Abstract
Background:
Gallium-68 Dotatate binds preferentially to somatostatin receptor (sstr) subtype-2 (sstr-2) on inflammatory cells. We aimed at investigating the potential clinical use of sstr-targeted imaging for the detection of myocardial inflammation.
Methods:
13 patients with suspected cardiac sarcoidosis (CS) based on clinical history and myocardial uptake on recent fluorine-18 fluorodeoxyglucose (FDG) PET, were enrolled to undergo Dotatate PET after FDG PET (median time 37 days [IQR 25 – 55]). Additionally, we investigated ex-vivo the immunohistochemistry expression of sstr-2 in 3 explanted sarcoid hearts.
Results:
All FDG scans showed cardiac uptake (focal/multifocal = 6, focal on diffuse/heterogeneous = 7), and 46% (n=6) extra-cardiac uptake (mediastinal/hilar). In comparison, Dotatate scans showed definite abnormal cardiac uptake (focal/multifocal) in 4 patients, probably abnormal (heterogenous/patchy) in 3, and negative uptake in 6 cases. Similarly, 6 patients had increased mediastinal/hilar Dotatate uptake. Overall concordance of FDG and Dotatate uptake was 54% in the heart and 100% for thoracic nodal activity. Quantitatively, FDG maximum standardized uptake value was 5.0 times [3.8 – 7.1] higher in the heart, but only 2.25 times [1.7 – 3.0; P=0.019] higher in thoracic nodes relative to Dotatate. Ex-vivo, sstr-2 immunostaining was weakly seen within well-formed granulomas in all 3 examined sarcoid heart specimens with no significant staining of background myocardium or normal myocardium.
Conclusion:
Our preliminary data suggests that, compared to FDG imaging, somatostatin receptor-targeted imaging may be less sensitive for the detection of myocardial inflammation, but comparable for detecting extra-cardiac inflammation.
Keywords: sarcoidosis, somatostatin receptor, myocardial inflammation, PET
INTRODUCTION
Sarcoidosis is a complex multisystemic inflammatory disease involving the heart in ~25% of cases.1–3 Affected patients are at risk of arrhythmic complications and heart failure progression. Therapy to suppress the immune system is typically indicated in patients with active myocardial disease.4, 5 Thus, assessment of disease activity or inflammation in cardiac sarcoidosis is clinically relevant, yet it remains a challenging task to achieve by current technology. Positron emission tomography (PET) using fluorine-18 fluorodeoxyglucose (FDG) lacks specificity for the detection of myocardial inflammation since 1) fluctuations in myocyte metabolism (both physiologic and pathologic),6–8 and 2) tissue hypoxia/ischemia9, 10 are potent stimuli for glucose transporter expression thus potentially compromising the distinction of inflammatory from non-inflammatory mechanisms of uptake in the heart. This is particularly relevant when monitoring treatment response in primary inflammatory cardiomyopathies (such as cardiac sarcoidosis), in which a more specific approach is desired in order to avoid unnecessary immunotherapy and potentially harmful side effects. Consequently, cardiac sarcoidosis serves as a unique model for the investigation of more specific techniques capable of reliably assessing the presence and treatment response of inflammation in the heart.
Emerging data suggests that somatostatin receptor-targeted imaging with PET/CT may be a novel approach for detecting myocardial inflammation, and consequently sarcoidosis.11–14 Gallium-68 Dotatate is a PET radiopharmaceutical that binds preferentially to the somatostatin receptor (sstr) subtype-2 (sstr-2) on the cell surface and may provide a more specific marker of disease activity in subjects with suspected cardiac sarcoidosis due to the following: 1) sstr-2 receptors are over-expressed in inflammatory cells, including non-caseating granulomas as determined by immunohistochemistry,15, 16 2) unlike FDG, Dotatate imaging has an advantageous biodistribution for cardiac imaging given the lack of significant cardiac Dotatate uptake under baseline conditions,17, 18 and 3) small case series have shown Dotatate accumulation within inflammatory myocardium.12, 13
In this pilot study, we aimed to continue to investigate the potential role of sstr-targeted imaging with Dotatate in cardiac sarcoidosis as an alternative marker of myocardial inflammation to FDG PET imaging. We also used immunohistochemistry to assess the expression of sstr-2 (as a surrogate of Dotatate activity) in a small sample of explanted hearts with granulomatous involvement.
METHODS
A total of 13 adult patients with suspected active CS based on clinical history and imaging findings were prospectively enrolled to undergo Dotatate PET/CT after a clinically indicated FDG PET/CT scan. Inclusion criteria: 1) individuals aged 18 or older; 2) documentation of biopsy-proven sarcoidosis OR patients with typical findings on FDG PET and CMR without previous biopsy; 3) clinical suspicion of cardiac involvement defined as the presence of any of the following: high-degree A-V nodal block, reduced left or right ventricular systolic function, ventricular arrhythmias, and/or unexplained dyspnea or syncope; and 4) FDG-PET suggestive of active myocardial inflammation. Exclusion criteria: Patients taking Octreotide, or on total parenteral nutrition (per Dotatate package insert).
