Abstract
This study determined the predictive capabilities of pain intensity and disability on health care utilization (number of condition-specific health care visits, incident and chronic opioid use) and costs (total condition-specific and overall medical costs) in the year following an initial evaluation for musculoskeletal pain. We explored pain catastrophizing and spatial distribution of symptoms (i.e., body diagram symptom score) as mediators of these relationships. Two hundred eighty-three military service members receiving initial care for a musculoskeletal injury completed a region-specific disability measure, numeric pain rating scale, Pain Catastrophizing Scale (PCS) and body pain diagram. Pain intensity predicted all outcomes, while disability predicted incident opioid use only. No mediation effects were observed for either opioid use outcome, while pain catastrophizing partially mediated the relationship between pain intensity and number of health care visits. Pain catastrophizing and spatial distribution of symptoms fully mediated the relationship between pain intensity and both cost outcomes. The mediation effects of pain catastrophizing and spatial distribution of symptoms are outcome-specific, and more consistently observed for cost outcomes. Higher pain intensity may drive more condition-specific health care utilization and use of opioids, while higher catastrophizing and larger spatial distribution of symptoms may drive higher costs for services received.
Perspective:
This article examines underlying characteristics that help explain relationships between pain intensity and disability, and the outcomes of health care utilization and costs. Health care systems can use these findings to refine value-based prediction models by considering factors that differentially influence outcomes for health care use and cost of services.
Keywords: Value-based care, rehabilitation, psychological, mediation, outcomes
Introduction
Best practice management of musculoskeletal pain is changing rapidly in response to recent health care reform initiatives. First and broadly in health care, value-based purchasing has incentivized high quality, low cost care.4,7 As a result, health care utilization and costs have become important outcomes by which health care systems and payers measure treatment effectiveness. Second and specific to management of musculoskeletal pain, recent clinical practice guidelines and national pain research priorities have called for reductions in opioid use, with the goal of increasing non-pharmacological treatment options while decreasing opioid-related morbidity and mortality.31,45 Given these initiatives, health care systems, payers and providers have taken an interest in better understanding risk for high costs and resource utilization, as they often represent suboptimal outcomes of care.
For individuals with musculoskeletal pain conditions, higher pain intensity and disability are known to predict increased health care utilization and costs.3,20,39 However, the process by which they influence the use of health care services and costs remains largely unknown. In particular, we don’t know whether pain intensity and disability are directly related to health care utilization and cost outcomes, or if other factors relevant to the experience of pain and disability (i.e. psychosocial characteristics or distribution of symptoms) underlie these relationships. If we understood how these factors were related, we could build more accurate models to identify risk for high costs and utilization. In turn, healthcare systems could use this information to potentially improve value of services for musculoskeletal pain by addressing modifiable risk factors through treatment.
Pain catastrophizing and spatial distribution of symptoms (i.e. reported across anatomical locations) are unique characteristics related to the pain experience46, that are independently associated with pain intensity12,34,41, disability12,34 and higher risk of failing to maintain treatment gains25, all of which could directly lead to the need for continued care and persistent high cost health care.44 In our prior cross-sectional work, both pain catastrophizing and spatial distribution of symptoms were important contributors to pain intensity, prompting further investigation in longitudinal analyses.9 If these characteristics explained how pain intensity and disability were related to utilization and cost outcomes, they would become important targets of value-based health care policy and clinical practice to improve care for musculoskeletal pain. To our knowledge studies have not yet examined empirically whether pain catastrophizing and spatial distribution of symptoms mediate relationships between established predictors (i.e., pain intensity and disability) and measures of health care value (i.e., utilization of services and costs).
In this retrospective cohort study, we evaluated baseline pain intensity and self-reported disability as predictors of health care utilization and costs in the year following a primary care evaluation for musculoskeletal pain in a closed, single-payer health system. We selected health care utilization and cost outcomes relevant to ongoing value-based payment reforms (i.e., bundled or capitated payment models) and pain care initiatives emphasizing reduced reliance on pharmacological management.29–31 Health care utilization outcomes included: 1) high number of health care visits for the index pain condition (i.e., condition-specific visits), 2) incident opioid use, and 3) development of chronic opioid use. For health care costs, we examined: 1) total medical costs for treatment for the index pain condition (i.e., condition-specific costs), and 2) total overall medical costs. We explored utilization and cost outcomes separately because emerging evidence suggests predictors may differ across these outcomes in populations with musculoskeletal pain.1,20,21,35 To address our primary research question, we used multiple mediation analyses to explore the mediation effects of pain catastrophizing and spatial distribution of symptoms derived from a previously validated body diagram score. We hypothesized that both potential mediators would help to explain the prediction of utilization and cost outcomes by pain intensity and disability.
Methods
Design
This study was an analysis of medical record data from military service members receiving initial care for a musculoskeletal injury in a multidisciplinary care clinic at Madigan Army Medical Center, a large U.S. Department of Defense (DoD) hospital. Soldiers returning from a 1-year combat deployment in Afghanistan passed through a mandatory medical screening process with the intent to identify any persistent medical issues, including musculoskeletal injuries that were not adequately addressed during the deployment. Soldiers found to have a primary musculoskeletal complaint that could require further medical care were referred directly to a musculoskeletal clinic established within the physical therapy department. Subjects filled out medical history questionnaires, pain diagrams, and self-reported disability and psychosocial status forms as part of their initial assessment. Health care use and cost data for the year preceding and following the assessment were then extracted for each subject through the Military Health System Data Repository (MDR).33 Ethics approval was obtained from the Western Regional Medical Command Institutional Review Board (IRB). Need for individual consent was waived by the IRB, as the data were collected as part of routine clinical practice.
