Abstract
Urinary tract infections are a severe public health problem. The emergence and spread of antimicrobial resistance among uropathogens threatens to further compromise the quality of life and health of people who develop acute and recurrent upper and lower urinary tract infections. The host defense mechanisms that prevent invasive bacterial infection are not entirely delineated. However, recent evidence suggests that versatile innate immune defenses play a key role in shielding the urinary tract from invading uropathogens. Over the last decade, considerable advances have been made in defining the innate mechanisms that maintain immune homeostasis in the kidney and urinary tract. When these innate defenses are compromised or dysregulated, pathogen susceptibility increases. The objective of this review is to provide an overview of how basic science discoveries are elucidating essential innate host defenses in the kidney and urinary tract. In doing so, we highlight how these findings may ultimately translate into the clinic as new biomarkers or therapies for urinary tract infection.
Keywords: Urinary tract infection, Innate immunity, Pyelonephritis, Pattern Recognition Receptors, Cytokines, Antimicrobial Peptides
Introduction
Bacterial urinary tract infections (UTIs) constitute a common cause of significant morbidity in all pediatric patients. The most common bacterial cause of UTI in children is uropathogenic Escherichia coli (UPEC), which accounts for approximately 80% of cases [1]. Acutely, ascending UTI can lead to pyelonephritis, acute kidney injury, renal abscess formation, and bacteremia. Chronically, UTIs can result in renal scarring, proteinuria, hypertension, and chronic kidney disease. Mounting evidence points toward complex roles of the innate immune system in UTI, functioning acutely to eliminate invading uropathogens and chronically to drive renal parenchymal injury and scarring. The purpose of this educational review is to highlight our current understanding of the contributions of innate immunity during UTI.
Overview of the innate immune system
In contrast to the adaptive immune response, the innate immune system generates a more rapid response to microbial challenge. When the innate immune system is compromised or dysregulated, signs of progressive inflammation and infection become clinically apparent [2]. In general, the innate immune system is composed of (1) pattern recognition receptors like toll-like receptors (TLR); (2) plasma proteins, chemokines, and cytokines; (3) cellular components like epithelial cells, bone marrow-derived phagocytes, dendritic cells, and natural killer cells; (4) toxic molecules such as reactive oxygen and reactive nitrogen intermediates; and (5) antimicrobial peptides (AMPs). Additionally, normally present local microbiota in the urogenital system and intestinal tract serve as another source of innate immunity, altering the pH of the local environment and producing their own antimicrobial products to help control UTI, as well as simply acting as competitive inhibitors of more virulent bacterial strains such as UPEC. When innate immune cells encounter potential pathogens, they activate intracellular signaling cascades that lead to the production of antimicrobial mediators, cytokines, and chemokines that orchestrate a local immune response. Epithelial cells make essential contributions to innate immunity by serving as physical barriers, communicating with hematopoietic cells, producing cytokines and chemokines, and secreting antimicrobial proteins and peptides that kill invading pathogens [3–7].
Molecular Targets of Innate Immunity
A. Pattern Recognition Receptors:
One of the most important elements in host-pathogen interactions is the ability of the host to identify external pathogens from self and elicit an appropriate response. Pattern recognition receptors (PRR) are one such host mechanism that detects pathogen- or damage-associated molecular patterns (PAMPs or DAMPs, respectively) and activates the innate immune response. PRR tend to be located on antigen-presenting immune cells like dendritic cells and macrophages. However, PRR can also be expressed by other immune and non-immune cells. These receptors generally localize to cell surfaces but they can also be intracellular, located in either the cytoplasm or in endosomes. The type of PRR activated is both pathogen- and location-specific [8]. Broadly, PRR activation initiates cellular signaling cascades that trigger transcription of genes involved in host defense. Specifically, PRR activate Nuclear Factor kappa B (NF-κB) signaling, downstream cytokine and chemokine expression, and inflammatory cell recruitment for phagocytosis and bacterial clearance [9].
TLRs are one of the most important and thoroughly investigated families of PRRs. TLRs are characterized by the presence of a large extracellular domain of leucine-rich repeats, a transmembrane segment, and a cytoplasmic Toll/Interleukin—1 receptor-like (TIR) domain which helps mediate the interaction between ligand binding and intracellular signaling proteins. TLRs play an essential role in the innate recognition of microbial components and have been heavily equated with initiating innate defense in the setting of UTIs [10, 11]. When uropathogens enter the urinary tract, they trigger a conformational change in the receptor to activate defined adaptor molecules that mediate different cascading responses, including the release of chemokines, interferons, interleukins (IL), antimicrobial substances, and proinflammatory cytokines (Figure 1). The molecular activation of different TLR-family members has been reviewed in detail elsewhere [10, 11].
