Abstract
Negative urgency, defined as a tendency to act rashly under extreme negative emotion, is strongly associated with tobacco use. Despite the robust evidence linking negative urgency and tobacco use and accumulating evidence suggesting that localized, segregated brain regions such as the nucleus accumbens (NAcc), insula, and amygdala are related to negative urgency, resting state functional connectivity (rsFC) of negative urgency in tobacco use has not yet been examined. This study included 34 daily tobacco users and 62 non-users matched on age, gender, race/ethnicity, and lifetime psychiatric diagnosis from a publicly available neuroimaging dataset collected by the Nathan Kline Institute-Rockland Project. Using the bilateral NAcc, insula, and amygdala as seed regions, seed-based rsFC analyses were conducted on the whole brain. In the whole sample, negative urgency was positively correlated with rsFC between the left insula and right dorsal anterior cingulate cortex (dACC). Compared to non-users, tobacco users had a stronger rsFC strength between the right amygdala and right middle temporal gyrus. In tobacco users, negative urgency was negatively associated with rsFC between the left NAcc and right dACC and between the left NAcc and right dorsolateral prefrontal cortex; these relationships were positive in non-users. Identifying functional connectivity implicated in negative urgency and tobacco use is the crucial first step to design and test pharmacological and physiological interventions to reduce negative urgency related tobacco use.
Keywords: Negative urgency, impulsivity, tobacco use, resting state functional connectivity, cingulo-insular, cortico-striatal
Introduction
Negative Urgency, defined as the tendency to act rashly under extreme negative emotion (Cyders & Smith, 2007; Whiteside & Lynam, 2001), is one of five impulsivity traits in the UPPS-P model, the multidimensional model of impulsive personality traits. Negative urgency is related to addictive behaviors, such as tobacco use (e.g., Billieux et al., 2007; Doran et al., 2008, 2009; Lee et al., 2015), problematic alcohol use (e.g., Coskunpinar, Dir, & Cyders, 2013), and other drug use (e.g., Settles et al., 2012). Although well documented to be a robust risk factor for a wide range of addictive behaviors, and despite its clinical relevance, brain function underlying negative urgency is not yet well understood.
Neural correlates of negative urgency
Neuroimaging studies of negative urgency highlight the importance of brain regions involved in reward, salience, and emotion processing, including the nucleus accumbens (NAcc), insula, and amygdala.
Reward regions.
First, disrupted NAcc function is associated with higher negative urgency. The NAcc is a part of the reward pathway in the brain implicated in addictive behaviors, specifically in tobacco dependence (Brody, 2006). In a study using a reward-modulated version of the Go/No-Go task (Wilbertz et al., 2014), negative urgency moderated the relationship between the blood-oxygen level dependent (BOLD) responses from NAcc and response inhibition. Individuals with low or medium negative urgency showed better task performance with greater NAcc response than those high in negative urgency. Structurally, negative urgency is related to smaller gray matter volume in the left NAcc (Muhlert & Lawrence, 2015). The relationship between negative urgency and the NAcc suggests that altered structure/function of the NAcc may underlie negative urgency-based reward-oriented behaviors.
Salience regions.
Second, negative urgency is related to insula function, even more so when individuals exert cognitive control during negative emotional states. The insula is a key node in the salience network and is implicated in a wide range of functions including affective processing and cognition (Uddin, Nomi, Hebert-Seropian, Ghaziri, & Boucher, 2017). Higher negative urgency was associated with weaker activation in the right anterior insula during response inhibition without emotional manipulation (Wilbertz et al., 2014). However, in an emotional Go/No-Go task using negative emotional stimuli, the high negative urgency group showed greater activation in the bilateral anterior insula during response inhibition, and response inhibition accuracy was positively correlated with activations in prefrontal inhibitory regions including anterior insula (Chester et al., 2016). Further, only the right anterior insula mediated the relationship between negative urgency and alcohol consumption in the high negative urgency group: greater right anterior insula activation in response to negative emotional stimuli predicted greater future alcohol consumption in this group. In a study that compared cocaine users and controls, negative urgency was more strongly and positively related to the functional connectivity between the right dorsolateral prefrontal cortex (dlPFC) and the right insula during the negative emotion maintenance in cocaine users than in the controls (Albein-Urios et al., 2012). These connections suggest that negative urgency may be influenced in part by increased salience to reward cues, especially under emotional provocation.
Emotional processing.
Third, the amygdala has been theorized as a key region for negative urgency (Cyders & Smith, 2008; Smith & Cyders, 2016). The amygdala is a key node of the limbic system and is important for a number of emotional processes, including fear and motivation (LeDoux, 2007). Negative urgency is related to hyperactivation of the amygdala in response to negative emotional stimuli (Cyders et al., 2015) or when individuals who are higher in negative urgency (e.g., cocaine users with personality disorders) attempt to regulate their emotions in response to negative emotional stimuli (Albein-Urios et al., 2013). Notably, negative urgency mediated the relationship between left amygdala BOLD responses to negative emotional stimuli and risk taking (Cyders et al., 2015). Increased left amygdala response was related to higher negative urgency, which in turn was related to greater risk-taking. Interestingly, during the re-appraisal of negative emotion, cocaine users with personality disorders had a positive relationship between negative urgency and amygdala activation, whereas controls and cocaine users without comorbidity did not (Albein-Urios et al., 2013). However, negative urgency has also been related to weaker or disrupted connectivity in amygdala-related circuits. Negative urgency was negatively related to the functional connectivity between the right inferior frontal gyrus and amygdala in the re-appraisal of negative emotion in control subjects but unrelated in cocaine users (Albein-Urios et al., 2012). Also, individuals with alcohol dependence showed a negative correlation between negative urgency and resting state functional connectivity (rsFC) strength in the amygdala-striatum network, thought to be an “impulsive system” (Zhu, Cortes, Mathur, Tomasi, & Momenan, 2015). Thus, negative urgency appears to be reflected, in part, by hyeractivity in the amygdala in response to negative emotional cues.