For the ex-vivo experiment, we searched our pathology archives and identified 3 explanted heart specimens of patients who either died with or underwent transplantation for sarcoid cardiomyopathy. The Human Research Committee approved this study. All subjects signed informed consent for the prospective portion of the study.
FDG PET/CT Protocol
All patients received written instructions to adhere to a specific high fat, high protein, no carbohydrate diet for 24 hours and were advised to fast for 12 hours prior to the procedure. All patients underwent evaluation of regional myocardial perfusion and metabolic imaging using an integrated hybrid protocol that includes single photon emission computer tomography (SPECT) myocardial perfusion imaging and FDG PET. First, myocardial perfusion SPECT/CT (Symbia T6, Siemens Healthcare, Hoffman Estates, Chicago, IL) imaging was performed ~45 minutes after the intravenous injection of technetium-99m Sestamibi (~740 Mbq [20 mCi]) at rest. Subsequently, FDG was administered intravenously (~10 mCi / 370 MBq) and after a 90-minute uptake time, a dedicated 10-minute 3-dimensional cardiac PET acquisition was followed by a limited whole-body PET (Discovery RX or LightSpeed VCT 64, GE Healthcare, Milwaukee, WI, USA) obtained from the base of the skull to the kidneys. Non-contrast CT was performed over the same range for attenuation correction and co-localization of FDG activity.
Dotatate PET/CT Protocol
Neither dietary restriction nor fasting was required prior to the procedure. Dotatate was injected intravenously at the recommended activity of 2 MBq/kg (0.054 mCi/kg) up to 200 MBq (5.4 mCi). After a 60-minute uptake period, a 3-dimensional static PET acquisition of the chest was acquired for 30 minutes. A low-dose CT of the chest was also performed for attenuation correction and co-localization of Dotatate activity.
Image Processing and Interpretation of FDG and Dotatate PET/CT Scans:
All PET images were reconstructed with attenuation correction (Matrix 128×128, OSEM, subsets 28, iteration 2, post filter 6.0 mm), processed and reviewed using MIM Software Inc. (Cleveland, OH, USA). Radiotracer uptake was qualitatively and quantitatively assessed in the myocardium, and extra-cardiac structures, with a focus on hilar and mediastinal nodes.
Qualitative Assessment:
Uptake in the heart was visually inspected and described as negative/absent, or positive/present. When present, uptake was further graded as: 1) diffuse/heterogenous; 2) focal or multifocal; 3) focal on diffuse. Extra-cardiac uptake was graded binarily as present or absent. Dotatate PET display was set to a narrower standardized uptake value (SUV) window width (0-3) compared to FDG (0-5).
Quantitative assessment:
A circular region of interest (ROI) was placed over the axial PET image slice exhibiting maximum cardiac and extra-cardiac uptake to derive maximum standardized uptake values (SUVmax).
Immunohistochemistry Analyses
Immunohistochemical analysis was performed on cardiac tissue from 3 patients with active sarcoid in the explanted heart, on lymph node tissue from one patient with sarcoid in a mediastinal lymph node biopsy and in one normal control heart using standard immunohistochemical methods. Slides were created from formalin-fixed, paraffin embedded samples, incubated with an anti-somatostatin receptor subtype2 antibody (UMB-1; Abcam, Cambridge, MA, USA) at a 1:250 dilution, and counterstained with hematoxylin. As a positive control for the antibody, a section from human pancreas (islets) was used. Tissue sections were categorized as positive or negative for sstr-2 immunoreactivity depending on the presence or absence of immunostaining.
Statistical analyses
We analyzed the data using STATA (version 13.1). Continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range [IQR], T-test and Wilcoxon were performed to compare differences between groups depending on the normal or non-normal distribution of the data respectively. Association between categorical variables was measured using Fisher’s exact test and results are presented as percentages. All statistical tests were 2-tailed, and a P value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics and imaging findings for the study cohort are summarized in Tables 1 and 2 respectively.
Table 1.
Baseline characteristics
| Baseline Characteristics | Total (N = 13) |
|---|---|
| Age, years ± SD | 58 ± 10 |
| Males, n (%) | 11 (85) |
| Whites, n (%) | 13 (100) |
| History of extracardiac sarcoidosis, n (%) | 10 (77) |
| Immunosuppression therapy at time of FDG, n (%) | 4 (31) |
| Immunosuppression therapy at time of Dotatate, n (%) | 5 (38) |
| Left ventricular ejection fraction, % ± SD | 59 ± 15 |
| LVEF < 50%, n (%) | 3 (23) |
| Implantable cardioverter defibrillator/pacemaker, n (%) | 8 (61) |
| Hight degree atria-ventricular block, n (%) | 4 (31) |
| Ventricular tachycardia, n (%) | 2 (15) |
| Dyspnea, n (%) | 6 (46) |
| Near syncope, n (%) | 1 (8) |
Table 2.