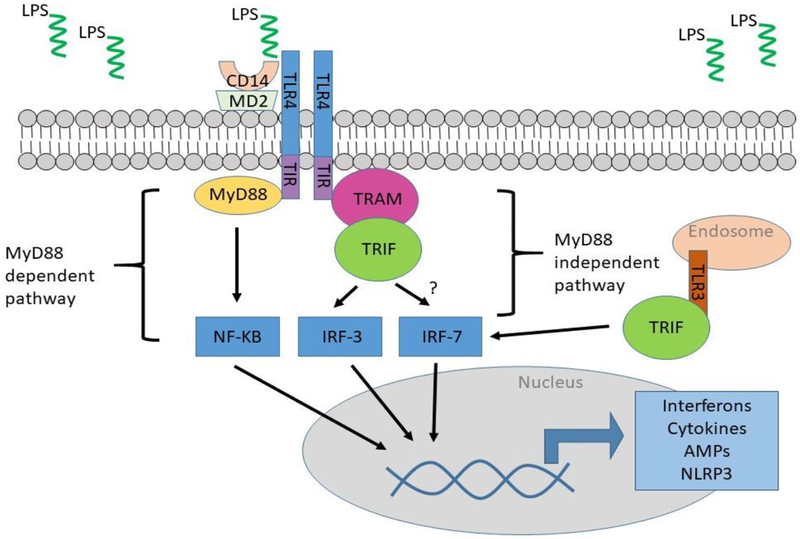
Figure 1.
TLR4 signaling cascade. TLR4 activation by Lps requires co-receptors CD14 and MD2. Signaling cascades are MyD88-dependent (via NF-κB) and MyD88-independent (via IRF-3 and IRF-7) and result in interferon, cytokine, antimicrobial peptide (AMP), and inflammasome expression.
TLR2, TLR4, TLR5, and TLR11 have been shown to regulate UTI susceptibility [10, 11]. Of these, the best studied is TLR4, whose ligand is the Gram-negative bacterial cell wall component, lipopolysaccharide (Lps). TLR4 controls the earliest steps of the mucosal response towards UPEC. Certain inbred mouse strains harbor a point mutation in the TIR-domain of TLR4 that renders them resistant to endotoxin, but highly susceptible to Gram-negative infections including UTI [12]. Subsequent studies have shown that TLR4 expression on bladder epithelial cells as well as innate immune cells is required to successfully combat invasive UPEC infection [13–15]. In the kidney, TLR4 and TLR5 signaling in renal collecting duct cells play key roles in UPEC clearance during pyelonephritis [16, 17]. The following are excellent reviews on TLR-signaling and innate defense of the urinary tract [10, 11, 18].
While TLR signaling activates the host innate immune response, it can also play a pathologic role by activating inflammatory responses that damage local tissues. Specifically, TLR signaling may play a role in initiating and perpetuating renal damage in the setting of UTI. Fortunately, there are innate regulatory mechanisms to fine-tune TLR signaling in order to ensure appropriate response selectively. These include reliance on co-receptors, receptor folding, post-translational modifications, cleavage, intracellular trafficking, and the presence of negative regulators [19].
Additional evidence in human and UPEC genomes attests to the central role of Lps-TLR4 as a critical ligand-receptor interaction during UTI pathogenesis. In humans, polymorphisms in the TLR4 gene have been associated with the development of recurrent UTI [20, 21]. In UPEC, mutations in operons for Lps biosynthesis suppress the ability of bladder epithelial cells to secrete cytokines and chemokines. Moreover, certain UPEC genomes contain TLR4-TIR domain containing proteins (Tcps) that suppress TLR signaling. In one study, approximately 40% of UPEC isolated from patients with acute pyelonephritis had these Tcps, as compared to between 16-21% in patients with asymptomatic bacteriuria and cystitis. In experimental UTI, bacteria encoding Tcp had better survival and resulted in worse renal pathology [22]. As such, Tcps act as bacterial virulence factors to suppress innate immunity and increase UTI susceptibility. Together, these observations link defects in TLR4 expression and signaling to heightened UTI risk.
B. Interferon Regulatory Factors
Interferon Regulatory Factor (IRF)-3 and IRF-7 are transcription factors induced during UTI as a result of TLR activation [23, 24] (Figure 1). While both act as transcriptional activators, IRF-3 and IRF-7 exert opposing effects during UTI. IRF-3 is dependent on TLR4 activation and important in regulating the antibacterial response [23]. Mice that lack Irf3 develop severe acute pyelonephritis in experimental UTI. This is accompanied by massive tissue damage, significant mucosal and urine neutrophil accumulation, and increased tissue and urine bacterial burden [23]. In contrast, IRF-7 appears to drive the inflammatory response. Accordingly, Irf7 KO mice experience lower neutrophil recruitment and UPEC burden than Irf3 KO and wild type mice in response to experimental UTI. Evaluation of gene profiles between Irf3 and Irf7 KO mice confirms that the hyperinflammatory response during UTI is driven by IRF-7 [23, 24]. Thus, it appears that IRF-3 and IRF-7 expression balance each other to mount an effective, limited innate immune response to bacterial infection.