Functional connectivity evidence with negative urgency
The majority of neuroimaging studies examining negative urgency has primarily focused on the identification of segregated, localized brain regions using structural characteristics or task-based BOLD responses. rsFC analysis can augment recent neuroimaging findings of negative urgency beyond anatomically-connected patterns (Buckner, 2010). Specifically, Buckner (2010) asserted that rsFC combines influences of anatomically-connected patterns and synaptic modifications derived by an individual’s prior experiences, and that this could provide meaningful information about individual differences in brain circuit function. Negative urgency is a stable personality trait independent of frequency and intensity of emotional states (Cyders & Coskunpinar, 2010) and describes how an individual experiences and behaves under emotionally charged circumstances. Given recent findings suggesting individual differences in gray matter patterns and changes in regional BOLD signals during cognitive tasks as underlying negative urgency, it is reasonable to speculate that negative urgency, which can shape an individual’s experiences, could be driven by individual differences in resting brain function.
rsFC measures a temporal correlations of activity in different brain regions using low frequency, spontaneous fluctuations of BOLD signals in the absence of a specific task, and previous literature has demonstrated a temporal correlation of BOLD signals among brain regions that are functionally-related and thought to reflect intrinsic relationships in the brain (Biswal, Kylen, & Hyde, 1997; Biswal et al., 1995). Identifying resting-state brain circuits related to negative urgency would explain how functional relationships between key regions drive actions in emotional states and provide novel insights to patterns of activation during urgency-related tasks. Further, it would provide a more system-level approach in examining negative urgency-related resting brain circuits related to maladaptive behaviors that increase health risks.
To our knowledge, only three studies have examined negative urgency as related to rsFC (Contreras-Rodríguez et al., 2015; Hoptman, Antonius, Mauro, Parker, & Javitt, 2014; Zhu et al., 2015). These studies examined negative urgency using rsFCs related to general self-control, a related but broader construct encompassing negative urgency, or by comparing rsFC strengths between their study groups. However, to our knowledge, no study has examined how negative urgency is related to rsFCs that represent more specific underpinnings of negative urgency. The current study is novel because it focused on seed-based rsFC using a priori negative urgency-related brain regions as seed regions: NAcc, insula, and amygdala. This investigation provides an understanding of how spatially distinct brain regions work with each other in the behavioral expression of negative urgency-related tobacco use. The identification of underlying rsFC of negative urgency can provide prime targets for the design and testing of pharmacological and physiological interventions to mitigate the negative effects of negative urgency.
The current study
The current study utilized tobacco use as a candidate condition to identify negative urgency-related rsFC. Negative urgency is significantly associated with increased tobacco craving, specifically anticipated relief from negative affect (Billieux et al., 2007; Doran et al., 2009), higher negative affective states in response to tobacco cue exposure (Doran et al., 2008), and tobacco use frequency (Lee et al., 2015), yet there have been no rsFC studies linking negative urgency and tobacco use. Using a seed-based functional connectivity analysis in tobacco users and non-users, we examined three aims of the present study: 1) characterize rsFC associated with negative urgency in the whole sample; 2) examine how rsFC might differ between tobacco users and non-users; and 3) determine whether the relationship between negative urgency and rsFC differs by tobacco use status. We chose the bilateral NAcc, insula, and amygdala as a priori seed regions since these regions are most consistently associated with negative urgency in prior work. We hypothesized that 1) these seed regions would show connectivity patterns related to negative urgency in the whole group, 2) the connectivity patterns would differ across our groups, and 3) the relationships between negative urgency and connectivity patterns would differ across our groups. Examining whole-brain functional connectivity patterns of these seed regions was a necessary next step to identify rsFC correlates of negative urgency.
Materials and Methods
Data Source
This study included de-identified assessment and neuroimaging data collected by the Nathan Kline Institute (NKI)-Rockland Project (Nooner et al., 2012), and the data were accessed through the Collaborative Informatics and Neuroimaging Suite (COINS) at https://coins.mrn.org. The project was approved by the Institutional Review Board at NKI and Montclair State University and written informed consent was obtained from all participants included in the study. For this study, samples included 36 daily tobacco users and 72 non-users, which were matched based on age, gender, race, and lifetime psychiatric diagnosis (Table 1). Samples were selected based on following criteria: 1) between the ages of 18 – 65; 2) have structural and resting-state functional scans; 3) right-handedness; 4) negative urine drug screening results on a scan day, including benzodiazepines, cocaine, methadone, phencyclidine, barbiturates, opiates, and amphetamines (positive drug test on marijuana was permitted); 5) no history of neurological diagnosis; and 6) no incidental findings from MRI scans. Positive marijuana results on a scan day were not excluded because of the high co-occurrence of tobacco and marijuana use among the U.S. population, which has increased due to the recent marijuana legalization (Richter et al., 2004; Schauer, Berg, Kegler, Donovan, & Windle, 2015) and because the length of time marijuana use can be detected in urine sample can range between 3 days (if single use) to longer than 30 days (if chronic heavy use) after the actual use (Moeller, Lee, & Kissack, 2008). We attempted to be as inclusive as possible with our sample (i.e., did not exclude based on substance use, psychiatric diagnosis, or positive marijuana test) in order to increase the generalizability of our findings to the general population that commonly shows co-occurrence of tobacco use and other substance use or clinical problems (Chou et al., 2016). However, we matched tobacco users and non-users on these variables or controlled for them in all analyses.
Table 1.