Eligibility Criteria
Data were collected for all service members who were part of the 116th Brigade Combat Team returning from a deployment between August and September of 2011, and with a primary musculoskeletal complaint requiring further medical care. Eligibility criteria for inclusion in the analysis were broad and not limited by region of pain, number of painful sites, or chronicity of pain. We did not exclude any service members from this cohort.
Predictors
All demographic, predictor and mediator variables were collected at the baseline evaluation for the musculoskeletal pain complaint.
Pain Intensity.
Pain intensity was assessed with an 11-point numeric pain-rating scale (NPRS). Pain intensity ratings range from 0 (no pain) to 10 (worst imaginable pain). Subjects were asked to rate their “worst” and “best” pain levels over the past 24 hours. They were also asked to rate their current level of pain. All 3 pain ratings were averaged to get a composite pain intensity score. The NPRS has been shown to be a reliable method of pain intensity assessment (intraclass correlation coefficient [ICC] = 0.74, 0.76).9,24
Self-Report of Disability.
Based on anatomical region of pain, subjects completed the Shoulder Pain and Disability Index (SPADI) for shoulder or upper arm pain, the Oswestry Disability Index (ODI) for low back pain, the Neck Disability Index (NDI) for neck pain, and the Lower Extremity Functional Scale (LEFS) for knee or lower-limb pain.5,9,15,37 These questionnaires are valid measures of region-specific function commonly used in outpatient orthopedic rehabilitation settings.5,9,15,37 The NDI has shown moderate test-retest reliability (ICC=0.50), while the LEFS (r = 0.94 [95% lower limit confidence interval (CI) = 0.89]), SPADI (ICC range = 0.84-0.94) and ODI (r = 0.83-0.99) have good-to-excellent test-retest reliability.5,9,15,37 These region specific disability measures were z-score transformed for all analyses so that all subjects could be evaluated on a common metric.17,34 Prior to z-score transformation, to match the scoring design of the other disability questionnaires, we transformed LEFS scores so that higher scores represented poorer function.34
Outcomes
Healthcare cost and utilization data for 1 year prior to and after the initial visit to the musculoskeletal care clinic were extracted from the MDR, representing 24 months of data for each subject. The MDR captures and tracks all medical visits for all beneficiaries of the Department of Defense (DoD) healthcare system, retired, active military, and service family members alike. The Military Health System (MHS) is a single-payer closed system providing care in Military Treatment Facilities as well as purchased care in civilian network clinics, all around the world. Any medical visit, procedure, or pharmaceutical prescription, in a military or civilian setting worldwide where TRICARE insurance plan is the payer, is captured in the MDR.33
Condition-specific health care visits and medical costs.
Data were collected to create two outcomes captured during the full 1-year surveillance period after the initial clinic visit: condition-specific visits, and condition-specific costs. Condition-specific visits were all visits for any medical care related specifically to the musculoskeletal condition(s) reported at the initial evaluation in which Tricare was the payer including behavioral health, mental health, and complementary and integrative care (as covered by Tricare), occurring in both Military Treatment Facilities and Civilian clinics. Only visits associated with the musculoskeletal condition(s) reported at the initial evaluation were used in this analysis (i.e., ICD-9 diagnosis codes (ranges 700-900; pertaining specifically to musculoskeletal conditions for the lower and upper extremity and spine). Condition-specific costs were the total costs associated with all condition-specific visits (to include procedures).
Total number of opioid prescriptions.
Unique prescriptions were identified from the Pharmacy Data Transaction Service (PDTS) data source from within MDR. Prescriptions with American Hospital Formulary Service (AHFS) therapeutic class codes 280808 and 280812 were flagged as opioid fills. Of note, Tramadol was classified as an opioid for the purposes of this study, despite not being classified as an opioid until 2014, after the period in this study. Total unique fills and total days’ supply were calculated. The data collected did not allow for linking of prescriptions to specific musculoskeletal pain diagnoses nor were able to obtain information on morphine equivalent daily dose (MEDD).
Total medical costs
This included costs for all medical care, in any setting, received for any reason during the 1-year follow-up surveillance period.
Mediators
Body Diagram Score.
Subjects completed a body diagram to capture the spatial distribution of symptoms. Subjects were asked to indicate the location of their symptoms related to pain, numbness, and tingling with distinct marks. Body diagrams were scanned into PDF files, and a grid with 3 × 3-mm square boxes was electronically overlaid on the diagram. Boxes that contained any part of the distinct marking were counted for each symptom. Markings outside of the body diagram were not counted unless they entered a square that overlapped with part of the body diagram. This measurement technique has been shown to be valid and reliable for symptom diagram scoring.6 A composite symptom score was calculated by adding the number of boxes for pain, tingling, and numbness. We used a composite symptom score, rather than a pain-only score, based on prior work which demonstrated that a total symptom score was more closely associated with disability and pain intensity in this population.34 Interrater reliability (2-way random, absolute agreement) of composite symptom distribution is excellent (ICC = 0.98; 95% CI: 0.96, 0.99).34
Pain Catastrophizing.