Genetic variants of IRF3 and IRF7 expression may affect human UTI susceptibility. Clinical evaluation has found that children with recurrent acute pyelonephritis have a hypomorphic IRF3 promoter compared to children with asymptomatic bacteriuria [23]. In contrast, IRF7 promoter polymorphisms conferring lower IRF7 expression were protective against recurrent acute pyelonephritis in children and more commonly associated with asymptomatic bacteriuria [24]. In addition, suppressing Irf7 expression in pyelonephritis-susceptible Irf3 KO mice was actually protective of UTI and renal tissue damage [24]. In fact, Irf7 suppression was comparable to antibiotic therapy in regards to preventing renal abscess formation, suggesting IRF-7 could serve as a target of immunotherapy in limiting UTI pathogenesis [24].
C. Chemokines and Cytokines
Cytokine signaling plays a seminal role in coordinating the innate immune response during UTI [25–27]. Cytokines broadly refer to small proteins made intracellularly that are released to enable cell-to-cell communication through autocrine, paracrine, and/or endocrine actions. Interleukins (ILs) are cytokines typically made by inflammatory cells with action on inflammatory cells, while chemokines are cytokines with chemotactic properties. Interestingly, mucosa-derived cytokine expression was first observed in the setting of UTI [28]. As highlighted below, extensive investigation has been performed on the roles of IL-6 and IL-8 during UTI.
Studies suggest that IL-6 plays a role in UTI prevention through multiple mechanisms [29, 30]. IL-6 expression localizes to the bladder urothelium and induces AMP expression to facilitate UPEC clearance [27, 29] (Figure 2). Additionally, IL-6 regulates monocyte proliferation and alters iron homeostasis to impede intramacrophage UPEC growth [31, 32]. When IL-6 knockout mice are subjected to experimental UTI, they experience higher mortality rates, more severe renal histopathology, and higher renal UPEC burden than wild type controls [29, 30]. During early cystitis, IL-6 deficiency leads to increased numbers of UPEC intracellular bacterial communities (IBC), which allow UPEC to evade the innate immune system and contribute to UTI chronicity [29]. This body of work suggests that IL-6 signaling may be developed as a target to treat acute UTI or prevent recurrent infections.
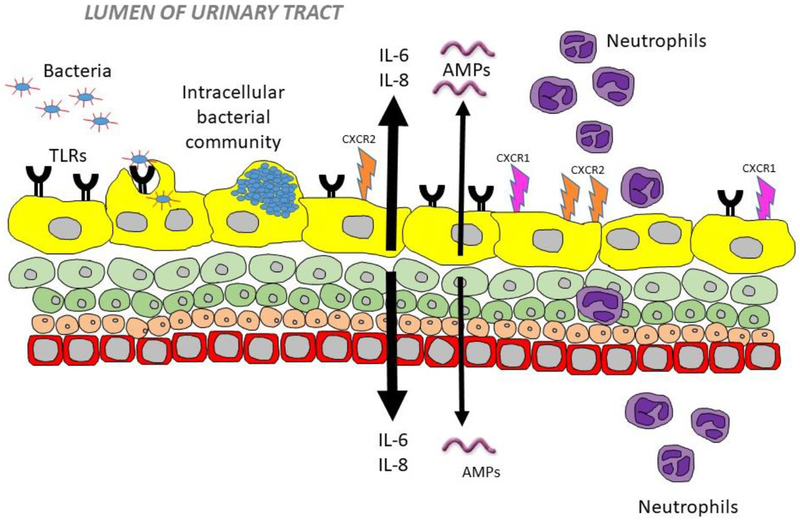
Figure 2.
Urothelial innate immunity. Bacteria bind to TLRs and form intracellular bacterial communities. UTI induces urothelial expression of the cytokine IL-6, which is involved in AMP expression, and the chemokine IL-8, which initiates neutrophil trafficking into the urinary space. Cell surface receptors CXCR1 and CXCR2 are expressed on urothelial cells to enhance neutrophil migration
Recent findings also suggest that IL-6 may be a novel UTI biomarker. Murine UTI models demonstrate that serum and urinary concentrations of IL-6 increase during UTI in a TLR4-dependent manner [29, 33]. Clinically, serum and urine concentrations of IL-6 correlate to the degree of UTI severity. Specifically, children with pyelonephritis have higher IL-6 serum and urine concentrations than children with cystitis. In the pyelonephritis cohort, children with acquired renal scars had higher IL-6 concentrations than those without scars [34–36]. Thus, future prospective studies can evaluate the role of IL-6 as an acute UTI biomarker (cystitis vs. pyelonephritis) or a prospective marker for chronic renal injury and scarring.
Like IL-6, IL-8 transcription is linked to TLR4 activation. IL-8 (also known as CXCL8) belongs to the CXC family of chemokines. IL-8 plays an important role in inflammatory cell chemotaxis, particularly neutrophil recruitment during UTI [13, 34, 37]. IL-8 is expressed both by infected epithelial and circulating cells, as well as recruited immune cells. Neutrophils migrate towards gradients of IL-8 to phagocytose and kill bacteria. In the urinary tract, IL-8 facilitates transepithelial neutrophil migration in the urothelium, permitting neutrophil trafficking from the blood through tissue and across the mucosa to enter the urine [37]. As a result, IL-8 level correlate with the presence of urinary leukocytes and is responsible for the pyuria seen in UTI [18] (Figure 2).
