarded, and when the amino acid substrate is not glycine but L-serine, for example, the binding of succinyl-CoA is non-productive [43]. In fact, a lag phase was observed in the time course for L-serine external aldimine formation, indicating a hysteretic behavior in ALAS and that wild-type ALAS undergoes conformational rearrangements with a slower rate than that of the corresponding chemical reaction. However, when Thr148 in mALAS2 was replaced with alanine, hysteresis could not be detected in the T148A-catalyzed L-serine external aldimine formation. Based on these findings along with structural models for binding of succinyl-CoA to the glycine external aldimine of R. capsulatus ALAS, Stojanovski et al. [43] reasoned that the ability of succinyl-CoA to accelerate the rate of the ALAS-catalyzed deprotonation of the glycine external aldimine stems from placing the pro-R proton to the α-carbon bond in an optimal position for deprotonation. Specifically, the carbonyl group of succinyl-CoA, by interacting with the Schiff base nitrogen, minimizes fluctuations in the positioning of the C-α glycine to the pro-R proton bond, maintains this labile bond perpendicular to the plane of the PLP ring and stabilizes the closed conformation of ALAS. Superimposition of the crystal structures of two glycine-bound R. capsulatus ALAS monomers indicated that the positioning of the glycine α-carbon varied in relation to the plane of the PLP ring, but it did not vary in the R. capsulatus ALAS succinyl-CoA-bound monomers [43]. This reinforced the idea that minimizing fluctuations in the positioning of the glycine α-carbon in relation to the plane of the PLP ring is essential for an effective abstraction of the glycine pro-R proton by the catalytic lysine [38] to occur [43]. Also, the inability of ALAS to stabilize the positioning of the glycine α-carbon in the absence of succinyl-CoA is very likely physiologically relevant in preventing undesirable side reactions [43]. From a structural perspective, there are clear differences in the positioning of the amino acid substrate carboxyl group. While the carboxyl group of the physiological substrate glycine forms a salt bridge with R439 in mALAS2 [51], the α-carboxyl group of L-serine is predicted to interact with H207 [43] (Fig. 4C). Thus, presumably L-serine sterically clashes with active site amino acids during the serine-wild-type ALAS external aldimine formation, but once the serine-aldimine is formed the enzyme assumes a closed conformation similar to that adopted with its physiological substrates.
A possible interpretation for the role of mALAS2 Thr148 in modulating the amino acid substrate specificity of mALAS2 relates to its direct interaction with the amino acid substrate. While the α-carboxylate of the glycine external aldimine is oriented away from Thr148, the L-serine α-carboxylate is at a distance of approximately 2Å from the Thr148 side chain methyl group in the serine external aldimine (Fig. 4C). This orientation is not conducive to a rapid formation of the serine external aldimine in mALAS2, probably because of the previously noted steric clashes between L-serine and T148 [43]. When Thr148 is replaced with alanine, L-serine and succinyl-CoA can be easily accommodated and properly oriented in the active site of the T148A variant, so that not only the external aldimine can be formed, but also the subsequent Claisen condensation with succinyl-CoA can occur.
3.3. Adenosyl-binding site lysine versus substrate binding and catalysis
Despite the wide network of interactions between the succinyl-moiety of succinyl-CoA and ALAS, it is the CoA, rather than the succinyl moiety, that facilitates binding of succinyl-CoA to the active site of mALAS2 [52]. The adenosyl group of succinyl-CoA resides in a predominantly hydrophobic pocket, located on the active site surface [40]. Substrate protection studies revealed that succinyl-CoA binding does minimize the exposure of the active site interior to the solvent. Within the adenosyl-binding pocket, Lys156 of R. capsulatus ALAS (Lys221 in mALAS2; Table 1) forms a hydrogen bond with the ribose group of succinyl-CoA (Fig. 5A). The absence of this hydrogen bond in the active site of mALAS2 in a mutated enzyme with valine rather than lysine at position 221 reduced the specificity constant for succinyl-CoA (Fig. 5B). Of note, the increased value of the K221V variant did not result from weaker binding affinity, but instead from hindered individual catalytic rates, including those of quinonoid intermediate II formation and decay [52]. Thus, Lys221 in the adenosyl-binding site of mALAS2 contributes to binding and orientation of succinyl-CoA for effective catalysis.
FIGURE 5. Adenosyl-binding site K221 modulates substrate binding and catalysis in mALAS2.
A. K156 (gray) of R. capsulatus ALAS coordinates the positioning of the adenosyl group of succinyl-CoA (magenta). On opposite sides of K156 are β-strand 134–138 and α-helix 143–150 (cyan), which are connected by a linker that contains the catalytic His142. R. capsulatus ALAS K156 and H142 (in black font) correspond to K221 and H207 in mALAS2 (in blue font) (Table 1). Image created with Pymol using PDB file 2BWO. B. Specificity constants of mALAS2 and K221 variant towards succinyl-CoA. [Adapted from [52] Stojanovski, B.M. and Ferreira, G.C., FEBS Open Bio 5 (2015) 824–832. Published under a Creative Commons Attribution (CC BY) License.]
3.4. How does ALAS establish a catalytic balance between the forward and reverse reactions?
Detailed examination of the crystal structure of R. capsulatus ALAS [40] enabled Stojanovski et al. [43, 48] to identify an alternative channel, distinct from that occupied by succinyl-CoA, that may serve as a path of entry for glycine to and exit for ALA from the active site. The architecture of this channel is delineated by amino acids from the N-terminal (i.e., R. capsulatus ALAS amino acids 1–24) and C-terminal (i.e., R. capsulatus ALAS amino acids 337–352; Table 1) domains of one monomer and by the glycine-rich stretch from the opposite monomer (i.e., R. capsulatus ALAS amino acids 77’−89’). Asn85’ (Asn150’ of mALAS2; Table 1) in the glycine-rich stretch contributes to mediating the opening and closing of this channel (Fig. 6). In the closed conformation, Asn85’ is within an H-bond distance from Gln359 of the flexible active site loop [(Gln424 of mALAS; Table 1), while it is reoriented away from the active site loop in the closed conformation (Fig. 7). Therefore, disruption of the interaction between Asn85’and Gln359 in R. capsulatus ALAS (Asn150’ and Gln424 in mALAS2; Table 1) causes the channel to open.