Pain catastrophizing was measured with the Pain Catastrophizing Scale (PCS), a 13-item, 52-point questionnaire measuring 3 aspects of the pain experience: rumination, magnification, and helplessness.42 Each item on the PCS is scored from 0 (not at all) to 4 (all the time), with higher scores indicating higher levels of catastrophizing. Psychometric studies suggest acceptable levels of reliability (subscale Cronbach alpha range: 0.75-0.91) and validity.28,40 Factor analysis suggests a hierarchical factor structure, with 3 individual subscales of the PCS (rumination, magnification, and pessimism) contributing to an overall factor of pain catastrophizing.40,42
Covariates
Age, sex, use of opioids (yes/no) in the year prior to evaluation, comorbidity count at baseline and surgery during follow-up were considered as covariates since prior studies have shown that these factors can influence pain intensity and disability in patients with musculoskeletal pain.10,16,19,32,36 We considered comorbidities relevant to both military populations and individuals with musculoskeletal pain conditions, that have demonstrated effects on clinical outcomes in prior analyses.26,38,43 All comorbidities were identified through clinical encounters with relevant ICD-9 diagnosis codes present in the MDR, and represented conditions present in the year leading up to the initial evaluation. Comorbid conditions included: insomnias, circadian sleep disorders, hypersomnias, parasomnias, sleep apnea, sleep-related movement disorders, isolated sleep symptoms, other sleep disorders, arthropathies, chronic pain diagnoses, metabolic syndromes, mental health disorders (including depressive disorders), post-traumatic stress disorder (PTSD), cardiovascular disease, neoplasms, and substance abuse disorders. These conditions were counted for each subject and included in the analysis as a count score. We isolated PTSD from other mental health disorders and separately considered sleep disorders because they are especially informative in this population. See Appendix 1 for a list of all mental health disorders included in that category. Because comorbid conditions were extracted from data collected during deployment, access to care and type of services available is particularly relevant. The exact type of care available during deployment for this specific unit and setting is unknown, however forward deployed services available to most units of this type include: rehabilitation services, urgent care, psychological resiliency centers, and in some areas, forward deployed pain management and treatment teams. In addition, field surgeons (deployed physicians and physician’s assistants) co-located with the troops as well as at the Combat Support Hospitals in theater could have also rendered care.
Statistical Analysis
We developed separate multiple mediator models using the Hayes PROCESS macro18 in SPSS (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY) (Model 4) with bias-corrected bootstrapping (5000 samples) to predict 3 measures of health care utilization and 2 measures of health care costs. Health care utilization measures included the following outcomes at 12 months: 1) high number of health care visits for the index musculoskeletal pain condition, 2) incident opioid use, and 3) chronic opioid use. PROCESS uses ordinary least squares (OLS) regression to estimate mediation effects, except when the outcome variable is dichotomous, in which case the model is estimated with logistic regression. Because this method cannot appropriately model count data, we dichotomized all utilization measures (visits and opioid counts) into clinically and policy-relevant variables. We defined incident opioid use as having at least one opioid prescription during the follow-up year, while chronic opioid use was defined as having 3 or more opioid prescriptions during follow-up based on prior analyses which have used a similar criterion for chronic opioid use.13,47 For health care visits, we defined high health care use for the musculoskeletal pain condition as having a total number of visits for the index pain condition in the top 30% of the sample distribution. Prior analyses have used similar thresholds to define high cost utilizers for musculoskeletal pain21, and we valued a liberal threshold for identifying high cost utilizers as the top 30% of health care users account for approximately 90% of all health care costs in the US.11 Dichotomizing these utilization measures would allow us to 1) understand how well pain intensity and function predicted important measures of health care use related to musculoskeletal pain, and 2) determine whether catastrophizing and distribution of symptoms helped to explain these relationships.
Health care costs measures included the following outcomes at 12 months: 1) total costs associated with treatment of the index musculoskeletal pain condition, and 2) total overall medical costs. We kept cost measures continuous, however log-transformed them prior to analysis to reduce skewness and conform to the normality assumption of OLS regression.22,23
The independent variables (i.e., predictors) of pain intensity and disability (z-scored region-specific disability measure(s) and averaged) were modeled separately for each outcome (10 models total). For each analysis, covariates included age, gender, number of comorbidities, and incidence of musculoskeletal surgery during follow-up. For analyses that included opioid use as the dependent variable, we also included prior use of opioids as a covariate in the model since prior opioid use is a strong predictor of future opioid use.8,35 The mediators of interest for each model included PCS score and body diagram score.
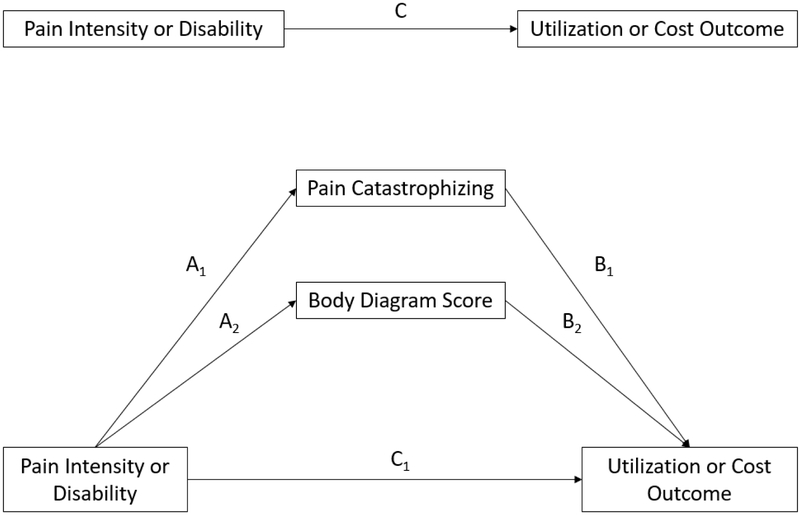
The multiple mediation framework is depicted in Figure 1. Certain pre-conditions were necessary to enable a test of mediation2, specifically the regression coefficient for the predictor in each base model had to be statistically significant after accounting for covariates (significant total effect, path C in Figure 1). Mediation was confirmed if the bias-corrected 95% confidence interval for the indirect effect (A1xB1 or A2xB2) did not include zero. Complete mediation was confirmed if the bias-corrected 95% confidence interval for the direct effect (C1) included zero.
Figure 1.
Multiple mediator model testing mediation effects of pain catastrophizing and body diagram score on relationship between predictor (pain intensity or disability) and outcome (measures of utilization and costs). Path AxxBx represents the indirect effect(s). Path C1 represents the direct effect. Path C represents the total effect. Covariates were included in each model but are not depicted in the figure for clarity.