Sample Characteristics
| Tobacco Users (n = 34) |
Non-Users (n = 62) |
t or x2 | p | |
|---|---|---|---|---|
| Age, mean (SD) | 34.15 (12.68) | 35.31 (14.14) | 1.16 | .68 |
| Gender, n | .01 | .94 | ||
| Male : Female | 14 : 20 | 25 : 37 | ||
| Race, n (%) | 5.44 | .25 | ||
| White | 27 (79%) | 41 (66%) | ||
| African American | 6 (18%) | 17 (27%) | ||
| Other races | 1 (3%) | 4 (7%) | ||
| Negative Urgency, mean (SD) | 2.09 (0.67) | 1.89 (0.55) | −1.45 | .15 |
| Lifetime Psychiatric Disorder Diagnosis, n* | .65 | .42 | ||
| Major Depressive Disorder | 2 | 2 | ||
| Panic Disorder w/o Agoraphobia | 1 | 1 | ||
| Attention-Deficit/Hyperactivity Disorder | 1 | 1 | ||
| Phobia | 1 | 0 | ||
| Lifetime Substance Abuse/Dependence Diagnosis, n* | 19.15 | < .001 | ||
| Alcohol | 11 | 7 | ||
| Cannabis | 17 | 4 | ||
| Cocaine | 4 | 2 | ||
| Sedative/Hypnotic/Anxiolytic | 1 | 1 | ||
| Past Year Alcohol Use Frequency, n (%) | 9.36 | .23 | ||
| No past year use | 5 (15%) | 17 (27%) | ||
| Less than monthly | 6 (18%) | 21 (34%) | ||
| Monthly | 11 (32%) | 13 (21%) | ||
| Weekly | 9 (26%) | 11 (18%) | ||
| Daily | 1 (3%) | 0 (0%) | ||
| Missing data | 2 (6%) | - | ||
| Past Year Marijuana Use Frequency, n (%) | 9.90 | .36 | ||
| No past year use | 15 (44%) | 51 (82%) | ||
| Less than monthly | 6 (15%) | 5 (8%) | ||
| Monthly | 3 (8%) | 3 (5%) | ||
| Weekly | 4 (12%) | 1 (2%) | ||
| Daily | 5 (14%) | 2 (3%) | ||
| Missing data | 2 (6%) | - | ||
| Age of first tobacco use, mean (SD) | 15.13 (2.93) | - | ||
| Frequency of tobacco use per day in the past 6-month, mean (SD) | 9.07 (8.07) | - | ||
| Years of use, mean (SD) | 16 Yrs 0 Mos (12 Yrs 7 Mos) |
- | ||
| FTND scores, mean (SD) | 2.30 (2.04) | - |
Notes.
Diagnoses include participants diagnosed with multiple psychiatric disorders.
Tobacco users had to be daily tobacco users and free of current substance dependence/abuse except for tobacco. Non-users had to be free of current substance dependence/abuse including tobacco, endorse no history of lifetime tobacco use, and were matched with tobacco users for age, gender, race/ethnicity, and any psychiatric diagnosis. During the preprocessing, two tobacco users and ten non-users were excluded because of excessive head motion and small ventricle size that precluded extraction of nuisance signals originating from ventricles (see preprocessing details). The final samples included in the group analyses were 34 daily tobacco users (mean age = 34.15 years, SD = 12.68; 59% females; 79% Whites) and 62 non-users (mean age = 35.31 years, SD = 14.14; 60% females; 66% Whites; see Table 1). Of these individuals, five tobacco users and three non-users had a positive marijuana drug screen on the scan day, and eight tobacco users and eight non-users had missing drug screen data.
Tobacco Use
Individuals were classified as tobacco users if they were daily tobacco users in the past year (i.e., Responded “Once per day” or more frequent tobacco use to the item “In the past year, what was your typical pattern of use?”). Individuals were classified as non-tobacco users if they endorsed no lifetime history of tobacco use (i.e., Responded “No” to the item “Have you ever used Tobacco?”). Among tobacco users, tobacco use was characterized via age of first tobacco use (M = 15.13, SD = 2.93), length of tobacco use (M = 16 years, SD = 12 years 7 months), past 6-month frequency of tobacco use per day (M = 9.07, SD = 8.07), and nicotine dependence measured by the Fagerström Test of Nicotine Dependence (FTND; Cronbach’s α = .61; M = 2.30, SD = 2.04; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Negative Urgency
The UPPS-P Impulsive Behavior Scale (UPPS-P; Lynam, Smith, Whiteside, & Cyders, 2006) is a 59-item self-report scale that measures five sub-facets of trait impulsivity. Only the negative urgency subscale was utilized in this study (Cronbach’s α = .85; mean (SD): tobacco users = 2.09 (0.67), non-users = 1.89 (0.55)) because negative urgency is the trait most highly linked to tobacco use (Lee et al., 2015) and ample evidence from past neuroimaging studies were able to provide clear a priori determination of seed regions. Higher mean score indicated higher negative urgency, which ranges from 1 to 4.
MRI Data Acquisition and Preprocessing
The fMRI data were collected from a 3.0T SIEMENS MAGNETOM Trio Tim scanner. The T1-weighted anatomical image was acquired for each subject using the magnetization-prepared rapid gradient echo (MPRAGE) sequence (repetition time (TR)/echo time (TE) =1900/2.52ms, Flip Angle (FA) = 9°, slice thickness = 1.0mm, field of view (FOV) = 250mm, 176 slices, voxel size =1.0 mm3, 256 × 246 matrix). The 5-minute resting-state image was acquired for each subject using an echo-planner imaging (EPI) sequence (TR/TE = 2500/30ms, FA = 80°, FOV = 216mm, slice thickness = 3.0mm, voxel size = 3.0 mm3, 38 interleaved slices, transversal orientation, 72 × 72 matrix).
Standard preprocessing steps were employed using AFNI ver.16.3.00 (Cox, 1996) and FreeSurfer (https://surfer.nmr.mgh.harvard.edu). The resting-state fMRI (rsfMRI) data were slice-time corrected, despiked, and volume registered. The MPRAGE anatomical data was skull-stripped and aligned to rsfMRI. In FreeSurfer, the anatomical data were segmented into the whole brain, white matter, grey matter, and the four large ventricles. The AFNI ANATICOR (Jo, Saad, Simmons, Milbury, & Cox, 2010) program was used to remove potential noise artifacts using anatomically modeled signals by deriving signals emanating from white matter and cerebrospinal fluid. Six motion parameters, six motion derivative parameters, and non-grey matter signals (i.e., from white matter and four large ventricles) were included as nuisance regressors to minimize noise artifact. Global signal (i.e., an average signal of an entire brain) was not included as an additional regressor due to concern that this would artificially alter interregional correlations and the resulting interpretation of the functional connectivity (Saad et al., 2012). ANATICOR erodes four ventricle masks by one voxel along each of the three directions to reduce the chance of including grey matter signals as signals from ventricles (Jo et al., 2010). Therefore, this procedure may erode the whole ventricle when subjects have a very small ventricle resulting in no signal from that specific ventricle mask leading to the automatic failure of processing pipeline. The preprocessed rsfMRI data were smoothed with a 6mm FWHM kernel to reduce noise. Bandpass filtering of the regressed time series retained frequencies between 0.01 Hz and 0.08 Hz (Satterthwaite et al., 2013).