FIGURE 6. Alternative channel leading to the active site of R. capsulatus ALAS in the open (A) and closed (B) conformations.
In the closed conformation, the repositioning of the side chain of Asn85’ (yellow sticks) results in closure of the channel. The N-terminal domain is shown in cyan, the catalytic domain in gray, and the C-terminal domain in magenta, while the opposite monomer is shown in yellow. The image was created by aligning the A and B monomers of PDB file 2BWN with those of 2BWO using PyMOL. [Reproduced from [48]; originally published in the Journal of Biological Chemistry. Stojanovski, B.M. and Ferreira, G.C. J. Biol. Chem. 2015; 290: 30750–30761. © the American Society for Biochemistry and Molecular Biology]
FIGURE 7. Side chain of Asn85’ becomes reoriented during the closed (cyan) to open (gray) conformational transition in R. capsulatus ALAS.
In the closed conformation (PDB: 2BWO), the distance between Asn85’/Oδ1 from chain A and Gln359/Nε1 from chain B is 2.5 Å. In the open conformation (PDB: 2BWN), the distance increases to 5.3 Å. Asn85’ in R. capsulatus ALAS corresponds to Asn150’ in mALAS2 (Table 1). Succinyl-CoA is shown in magenta. The image was created by aligning the A and B monomers of PDB file 2BWN with those of 2BWO using PyMOL. R. capsulatus amino acid numbering is in black font, while that form mALAS2 is in blue font (Table 1). [Reproduced from [48]; originally published in the Journal of Biological Chemistry. Stojanovski, B.M. and Ferreira, G.C. J. Biol. Chem. 2015; 290: 30750–30761. © the American Society for Biochemistry and Molecular Biology]
ALAS belongs to the α-oxoamine synthase (AOS) family of PLP-dependent enzymes whose members share a conserved structural fold and a common catalytic mechanism that entails the condensation reaction between an amino acid substrate and acyl-CoA [48]. Comparison between all available crystal structures of AOS enzymes revealed that in the glycine-rich stretch of other AOS enzymes, histidine and phenylalanine occupy the position analogous to Asn150 in mALAS2 [48]. To attempt at understanding the evolutionary pressure that favored the selection of Asn at position 150 as opposed to the more commonly observed histidine and phenylalanine residues that are found at the analogous structural positions in other AOS enzymes, Stojanovski and Ferreira characterized the functional and catalytic properties of the N150H and N150F mALAS2 variants [48]. The main conclusion that emerged from this study was that Asn150 has an essential role in establishing a catalytic balance between the rates of the reversible reaction. Indeed, the N150H and N150F mutations in mALAS2 significantly reduced the rate of quinonoid intermediate formation in the forward direction (i.e., Fig. 2, III), while increasing the reverse reaction rate (i.e., towards the VII intermediate in Fig. 2) [48]. To rationalize the observed shift in the catalytic balance, two possible explanations were provided. First, given that the side chain of this conserved asparagine regulates the opening and closing of the alternative channel, it was postulated that its replacement with the bulkier side chains of histidine and phenylalanine results in partial obstruction of the channel and slowing of ALA release from the active site (Figs. 6 and 7). A second, but not mutually exclusive explanation, is that the active sites of the Asn150 variants provide a catalytic environment that lowers the energetic barrier required for the chemical transformation of ALA back into the quinonoid intermediate. Regardless of the molecular details that are responsible for the shift in the catalytic balance, the authors of this study concluded that evolutionary pressure favored the selection of Asn150 in order to optimize the forward reaction at the expense of the reverse reaction. Simply, by favoring the forward reaction, mALAS2 was bestowed with selectivity toward ALA production, while minimizing its non-productive breakdown into glycine and succinyl-CoA, and ultimately securing sufficient quantities of ALA that are necessary to sustain heme biosynthesis in differentiating erythroid cells.
Curiously, ALAS in Streptomyces nodosus and Streptomyces aizunensi can catalyze a second PLP-dependent reaction, the cyclization of ALA-CoA to produce 2-amino-3-hydroxycyclopent-2-en-1-one five membered rings, or C5N units [53, 54]. These oxoamine C5N units or scaffolds are precursors in the biosynthesis of actinomycete secondary metabolites, such as asukamycin [53, 54]. The enzyme responsible for conversion of the ALA-CoA thioester into C5N has been designated cyclizing ALAS or ALA-CoA cyclase [53, 54]. Recently, Liu et al. [53] investigated the bifunctionality of ALA-CoA cyclase and “classical” ALAS. The “classical” enzyme could cyclize ALA-CoA, even if much less efficiently than ALA-CoA cyclase. Liu et al. [53] postulated that differences regarding accessibility of the alternate channel to the active site of the enzyme might be at the core of the dissimilar activity values between ALAS and ALA-CoA cyclase.
4. The structural and dynamic properties governing the role of the active site loop
A conformational change, dictated by the movement of an active site loop, returns ALAS to the open conformation during the release of ALA [36, 44, 46]. The rate of ALA release is controlled by a hysteretic kinetic mechanism, also consistent with conformational changes governing the dissociation of ALA from ALAS [43]. The observed hysteretic behavior reflects the structural rearrangements during ALA external aldimine formation. Either generation of the external aldimine or dissociation of ALA from PLP can induce conformational strain within the active site and destabilize the closed conformation [43]. The extent to which segmental rearrangements and motions of the active site loop limit product release was investigated by performing molecular dynamics (MD) simulation analyses of the wild-type mALAS2 dimer and a mALAS2 variant harboring seven simultaneous mutations in the active site loop and with significantly increased values for the kcat and specificity constants for both substrates (aka hyperactive heptavariant [47]) [55]. During the MD simulations, a transient β-strand clearly emerges in the active site loop of the heptavariant, followed by its collapse within 200 ns. Upon its formation, the β-strand interacts with two preexisting β-strands giving an anti-parallel three-stranded β-sheet (Fig. 8 and Table 2), which might contribute to the increased rigidity of the active site loop and destabilization of the closed conformation. Thus, the newly generated anti-parallel three-stranded β-sheet most likely endows the heptavariant with a more stable open conformation than that of wild-type mALAS2. Further, the lowered flexibility intrinsic to the active site loop of the heptavariant probably results from inducing a loop-to-β-strand conversion. All of these structural and dynamic changes concur with a kinetic mechanism involving a faster closed-to-open conformation transition and ALA release for the heptavariant than the wild-type enzyme [55].