IL-8 has two cell surface receptors: CXCR1 (IL-8RA) and CXCR2 (IL-8RB). CXCR1 is more specific for binding IL-8, whereas CXCR2 is more promiscuous and can bind to multiple CXC chemokines. Both CXCR1 and CXCR2 are expressed on urothelial cells (Figure 2). Their expression increases in the setting of infection to enhance IL-8-dependent neutrophil migration [18, 37]. CXCR1 in particular is thought to be essential for increased neutrophil migration across infected cells in vitro, as antibodies specific to CXCR1 inhibit this process compared to antibodies against CXCR2. This has been confirmed in vivo where neutrophils in mice lacking the murine homologue of the CXCR1 receptor are unable to migrate across the urothelial border into the bladder lumen and thus accumulate in the subepithelial layer [37]. Consequently, these mice are unable to clear UPEC from their bladder and kidneys with resulting renal abscess formation, scarring, and bacteremia [38, 39]. Children prone to acute pyelonephritis have also been found to have reduced CXCR1 levels as compared to age-matched controls [38]. In part, this may be due to CXCR1 and CXCR2 polymorphisms, suggesting that variations in these genes may provide predictive markers to determine UTI susceptibility [40].
D. Antimicrobial Peptides
Host defense peptides and proteins, also known as AMPs, are short, cationic oligopeptides that are evolutionarily conserved. AMPs represent a diverse class of molecules, including defensins, cathelicidin, ribonucleases, and metal-binding proteins. The field of study of AMPs has been steadily expanding over the past decade, suggesting that these molecules have captured the interest of a number of research programs investigating different disease states [41]. In humans, these peptides are expressed by epithelial tissues that often come into contact with pathogenic microorganisms, such as the bladder urothelium and renal collecting duct (Table 1). Additionally, AMPs are expressed in immune cells that are recruited to sites of injury and infection. Table 1 summarizes the epithelial-derived AMPs expressed in the human urinary tract and highlights studies showing their antibacterial activity and biological relevance.
Table 1.
Epithelial Antimicrobial Peptides and Proteins Produced in the Urinary Tract
| Name | Classification | Cellular Source | Biological Relevance |
|---|---|---|---|
| α-and β-defensins | AMP | Bladder Urothelium and Kidney Intercalated and Principal Cells | Defb1−/− mice exhibit increased rates of spontaneous bacteriuria [66]. |
| Cathelicidin | AMP | Bladder Urothelium and Kidney Intercalated Cells | Increased kidney UPEC burden in Camp−/− mice after experimental UTI [67]. |
| Ribonucleases | AMP | Bladder Urothelium and Kidney Intercalated Cells | RNase 4 and 7 neutralization promotes UPEC growth in human urine [68, 55]. |
| Lipocalin 2 | Siderophore | Bladder Urothelium and Kidney Intercalated Cells | Increased bladder UPEC burden in Lcn2−/− mice after experimental UTI [54, 69]. |
| Hepcidin | Iron Regulation | Nephron and Collecting Duct | Increased bladder/kidney UPEC burden in mice after experimental UTI [70, 71]. |
| Uromodulin | Glycoprotein | Loop of Henle | Increased bladder UPEC burden in Thp−/− mice after experimental UTI [72]. |
The most direct application of AMPs to clinical medicine is their development as novel antimicrobials for UTI as well as other common infections. Limitations in their direct delivery have been addressed by methods to induce endogenous AMP production, such as using short chain fatty acid derivatives like butyrate, vitamin D3 derivatives, and estrogens [42–44]. Recent data also suggest that AMPs can be developed as UTI prognostics or diagnostics. Auxiliary studies to the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial show that genetic copy number variation of in the alpha defensin DEFA1A3 locus in children with vesicoureteral reflux predict recurrent UTI [45]. AMPs have also been employed as an adjunct tool in addition to leukocyte esterase for diagnosing UTI in children [46]. Urinary levels of NGAL correlate negatively with recurrent UTI risk, and NGAL has been suggested as an additional biomarker for UTI in children [47, 48]. The role of AMPs in UTI has been reviewed extensively elsewhere [2, 5, 49].
Cellular Mechanisms of Innate Immunity
The innate immune response to UTI is orchestrated through close collaboration between epithelial cells and leukocytes, summarized in Table 2 and illustrated in Figure 3. The urothelial cells lining the bladder, ureter, and renal pelvis, as well as the intercalated cells (IC) of the renal collecting duct have been implicated in early innate defense against uropathogens. This occurs through a number of mechanisms including: (1) detection of bacteria by PRR expression; (2) AMP production; (3) expulsion of intracellular bacteria; (5) production of cytokines such as IL-6; (6) production of IL-8 and other chemokines that promote phagocyte recruitment; (7) barrier function; and (8) regulated exfoliation and regeneration [38, 50–52].