In addition, time points with excessive head motion were censored out if framewise displacement, the Euclidean norm of head motion from one time-series volume to the next, exceeded 0.3mm, with additional removal of time points before and after the excessive head movement. Also, time points were removed if more than 10% of voxels across the brain were outliers at a specific time point (AFNI command 3dToutcount). Individuals were excluded when more than 30% of time series data were excluded due to failing to meet these two criteria. All preprocessing steps were done in the original space of each subject’s brain. The voxel was resized to 2.0 mm3.
Seed-based rsFC analyses
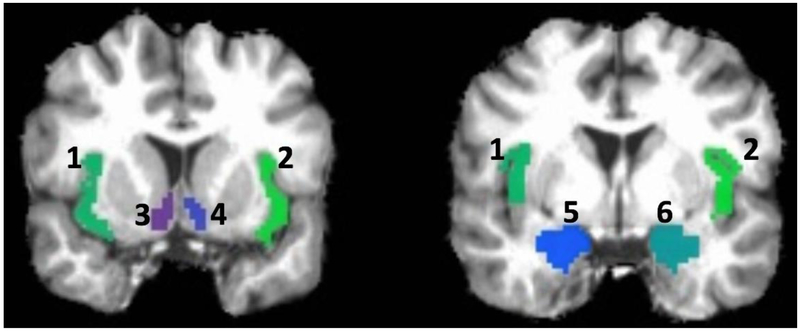
In FreeSurfer, anatomical parcellation developed by Destrieux, Fischl, Dale, & Halgren (2010) was used to parcellate cortical and subcortical brain regions. Bilateral NAcc, insula, and amygdala, totaling six seed regions, were identified (Fig. 1). Insula seeds were created by combining four insula regions, which included the anterior segment of the circular sulcus of the insula, superior segment of the circular sulcus of the insula, short insular gyri, and long insular gyrus and central sulcus of the insula. The seed-based whole brain functional connectivity analyses were conducted for each seed region separately. Anatomical seeds were resampled to the resolution of the functional time series, and a mean functional time series was calculated from all voxels in each seed region. This averaged time series in the seed region was correlated with the time series of all voxels across the entire brain after removing censored time points. This whole-brain functional connectivity map was then Fischer’s Z transformed, resulting in a whole-brain functional connectivity map for each seed. Finally, each subject’s functional connectivity maps were warped into the standard Talairach space with an affine transformation (Talairach & Tounoux, 1988).
Figure 1.
Seed regions.
Note. 1 = Left insula; 2 = Right insula; 3 = Left NAcc; 4 = Right NAcc; 5 = Left Amygdala; 6 = Right Amygdala; Seed regions illustrated here is based on a single brain in its anatomical space before warping it into the standard Talairach space.
Results
Self-report results
The tobacco users and non-users did not differ on age, gender, and race (see Table 1). Interestingly, negative urgency did not differ between groups. Negative urgency was higher among individuals with positive or missing marijuana drug test results on a scan day (t(94) = −2.06, p = .04), lifetime psychiatric diagnosis (t(94) = −2.51, p = .01), and lifetime substance abuse/dependence diagnosis (t(94) = −1.99, p < .05) in the whole sample and among individuals with lifetime psychiatric diagnosis (t(60) = −3.66, p = .001) in non-users (see Table 2). In tobacco users, negative urgency was not associated with age of first tobacco use, frequency of tobacco use per day in the past 6 months, length of tobacco use, and the FTND scores.
Table 2.
Relationship among negative urgency and sample characteristics
| All samples (n = 96) |
Tobacco users (n = 34) |
Non-users (n = 62) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| r | t | P | r | t | P | r | t | P | |
| Age | −.10 | .32 | −.24 | .18 | −.02 | .88 | |||
| Gender | −1.19 | .24 | .11 | .91 | −1.75 | .09 | |||
| Race | .40 | .69 | .48 | .63 | −.12 | .90 | |||
| Positive or missing marijuana drag test results on a scan day | −2.06 | .04* | −1.99 | .06 | −.41 | .69 | |||
| Lifetime Psychiatric diagnosis | −2.51 | .01* | .06 | .95 | -3.66 | .001* | |||
| Lifetime Substance abuse/dependence diagnosis | −1.99 | .05* | −1.85 | .07 | −.11 | .91 | |||
| Age of first tobacco use | −.11 | .57 | |||||||
| Frequency of tobacco use per day in the past 6-month | −.13 | .49 | |||||||
| Length of tobacco use | −.16 | .38 | |||||||
| FTND scores | −.28 | .11 | |||||||
Note. r = Pearson’s r; t = Independent samples t-test; Race was coded as white and non-white
Seed-based rsFC analyses
Relationship between negative urgency and rsFC strength
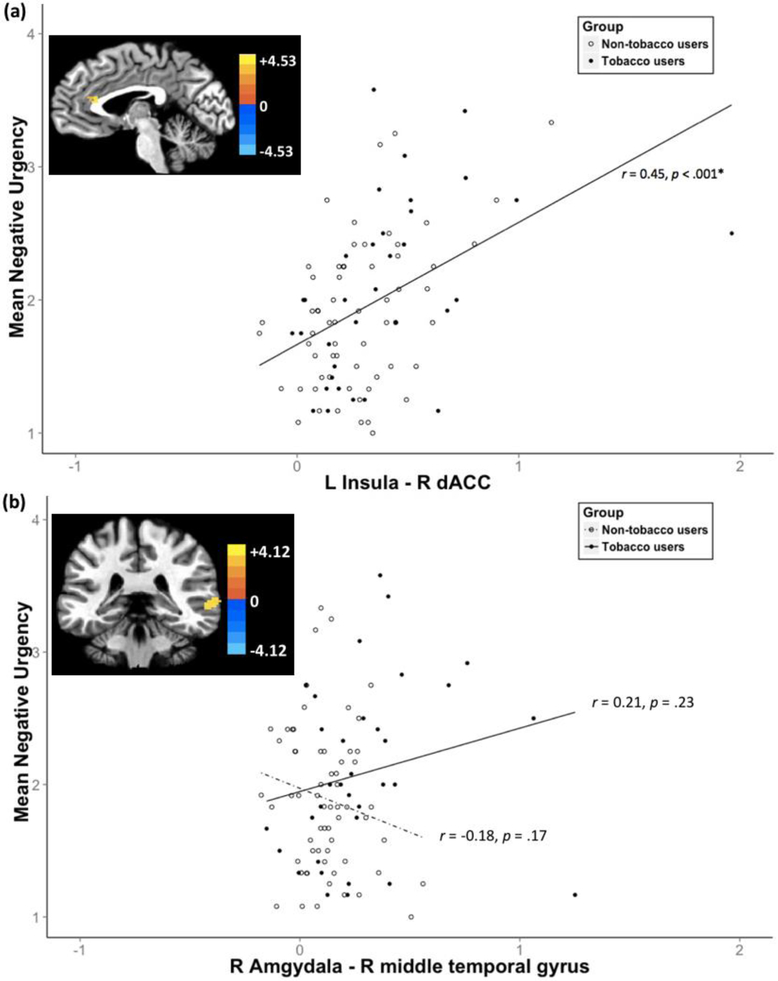
In the whole sample, negative urgency was positively related with stronger rsFC between the left insula and a cluster in right dorsal anterior cingulate cortex (dACC; r = .45, p < .001) (see Table 3, Fig. 2(a)). The relationship between negative urgency and the rsFC was examined by extracting the significant cluster. This relationship remained significant when each group was examined separately (tobacco users: r = .43, p = .011; non-users: r = 0.45, p < .001).