FIGURE 8. Dynamic properties of ALAS: A β-strand emerges in the active site loop. A. mALAS2, chain A. B. Hyperactive, heptavariant of mALAS2, chain A.
The C-terminal regions covering the active site loop (red), strand β13 (blue), and β11-α13-β12 structural motif of the dimeric structures of wild-type mALAS2 (ALAS-WT-chA) and heptavariant (mALAS2-Heptavariant-chA) were selected at 100 ns to illustrate the dynamics undergone by these proteins. A β-strand (magenta) is clearly present within the mutated active site loop (mALAS2-Heptavariant-chA in B). Note that a greater number of hydrogen bonds between the active site loop (residues Y500-E514) and the β13 strand (residues L515-L518) was observed within the heptavariant trajectory (2.6 ± 0.75) than within the wild-type mALAS2 trajectory (1.7 ± 0.6) [55]. [The amino acid numbering is according to that for the precursor form of mALAS2.] (Adapted from [55]).
Table 2.
C-terminal amino acids of selected ALASs
Red, active site loop amino acids; red italics, amino acids involved in β-strand formation (see text); blue, C-terminal extension amino acids; black and blue italics, gain-of-function domain. Mutations of amino acids denoted with red stars produce truncated or lengthened variants of hALAS2 associated with XLPP. Note that the mALAS2 numbering refers to the 587-amino acid precursor protein (and not the 509-amino acid recombinant mature protein, as used in Table 1). Precursor mALAS2 refers to the protein as synthesized in cytosolic ribosomes, while mature mALAS2 refers to mALAS2 upon import into the mitochondrion and cleavage of its N-terminal presequence.
Additional MD studies and data analyses permitted Na et al. [56] to demonstrate anti-correlation between the structural and dynamic behavior of the ALAS2 active site loop and the disordered C-terminal extension (Table 2). Conceivably, binding of a ligand to the highly disordered C-terminal extension could induce this region to fold and, thus, modulate the flexibility of the mALAS2 active site loop. Since an increase in flexibility of the active site loop appears to be accompanied with a decrease in the reaction rate of mALAS2, and the dynamics of the mALAS2 active site and those of the C-terminal extension are anti-correlated, then a ligand restricting the motion of the C-terminal extension would produce a decrease in the mALAS2 reaction rate. Because all identified XLPP-causing mutations reside in the hALAS2 C-terminal extension (Table 2) and the resulting defective hALAS2 variants have enhanced reaction rates [22, 57], discovery of such a ligand would represent a good lead for the development of a new drug against XLPP. This would be significant given that the present treatment options are confined to relieving the photosensitivity symptoms associated with XLPP. For example, afamelanotide, a synthetic analog of the α-melanocyte-stimulating hormone [58–60], increases skin pigmentation by increasing melanin production in melanocytes, which leads to reduced light penetration and thus greater sunlight tolerance in XLPP patients. By contrast, the discovery of a ligand that reduces the synthesis of ALA and, consequently, porphyrin, should impact the root cause of XLPP.
Notable differences were also identified in the dynamic behavior of the two protomers in the mALAS2 and heptavariant dimeric forms [55]. While there was a reduction in the dynamics of the heptavariant chain B in relation to those of the wild-type mALAS2 chain B, the inverse was observed when the dynamics of the heptavariant chain A were compared to those of the wild-type mALAS2 chain A. Na et al. [55] suggested that this functional non-equivalence or asymmetry arises from allosteric regulation in dimer assembly, and not by the mutations per se, given that both proteoforms in dimeric mALAS2 possessed the same seven mutations. An asynchronous allosteric regulation of the active site, in which the occupancy of, or release from, one active site affects the function of the other, could explain the distinct dynamic behavior of proteoforms within the dimeric structure. Therefore, two active sites of a dimer can act in “opposite phases”, with the closure of one of the active sites promoting the opening of the other [55]. Asymmetry in structure and active site reactivity has been identified in various homodimeric enzymes [61–63], including glutamate-1-semialdehyde aminomutase (GSAM) [64], a PLP-dependent enzyme, which catalyzes the isomerization of glutamate-1-semialdehyde into ALA in plants, green algae and most bacteria [64–66]. Although Turbeville et al. [67] demonstrated functional asymmetry for the active sites of an engineered, single polypeptide chain mALAS2 dimer, asymmetry in ALAS active site reactivity in solution has not been identified [52]. Until determination of the asymmetry of the active site loop conformation between the two ALAS subunits is technically feasible, the possibility of allosterically controlled intersubunit communication in ALAS2 as another mechanism of regulation of the first step of heme synthesis in differentiating erythroid cells cannot be dismissed.
ALAS1 and ALAS2: Concluding remarks and future directions
Hallmarks of the ALAS catalytic pathway include the stereoelectronic control exerted by the PLP cofactor and the positioning and orientation of the active site amino acids in relation to the plane of the PLP ring. The former makes it possible for ALAS to catalyze the cleavage of two of the four glycine C-α bonds without requiring some intermediate torsion about the aldimine linkage, yet adhering to the Dunathan hypothesis that the α-carbon bond of the external aldimine to be cleaved needs to be oriented perpendicular to the plane of the cofactor pyridinium ring. With the latter, substrate specificity, succinyl-CoA gating, facile catalysis and catalytic balance are achieved. The active site lysine covalently bound to the PLP cofactor assumes a second role as the catalytic residue involved in the abstraction of the pro-R proton of glycine and protonation of the ALA quinonoid intermediate. An active site histidine catalyzes the decarboxylation of the β-ketoacid intermediate by acting as an acid catalyst and donating a proton to the β-keto group.