Missing data and sensitivity analyses
The PCS and body diagram scores were missing for 36 (12.7%) and 21 (7.4%) subjects, respectively with 5 subjects missing both items. The only other missing predictor variables were function scores, which were missing for 9 (3.2%) subjects. Total health care costs were missing for 5 (1.8%) subjects and number of musculoskeletal pain specific visits were missing for 4 (1.4%) subjects. Little’s test indicated missingness was completely at random (MCAR, chi-square=43.48, p=0.96) and therefore all primary analyses were conducted with listwise deletion of subjects with missing data. However, to confirm no significant bias due to missingness, we preformed follow-up analyses by imputing missing data using the expectation-maximization (EM) algorithm to assess the sensitivity of the findings.
Results
This analysis included 283 individuals [n=263 males (92.9%); mean age 31.85 (SD=8.65)]. This was the entire sample that presented for care in the clinic during August or September 2011, without any exclusions. Descriptive information for the sample is provided in Table 1 and Supplemental Table 1. Results of the multiple mediation analyses are listed in Table 2. The table lists regression coefficients and their associated path, which can be referenced in Figure 1. When a precondition for mediation was not met (i.e., predictor was not associated with outcome after controlling for covariates), we did not include the results of that analysis in the table.
Table 1.
Descriptive information for study cohort (n=283)
| Covariates | Value* | |
|---|---|---|
| Age (years) | 31.85 ± 8.65 (19-55) | |
| Sex | Male | 263 (92.9) |
| Female | 20 (7.1) | |
| Anatomical region | Low back | 121 (42.8) |
| Neck | 71 (25.1) | |
| Lower extremity | 80 (28.3) | |
| Upper extremity | 163 (57.6) | |
| Prior opioid use | Yes | 72 (25.4) |
| No | 211 (74.6) | |
| Comorbid conditions | 0 | 248 (87.6) |
| 1 | 14 (4.9) | |
| 2 | 7 (2.5) | |
| 3 | 5 (1.8) | |
| 4 | 4 (1.4) | |
| Surgery during follow-up | Yes | 40 (14.1) |
| No | 243 (85.9) | |
| Predictors | ||
| Pain Intensity (numeric rating scale) | 4.08 ± 1.61 (0-9) | |
| Disability score | ODI (n=121) | 17.18 ± 17.68 (0-93) |
| NDI (n= 71) | 14.96 ± 15.67 (0-76) | |
| LEFS (n=80) | 36.87 ± 25.51 (0-80) | |
| SPADI (n=163) | 20.34 ± 17.75 (0-103) | |
| Mediators | ||
| Pain Catastrophizing Scale (PCS) score (n=247) | 12.45 ± 8.88 (0-38) | |
| Body diagram score (squares) (n=262) | 14.61 ± 15.38 (0-94) | |
| Utilization outcomes | ||
| Visits for index musculoskeletal pain condition (n=279) | 3.97 ± 8.89 (0-63) | |
| Opioid prescriptions after initial evaluation | 1.34 ± 3.26 (0-25) | |
| Incident opioid use | Yes | 93 (32.9) |
| No | 190 (67.1) | |
| Chronic opioid use (≤ 3 opioid prescriptions) | Yes | 41 (14.5) |
| No | 242 (85.5) | |
| Cost outcomes | ||
| Condition-specific medical costs | $1,020.20 ±$2,556.94 ($0-$16,032.83) | |
| Total overall medical costs (n=278) | $2,763.38 ± $5,463.86 ($57.12-$30,794.01) | |
Values are mean ± standard deviation (range) for continuous variables, and count (%) for categorical variables. ODI = Oswestry Disability Index, NDI = Neck Disability Index, LEFS = Lower Extremity Functional Scale, SPADI = Shoulder Pain and Disability Index
Table 2.
Standardized regression coefficients for multiple mediator analyses
| Path A |
Path B |
Total Effect (Path C) |
Direct Effect (Path C’) |
Indirect Effect (Path AxB) |
95% CI | |
|---|---|---|---|---|---|---|
| Condition-specific visits | ||||||
| Pain intensity (n=229) | 0.405* | 0.273* | ||||
| Pain catastrophizing | 2.323* | 0.049* | 0.114* | 0.012 - 0.228 | ||
| Body diagram | 2.640* | 0.007 | 0.018 | −0.034 - 0.081 | ||
| Incident opioid use | ||||||
| Pain intensity (n=231) | 0.196* | 0.273* | ||||
| Pain catastrophizing | 2.296* | −0.032 | −0.073 | −0.239 - 0.065 | ||
| Body diagram | 2.776* | −0.001 | −0.004 | −0.115 - 0.058 | ||
| Disability (n=226) | 0.479* | 0.481* | ||||
| Pain catastrophizing | 1.364* | −0.005 | −0.006 | −0.097 - 0.081 | ||
| Body diagram | 0.966 | 0.004 | 0.004 | −0.037 - 0.040 | ||
| Chronic opioid use | ||||||
| Pain intensity (n=231) | 0.389* | 0.366* | ||||
| Pain catastrophizing | 2.296* | 0.035 | 0.080 | −0.060 - 0.229 | ||
| Body diagram | 2.776* | −0.021 | −0.057 | −0.206 - 0.058 | ||
| Condition-specific costs | ||||||
| Pain intensity (n=231) | 0.104* | 0.007 | ||||
| Pain catastrophizing | 2.332* | 0.027* | 0.064* | 0.013 - 0.121 | ||
| Body diagram | 2.816* | 0.012 | 0.033* | 0.001 - 0.067 | ||
| Total health care costs | ||||||
| Pain intensity (n=228) | 0.059* | 0.025 | ||||
| Pain catastrophizing | 2.291* | 0.008* | 0.019* | 0.001 - 0.040 | ||
| Body diagram | 2.617* | 0.006* | 0.015* | 0.003 - 0.028 |
Indicates p < 0.05. Predictor variables for each model are indicated in italics. Note: All analyses included covariates age, sex, comorbidity count at baseline and surgery during follow-up. Analyses with opioid use outcomes also included prior opioid use status in the previous year as a covariate.