FDG and Dotatate findings for the study cohort
| ID | Prior Immunotherapy and Start Date | Cardiac FDG uptake (SUVmax) | Nodal FDG uptake (SUVmax) | Days to Dotatate | Cardiac Dotatate uptake (SUVmax) | Nodal Dotatate uptake (SUVmax) | Cardiac concordance | Nodal concordance |
|---|---|---|---|---|---|---|---|---|
| 1 | Methotrexate 2.5 mg weekly > 1 year | Focal (6.58) | Increased (8.4) | 139 | Focal (1.33) | Increased (1.86) | Concordant | Concordant |
| 2 | None | Multifocal (6.89) | Increased (11.47) | 35 | Multifocal (1.70) | Increased (2.74) | Concordant | Concordant |
| 3 | None | Focal on diffuse (10.97) | Increased (22.6) | 70 | Focal (2.05) | Increased (2.40) | Concordant | Concordant |
| 4 | None | Focal (4.15) | Increased (7.01) | 37 | Focal (1.09) | Increased (2.46) | Concordant | Concordant |
| 5 | None | Focal on diffuse (2.77) | Negative (2.10) | 22 | Heterogeneous (1.34) | Negative (0.97) | Concordant | Concordant |
| 6 | None | Focal on diffuse (5.22) | Negative (4.14) | 37 | Heterogenous (2.21) | Negative (1.84) | Concordant | Concordant |
| 7 | None | Multifocal (11.48) | Negative (2.01) | 51 | Diffuse intense (1.84) | Negative (1.19) | Concordant | Concordant |
| 8 | Prednisone 20 mg daily 5 days before Dotatate | Focal on diffuse (6.50) | Increased (4.47) | 61 | Negative (0.91) | Increased (1.79) | Discordant | Concordant |
| 9 | Methotrexate 15 mg weekly > 1 year | Focal on diffuse (9.18) | Increased (5.95) | 13 | Negative (0.99) | Increased (2.01) | Discordant | Concordant |
| 10 | Prednisone 15mg daily 5 months before FDG | Focal (2.33) | Negative (1.08) | 15 | Negative (1.28) | Negative (1.03) | Discordant | Concordant |
| 11 | Prednisone 15 mg daily 25 days before FDG | Focal on diffuse (7.85) | Negative (1.59) | 16 | Negative (1.11) | Negative (1.24) | Discordant | Concordant |
| 12 | None | Focal (5.91) | Negative (2.85) | 27 | Negative (1.19) | Negative (1.54) | Discordant | Concordant |
| 13 | None | Focal on diffuse (5.47) | Negative (2.0) | 55 | Negative (0.67) | Negative (1.38) | Discordant | Concordant |
| Total | 38% | 6.5 [5.2-7.9] | 4.1 [2.0-7.0] | 37 [25-55] | 1.28 [1.1-1.7] | 1.8 [1.2-2.0] | 54% concordance | 100% concordance |
Values are median [interquartile rang]
SUVmax = Maximum standard uptake value
Cardiac FDG uptake was focal or multifocal in 6 patients, and focal on diffuse in 7 patients. In comparison, Dotatate uptake in the heart was considered definitely abnormal in 4 patients (focal/multifocal), probably abnormal in 3 subjects (heterogenous/diffuse), and negative in the remaining 6. Outside the heart, increased uptake was evident in mediastinal and hilar nodes with both FDG and Dotatate in the same 6 subjects.
As a result, the overall concordance of FDG and Dotatate uptake was 54% in the heart but 100% for thoracic nodal (extra-cardiac) activity. Specifically, 4 patients had concordant cardiac (Figures 1 and 2) and nodal uptake (Figure 3), 3 had concordant cardiac uptake without nodal uptake (Figure 4), and the remaining 6 had discordant cardiac uptake with concordant nodal uptake in 2 cases (Figures 5).
Figure 1.
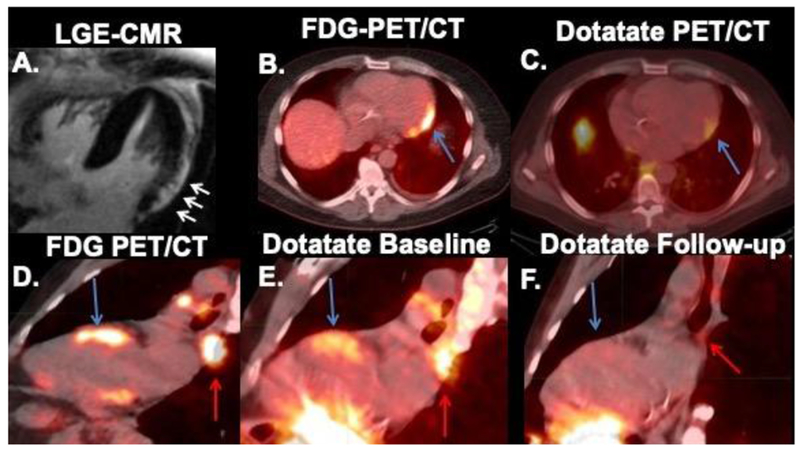
Abnormal cardiac Dotatate uptake in 2 different patients. First patient (top row) had cardiac magnetic resonance (CMR) showing late gadolinium enhancement (LGE) in the subepicardial aspect of the left ventricular lateral wall (A) with concordant FDG (B) and Dotatate (C) uptake (4.6 months post-FDG) (blue arrows). The second patient (bottom row) showed concordant FDG (D) and Dotatate (E) uptake in the anterior septum (blue arrows) and mediastinal nodes (red arrows) at baseline. Please note interval resolution of activity in heart and nodes 5 months later on follow-up Dotatate (F).