Protein dynamics are essential to the ultimate function of ALAS. Conformational changes with “ordering” of structural elements, elicited by incorporation of the PLP cofactor, shape the ALAS active site for substrate binding and catalysis [41]. Similarly, protein conformational changes go hand in hand with the various steps of the ALAS reaction. Binding of succinyl-CoA induces the transition from the open to the closed conformation, which is accompanied by an over 105-fold increase in the rate associated with removal of the glycine pro-R proton [44]. The subsequent chemical steps, decarboxylation and protonation leading to ALA formation, also occur in the closed conformation [36]. Return to the open conformation, coincident with ALA release, is the slowest step of the overall ALAS-catalyzed reaction [2, 36, 47]. In particular, segmental rearrangements and motions of an active site loop control accessibility to and from the ALAS active site [47, 55]. While inducing a transient loop-to-β-strand conversion lowers the flexibility intrinsic to the active site loop [55] and stabilizes a more open conformation conducive to faster product release, defining whether the individual chemical steps coincide with the dynamics of the active site loop remains an open question. Whether the detected functional asymmetry in the ALAS homodimer reflects asymmetry of the active site loop conformation between the two ALAS subunits and corresponds to an allosterically controlled intersubunit communication in ALAS2 also remains unanswered. The finding that the dynamics of the mALAS2 active site loop is anti-correlated with the dynamics of the C-terminal extension (Table 2; unpublished results) and our proposal that this region is an allosteric effector-binding site [1, 2, 68] lead us to hypothesize that a ligand inducing the C-terminal extension to fold could represent a potential new therapy for XLPP. Such an allosteric effector is still to be discovered.
Even though most of the studies on the ALAS catalytic pathway have focused on mALAS2 as the prototype ALAS, the likelihood for an identical mechanism for ALAS1 is very high. First, alignment of the amino acid sequences for ALAS1 and ALAS2 indicates that differences reside solely within the N- and C-termini, and the conserved central catalytic domain has a high degree of similarity. Munakata et al. [69] were among the first investigators to demonstrate that the “papain-resistant core domain” was conserved among all ALASs (including ALAS1 and ALAS2 from different organisms) and possessed catalytic activity similar to that of the whole enzyme. Second, comparative genomics permit us to infer that ALAS1 and ALAS2 were formed in ancestral vertebrates around 550 million years ago (Fig. 9). The genes for ALAS1 and ALAS2, and glycine C-acetyltransferase (GCAT) appear to have arisen from two major gene duplication events during evolution of eukaryotes over 1 billion years ago (Fig. 9). Homology in amino acid sequences and similar reaction specificities as well as fold types derived from three-dimensional structures have been criteria used to classify PLP-dependent enzymes [70–73]. Members of the AOS family share a common fold (type I fold) and chemical reaction mechanism [40, 46, 74–78]. Perhaps not surprisingly, GCAT, also known as 2-amino-3-ketobutyrate coenzyme A ligase, and ALAS belong to the same family of PLP-dependent enzymes: the AOS family [1]. The subtleties hidden in the different N- and C-termini of ALAS1 and ALAS2 that certainly endow these two proteins with distinct cellular roles are still to be unveiled.
FIGURE 9. Evolution of ALAS genes: Using comparative genomics to study the evolutionary origin of ALAS1 and ALAS2.
Two major gene duplication events gave rise to GCAT, ALAS1 and ALAS2 during evolution of eukaryotes over 1 billion years. ALAS1 and ALAS2 were formed in ancestral vertebrates around 550 million years ago. The graph is based on Ensembl gene tree of maximum likelihood (ML) methods (Ensembl Release 92; Genomicus v92.01). TreeBeST computed the consensus tree based on consensus topology by minimizing the number of duplications and losses inferred and having the highest bootstrap support. Branch lengths are estimated with the phylogenetic analyses program phyml [79] for the final consensus tree based on the DNA alignments. [GCAT encodes glycine C-acetyltransferase.]
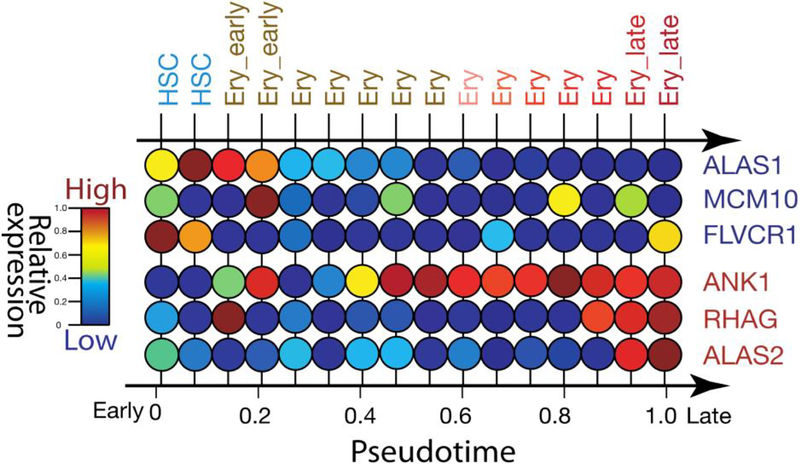
With the rapid growth of single-cell technologies, questions related to expression of ALAS1 and ALAS2 in developing erythroid cells can now be addressed without the hindrance associated with cell population heterogeneity. Figure 10 illustrates the differential expression of ALAS1 and ALAS 2 in erythroid precursor cells as discerned from single-cell RNA sequencing (RNAseq) of erythroid progenitor cells isolated from human bone marrow. Defining key differences in erythropoiesis in healthy and erythropoietic porphyria patients, and searching for “druggable” targets in these patients to restore normal heme metabolism, red blood cell development and function will be certainly investigated.
FIGURE 10. Single-cell RNAseq resolves differential expression of ALAS1 and ALAS2 in erythroid progenitors.