Visits for index musculoskeletal pain condition
Disability was not a significant predictor of musculoskeletal-specific visits after accounting for covariates, therefore we did not explore mediation further for disability. We observed a significant direct effect of pain intensity on number of visits (β=0.273, 95% CI: 0.034-0.512), as well as a significant indirect effect through PCS score (β=0.114, 95% CI: 0.018-0.234), suggesting the relationship between pain intensity and visits is partially mediated by catastrophizing. The direct effect of pain intensity accounted for approximately 67% of the total effect, while the indirect effect of PCS score accounted for approximately 28% of the total effect.
Incident opioid use
Both pain intensity and disability had significant direct effects (β=0.380, 95% CI: 0.054-0.706; and β=0.481, 95% CI: 0.072-0.0886, respectively) on incident opioid use after controlling for covariates, including prior opioid use. However, we observed no significant indirect effects of PCS score or body diagram, indicating no mediation.
Chronic opioid use
Pain intensity, but not disability, had a significant total effect on chronic opioid use, therefore we did not explore mediation effects further for disability. Pain intensity had a significant direct effect on chronic opioid use (β=0.366, 95% CI: 0.041-0.692) with no significant indirect effects of PCS score or body diagram, indicating no mediation.
Condition-specific medical costs
Pain intensity, but not disability, had a significant total effect on pain-related costs, therefore we did not explore mediation effects further for disability. Pain intensity did not have a significant direct effect on costs, however we observed significant indirect effects through both PCS score (β=0.064, 95% CI: 0.011-0.120) and body diagram score (β=0.033, 95% CI: 0.001-0.067) indicating full mediation of the relationship between pain intensity and condition-specific costs.
Total overall medical costs
Pain intensity, but not disability, also had a significant total effect on total medical costs, therefore we did not explore mediation effects further for disability. Pain intensity did not have a significant direct effect on total costs, however both PCS score (β=0.019, 95% CI: 0.001-0.041) and body diagram score (β=0.015, 95% CI: 0.003-0.028) had significant indirect effects indicating full mediation of the relationship between pain intensity and total costs.
Sensitivity analyses
Sensitivity analyses using the imputed dataset replicated the findings of each model in the complete case analyses. Therefore, we do not separately report detailed results of the sensitivity analyses.
Discussion
Similar to prior studies3,14,20, we found that baseline pain intensity and disability can predict important metrics of health care use and cost outcomes 12 months later among military service members with musculoskeletal pain. Our analyses provide novel insights by clarifying the mediating role of psychological characteristics and spatial distribution of symptoms for these relationships. One key finding is that mediation effects were outcome-specific, and more consistently observed for cost outcomes compared to utilization outcomes. For example, pain intensity at intake had a strong and direct relationship with 12-month opioid use that was not mediated by pain catastrophizing or spatial distribution of symptoms. This means that for this sample, catastrophizing and spatial distribution of symptoms did not influence the positive relationship between intake pain intensity and subsequent opioid use. Conversely, pain intensity had no direct effect on 12-month condition-specific or total costs, with these relationships fully mediated by pain catastrophizing and spatial distribution of symptoms. This means that for this sample high concomitant catastrophizing and symptom distribution at intake fully explain why higher pain intensity is related to higher costs of health care over the next 12 months. Collectively, these results suggest that higher pain intensity at intake in particular may drive higher volume of health care, including use of opioids, while characteristics of the pain experience like elevated catastrophizing and larger spatial distribution of symptoms may drive use of higher cost services.
The implications of these findings are that optimal prediction models for health care utilization outcomes might differ from those for cost outcomes. This could be counterintuitive if in building predictive models it was assumed that utilization and cost will always be highly correlated. For instance, health care systems and providers interested in optimizing risk models for incident and chronic opioid use should incorporate baseline pain intensity given the lack of mediation by pain catastrophizing and spatial distribution of symptoms. Conversely, optimal prediction models for medical costs should incorporate pain catastrophizing and spatial distribution of symptoms since pain intensity and disability had no direct effects on medical costs. Outcome specificity, even for closely related outcomes such as these, is supported by prior research20 and further emphasized by these current findings. Importantly, our results lend direction to health care systems and providers that might prioritize identifying risk for high utilization of specific services (i.e., opioid use), as well as those that want to differentiate risk of high utilization volume from risk of high costs (i.e., in response to shared-risk payment models that incentivize high quality, low cost care).