Table 3.
Significant rsFCs.
| Seed ROI | Cluster anatomical location | Cluster voxel size |
Primary peak location in Talairach space (x, y, z) |
Peak z-score |
|---|---|---|---|---|
| Correlation between negative urgency and rsFC strength in overall group | ||||
| Left Insula | Right dACC | 77 | −3, +27, +18 | 4.53 |
| Group differences between tobacco users and non-tobacco users | ||||
| Right Amygdala | Right Middle Temporal Gyrus | 84 | +57, −33, +0 | 4.12 |
| Differences in correlations between negative urgency and rsFC strength between groups | ||||
| Left NAcc | Right dACC | 262 | +11, +19, +30 | −4.57 |
| Left NAcc | Right dlPFC | 165 | +31, +25 +38 | −4.64 |
Note. Voxel level threshold p = .001; cluster level threshold α < .05. Voxel size = 2.0 mm3
Figure 2.
(a) Positive correlation between negative urgency and rsFC strength between Left Insula and Right dACC; (b) Group differences in rsFC strength between Right Amgydala and Right Middle Temporal Gyrus
Note. Voxel level threshold p = .001; cluster level threshold α < .05. The color bar represents z-scores.
Group differences between tobacco users and non-tobacco users
Compared to non-users, tobacco users showed stronger rsFC between the right amygdala and a cluster in right middle temporal gyrus (see Table 3, Fig. 2(b)). When examining the relationship between negative urgency and the extracted significant rsFC cluster, negative urgency was not related to rsFC strength between the right amygdala and right middle temporal gyrus (tobacco users: r = 0.21, p = .23; non-users: r = 0.18, p = .17).
Differential relationship between negative urgency and rsFC strength by tobacco use status
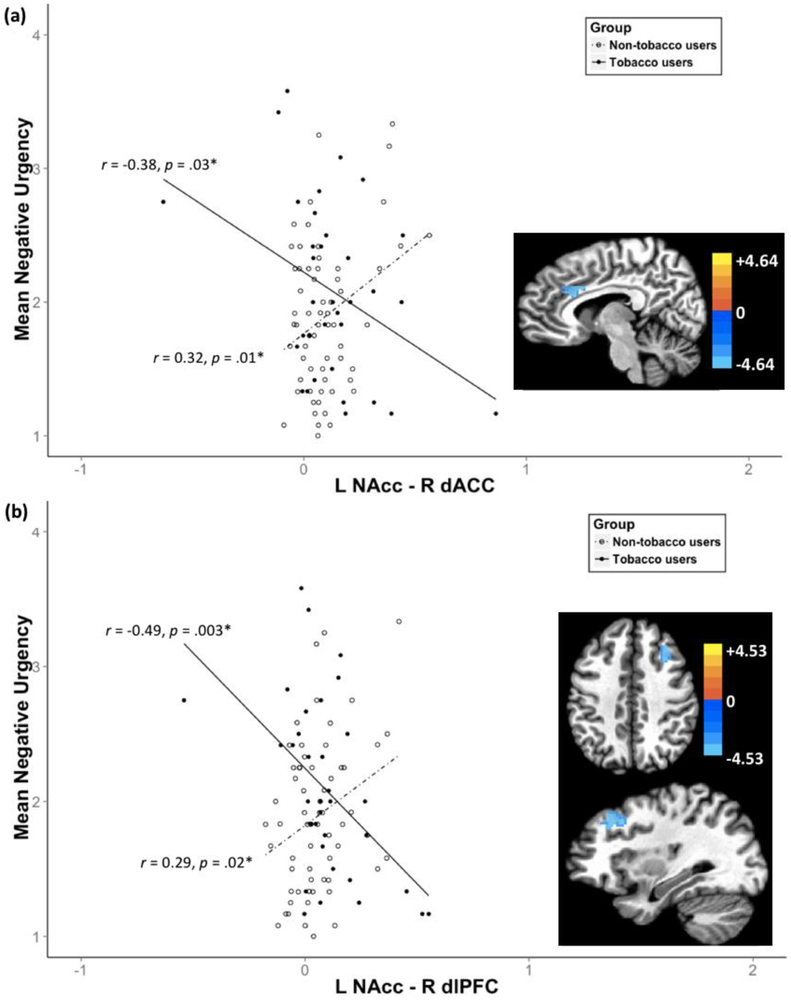
Negative urgency was differentially related to rsFC strength between the left NAcc and a cluster in right dACC (see Table 3, Fig. 3(a)) and between the left NAcc and a cluster in right dlPFC (see Table 3, Fig. 3(b)) by tobacco use status. Tobacco users showed a negative relationship between negative urgency and rsFC strength in the left NAcc-right dACC (r = −0.38, p = .03), whereas non-users showed a positive relationship (r = 0.32, p = .01; see Fig. 3(a)). Similarly, tobacco users showed a negative relationship between negative urgency and rsFC strength in the left NAcc and the right dlPFC (r = −0.49, p = .003), whereas non-users showed a positive relationship (r = 0.29, p = .02; see Fig. 3(b)). In tobacco users, the tobacco use variables were not related to any rsFC strengths.