Figure 2.
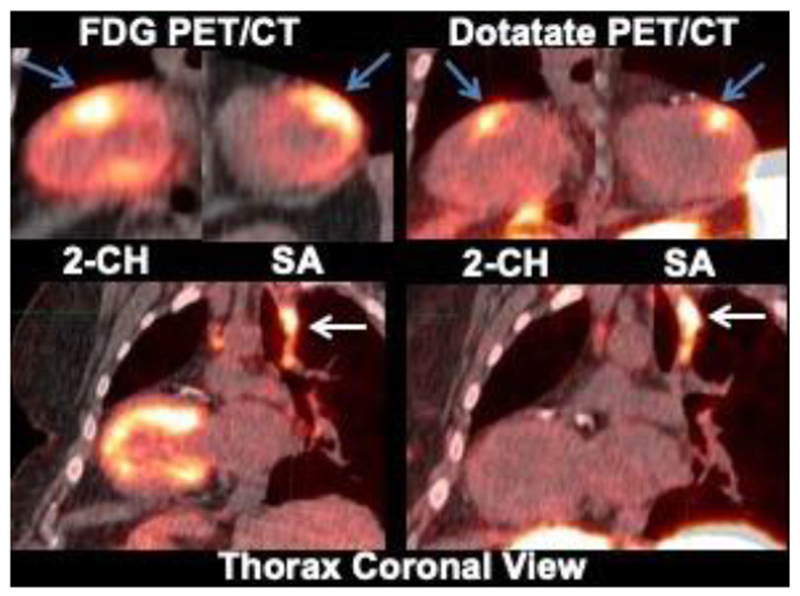
Case of focal on diffuse FDG activity matching LV Dotatate uptake.
Please notice that Dotatate clearly shows focal uptake in the anterior wall (blue arrows). Concordant hilar nodal uptake (white arrow) is also seen with both FDG and Dotatate. Two-chamber view = 2-CH; Short axis = SA
Figure 3.
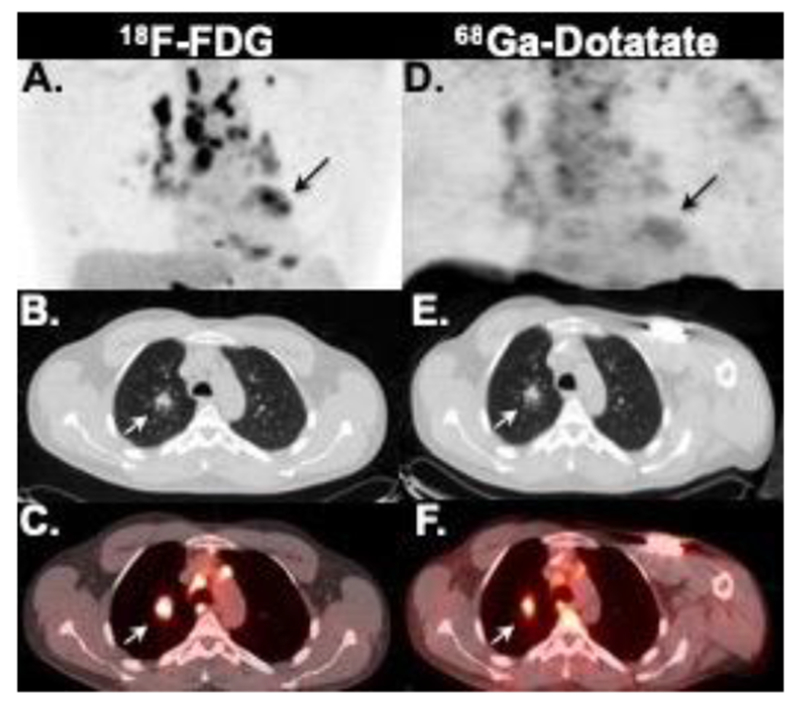
Case of extensive mediastinal, hilar, pulmonary (white arrow), as well as cardiac (black arrow) inflammation seen on both FDG (A-C) and Dotatate (D-E) on maximum intensity projection (A and D), axial chest CT (D and E) and fused PET/CT (C and F) images.
Figure 4.