Table 2:
Cellular Effectors of Innate Immunity During UTI
| Cell | Mechanism |
|---|---|
| Urothelial cells | • Detect UPEC by expressing PRRs [50] • UPEC expulsion [52] • Secrete AMP [5] • Release chemokines that promote neutrophil chemotaxis [38] • Exfoliation and regeneration [51, 73] |
| Intercalated cells | • Detect ascending bacteria in a TLR4 and TLR5 dependent manner [16, 17] • Secrete AMP, some of which are regulated by insulin/PI-3 kinase signaling [5, 54, 55] |
| Monocyte derived phagocytes | • Regulate neutrophil recruitment during pyelonephritis [57] • Phagocytose and kill bacteria [57] • Regulated by medullary sodium gradient in an NFAT5-dependent manner [57] |
| Neutrophils | • Phagocytosis and bactericidal activity [58] • Prolonged recruitment and/or activation may promote tissue injury and infection chronicity [38, 39, 59] |
| Inflammatory monocytes | • Recruited to the infected bladder and kidney from the peripheral blood and bone marrow • Secrete TNF-α , which indirectly promotes neutrophil recruitment during acute cystitis [60] |
| Resident macrophages | • Secrete Cxcl2 in a TNF-α dependent manner, which promotes secretion of matrix metalloproteinase essential for neutrophil transmigration across the urothelium [60] |
| Natural Killer cells | • Promote bacterial clearance by secreting TNF-α [62] |
| Natural Killer-T cells | • Promote bacterial clearance by cytokine secretion in response to activating glycolipids [61] |
| Mast cells | • Recruit neutrophils by producing TNF-α [64, 65] • Release cytolytic enzymes that trigger exfoliation of bladder umbrella cells [51] |
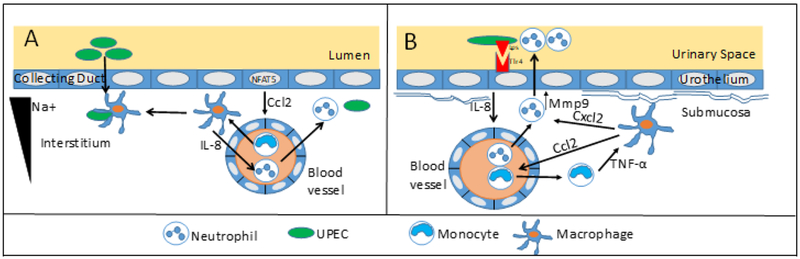
Figure 3.
Leukocyte-epithelial cell interactions orchestrate the innate immune response to UPEC (A) Tubular cells establish the medullary Na+ gradient and secrete the chemokine Ccl2 in a NFAT5 dependent manner. [57] Ccl2 recruits circulating monocytes, which differentiate into macrophages These macrophages increase phagocytosis, IL-8 dependent neutrophil recruitment, and antimicrobial activity in the presence of sodium. This network of myeloid cells serves a critical role in limiting interstitial spread of bacteria. (B) Urothelial cells express TLR4 that recognizes bacterial LPS, resulting in production of IL-8 that elicits neutrophil chemotaxis. Efficient neutrophil transepithelial migration relies on resident macrophages, which recruit circulating monocytes in a Ccl2-dependent manner. Monocyte TNF-α stimulates macrophages to secrete Cxcl2. Cxcl2 stimulates neutrophil production of Mmp9, which degrades matrix and promotes efficient transepithelial neutrophil migration to the urinary space.[37, 60]
Recent genetic studies have established an essential role for IC during UTI. In one approach, IC depletion was achieved through deletion of the Car2 gene encoding carbonic anhydrase 2, which serves a critical function in maintaining electrochemical gradients in the IC [53]. Car2 knockout mice exhibit a 4-fold decrease in IC number and are more susceptible to pyelonephritis following transurethral UPEC inoculation [53]. Alternatively, the consequences of IC depletion on UTI susceptibility were investigated by deletion of an essential transcription factor for IC development, Tcfcp2l1 [54]. Like Car2 knockouts, Tcfcp2l1 deficiency leads to increased bacterial burden following transurethral UPEC inoculation. Using a condition knockout strategy, we have recently demonstrated that the insulin receptor (IR) is essential for AMP production and UPEC clearance by IC [55]. IR deletion does not impact IC number; rather, insulin is responsible for AMP production by IC in a manner dependent on the phosphatidylinositol-3-kinase signaling pathway [55, 56].
Essential interactions between tubular epithelial cells and mononuclear phagocytes have been implicated in forming an intercellular network in the kidney (Figure 3A) [57]. Renal medullary epithelial cells express a transcription factor, NFAT5, that regulates the secretion of chemokines in response to increasing extracellular sodium. The medullary sodium gradient, which classically functions to promote urine concentration, is also required for monocyte-derived mononuclear phagocytes (MNP) to localize to the renal medulla. The hypersaline microenvironment of the medulla also drives bactericidal and neutrophil chemotactic activities of MNPs. Disruption of the medullary sodium gradient in mice and patients with nephrogenic diabetes insipidus results in increased UTI susceptibility [57]. This elegant work, supported by human data, illustrates the manner in which the unique physiology of the kidney can modulate the innate immune response.