Figure 3.
Differential relationship between negative urgency and rsFC strength between (a) the Left NAcc - Right dACC and (b) the Left NAcc - Right dlPFC by tobacco use status
Note. Voxel level threshold p = .001; cluster level threshold α < .05. The color bar represents z-scores.
Discussion
The purpose of the present study was to identify rsFC associated with negative urgency in a sample of community-based adults, examine how rsFC might differ between tobacco users and non-users, and examine if the relationship between negative urgency and rsFC would differ by tobacco use status. Using the NAcc, insula, and amygdala as a priori seed regions, we detected four significant results: 1) higher negative urgency was related to the stronger rsFC between the left insula and right dACC in the whole sample; 2) tobacco users had a stronger relationship in the rsFC between right amygdala and right middle temporal gyrus; and the relationship between negative urgency and rsFCs differed as a function of tobacco use status for rsFCs between 3) the left NAcc and right dACC and 4) the left NAcc and right dlPFC.
Interestingly, rsFC between left insula and right dACC was related to negative urgency regardless of tobacco use status, while rsFCs between the left NAcc and prefrontal regions showed differential relationships to negative urgency by tobacco use status, suggesting negative urgency is represented distinctly with rsFC patterns of different regions in tobacco use. Given the limited research on rsFC in negative urgency, the current results provide novel and meaningful extensions of previous research from region-specific to circuit-level correlates of negative urgency. Further, this study overcame limitations of existing connectivity studies with negative urgency that had previously utilized seed regions related to general self-control or identified group differences (Contreras-Rodríguez et al., 2015; Hoptman et al., 2014; Zhu et al., 2015) by instead utilizing regions related specifically to negative urgency based on previous findings.
Left insula – right dorsal ACC functional connectivity
Negative urgency was positively related to rsFC between the left insula and right dACC in the whole sample regardless of tobacco use status. Both the insula and the dACC are key nodes of the salience network (Seeley et al., 2007). The salience network identifies and selects a stimulus among a myriad of emotional, homeostatic, or cognitive inputs based on personal relevance or saliency, which an individual subsequently acts upon. This network is highly relevant to negative urgency, as individuals with greater negative urgency are more likely to act impulsively in response to subjective relevance or saliency of negative emotions. For example, tobacco users with greater negative urgency show heightened tobacco craving related to negative affect when they were exposed to personally salient stimuli, such as a lit cigarette of their usual brand (Doran et al., 2008, 2009). The degree of connectivity within the salience network may be important in an individual’s level of negative urgency, given the overlap of detecting personally relevant or salient stimuli (e.g., negative emotions) in the expression of negative urgency. Thus, it would be important to determine if salience network connectivity underlies negative urgency, negative urgency underlies salience network connectivity, or if there is a reciprocal relationship between these two factors to understand how these relationship affects negative urgency-related behaviors such as tobacco use in response to negative emotions.
Right amygdala – right middle temporal gyrus functional connectivity
Tobacco users showed stronger right amygdala – right middle temporal gyrus rsFC strength than non-users. A role of the amygdala in negative urgency has been theorized (Cyders & Smith, 2008; Smith & Cyders, 2016) and has gained empirical support (Albein-Urios et al., 2012, 2013, Cyders et al., 2014, 2015; Zhu et al., 2015). However, the relation of middle temporal gyrus to negative urgency or tobacco use is largely unknown, and this region is thought to be involved in semantic processing, such as processing of knowledge, properties, and facts about words or objects (e.g., Cabeza & Nyberg, 2000; Chao, Haxby, & Martin, 1999). The stronger rsFC in tobacco users may mean that processing of tobacco-related stimuli is related to the initial emotional processing in the amygdala, which warrants future investigation. Importantly, negative urgency was not significantly related to this rsFC in either group, so intervening on this circuit to reduce negative urgency tendencies may not be a viable treatment target for negative urgency. However, future work should examine whether this lack of relationship is also present during task performance, especially one that mimics rash action under negative emotions. If negative urgency is related to this circuit under task performance, the viability of targeting it to reduce negative urgency could then be revisited.
Left NAcc – prefrontal functional connectivity
Two left NAcc-based rsFC findings (left NAcc – right dACC and left NAcc – right dlPFC) showed differential relationships with negative urgency by tobacco use status. Both showed a negative relationship between negative urgency and rsFC strength in tobacco users and a positive relationship in non-users. Drug addiction, such as tobacco use, is characterized by an imbalance between reward-related regions like the NAcc and cognitive control-related regions such as the dACC and dlPFC, in which brain regions involved in cognitive control are disrupted and overridden by reward-related regions (Goldstein & Volkow, 2012; Volkow, Wang, Fowler, Tomasi, & Telang, 2011). The dACC and dlPFC engage in different cognitive control processes: while the dACC is involved in conflict monitoring, the dlPFC is involved in implementing control (Bush, Luu, & Posner, 2000; MacDonald, Cohen, Stenger, & Carter, 2000). Further, previous studies show heightened tobacco cue reactivity in both dACC and dlPFC among tobacco users (Janes et al., 2010; Luijten et al., 2011; Zhang et al., 2011). The NAcc activates in response to or in anticipation of reward-related or drug cues after learning the cue – reward association (Kühn & Gallinat, 2011; Schultz, Dayan, & Montague, 1997; Volkow et al., 2011). Both the dACC and dlPFC likely communicate with the NAcc to monitor and exert control when a tobacco cue is detected. Given these regions’ involvement in drug addiction and robust relationship of negative urgency to various substance use, it would be interesting to examine if these results generalize to other substances.
The imbalance between top-down control and reward processing in drug addiction may be attributed to negative urgency as, in our sample, tobacco users higher in negative urgency showed weaker connectivity strengths between the left NAcc and right dACC as well as the left NAcc and right dlPFC, which suggests that having weaker connectivity pattern may exacerbate the behavioral expression of negative urgency, such as tobacco use in response to negative emotion. On the other hand, non-users higher in negative urgency showed stronger connectivity strength between the left NAcc-related rsFCs suggesting more effective communication between regions involved in reward processing and cognitive control, thereby potentially protecting individuals from using tobacco in response to negative emotions. Thus, this could suggest interventions that strengthen connectivity between the left NAcc and right prefrontal regions might be examined for their ability to alleviate negative urgency-related behaviors.