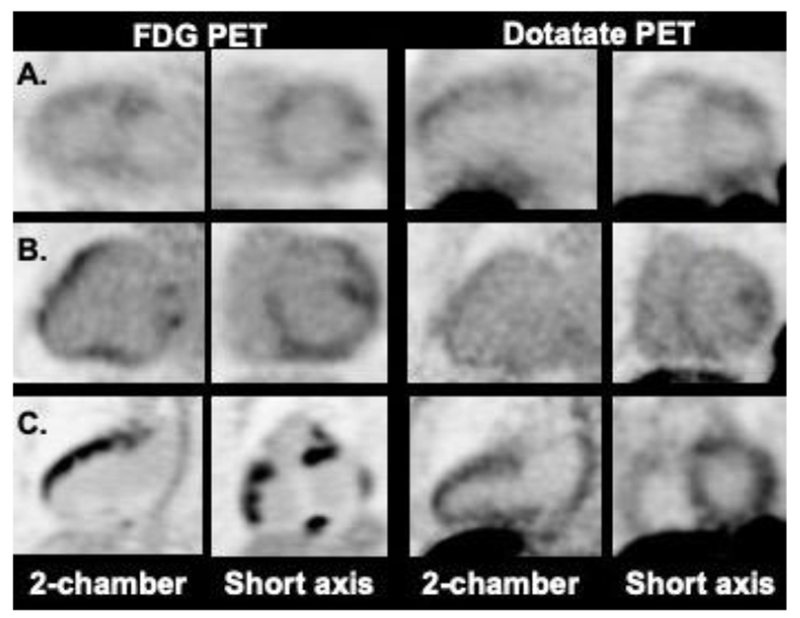
Probably abnormal cardiac Dotatate uptake. Cases A and B showed a similar pattern of FDG and Dotatate uptake in the LV (heterogeneous/patchy). Case C, in contrast, showed multifocal FDG uptake in the left and right ventricles, whereas, Dotatate uptake was moderately diffusely increased in both LV and RV.
Figure 5.
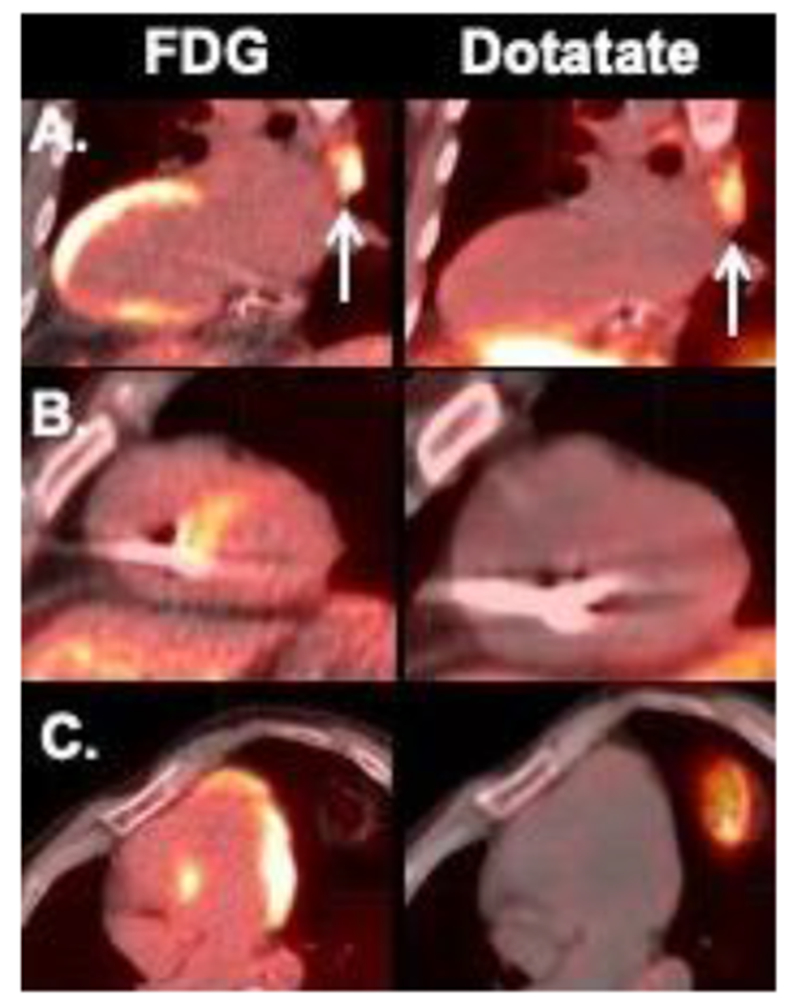
Discordant Dotatate (negative) and FDG uptake in the heart. Of note, case A showed increased mediastinal nodal activity in both FDG and Dotatate scans (arrows), whereas no extra-cardiac activity was seen in cases B and C.
Patients with discordant cardiac exams were more likely to be on immunomodulator therapy prior to imaging with both FDG and Dotatate (4/6 [67%] vs 1/7 [14%]; P=0.1). Only one subject was started on prednisone between FDG and Dotatate scanning (Table 2, subject ID 8).
Only one patient underwent a repeat Dotatate PET/CT scan to investigate treatment response in our study. The follow-up scan showed near complete resolution of Dotatate uptake in the heart and mediastinum after 5 months of high-dose prednisone therapy (Figure 1 E–F).
Quantitatively, SUVmax was confirmed to be significantly higher in the heart (1.70 [1.33 – 2.05] vs. 1.05 [0.91 —1.19]; P= 0.01) and thoracic nodes (2.21 [1.86 – 2.46] vs. 1.24 [1.03 – 1.54]; P=0.004) of patients with visually increased Dotatate uptake compared to those with negative uptake in the heart and thoracic nodes respectively. Compared to Dotatate, SUVmax derived from FDG was significantly higher in the heart (6.5 [IQR 5.2-7.9] vs. 1.28 [1.1-1.7]; P=0.0015) and thoracic nodes (4.1 [2.0-7.0] vs. 1.8 [1.2-2.0]; P=0.0015). Consequently, FDG uptake was 5.0-fold [3.8 – 7.1] higher in the heart, but only 2.25-fold [1.7 – 3.0; P=0.019] higher in thoracic nodes relative to Dotatate.