Single cells with CD34+ markers were selected from a healthy human bone marrow donor for single cell RNAseq (data were retrieved from [80]). Every vertical line represents a single cell expression of erythroid-related genes, including those for ALAS. Pseudotime represents a dimension for single cell developmental progression, analogous to the ‘real’ time measured in conventional laboratory settings. For this set of erythropoiesis-related single cell data, the first tSNE (t-distributed Stochastic Neighbor Embedding) factor was computed from all single cell gene expression patterns and used as developmental pseudotime, if the expression levels were of sufficient quality and quantity based on the study of [80]. Specifically, developmental pseudotime from “early” to “late” stages was calculated with cell lineage specificity derived from over 8000 genes expression patterns during human hematopoiesis in Differentiation Map (DMAP). Single cell expression levels were aligned horizontally from early to late of erythropoiesis. The polarity of this reconstructed temporal sequences was determined as “early” if known early progenitors genes, e.g., ALAS1, were enriched, and as “late” if mature erythroid genes were enriched. The gene expression levels were normalized from the lowest to the highest expression as measured by FPKM (Fragments Per Kilobase of transcript per Million mapped reads), on a scale from 0 to 1, and represented by a heatmap scheme. ALAS1 is expressed at higher levels in early progenitor cells compared to late-staged cells, while the expression of ALAS2 increases during the course of differentiation. ALAS1, MCM10 and FLVCR1 gene expressions patterns are enriched in early progenitors; their gene products are known to be present in early erythropoiesis. ALAS2, ANK1 and RHAG gene expressions are enriched in late progenitors; their gene products are known to be present in mature erythroid cells. Abbreviations: HSC, hematopoietic stem cell; Ery, erythroid progenitor cells; MCM10, MiniChromosome Maintenance 10 replication initiation factor; FLVCR1, feline leukemia virus subgroup C cellular receptor 1; ANK1, ankyrin 1; RHAG, Rh associated glycoprotein.
Funding
The research reported in this study was supported in part by the National Institutes of Health (#GM080270 and #DK63191) and the American Heart Association, Greater Southeast Affiliate (grant # 10GRNT4300073) to G.C.F. RHYJ and GCF received support from the American Cancer Society (ACS-IRG; grant # IRG-14-189-19).
Abbreviations:
- ALA
5-aminolevulinic acid
- ALAS1
non-specific isoform of 5-aminolevulinate synthase
- ALAS2
erythroid-specific isoform of 5-aminolevulinate synthase
- hALAS2
erythroid-specific isoform of human 5-aminolevulinate synthase
- mALAS2
erythroid-specific isoform of murine 5-aminolevulinate synthase
- MD
molecular dynamics
- PLP
pyridoxal 5’-phosphate
- XLPP
X-linked protoporphyria
- XLSA
X-linked sideroblastic anemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fratz EJ, Stojanovski BM, Ferreira GC, Toward Heme: 5-Aminolevulinate Synthase and Initiation of Porphyrin Synthesis, in: Ferreira GC, Kadish KM, Smith KM, Guilard R (Eds.) Handbook of Porphyrin Science, Heme Biochemistry, World Scientific Publishing Co. Pte. Ltd., Place Published, 2014, pp. 3–68. [Google Scholar]
- [2].Hunter GA, Ferreira GC, Molecular enzymology of 5-aminolevulinate synthase, the gatekeeper of heme biosynthesis, Biochim. Biophys. Acta, 1814 (2011) 1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ponka P, Koury MJ, Sheftel AD, Erythropoiesis, Hemoglobin Synthesis, and Erythroid Mitochondrial Iron Homeostasis, in: Ferreira GC, Kadish KM, Smith KM, Guilard R (Eds.) Handbook of Porphyrin Science, Erythropoiesis, Heme and Applications to Biomedicine, World Scientific Publishing Co. Pte. Ltd., Place Published, 2014, pp. 42–84. [Google Scholar]
- [4].Bottomley SS, May BK, Cox TC, Cotter PD, Bishop DF, Molecular defects of erythroid 5-aminolevulinate synthase in X-linked sideroblastic anemia., J. Bioenerg. Biomembr, 27 (1995) 161–168. [DOI] [PubMed] [Google Scholar]
- [5].Cotter PD, Drabkin HA, Varkony T, Smith DI, Bishop DF, Assignment of the human housekeeping δ-aminolevulinate synthase gene (ALAS1) to chromosome band 3p21.1 by PCR analysis of somatic cell hybrids, Cytogen. Gen. Res, 69 (1995) 207–208. [DOI] [PubMed] [Google Scholar]
- [6].Gotoh S, Nakamura T, Kataoka T, Taketani S, Egr-1 regulates the transcriptional repression of mouse delta-aminolevulinic acid synthase 1 by heme, Gene, 472 (2011) 28–36. [DOI] [PubMed] [Google Scholar]
- [7].Wu N, Yin L, Hanniman EA, Joshi S, Lazar MA, Negative feedback maintenance of heme homeostasis by its receptor, Rev-erbalpha, Genes Dev, 23 (2009) 2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Munakata H, Sun JY, Yoshida K, Nakatani T, Honda E, Hayakawa S, Furuyama K, Hayashi N, Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase, J Biochem, 136 (2004) 233–238. [DOI] [PubMed] [Google Scholar]
- [9].Tian Q, Li T, Hou W, Zheng J, Schrum LW, Bonkovsky HL, Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells, J Biol Chem, 286 (2011) 26424–26430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kramer MF, Gunaratne P, Ferreira GC, Transcriptional regulation of the murine erythroid-specific 5-aminolevulinate synthase gene, Gene, 247 (2000) 153–166. [DOI] [PubMed] [Google Scholar]
- [11].Zhang FL, Shen GM, Liu XL, Wang F, Zhao HL, Yu J, Zhang JW, Hypoxic induction of human erythroid-specific delta-aminolevulinate synthase mediated by hypoxia-inducible factor 1, Biochemistry, 50 (2011) 1194–1202. [DOI] [PubMed] [Google Scholar]