Svanborg and colleagues established the importance of neutrophils for UTI eradication [58]. Recruitment of neutrophils was shown to be reliant on bacterial Lps, as Lps-resistant mice lacked neutrophil recruitment and this was associated with bacterial persistence in the bladder and kidneys. When neutrophils were depleted from Lps-sensitive mice prior to infection, UPEC clearance from the urinary tract was impaired [58]. In addition to their essential role in bacterial killing, activated neutrophils are capable of causing extensive parenchymal injury in the infected urinary tract. Neutrophil-derived cyclooxygenase-2 (COX-2) has been implicated as a driver of inflammation associated with severe, recurrent cystitis [59]. Neutrophils are responsible for severe tubulointerstitial nephritis in IL-8 receptor-deficient mice [38, 39]. Thus, the fine-tuning of the neutrophil response is essential to balance their bactericidal function against their propensity to cause tissue injury.
Monocytes and macrophages are well-suited to modulate neutrophil function during UTI. Elegant experiments from the Engel laboratory established that complex interactions between neutrophils, resident macrophages, and recruited inflammatory monocytes trigger neutrophil migration across the urothelium during cystitis (Figure 3B) [60], In response to UTI, inflammatory monocytes are recruited to the bladder submucosa and produce the cytokine, Tumor Necrosis Factor (TNF)-α. Next, TNF-α engages its receptor on resident macrophages, leading to secretion of the Cxcl2 chemokine. Finally, Cxcl2 engages the Ccr2 receptor on neutrophils, leading to production of the Mmp9 matrix metalloproteinase. Mmp9 degrades the extracellular matrix lining the urothelial basement membrane, triggering transmigration of neutrophils [60]. Further studies are required to determine if this licensing of neutrophil migration by monocyte-macrophage interactions is applicable during pyelonephritis.
The natural killer (NK) and NK-T lineages have been implicated in host defense during UTI. Invariant NK-T cells are activated by the ligand, α-galactosylceramide (α-GalCer). Administration of α-GalCer leads to reduced kidney bacterial burden during Gram-negative and Gram-positive UTI, associated with higher levels of IL-12, IFN-γ, and TNF-α compared to control glycolipids [61]. More recent experiments have implicated NK cells as critical responders to UPEC, by recognition of bacterial type I pili and production of TNF-α [62]. Optimal NK cell and neutrophil recruitment during UTI requires urothelial cell secretion of the chemokine, Stromal Cell-Derived Factor 1, another example of the collaborative network linking epithelial cells and leukocytes in urinary tract defense [63].
A landmark historical study established that mast cells (MC) are critical mediators of neutrophil activation during Gram-negative bacterial infections through production of TNF-α in a manner dependent on the bacterial fimbrial protein, FimH [64]. Likewise, MC produce TNF-α following UPEC exposure in a FimH-dependent manner, and MC-deficient mice display impaired UPEC clearance during experimental UTI associated with reduced neutrophil recruitment [65]. A recent study has revealed a critical role for MC in triggering exfoliation of bladder umbrella cells during acute cystitis [51]. Following transurethral inoculation of UPEC, umbrella cells secrete the cytokine, IL-1β, which induces MC to migrate to a position immediately deep to the umbrella cell. These MC degranulate locally, and umbrella cells endocytose MC granules containing chymase. Once chymase reaches the umbrella cell cytosol, it triggers cleavage and activation of caspase-1, which mediates cytolysis and exfoliation [51]. Thus, MC serve multiple essential roles in coordinating the innate immune response.
Conclusions
The innate immune system plays an essential role in the prevention of recurrent and invasive UTI, but failure to dampen the innate immune response may lead to irreparable parenchymal injury. This raises the prediction that, in select cases, modulation of the innate immune system may facilitate clinical management of UTI in the future. A potential future strategy in the prevention of intractable UTIs or multi-drug resistant UTIs will be to combine antimicrobial therapy with innate immune modulators. Unlike conventional antibiotics, such immunomodulatory therapies will not be universally applicable. Instead, they will need to be tailored to each patient and take into account the patient’s age, genetic profile, immune competence, pathogen virulence and antibiotic susceptibility. For these novel strategies to be safe and effective, a thorough comprehension of the host innate immune response will be required.
Key summary points.
A well-coordinated and effective innate immune response balances the need to promptly clear invading pathogens while minimizing inflammatory responses that disrupt epithelial barriers and cause cellular injury.
Cross-talk between different innate immune mechanisms coordinates the recruitment or activation of leukocytes, transcription factor activation, and the release of epithelial peptides and proteins to facilitate pathogen clearance.
Genetic mutations in innate immune effectors, like TLRs, transcription factors, and AMPs, may increase UTI risk.
Novel preclinical strategies have been described that can enhance innate immune defenses of the kidney and urinary tract to prevent and eradicate UTI.
Multiple choice questions (answers after reference list).