Ex-vivo (Figure 6), all 3 explanted heart specimens with pathology-proven sarcoidosis showed positive but weak immunostaining for sstr-2 within the granulomas (Figure 6 C–D). Somewhat more intense sstr-2 immunostaining was seen in granulomas from a mediastinal lymph node specimen (Figure 6B). In contrast, significant sstr-2 expression was not seen in a normal myocardium specimen (Figure 6A) or in the background myocardium in the sarcoid specimens.
Figure 6.
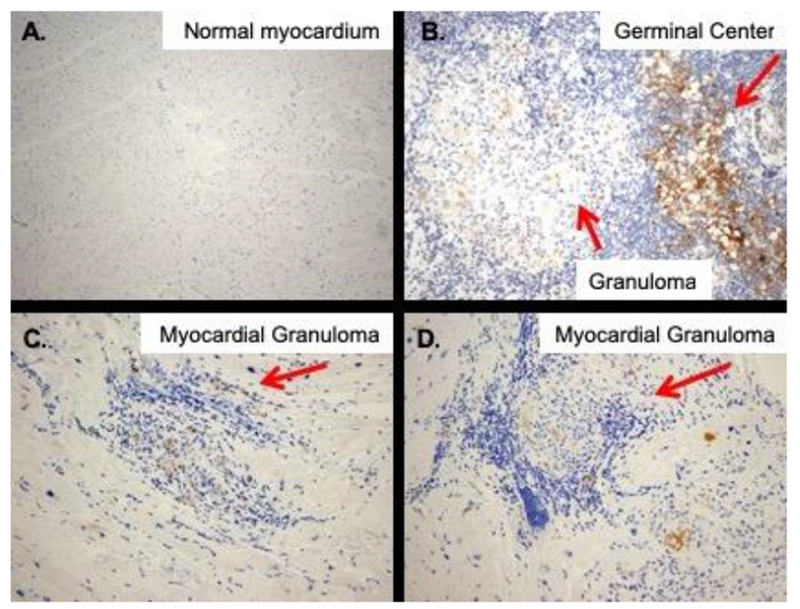
Expression of somatostatin receptor subtype-2 by immunohistochemistry. No staining is seen in normal myocardium (A), whereas, weak-to-moderate-staining is seen within well-formed granulomas (arrows) in a mediastinal lymph node (B), and two sarcoid heart specimens (C&D). (Original magnification ×100 for A, and 200× for B-D)
DISCUSSION
Our pilot study confirms prior observations that sstr-targeted imaging is feasible for the detection of myocardial inflammation, and provides new evidence suggesting that this novel approach may be less sensitive, though potentially more specific, than FDG based on the following findings: 1) myocardial retention, indicative of inflammation, was detected by Dotatate in 54% of the patients; 2) thoracic nodal uptake was highly concordant between FDG and Dotatate implying that both radiotracers accumulate well in extra-cardiac inflammatory tissue; 3) FDG uptake, particularly in the heart, was invariably greater than with Dotatate; 4) expression of sstr-2, although low, was confirmed ex-vivo in pathology-proven sarcoid heart specimens; 5) dietary-induced metabolic switch is not required with Dotatate, which in itself, should further reduce the number of false positive findings and non-diagnostic scans.
Detection of granulomatous myocarditis on endomyocardial biopsy (EMB) is the “gold standard” for establishing the diagnosis of myocardial inflammation related to sarcoidosis. Unfortunately, EMB has poor sensitivity (~20%) due to sampling error19,20 and is associated with potentially serious complications.21 Consequently, non-invasive testing with LGE-CMR and/or FDG-PET has largely replaced EMB as the diagnostic strategy of choice.22,23 However, they both suffer from logistic, technical and biologic limitations. LGE-CMR, for example, may help stablish the diagnosis of myocardial injury associated with sarcoidosis but is not a reliable marker of disease activity, and, thus, cannot be used for monitoring treatment. FDG, on the other hand, is a very sensitive marker of tissue inflammation. The problem, however, is that healthy myocytes can be remarkably FDG-avid as well, and the available glucose suppressive strategies are highly variable, with suppression rates ranging between 9% after a 6-hour fasting period,24 and 81–84% following strict, highly supervised dietary restrictions including prolonged fasting.25,26 This makes the distinction between physiologic and pathologic FDG uptake problematic in some cases (Figures 1C, 2, and 5). In addition, ischemia,10 tissue hypoxia,9 exercise, hypertrophy, and increased workload,27 can lead to myocardial glucose up-regulation through mechanisms other than inflammation, which may potentially yield further false positive scans.