One high priority health care setting where these findings could be tested and validated is the Veteran’s Health Administration (VHA). The MHS and VHA comprise the two largest health care systems (close to 20 million enrollees) continuously working towards improved integration of services for current and past military service members in the United States. Epidemiological evidence suggests high pain severity is prevalent among US military veterans27, and the VHA is focused on opportunities to reduce the personal and economic burden of chronic pain in this population.48 Secular effects such as service era and age, as well as a higher prevalence of comorbid conditions contribute to notable differences in VHA and DoD populations. However, many of these same soldiers transition out of DoD medical care and into VHA medical care following discharge. If the findings from our related sample hold, it would suggest the need to look beyond pain severity to include pain catastrophizing and distribution of pain when identifying veterans at risk for high health care costs. These predictive factors could also be used to better guide use of clinical resources in the MHS and VHA for those seeking care for musculoskeletal pain. These factors could also be included in future cohort studies and clinical trials from the newly-developed NIH/DoD/VA Pain Management Collaboratory, which has the stated goal of developing cost-effective, pragmatic clinical research on non-pharmacological approaches to pain management in military and veteran health care delivery organizations.49
This study design accounts for temporal associations by using baseline measures to predict 12-month utilization and cost outcomes. However, our design is incapable of evaluating the causal mechanisms that underlie these relationships. For this reason, our results are currently better suited to inform clinical decision making consistent with prognosis and prediction. Nevertheless, these findings provide important information that could ultimately improve upon the value of clinical services already available for musculoskeletal pain. For instance, clinical pathways and health care policies that focus only on improving pain intensity may limit future opioid use or visits, but may not reduce costs unless they also improve pain coping and decrease spatial distribution of symptoms. Controlling both utilization and costs may require health care systems to simultaneously address multiple potentially modifiable factors such as pain intensity, disability, pain coping, and spatial distribution of symptoms. These factors appear to drive utilization and/or costs and could be important leverage points in care pathways for musculoskeletal pain conditions, especially when they are modifiable (e.g. individuals with acute pain). However, even in situations where characteristics like pain intensity or pain distribution are not as easily modifiable (e.g., individuals with chronic pain), or not the direct target of treatment (e.g., psychosocial approaches), the prognostic utility of these characteristics remains relevant for informing the efficient distribution of health care resources.
If future studies confirm the causal relationships proposed in this study, enacting health care policies and promoting treatments that specifically target modifiable factors have great potential to improve the value of pain care. Currently, the routine clinical evaluation of pain coping and use of behavioral and/or psychological treatment options are often limited to tertiary pain management clinics. However, this study demonstrates the need to expand assessments of pain coping and pain distribution at the point of health care system entry for musculoskeletal pain (e.g. primary care settings). This expanded assessment seems especially important for refining prediction of utilization and costs. Moreover, these findings suggest behavioral and psychological treatments could be applied earlier in stepped care pathways for pain management, and not just primarily delivered in dedicated pain clinics. Future work would need to verify the relative benefit of this approach for patients with acute and chronic pain.
Our study has some notable strengths. First and unique to this study being completed in the military is the use of the MDR for the analyses. The MHS is a closed and single payer system, meaning the MDR has nearly complete utilization data on all eligible beneficiaries. This is an ideal data set for questions related to cost and utilization because of the closed, single payer characteristic.33 As a result, we are quite confident in our utilization and cost outcomes. We took the additional step of following up the analyses with an imputed dataset to account for the minimal amount of missing patient-reported, non-MDR data, and found very similar results. An additional strength is the use of longitudinal data, and in particular the availability of data for the year prior to the index date, which allowed us to identify prior use of opioids. We also included individuals with acute and chronic pain, which makes this model generalizable to most primary care settings that encounter heterogeneous patient populations. However, this may alternatively limit its direct application in settings that primarily manage populations with chronic pain. Finally, we modeled health care costs and utilization outcomes separately, which was warranted given the emerging evidence for differential predictors among these outcomes in patients with musculoskeletal pain.1,20,21,35 The consideration of differential predictors is important because some health care policies and practice guidelines may want to target risk for use of specific services, such as opioid use, while others might specifically focus on risk for high costs. By modeling these outcomes separately, our findings are more widely applicable.
There are some limitations readers should consider when interpreting our results. First, we conducted this study in a primarily male, military cohort in a multidisciplinary musculoskeletal clinic. Females are notably underrepresented in this cohort, therefore we caution readers on the application of these findings to female populations as they may have different health care utilization patterns and pain or psychological profiles. Patients were screened for the presence of any musculoskeletal disorders and sent directly to this clinic for definitive care. This model of accessing care may not be consistent with common delivery models outside of the military setting. In addition, among military service members, different factors and motivations may drive health care utilization and timing compared to civilian counterparts. In this analysis, absolute costs represent those incurred by the Department of Defense, which could differ compared to other civilian health systems. However, MDR data include health care utilization that occurs within military treatment facilities and civilian hospital networks covered by TRICARE, which improves generalizability of the findings.
A second limitation is that the sensitivity for identifying relevant medical care visits and procedures in medical records is only as good as the entry of the data by the providers. This limitation is common to any analysis using claims data and should be weighed against the benefits and limitations of other methods for assessing utilization, such as patient recall. Nevertheless, readers should interpret findings with this in mind. We additionally acknowledge that comorbid conditions were identified from health care utilization episodes that occurred during deployment, and may underrepresent actual comorbid conditions or health care needs due to the limited care options available during deployment. Although expected given the age and fitness level of the sample, we observed a very low prevalence of individual comorbid conditions. Third, the analytic technique required that we dichotomize count outcomes for visits and opioid use. Despite our inability to model count data, we believe that outcomes such as high health care use (top 30th percentile), incident use of opioids and chronic use of opioids are outcomes with high clinical relevance that are frequently targeted by national initiatives and health care policies aiming to improve musculoskeletal pain treatment outcomes. Fourth, the pain intensity measure available for this analysis asked individuals to rate their pain over the last 24 hours. This timeframe is commonly used in clinical assessments of pain intensity, but may not account for the dynamic nature of pain intensity. Future studies of this design should evaluate other dimensions of the pain experience, and across longer timeframes.
An additional consideration is that these data were collected in 2011 and preceded the opioid crisis and subsequent change in prescribing guidelines. We speculate that characteristics such as pain intensity and catastrophizing would continue to be relevant drivers of health care utilization, including the use of opioids, however these hypotheses would require testing with more current data. A related limitation is that we cannot distinguish between appropriate and inappropriate use of any of the services, including the use or dosage of opioid medication. Therefore, this model is useful for predicting general utilization and costs, but unable to distinguish risk for low-value or inappropriate care. Models that predict risk of inappropriate use, or low value care, would be especially relevant in the current health care system and should be a high priority for future research. Finally, other characteristics known to influence pain-related outcomes, such as depression, self-efficacy, and overall health may also mediate the relationships assessed in this study and should be investigated in future studies.