- [12].Rouault TA, The role of iron regulatory proteins in mammalian iron homeostasis and disease, Nat Chem Biol, 2 (2006) 406–414. [DOI] [PubMed] [Google Scholar]
- [13].Iwai K, Drake SK, Wehr NB, Weissman AM, LaVaute T, Minato N, Klausner RD, Levine RL, Rouault TA, Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: implications for degradation of oxidized proteins, Proc Natl Acad Sci U S A, 95 (1998) 4924–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paraskeva E, Hentze MW, Iron-sulphur clusters as genetic regulatory switches: the bifunctional iron regulatory protein-1, FEBS Lett, 389 (1996) 40–43. [DOI] [PubMed] [Google Scholar]
- [15].Bottomley SS, Fleming MD, Sideroblastic Anemias: Molecular Basis, Pathphysiology, and Clinical Aspects, in: Ferreira GC, Kadish KM, Smith KM, Guilard R (Eds.) Handbook of Porphyrin Science, Porphyrias and Sideroblastic Anemias, World Scientific Publishing Co. Pte. Ltd., Place Published, 2014, pp. 44–87. [Google Scholar]
- [16].Cotter PD, May A, Li L, Al-Sabah AI, Fitzsimons EJ, Cazzola M, Bishop DF, Four new mutations in the erythroid-specific 5-aminolevulinate synthase (ALAS2) gene causing X-linked sideroblastic anemia: increased pyridoxine responsiveness after removal of iron overload by phlebotomy and coinheritance of hereditary hemochromatosis, Blood, 93 (1999) 1757–1769. [PubMed] [Google Scholar]
- [17].Balwani M, Doheny D, Bishop DF, Nazarenko I, Yasuda M, Dailey HA, Anderson KE, Bissell DM, Bloomer J, Bonkovsky HL, Phillips JD, Liu L, Desnick RJ, Loss-of-Function Ferrochelatase and Gain-of-Function Erythroid-Specific 5-Aminolevulinate Synthase Mutations Causing Erythropoietic Protoporphyria and X-Linked Protoporphyria in North American Patients Reveal Novel Mutations and a High Prevalence of X-Linked Protoporphyria, Mol. Med, 19 (2013) 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karim Z, Gouya L, Deybach JC, Puy H, Heme Biosynthesis and Pathophysiology of Porphyrias, in: Ferreira GC, Kadish KM, Smith KM, Guilard R (Eds.) Handbook of Porphyrin Science, Porphyrias and Sideroblastic Anemias, World Scientific Publishing Co. Pte. Ltd., Place Published, 2014, pp. 90–118. [Google Scholar]
- [19].Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M, Corrigall AV, Meissner PN, Hift RJ, Marsden JT, Ma Y, Mieli-Vergani G, Deybach JC, Puy H, C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload, Am. J. Hum. Genet, 83 (2008) 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harigae H, Furuyama K, Hereditary sideroblastic anemia: pathophysiology and gene mutations, Int. J. Hematol, 92 (2010) 425–431. [DOI] [PubMed] [Google Scholar]
- [21].Manceau H, Gouya L, Puy H, Acute hepatic and erythropoietic porphyrias: from ALA synthases 1 and 2 to new molecular bases and treatments, Curr. Opin. Hemat, 24 (2017) 198–207. [DOI] [PubMed] [Google Scholar]
- [22].Ducamp S, Schneider-Yin X, de Rooij F, Clayton J, Fratz EJ, Rudd A, Ostapowicz G, Varigos G, Lefebvre T, Deybach JC, Gouya L, Wilson P, Ferreira GC, Minder EI, Puy H, Molecular and functional analysis of the C-terminal region of human erythroid-specific 5-aminolevulinic synthase associated with X-linked dominant protoporphyria (XLDPP), Human molecular genetics, 22 (2013) 1280–1288. [DOI] [PubMed] [Google Scholar]
- [23].Peoc’h K, Nicolas G, Schmitt C, Mirmiran A, Daher R, Lefebvre T, Gouya L, Karim Z, Puy H, Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias, Mol. Gen. Metab, (2019) 10.1016/j.ymgme.2019.1001.1015. [DOI] [PubMed] [Google Scholar]
- [24].Fujiwara T, Harigae H, Pathophysiology and genetic mutations in congenital sideroblastic anemia, Pediat. Intl, 55 (2013) 675–679. [DOI] [PubMed] [Google Scholar]
- [25].Furuyama K, Yamamoto M, Differential Regulation of 5-Aminolevulinate Synthase Isozymes in Vertebrates, in: Ferreira GC, Kadish KM, Smith KM, Guilard R (Eds.) Handbook of Porphyrin Science: Erythropoiesis, Heme and Applications to Biomedicine, World Scientific Publishing Co. Pte. Ltd., Place Published, 2014, pp. 1–39. [Google Scholar]
- [26].Kafina MD, Paw BH, Intracellular iron and heme trafficking and metabolism in developing erythroblasts, Metallomics, 9 (2017) 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kühn LC, Iron regulatory proteins and their role in controlling iron metabolism, Metallomics, 7 (2015) 232–243. [DOI] [PubMed] [Google Scholar]
- [28].Kikuchi G, Kumar A, Talmage P, Shemin D, The Enzymatic Synthesis of δ-Aminolevulinic Acid, J. Biol. Chem, 233 (1958) 1214–1219. [PubMed] [Google Scholar]
- [29].Gibson KD, Laver WG, Neuberger A, Initial stages in the biosynthesis of porphyrins. 2. The formation of δ-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes, Biochem. J, 70 (1958) 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abboud MM, Jordan PM, Akhtar M, Biosynthesis of 5-Aminolevulinic Acid: Involvement of a Retention-Inversion Mechanism, J. Chem. Soc. Chem. Comm, 276 (1974) 643–644. [Google Scholar]
- [31].Nandi DL, Studies on delta-aminolevulinic acid synthase of Rhodopseudomonas spheroides. Reversibility of the reaction, kinetic, spectral, and other studies related to the mechanism of action, J. Biol. Chem, 253 (1978) 8872–8877. [PubMed] [Google Scholar]
- [32].Zaman Z, Jordan PM, Akhtar M, Mechanism and stereochemistry of the 5-aminolaevulinate synthetase reaction, Biochem. J, 135 (1973) 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Toney MD, Controlling reaction specificity in pyridoxal phosphate enzymes, Biochim. Biophys, Acta (BBA) - Proteins and Proteomics, 1814 (2011) 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rossignoli G, Phillips RS, Astegno A, Menegazzi M, Voltattorni CB, Bertoldi M, Phosphorylation of pyridoxal 5′-phosphate enzymes: an intriguing and neglected topic, Amino Acids, 50 (2018) 205–215. [DOI] [PubMed] [Google Scholar]