Because of all these reasons, a more specific method to detect myocardial inflammation is desirable, and sstr-targeted imaging with Dotatate PET/CT may have the potential to overcome many of the aforementioned limitations. Dotatate binds preferentially to sstr-2 in vivo, a receptor that has been shown to be over-expressed by inflammatory cells, including activated macrophages.15, 16 Moreover, Dotatate has an advantageous biodistribution for cardiac imaging as it lacks significant cardiac uptake under baseline conditions, suggestive of low-level sstr-2 expression in normal myocardium.17, 18
However, the literature on sstr-targeted imaging for myocardial inflammation remains scarce and limited to Europe and Asia with mixing results. For example, the first series investigating myocardial sstr-targeted imaging was a Danish study that included 19 patients who underwent Ga-68 Dotanoc (an analog to Dotatate) and FDG.13 The authors found that Dotanoc was positive in 3 out of 3 patients with suspected cardiac sarcoidosis and negative in 16 out of 16 patients without the condition, implying an exceedingly high (~100%) diagnostic accuracy for this technique compared to FDG (78%).13 In a different study in Germany, 15 patients underwent CMR and Dotatoc (another sstr-analog) for evaluation of suspected cardiac sarcoidosis.28 They found regional myocardial sstr activity in 7/15 patients, whereas LGE was present in 10/15 patients. 28 In contrast, a subsequent German study found myocardial Dotatoc accumulation in only 1 out of 17 sarcoidosis patients with suspected cardiac involvement compared to 10/17 showing LGE on CMR.29
Interestingly, the diagnostic performance of sstr PET imaging appears to be higher and more consistent across studies for the detection of extra-cardiac inflammation. For instance Nobashi et al, found that Dotatoc was positive in 19 out of 20 Japanese patients with systemic sarcoidosis,30 whereas, Sharma el al. reported Dotanoc accumulation in 25/27 symptomatic and 2/12 asymptomatic pulmonary sarcoid patients in India, for an overall sensitivity of 92.5% and specificity of 83.3%.31
Our findings are in certain agreement with prior studies. On one hand, Dotatate accumulation in the heart was seen in about half of patients with myocardial FDG activity, whereas, the concordance rate of both tracers for detecting extra-cardiac inflammation within thoracic nodes was very high. Remarkably, we observed that the FDG-to-Dotatate ratio was 2 times higher in the heart than in thoracic nodes, suggesting that 1) mechanisms other than inflammation may be contributing to FDG accumulation in the heart and/or 2) relative to inflamed nodes, accumulation within inflamed myocardium may be greater with FDG than with Dotatate. While our ex-vivo analyses were qualitative and limited to only 3 heart sarcoid specimens and 1 mediastinal lymph node, they provided additional hints by showing weak staining of the sstr-2 antibody within myocardial granulomas, but somewhat higher staining in the germinal center of a sarcoid lymph node, suggesting that sstr-2 density may be higher in lymphoid tissue. Thus, it is conceivable that sstr-targeted PET imaging may be a more sensitive technique to detect extra-cardiac inflammation than myocardial inflammation. These findings highlight the need for further studies.
Study Limitations
There are several limitations in our study that are worth-mentioning. The sample size was small, and we only included individuals with myocardial FDG uptake, therefore, sensitivity and specificity could not be determined. Likewise, the ex-vivo analysis was limited to only 3 heart sarcoid specimens and 1 lymph node, and thus it cannot be considered hard evidence to support the in-vivo findings. This should be the aim of future investigations. The median time elicited between FDG and Dotatate scanning was 37 days, and 38% of subjects were on immunotherapy at time of evaluation, which may have potentially affected the study results, in particular the level of Dotatate activity in relation to FDG in the group with discordant cardiac uptake. Another limitation is the lack of a gold standard for the diagnosis of inflammation. Consequently, we could not determine with certainty if the scan results corresponded to true positive and/or true negative findings of myocardial inflammation. Finally, the potential of Dotatate to serve as a marker of therapy response was only tested on one subject (Figure 1 E–F), and thus, remains to be determined.
CONCLUSION
In summary, detection of myocardial inflammation is possible with sstr-targeted imaging with Dotatate. However, the sensitivity of this technique, while not directly tested, appears to be lower for the detection of myocardial inflammation, but comparable to detect extra-cardiac inflammation, compared to FDG imaging. While still preliminary, our findings call for further studies to enhance our understanding of this novel diagnostic approach, especially since Dotatate is already an FDA-approved radiopharmaceutical, and thus readily available for clinical application.
Supplementary Material
New Knowledge Gained:
We found that somatostatin receptor-targeted imaging may be less sensitive for the detection of myocardial inflammation, but comparable for identifying extra-cardiac inflammation, as compared to FDG imaging. On the other hand, our ex-vivo data suggests the potential specificity of somatostatin receptor-targeted imaging for detecting active granulomas.
Acknowledgment
This work was supported by a grant from the Radiological Society of North America Research & Education Foundation Board of Trustees (RF1632), Institutional Funds, and a Training Grant from the National Institutes of Health (1T32HL094301).