Health care for musculoskeletal pain conditions is now more acutely focused on minimizing costs and reducing opioid use, which has created the need to better understand the characteristics associated with these outcomes. Our findings suggest that pain intensity, pain catastrophizing and spatial distribution of symptoms are associated with future utilization and costs in an outcome-specific manner. These results provide a foundation for future causal mediation analyses, give health care systems direction for developing value based care pathways, and help to identify important characteristics that could elevate risk of low value care (e.g. chronic opioid use).
Supplementary Material
Table 3.
Summary findings from multiple mediation analyses
| Outcome | Predictors | Mediator | Full or Partial Mediation |
|---|---|---|---|
| Condition-specific visits | Pain Intensity | PCS score | Partial |
| Incident opioid use | Pain Intensity | None | N/A |
| Disability | None | N/A | |
| Chronic opioid use | Pain Intensity | None | N/A |
| Condition-specific costs | Pain Intensity | PCS score, Body Diagram score | Full |
| Total health care costs | Pain Intensity | PCS score, Body Diagram score | Full |
PCS = pain catastrophizing scale
Acknowledgments
Research funding: Dr. Lentz received support for this project from the Foundation for Physical Therapy with Promotion of Doctoral Studies (PODS I & II) Awards. The National Center for Complementary and Integrative Health provided funding through UG3AT009790 (Dr. George for manuscript planning and writing) and UG3AT009763 (Dr. Rhon for manuscript planning and writing) awards.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Disclosures: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Madigan Army Medical Center, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, Department of Defense, or the U.S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antaky E, Lalonde L, Schnitzer ME, Martin É, Berbiche D, Perreault S, Lussier D, Choinière M: Identifying heavy health care users among primary care patients with chronic non-cancer pain. Can J Pain 1:22–36, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51:1173–82, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Becker A, Held H, Redaelli M, Strauch K, Chenot JF, Leonhardt C, Keller S, Baum E, Pfingsten M, Hildebrandt J, Basler H-D, Kochen MM, Donner-Banzhoff N: Low back pain in primary care: costs of care and prediction of future health care utilization. Spine 35:1714–20, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Berwick DM, Nolan TW, Whittington J: The triple aim: care, health, and cost. Health Aff Proj Hope 27:759–69, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Binkley JM, Stratford PW, Lott SA, Riddle DL: The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther 79:371–83, 1999. [PubMed] [Google Scholar]
- 6.Bryner P: Extent measurement in localised low-back pain: a comparison of four methods. Pain 59:281–5, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Burwell SM: Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med 372:897–9, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Calcaterra SL, Scarbro S, Hull ML, Forber AD, Binswanger IA, Colborn KL: Prediction of Future Chronic Opioid Use Among Hospitalized Patients. J Gen Intern Med 33:898–905, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleland JA, Childs JD, Whitman JM: Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil 89:69–74, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Cleveland RJ, Luong M-LN, Knight JB, Schoster B, Renner JB, Jordan JM, Callahan LF: Independent associations of socioeconomic factors with disability and pain in adults with knee osteoarthritis. BMC Musculoskelet Disord 14:297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen S, Yu W: The Concentration and Persistence in the Level of Health Expenditures over Time: Estimates for the U.S. Population, 2008–2009 Statistical Brief #354. [Internet], Rockville, MD: Agency for Healthcare Research and Quality; 2012. January Available from: http://www.meps.ahrq.gov/mepsweb/data_files/publications/st354/stat354.pdf [Google Scholar]
- 12.Dave AJ, Selzer F, Losina E, Klara KM, Collins JE, Usiskin I, Band P, Dalury DF, Iorio R, Kindsfater K, Katz JN: Is there an association between whole-body pain with osteoarthritis-related knee pain, pain catastrophizing, and mental health? Clin Orthop 473:3894–902, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVries A, Koch T, Wall E, Getchius T, Chi W, Rosenberg A: Opioid use among adolescent patients treated for headache. J Adolesc Health Off Publ Soc Adolesc Med 55:128–33, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Engel CC, von Korff M, Katon WJ: Back pain in primary care: predictors of high health-care costs. Pain 65:197–204, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Fairbank JC, Pynsent PB: The Oswestry Disability Index. Spine 25:2940–2952; discussion 2952, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Fillingim RB: Individual differences in pain responses. Curr Rheumatol Rep 7:342–7, 2005. [DOI] [PubMed] [Google Scholar]
- 17.George SZ, Beneciuk JM, Lentz TA, Wu SS, Dai Y, Bialosky JE, Zeppieri G: Optimal Screening for Prediction of Referral and Outcome (OSPRO) for Musculoskeletal Pain Conditions: Results from the Validation Cohort. J Orthop Sports Phys Ther 48:460–75, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes A: Introduction to Mediation, Moderation, and Conditional Process Analysis: Second Edition: A Regression-Based Approach [Internet], 2nd ed. New York, NY: Guilford Press; 2017. [cited 2019 January 7], Available from: https://www.guilford.com/books/Introduction-to-Mediation-Moderation-and-Conditional-Process-Analysis/Andrew-Hayes/9781462534654 [Google Scholar]