- Which of the following secreted proteins directly promotes neutrophil chemotaxis across the urothelium during UTI?
- IL-12
- TNF-α
- IL-8
- IL-6
- IL-1β
- Which of the following cell types is NOT a source of TNF-α during UTI?
- Neutrophils
- Resident macrophages
- Mast cells
- Natural killer cells
- What are the roles of mast cells during UTI?
- Promote exfoliation of bladder umbrella cells
- Secrete TNF-α, which promotes recruitment of circulating neutrophils
- Neither a nor b is correct.
- Both a and b are correct.
- How do intercalated cells contribute to host defense against ascending UTI?
- Secrete TLR4 and TLR5, which bind to bacterial ligands and prevent kidney invasion
- Release antimicrobial peptides in an insulin dependent manner
- Exfoliate into the collecting duct lumen following UPEC binding
- Release chemokines that promote recruitment of circulating T and B lymphocytes
- Which of the following is a form of innate immunity?
- Biological shielding through use of local microbiota
- Induction of AMP expression in the urinary stream
- Production of chemokines and chemokines
- Toll-like receptors
- All of the above
Answers.
c
a
d
b
e
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- [1].Shaikh N, Morone NE, Bost JE, Farrell MH (2008) Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 27:302–308 [DOI] [PubMed] [Google Scholar]
- [2].Zasloff M (2007) Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol 18:2810–2816 [DOI] [PubMed] [Google Scholar]
- [3].Spencer JD, Schwaderer AL, Becknell B, Watson J, Hains DS (2014) The innate immune response during urinary tract infection and pyelonephritis. Pediatr Nephrol 29:1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hato T, Dagher PC (2015) How the Innate Immune System Senses Trouble and Causes Trouble. Clin J Am Soc Nephrol 10:1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Becknell B, Schwaderer A, Hains DS, Spencer JD (2015) Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat Rev Nephrol 11:642–655 [DOI] [PubMed] [Google Scholar]
- [6].Bogdan C, Rollinghoff M, Diefenbach A (2000) Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 12:64–76 [DOI] [PubMed] [Google Scholar]
- [7].Stapleton AE (2014) Urinary tract infection pathogenesis: host factors. Infect Dis Clin North Am 28:149–159 [DOI] [PubMed] [Google Scholar]
- [8].Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820 [DOI] [PubMed] [Google Scholar]
- [9].Chowdhury P, Sacks SH, Sheerin NS (2004) Minireview: functions of the renal tract epithelium in coordinating the innate immune response to infection. Kidney Int 66:1334–1344 [DOI] [PubMed] [Google Scholar]
- [10].Song J, Abraham SN (2008) TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol 11:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Behzadi E, Behzadi P (2016) The role of toll-like receptors (TLRs) in urinary tract infections (UTIs). Cent European J Urol 69:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088 [DOI] [PubMed] [Google Scholar]
- [13].Weichhart T, Haidinger M, Horl WH, Saemann MD (2008) Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest 38 Suppl 2:29–38 [DOI] [PubMed] [Google Scholar]
- [14].Schilling JD, Martin SM, Hunstad DA, Patel KP, Mulvey MA, Justice SS, Lorenz RG, Hultgren SJ (2003) CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect Immun 71:1470–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ (2003) Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 100:4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bens M, Vimont S, Ben Mkaddem S, Chassin C, Goujon JM, Balloy V, Chignard M, Werts C, Vandewalle A (2014) Flagellin/TLR5 signalling activates renal collecting duct cells and facilitates invasion and cellular translocation of uropathogenic Escherichia coli. Cell Microbiol 16:1503–1517 [DOI] [PubMed] [Google Scholar]
- [17].Chassin C, Vimont S, Cluzeaud F, Bens M, Goujon JM, Fernandez B, Hertig A, Rondeau E, Arlet G, Hornef MW, Vandewalle A (2008) TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol 19:2364–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ragnarsdottir B, Fischer H, Godaly G, Gronberg-Hernandez J, Gustafsson M, Karpman D, Lundstedt AC, Lutay N, Ramisch S, Svensson ML, Wullt B, Yadav M, Svanborg C (2008) TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections. Eur J Clin Invest 38 Suppl 2:12–20 [DOI] [PubMed] [Google Scholar]
- [19].Leifer CA, Medvedev AE (2016) Molecular mechanisms of regulation of Toll-like receptor signaling. J Leukoc Biol 100:927–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Castro-Alarcon N, Rodriguez-Garcia R, Ruiz-Rosas M, Munoz-Valle JF, Guzman-Guzman IP, Parra-Rojas I, Vazquez-Villamar M (2019) Association between TLR4 polymorphisms (896 A>G, 1196 C>T, - 2570 A>G, - 2081 G>A) and virulence factors in uropathogenic Escherichia coli. Clin Exp Med 19:105–113 [DOI] [PubMed] [Google Scholar]