Limitations
Despite novel findings in the current study, some limitations exist. First, the groups did not differ in negative urgency, unlike previous studies (Lee et al., 2015; Spillane, Smith, & Kahler, 2010). Given a high proportion of the current sample indicated substance use and psychiatric diagnosis and that these variables were matched between group, similar levels of negative urgency between groups is not completely surprising. Second, this study did not exclude participants with a missing drug test or who tested positive for recent marijuana use, although we did control for this in all analyses. Third, the current study includes participants with a wide age range (19–63 years). Normal aging has shown differing levels of recruitment in resting-state networks (Mowinckel, Espeseth, & Westlye, 2012). For this reason, age was matched between the groups. Fourth, the present study sample is comprised of tobacco users, which may include users of various tobacco products; thus, any effect of various tobacco products cannot be determined in this study. However, most tobacco users in the current study consumed their tobacco products by smoking (n = 30; n = 2 orally, n = 2 missing data). Lastly, groups with unequal sample sizes were compared. From the existing data, we identified 36 daily tobacco users meeting our criteria. We included more individuals in the non-user group to increase power and matched the proportion of non-users to tobacco users by age, gender, race, and lifetime psychiatric diagnosis, and these variables did not differ by group (Table 1).
Future Directions
Despite these limitations, the current study extends previous neuroimaging findings, which have mainly focused on how negative urgency is related to localized, segregated brain regions, by examining seed-based rsFC. Future studies should examine whether negative urgency-related rsFC found in this study also exists in samples with other drug use. Such research can lead to the development of a more transdiagnostic approach to understanding negative urgency’s contributions to drug addiction. This approach would guide the identification of focal biomarkers to mitigate the negative effects of negative urgency for addiction and help research to leverage identified biomarker to design and test pharmacological or physiological interventions to reduce drug addiction. For example, these efforts could improve maladaptive behaviors manifested by negative urgency, especially for psychosocial treatment-resistant patients.
Conclusion
The present study identified rsFCs related to negative urgency and tobacco use that involve key regions in salience, reward processing, and emotional processing. These study findings provide evidence for negative urgency-related rsFCs that can be leveraged and targeted in future research and the development and testing of novel treatment targets for negative urgency-related tobacco use.
Acknowledgements
The authors thank the Nathan Kline Institute for data sharing and Drs. Marian Logrip and Jesse Stewart for their helpful comments on an earlier draft of this manuscript. This project was based on an unpublished Master’s Thesis by the first author.
Funding
This study was funded by the National Institute on Alcoholism and Alcohol Abuse T32 grant (AA07462, PI: Czachowski) for Miji Um under the mentorship of Melissa A. Cyders and a K01 (K01AA020102, PI: Cyders). This work was also made possible through data sharing of the Rockland project by Nathan Kline Institute with support of several grants for data collection from the National Institutes of Health (NIMH BRAINS R01MH094639, PI: Milham, NIMH U01MH099059, PI: Milham; NIMH R01MH101555, PI: Craddock; NIA R01AG047596, PI: Colcombe). The listed funding agencies were not involved in study design, collection, analysis, and interpretation of the data. The opinions expressed in this manuscript are stated by the authors and do not reflect the views of listed funding agencies.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article contained analyses on de-identified data and thus was except from human subjects’ review.
Informed consent
Informed consent was obtained from all individual participants included in the study at the Nathan Kline Institute.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Albein-Urios N, Verdejo-Román J, Asensio S, Soriano-Mas C, Martínez-González JM, & Verdejo-García A (2012). Re-appraisal of negative emotions in cocaine dependence: dysfunctional corticolimbic activation and connectivity. Addiction Biology, 19, 415–426. 10.1111/j.1369-1600.2012.00497.x [DOI] [PubMed] [Google Scholar]
- Albein-Urios N, Verdejo-Román J, Soriano-Mas C, Asensio S, Martínez-González JM, & Verdejo-García A (2013). Cocaine users with comorbid Cluster B personality disorders show dysfunctional brain activation and connectivity in the emotional regulation networks during negative emotion maintenance and reappraisal. European Neuropsychopharmacology, 23, 1698–1707. 10.1016/j.euroneuro.2013.04.012 [DOI] [PubMed] [Google Scholar]
- Billieux J, Van der Linden M, & Ceschi G (2007). Which dimensions of impulsivity are related to cigarette craving? Addictive Behaviors, 32(6), 1189–1199. 10.1016/j.addbeh.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, & Hyde JS (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine, 10, 165–170. 10.1002/(SICI)1099-1492(199706/08)10 [DOI] [PubMed] [Google Scholar]
- Brody AL (2006). Functional brain imaging of tobacco use and dependence. Journal of Psychiatric Research, 40, 404–418. 10.1016/j.jpsychires.2005.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2010). Human functional connectivity: new tools, unresolved questions. PNAS, 107(24), 10769–10770. 10.1073/pnas.1005987107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Cabeza R, & Nyberg L (2000). Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience, 12(1), 1–47. 10.1162/08989290051137585 [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, & Martin A (1999). Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience, 2(10), 913–919. 10.1038/13217 [DOI] [PubMed] [Google Scholar]
- Chester DS, Lynam DR, Milich R, Powell DK, Andersen AH, & DeWall CN (2016). How do negative emotions impair self-control? A neural model of negative urgency. NeuroImage, 132, 43–50. 10.1016/j.neuroimage.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SP, Goldstein RB, Smith SM, Huang B, Ruan WJ, Zhang H, … Grant BF (2016). The epidemiology of DSM-5 nicotine use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditons-III. Journal of Clinical Psychiatry, 77(10), 1404–1412. 10.4088/JCP.15m10114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodríguez O, Albein-Urios N, Vilar-López R, Perales JC, Martínez-Gonzalez JM, Fernández-Serrano MJ, … Verdejo-García A (2015). Increased corticolimbic connectivity in cocaine dependence versus pathological gambling is associated with drug severity and emotion-related impulsivity. Addiction Biology, 1–10. 10.1111/adb.12242 [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, & Cyders M. a. (2013). Multidimensionality in Impulsivity and Alcohol Use: A Meta-Analysis Using the UPPS Model of Impulsivity. Alcoholism: Clinical and Experimental Research, 37(9), 1441–1450. 10.1111/acer.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI Clustering and False-Positive Rates. PNAS, 114(17), E3370–E3371. 10.1073/pnas.1614961114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Reynolds RC, & Taylor PA (2016). AFNI and Clustering: False Positive Rates Redux, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi KA, & Kareken DA (2015). Negative Urgency Mediates the Relationship between Amygdala and Orbitofrontal Cortex Activation to Negative Emotional Stimuli and General Risk-Taking. Cerebral Cortex, 25, 4094–4102. 10.1093/cercor/bhu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, & Kareken DA (2014). Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: FMRI evidence of emotion-based impulsivity. Alcoholism, Clinical and Experimental Research, 38(2), 409–17. 10.1111/acer.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, & Smith GT (2007). Mood-based rash action and its components: positive and negative urgency. Personality and Individual Differences, 43, 839–850. [Google Scholar]