- 19.Houde F, Cabana F, Léonard G: Does Age Affect the Relationship Between Pain and Disability? A Descriptive Study in Individuals Suffering From Chronic Low Back Pain. J Geriatr Phys Ther 2001 39:140–5, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Lentz TA, Beneciuk JM, George SZ: Prediction of healthcare utilization following an episode of physical therapy for musculoskeletal pain. BMC Health Serv Res 18:648, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeng DD, Stewart WF, Yan X, Boscarino JA, Mardekian J, Harnett J, Von Korff MR: Use of electronic health records for early detection of high-cost, low back pain patients. Pain Res Manag 20:234–40, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malehi AS, Pourmotahari F, Angali KA: Statistical models for the analysis of skewed healthcare cost data: a simulation study. Health Econ Rev 5:11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihaylova B, Briggs A, O’Hagan A, Thompson SG: Review of statistical methods for analysing healthcare resources and costs. Health Econ 20:897–916, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mintken PE, Glynn P, Cleland JA: Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg 18:920–6, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Moore E, Thibault P, Adams H, Sullivan MJL: Catastrophizing and pain-related fear predict failure to maintain treatment gains following participation in a pain rehabilitation program. Pain Rep 1:e567, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ: Sleep Disorders and Associated Medical Comorbidities in Active Duty Military Personnel. Sleep 36:167–74, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahin RL: Severe Pain in Veterans: The Impact of Age and Sex, and Comparisons to the General Population. J Pain Off J Am Pain Soc 18:247–54, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E: Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 20:589–605, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Piccinin MA, Sayeed Z, Kozlowski R, Bobba V, Knesek D, Frush T: Bundle Payment for Musculoskeletal Care: Current Evidence (Part 1). Orthop Clin North Am 49:135–46, 2018. [DOI] [PubMed] [Google Scholar]
- 30.Piccinin MA, Sayeed Z, Kozlowski R, Bobba V, Knesek D, Frush T: Bundle Payment for Musculoskeletal Care: Current Evidence (Part 2). Orthop Clin North Am 49:147–56, 2018. [DOI] [PubMed] [Google Scholar]
- 31.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians: Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 166:514–30, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Ramírez-Maestre C, Esteve R: The role of sex/gender in the experience of pain: resilience, fear, and acceptance as central variables in the adjustment of men and women with chronic pain. J Pain Off J Am Pain Soc 15:608–618.e1,2014. [DOI] [PubMed] [Google Scholar]
- 33.Rhon DI, Clewley D, Young JL, Sissel CD, Cook CE: Leveraging healthcare utilization to explore outcomes from musculoskeletal disorders: methodology for defining relevant variables from a health services data repository. BMC Med Inform Decis Mak 18:10, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhon DI, Lentz TA, George SZ: Unique Contributions of Body Diagram Scores and Psychosocial Factors to Pain Intensity and Disability in Patients With Musculoskeletal Pain. J Orthop Sports Phys Ther 47:88–96, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Rhon DI, Snodgrass SJ, Cleland JA, Sissel CD, Cook CE: Predictors of chronic prescription opioid use after orthopedic surgery: derivation of a clinical prediction rule. Perioper Med Lond Engl 7:25, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollman GB, Lautenbacher S: Sex differences in musculoskeletal pain. Clin J Pain 17:20–4, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Roy J-S, MacDermid JC, Woodhouse LJ: Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum 61:623–32, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Rundell SD, Gold LS, Hansen RN, Bresnahan BW: Impact of co-morbidities on resource use and adherence to guidelines among commercially insured adults with new visits for back pain. J Eval Clin Pract, 2017. [DOI] [PubMed] [Google Scholar]
- 39.Sabariego C, Brach M, Stucki G: Determinants of major direct medical cost categories among patients with osteoporosis, osteoarthritis, back pain or fibromyalgia undergoing outpatient rehabilitation. J Rehabil Med 43:703–8, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE: Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain 17:165–72, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Staud R, Price DD, Robinson ME, Vierck CJ: Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain Off J Am Pain Soc 5:338–43, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan MJL, Bishop SR, Pivik J: The Pain Catastrophizing Scale: Development and validation. Psychol Assess 7:524–32, 1995. [Google Scholar]
- 43.Suri P, Boyko EJ, Rundell SD, Smith NL, Goldberg J: Do medical conditions predispose to the development of chronic back pain? A longitudinal co-twin control study of middle-aged males with 11-year follow-up. BMC Musculoskelet Disord 19:362, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser EJ, Ramachenderan J, Davies SJ, Parsons R: Chronic Widespread Pain Drawn on a Body Diagram is a Screening Tool for Increased Pain Sensitization, Psycho-Social Load, and Utilization of Pain Management Strategies. Pain Pract Off J World Inst Pain 16:31–7, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Von Korff M, Scher AI, Helmick C, Carter-Pokras O, Dodick DW, Goulet J, Hamill-Ruth R, LeResche L, Porter L, Tait R, Terman G, Veasley C, Mackey S: United States National Pain Strategy for Population Research: Concepts, Definitions, and Pilot Data. J Pain Off J Am Pain Soc 17:1068–80, 2016. [DOI] [PubMed] [Google Scholar]
- 46.Walker BF, Losco CD, Armson A, Meyer A, Stomski NJ: The association between pain diagram area, fear-avoidance beliefs, and pain catastrophising. Chiropr Man Ther 22:5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Wilsey B, Bohm M, Weyrich M, Roy K, Ritley D, Jones C, Melnikow J: Defining risk of prescription opioid overdose: pharmacy shopping and overlapping prescriptions among long-term opioid users in medicaid. J Pain Off J Am Pain Soc 16:445–53, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Federal agencies partner for military and veteran pain management research [Internet], Natl. Inst. Health NIH 2017. [cited 2019 February 6]. Available from: https://www.nih.gov/news-events/news-releases/federal-agencies-partner-military-veteran-pain-management-research
- 49.NIH-DoD-VA Pain Management Collaborator [Internet]. [cited 2019. February 6], Available from: https://painmanagementcollaboratory.org [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.