- [35].Dunathan HC, Conformation and reaction specificity in pyridoxal phosphate enzymes, Proc. Natl. Acad. Sci. U.S.A, 55 (1966) 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hunter GA, Zhang J, Ferreira GC, Transient kinetic studies support refinements to the chemical and kinetic mechanisms of aminolevulinate synthase, J. Biol. Chem, 282 (2007) 23025–23035. [DOI] [PubMed] [Google Scholar]
- [37].Ferreira GC, Neame PJ, Dailey HA, Heme biosynthesis in mammalian systems: evidence of a Schiff base linkage between the pyridoxal 5’-phosphate cofactor and a lysine residue in 5-aminolevulinate synthase, Protein Sci, 2 (1993) 1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hunter GA, Ferreira GC, Lysine-313 of 5-Aminolevulinate Synthase Acts as a General Base during Formation of the Quinonoid Reaction Intermediates, Biochemistry, 38 (1999) 3711–3718. [DOI] [PubMed] [Google Scholar]
- [39].Yano T, Kuramitsu S, Tanase S, Morino Y, Kagamiyama H, Role of Asp222 in the catalytic mechanism of Escherichia coli aspartate aminotransferase: the amino acid residue which enhances the function of the enzyme-bound coenzyme pyridoxal 5’-phosphate, Biochemistry, 31 (1992) 5878–5887. [DOI] [PubMed] [Google Scholar]
- [40].Astner I, Schulze JO, van den Heuvel J, Jahn D, Schubert WD, Heinz DW, Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans, EMBO J, 24 (2005) 3166–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brown BL, Kardon JR, Sauer RT, Baker TA, Structure of the Mitochondrial Aminolevulinic Acid Synthase, a Key Heme Biosynthetic Enzyme, Structure, 26 (2018) 580–589.e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gong J, Hunter GA, Ferreira GC, Aspartate-279 in aminolevulinate synthase affects enzyme catalysis through enhancing the function of the pyridoxal 5’-phosphate cofactor, Biochemistry, 37 (1998) 3509–3517. [DOI] [PubMed] [Google Scholar]
- [43].Stojanovski BM, Hunter GA, Jahn M, Jahn D, Ferreira GC, Unstable Reaction Intermediates and Hysteresis during the Catalytic Cycle of 5-Aminolevulinate Synthase: IMPLICATIONS FROM USING PSEUDO AND ALTERNATE SUBSTRATES AND A PROMISCUOUS ENZYME VARIANT, J. Biol. Chem, 289 (2014) 22915–22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang J, Ferreira GC, Transient State Kinetic Investigation of 5-Aminolevulinate Synthase Reaction Mechanism, J. Biol. Chem, 277 (2002) 44660–44669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kaufholz A-L, Hunter Gregory A., Ferreira Gloria C., Lendrihas T, Hering V, Layer G, Jahn M, Jahn D, Aminolaevulinic acid synthase of Rhodobacter capsulatus: high-resolution kinetic investigation of the structural basis for substrate binding and catalysis, Biochem. J, 451 (2013) 205–216. [DOI] [PubMed] [Google Scholar]
- [46].Hunter GA, Ferreira GC, Pre-steady-state reaction of 5-aminolevulinate synthase. Evidence for a rate-determining product release, J. Biol. Chem, 274 (1999) 12222–12228. [DOI] [PubMed] [Google Scholar]
- [47].Lendrihas T, Hunter GA, Ferreira GC, Targeting the active site gate to yield hyperactive variants of 5-aminolevulinate synthase, J. Biol. Chem, 285 (2010) 13704–13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stojanovski BM, Ferreira GC, Asn-150 of Murine Erythroid 5-Aminolevulinate Synthase Modulates the Catalytic Balance between the Rates of the Reversible Reaction, J. Biol. Chem, 290 (2015) 30750–30761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gong J, Ferreira GC, Aminolevulinate synthase: functionally important residues at a glycine loop, a putative pyridoxal phosphate cofactor-binding site, Biochemistry, 34 (1995) 1678–1685. [DOI] [PubMed] [Google Scholar]
- [50].Thomas L, Junshun Z, A. HG, C. FG, Arg- 85 and Thr- 430 in murine 5- aminolevulinate synthase coordinate acyl- CoA- binding and contribute to substrate specificity, Protein Sci, 18 (2009) 1847–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tan D, Harrison T, Hunter GA, Ferreira GC, Role of arginine 439 in substrate binding of 5-aminolevulinate synthase, Biochemistry, 37 (1998) 1478–1484. [DOI] [PubMed] [Google Scholar]
- [52].Stojanovski BM, Ferreira GC, Murine erythroid 5- aminolevulinate synthase: Adenosyl- binding site Lys221 modulates substrate binding and catalysis, FEBS Open Bio, 5 (2015) 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu J, Kaganjo J, Zhang W, Zeilstra- Ryalls J, Investigating the bifunctionality of cyclizing and “classical” 5- aminolevulinate synthases, Protein Sci, 27 (2018) 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang W, Bolla ML, Kahne D, Walsh CT, A Three Enzyme Pathway for 2-Amino-3-hydroxycyclopent-2-enone Formation and Incorporation in Natural Product Biosynthesis, J. Am. Chem. Soc, 132 (2010) 6402–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Na I, DeForte S, Stojanovski BM, Ferreira GC, Uversky VN, Molecular dynamics analysis of the structural and dynamic properties of the functionally enhanced hepta-variant of mouse 5-aminolevulinate synthase, J. Biomol. Struct. Dyn, 36 (2018) 152–165. [DOI] [PubMed] [Google Scholar]