- Cyders MA, & Smith GT (2008). Emotion-based dispositions to rash action: positive and negative urgency. Psychological Bulletin, 134(6), 807–28. 10.1037/a0013341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, & Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Myers M, & Spring B (2008). Cue-elicited negative affect in impulsive smokers. Psychology of Addictive Behaviors, 22(2), 249–56. 10.1037/0893-164X.22.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, & Spring B (2009). Impulsivity and cigarette craving: Differences across subtypes. Psychopharmacology, 207(3), 365–373. 10.1007/s00213-009-1661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2012). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Review Neuroscience, 12(11), 652–669. 10.1038/nrn3119.Dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Antonius D, Mauro CJ, Parker EM, & Javitt DC (2014). Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: Relationship to Aggressive attitudes and behavior. American Journal of Psychiatry, 171(9), 939–948. 10.1176/appi.ajp.2014.13111553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2016). SPSS, Version 24.0. Armonk, NY: IBM Corp. [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de Frederick B, Chuzi S, Pachas G, … Kaufman MJ (2010). Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biological Psychiatry, 67(8), 722–729. 10.1016/j.biopsych.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, & Cox RW (2010). Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage, 52(2), 571–582. 10.1016/j.neuroimage.2010.04.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, & Gallinat J (2011). Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience, 33(7), 1318–1326. 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Current biology, 17(20), R868–R874. [DOI] [PubMed] [Google Scholar]
- Lee DC, Peters JR, Adams ZW, Milich R, & Lynam DR (2015). Specific dimensions of impulsivity are differentially associated with daily and non-daily cigarette smoking in young adults. Addictive Behaviors, 46, 82–85. 10.1016/j.addbeh.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M, & Franken IHA (2011). Neurobiological substrate of smoking-related attentional bias. NeuroImage, 54(3), 2374–2381. 10.1016/j.neuroimage.2010.09.064 [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, & Cyders MA (2006). The UPPS-P: Asessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University. [Google Scholar]
- MacDonald a W., Cohen JD, Stenger V. a, & Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 255(5472), 1835–1838. 10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- Moeller KE, Lee KC, & Kissack JC (2008). Urine drug screening: Practical guide for clinicians. Mayo Clinic Proceedings, 83(1), 66–76. 10.4065/83.1.66 [DOI] [PubMed] [Google Scholar]
- Mowinckel AM, Espeseth T, & Westlye LT (2012). Network-specific effects of age and in-scanner subject motion: A resting-state fMRI study of 238 healthy adults. NeuroImage, 63(3), 1364–1373. 10.1016/j.neuroimage.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Muhlert N, & Lawrence AD (2015). Brain structure correlates of emotion-based rash impulsivity. NeuroImage, 115, 138–146. 10.1016/j.neuroimage.2015.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, … Milham MP (2012). The NKI-Rockland sample: A model for accelerating the pace of discovery science in psychiatry. Frontiers in Neuroscience, 6(152), 1–11. 10.3389/fnins.2012.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, Kaur H, Resnicow K, Nazir N, Mosier MC, & Ahluwalia JS (2004). Cigarette smoking among marijuana users in the United States. Substance Abuse, 25(2), 35–43. 10.1300/J465v25n02_06 [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, & Cox RW (2012). Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2(1), 25–32. 10.1089/brain.2012.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, … Wolf DH (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage, 64(1), 240–256. 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, Berg CJ, Kegler MC, Donovan DM, & Windle M (2015). Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addictive Behaviors, 49(2015), 26–32. 10.1016/j.addbeh.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A Neural Substrate of Prediction and Reward. Science, 275, 1593–1598. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, & Smith GT (2012). Negative urgency: a personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. Journal of Abnormal Psychology, 121(1), 160–172. 10.1037/a0024948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, & Cyders MA (2016). Integrating affect and impulsivity: The role of positive and negative urgency in substance use risk. Drug and Alcohol Dependence, 163, S3–S12. 10.1016/j.drugalcdep.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane NS, Smith GT, & Kahler CW (2010). Impulsivity-like traits and smoking behavior in college students. Addictive Behaviors, 35(7), 700–705. 10.1016/j.addbeh.2010.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar streotaxic atlas of the human brain. 3-dimensional proportional system: an approach to cerebral imaging. G. Thieme. [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, & Boucher O (2017). Structure and Function of the Human Insula. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society, 34(4), 300–306. doi: 10.1097/WNP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, & Telang F (2011). Addiction: Beyond dopamine reward circuitry. PNAS, 108(31), 15037–15042. 10.1073/pnas.1010654108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, & Lynam DR (2001). The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences, 30(4), 669–689. 10.1016/S0191-8869(00)00064-7 [DOI] [Google Scholar]
- Wilbertz T, Deserno L, Horstmann A, Neumann J, Villringer A, Heinze HJ, … Schlagenhauf F (2014). Response inhibition and its relation to multidimensional impulsivity. NeuroImage, 103, 241–248. 10.1016/j.neuroimage.2014.09.021 [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, & Stein EA (2011). Anatomical differences and network characteristics underlying smoking cue reactivity. NeuroImage, 54(1), 131–141. 10.1016/j.neuroimage.2010.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, & Momenan R (2015). Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addiction Biology, 10.1111/adb.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]