Abbreviations
- PET
Positron emission tomography
- FDG
Fluorodeoxyglucose
- sstr-2
Somatostatin receptor (sstr) subtype-2
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosure
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from the johns hopkins hospital and massachusetts general hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 2.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–11. [DOI] [PubMed] [Google Scholar]
- 3.Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest 1993;103:253–8. [DOI] [PubMed] [Google Scholar]
- 4.Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2013;15:347–54. [DOI] [PubMed] [Google Scholar]
- 5.Hiramitsu S, Morimoto S, Uemura A, Kato Y, Kimura K, Ohtsuki M, et al. National survey on status of steroid therapy for cardiac sarcoidosis in japan. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders 2005;22:210–3. [PubMed] [Google Scholar]
- 6.Osborne MT, Hulten EA, Murthy VL, Skali H, Taqueti VR, Dorbala S, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan R, Song J, Wu Z, Guo M, Liu J, Li J, et al. Detection of myocardial metabolic abnormalities by 18f-fdg pet/ct and corresponding pathological changes in beagles with local heart irradiation. Korean J Radiol 2015;16:919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielniczuk LM, Birnie D, Ziadi MC, deKemp RA, DaSilva JN, Burwash I, et al. Relation between right ventricular function and increased right ventricular [18f]fluorodeoxyglucose accumulation in patients with heart failure. Circulation. Cardiovascular imaging 2011. ;4:59–66. [DOI] [PubMed] [Google Scholar]
- 9.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol 2011;58:603–14. [DOI] [PubMed] [Google Scholar]
- 10.Sibille L, Chambert B, Collombier L, Kotzki PO, Boudousq V. False positive 18f-fdg pet/ct in cardiac sarcoidosis. J Mol Biol & Mol Imaging 2015;2:1020. [Google Scholar]
- 11.Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, et al. Imaging of myocardial inflammation with somatostatin receptor based pet/ct - a comparison to cardiac mri. International journal of cardiology 2015;194:44–9. [DOI] [PubMed] [Google Scholar]
- 12.Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. European heart journal 2015;36:2404. [DOI] [PubMed] [Google Scholar]
- 13.Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)ga-dotanoc pet/ct and (18)f-fdg pet/ct pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smedema JP, van Kroonenburgh MJ, Snoep G, Backes W, Gorgels AP. Images in cardiovascular medicine. Cardiac sarcoidosis in a patient with hypertrophic cardiomyopathy demonstrated by magnetic resonance imaging and single photon emission computed tomography dual-isotope scintigraphy. Circulation 2004;110:e529–31. [DOI] [PubMed] [Google Scholar]
- 15.van Hagen PM. Somatostatin receptor expression in clinical immunology. Metabolism: clinical and experimental 1996;45:86–7. [DOI] [PubMed] [Google Scholar]
- 16.ten Bokum AM, Hofland LJ, de Jong G, Bouma J, Melief MJ, Kwekkeboom DJ, et al. Immunohistochemical localization of somatostatin receptor sst2a in sarcoid granulomas. European journal of clinical investigation 1999;29:630–6. [DOI] [PubMed] [Google Scholar]
- 17.Balon HR, Goldsmith SJ, Siegel BA, Silberstein EB, Krenning EP, Lang O, et al. Procedure guideline for somatostatin receptor scintigraphy with (111)in-pentetreotide. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2001;42:1134–8. [PubMed] [Google Scholar]
- 18.Bombardieri E, Ambrosini V, Aktolun C, Baum RP, Bishof-Delaloye A, Del Vecchio S, et al. 111in-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. European journal of nuclear medicine and molecular imaging 2010;37:1441–8. [DOI] [PubMed] [Google Scholar]
- 19.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302. [DOI] [PubMed] [Google Scholar]
- 20.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. The New England journal of medicine 2007;357:2153–65. [DOI] [PubMed] [Google Scholar]
- 21.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: A seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43–7. [DOI] [PubMed] [Google Scholar]
- 22.Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, et al. Usefulness of fasting 18f-fdg pet in identification of cardiac sarcoidosis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2004;45:1989–98. [PubMed] [Google Scholar]
- 23.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zurn C, Kramer U, et al. Cmr imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC. Cardiovascular imaging 2013;6:501–11. [DOI] [PubMed] [Google Scholar]
- 24.Maurer AH, Burshteyn M, Adler LP, Gaughan JP, Steiner RM. Variable cardiac 18fdg patterns seen in oncologic positron emission tomography computed tomography: Importance for differentiating normal physiology from cardiac and paracardiac disease. Journal of thoracic imaging 2012;27:263–8. [DOI] [PubMed] [Google Scholar]
- 25.Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18f-fdg uptake during pet: A randomized controlled trial. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2010;17:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial fdg uptake. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2011;18:926–36. [DOI] [PubMed] [Google Scholar]
- 27.Shao D, Tian R. Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol 2015;6:331–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, et al. Somatostatin receptor based pet/ct in patients with the suspicion of cardiac sarcoidosis: An initial comparison to cardiac mri. Oncotarget 2016;7:77807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail 2018;5:249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobashi T, Nakamoto Y, Kubo T, Ishimori T, Handa T, Tanizawa K, et al. The utility of pet/ct with (68)ga-dotatoc in sarcoidosis: Comparison with (67)ga-scintigraphy. Annals of nuclear medicine 2016;30:544–52. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Singh AD, Sharma SK, Tripathi M, Das CJ, Kumar R. Gallium-68 dota-noc pet/ct as an alternate predictor of disease activity in sarcoidosis. Nuclear medicine communications 2018;39:768–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