- [56].Na I, Catena D, Kong MJ, Ferreira GC, Uversky VN, Anti-Correlation between the Dynamics of the Active Site Loop and C-Terminal Tail in Relation to the Homodimer Asymmetry of the Mouse Erythroid 5-Aminolevulinate Synthase, Int. J. Mol. Sci, 19 (2018) 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tchaikovskii V, Desnick RJ, Bishop DF, Molecular expression, characterization and mechanism of ALAS2 gain-of-function mutants, Mole. Med, 25 (2019) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Balwani M, Erythropoietic Protoporphyria and X-Linked Protoporphyria: pathophysiology, genetics, clinical manifestations, and management, Mol. Gen. Metab, In Press (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lane AM, McKay JT, Bonkovsky HL, Advances in the management of erythropoietic protoporphyria - role of afamelanotide, Appl. Clin. Genet, 9 (2016) 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schneider-Yin X, Minder EI, Erythropoietic Protopoorphyria and X-linked Dominant Protoporphyria, in: Ferreira GC, Kadish KM, Smith KM, Guilard R(Eds.) Handbook of Porphyrin Science, World Scientific Publishing Co. Pte. Ltd., Place Published, 2014, pp. 299–328. [Google Scholar]
- [61].Orhanović S, Pavela- Vrančič M, Dimer asymmetry and the catalytic cycle of alkaline phosphatase from Escherichia coli, Eur. J. Biochem, 270 (2003) 4356–4364. [DOI] [PubMed] [Google Scholar]
- [62].Papaleo E, Renzetti G, Invernizzi G, Ásgeirsson B, Dynamics fingerprint and inherent asymmetric flexibility of a cold-adapted homodimeric enzyme. A case study of the Vibrio alkaline phosphatase, Biochim. Biophys. Acta - General Subjects, 1830 (2013) 2970–2980. [DOI] [PubMed] [Google Scholar]
- [63].Zocher G, Wiesand U, Schulz GE, High resolution structure and catalysis of O- acetylserine sulfhydrylase isozyme B from Escherichia coli, FEBS J, 274 (2007) 5382–5389. [DOI] [PubMed] [Google Scholar]
- [64].Campanini B, Bettati S, di Salvo ML, Mozzarelli A, Contestabile R, Asymmetry of the Active Site Loop Conformation between Subunits of Glutamate-1-semialdehyde Aminomutase in Solution, Biomed. Res. Inl, 2013:353270 (2013) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hennig M, Grimm B, Contestabile R, John RA, Jansonius JN, Crystal structure of glutamate-1-semialdehyde aminomutase: An α2-dimeric vitamin B6-dependent enzyme with asymmetry in structure and active site reactivity, Proc. Natl. Acad. Sci. (USA), 94 (1997) 4866–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Stetefeld J, Jenny M, Burkhard P, Intersubunit signaling in glutamate-1-semialdehyde-aminomutase, Proc. Natl. Acad. Sci. (U.S.A.), 103 (2006) 13688–13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Turbeville TD, Zhang J, Adams WC, Hunter GA, Ferreira GC, Functional asymmetry for the active sites of linked 5-aminolevulinate synthase and 8-amino-7-oxononanoate synthase, Arch. Biochem Biophys, 511 (2011) 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fratz EJ, Clayton J, Hunter GA, Ducamp S, Breydo L, Uversky VN, Deybach JC, Gouya L,Puy H, Ferreira GC, Human Erythroid 5-Aminolevulinate Synthase Mutations Associated with X-Linked Protoporphyria Disrupt the Conformational Equilibrium and Enhance Product Release, Biochemistry, 54 (2015) 5617–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Munakata H, Yamagami T, Nagai T, Yamamoto M, Hayashi N, Purification and Structure of Rat Erythroid-Specific d-Aminolevulinate Synthase, J. Biochem, 114 (1993) 103–111. [DOI] [PubMed] [Google Scholar]
- [70].Grishin NV, Phillips MA, Goldsmith EJ, Modeling of the spatial structure of eukaryotic ornithine decarboxylases, Protein Sci, 4 (1995) 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jansonius JN, Structure, evolution and action of vitamin B6-dependent enzymes, Curr. Opin. Struct. Biol, 8 (1998) 759–769. [DOI] [PubMed] [Google Scholar]
- [72].Percudani R, Peracchi AJBB, The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families, BMC Bioinformatics 10 (2009) 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schneider G, Käck H, Lindqvist Y, The manifold of vitamin B6 dependent enzymes, Structure, 8 (2000) R1–R6. [DOI] [PubMed] [Google Scholar]
- [74].Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, Eichler F, Brown RH Jr., Harmon JM, and Dunn TM, Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities, Proc. Natl. Acad. Sci. U. S. A, 106 (2009) 8186–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ikushiro H, Hayashi H, Mechanistic enzymology of serine palmitoyltransferase, Biochim. Biophys. Acta 1814 (2011) 1474–1480. [DOI] [PubMed] [Google Scholar]
- [76].Ikushiro H, Islam MM, Okamoto A, Hoseki J, Murakawa T, Fujii S, Miyahara I, Hayashi H, Structural Insights into the Enzymatic Mechanism of Serine Palmitoyltransferase from Sphingobacterium multivorum, J. Biochem, 146 (2009) 549–562. [DOI] [PubMed] [Google Scholar]
- [77].Schmidt A, Sivaraman J, Li Y, Larocque R, Barbosa JA, Smith C, Matte A, Schrag JD, Cygler M, Three-dimensional structure of 2-amino-3-ketobutyrate CoA ligase from Escherichia coli complexed with a PLP-substrate intermediate: inferred reaction mechanism, Biochemistry, 40 (2001) 5151–5160. [DOI] [PubMed] [Google Scholar]
- [78].Webster SP, Alexeev D, Campopiano DJ, Watt RM, Alexeeva M, Sawyer L, Baxter RL, Mechanism of 8-amino-7-oxononanoate synthase: spectroscopic, kinetic, and crystallographic studies, Biochemistry, 39 (2000) 516–528. [DOI] [PubMed] [Google Scholar]
- [79].Yang Z, PAML: A program package for phylogenetic analysis by maximum likelihood, Comput. Appl. Biosci, 13 (1997) 555–556. [DOI] [PubMed] [Google Scholar]
- [80].Zhao X, Gao S, Wu Z, Kajigaya S, Feng X, Liu Q, Townsley DM, Cooper J, Chen J, Keyvanfar K, Fernandez Ibanez M.d.P., Wang X, Young NS, Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells, Blood, 130 (2017) 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]