- [21].Karoly E, Fekete A, Banki NF, Szebeni B, Vannay A, Szabo AJ, Tulassay T, Reusz GS (2007) Heat shock protein 72 (HSPA1B) gene polymorphism and Toll-like receptor (TLR) 4 mutation are associated with increased risk of urinary tract infection in children. Pediatr Res 61:371–374 [DOI] [PubMed] [Google Scholar]
- [22].Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T (2008) Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med 14:399–406 [DOI] [PubMed] [Google Scholar]
- [23].Fischer H, Lutay N, Ragnarsdottir B, Yadav M, Jonsson K, Urbano A, Al Hadad A, Ramisch S, Storm P, Dobrindt U, Salvador E, Karpman D, Jodal U, Svanborg C (2010) Pathogen specific, IRF3-dependent signaling and innate resistance to human kidney infection. PLoS Pathog 6:e1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Puthia M, Ambite I, Cafaro C, Butler D, Huang Y, Lutay N, Rydstrom G, Gullstrand B, Swaminathan B, Nadeem A, Nilsson B, Svanborg C (2016) IRF7 inhibition prevents destructive innate immunity-A target for nonantibiotic therapy of bacterial infections. Sci Transl Med 8:336ra59 [DOI] [PubMed] [Google Scholar]
- [25].Abraham SN, Miao Y (2015) The nature of immune responses to urinary tract infections. Nat Rev Immunol 15:655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ (2010) Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6:e1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ (2001) Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol 166:1148–1155 [DOI] [PubMed] [Google Scholar]
- [28].Ragnarsdottir B, Svanborg C (2012) Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: host-pathogen interaction in urinary tract infections. Pediatr Nephrol 27:2017–2029 [DOI] [PubMed] [Google Scholar]
- [29].Ching CB, Gupta S, Li B, Cortado H, Mayne N, Jackson AR, McHugh KM, Becknell (2018) Interleukin-6/Stat3 signaling has an essential role in the host antimicrobial response to urinary tract infection. Kidney Int 93:1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khalil A, Tullus K, Bartfai T, Bakhiet M, Jaremko G, Brauner A (2000) Renal cytokine responses in acute Escherichia coli pyelonephritis in IL-6-deficient mice. Clin Exp Immunol 122:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dixit A, Bottek J, Beerlage AL, Schuettpelz J, Thiebes S, Brenzel A, Garbers C, Rose-John S, Mittrucker HW, Squire A, Engel DR (2018) Frontline Science: Proliferation of Ly6C(+) monocytes during urinary tract infections is regulated by IL-6 trans-signaling. J Leukoc Biol 103:13–22 [DOI] [PubMed] [Google Scholar]
- [32].Owusu-Boaitey N, Bauckman KA, Zhang T, Mysorekar IU (2016) Macrophagic control of the response to uropathogenic E. coli infection by regulation of iron retention in an IL-6-dependent manner. Immun Inflamm Dis 4:413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Agace W, Hedges S, Svanborg C (1992) Lps genotype in the C57 black mouse background and its influence on the interleukin-6 response to E. coli urinary tract infection. Scand J Immunol 35:531–538 [DOI] [PubMed] [Google Scholar]
- [34].Rodriguez LM, Robles B, Marugan JM, Suarez A, Santos F (2008) Urinary interleukin-6 is useful in distinguishing between upper and lower urinary tract infections. Pediatr Nephrol 23:429–433 [DOI] [PubMed] [Google Scholar]
- [35].Sheu JN, Chen MC, Chen SM, Chen SL, Chiou SY, Lue KH (2009) Relationship between serum and urine interleukin-6 elevations and renal scarring in children with acute pyelonephritis. Scand J Urol Nephrol 43:133–137 [DOI] [PubMed] [Google Scholar]
- [36].Mizutani M, Hasegawa S, Matsushige T, Ohta N, Kittaka S, Hoshide M, Kusuda T, Takahashi K, Ichihara K, Ohga S (2017) Distinctive inflammatory profile between acute focal bacterial nephritis and acute pyelonephritis in children. Cytokine 99:24–29 [DOI] [PubMed] [Google Scholar]
- [37].Godaly G, Hang L, Frendeus B, Svanborg C (2000) Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J Immunol 165:5287–5294 [DOI] [PubMed] [Google Scholar]
- [38].Frendeus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C (2000) Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J Exp Med 192:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hang L, Frendeus B, Godaly G, Svanborg C (2000) Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J Infect Dis 182:1738–1748 [DOI] [PubMed] [Google Scholar]
- [40].Han SS, Lu Y, Chen M, Xu YQ, Wang Y (2019) Association between interleukin 8-receptor gene (CXCR1 and CXCR2) polymorphisms and urinary tract infection: evidence from 4097 subjects. Nephrology (Carlton) 24:464–471 [DOI] [PubMed] [Google Scholar]
- [41].Mahlapuu M, Hakansson J, Ringstad L, Bjorn C (2016) Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front Cell Infect Microbiol 6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B (2006) Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A 103:9178–9183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Steinmann J, Halldorsson S, Agerberth B, Gudmundsson GH (2009) Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother 53:5127–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Park K, Kim YI, Shin KO, Seo HS, Kim JY, Mann T, Oda Y, Lee YM, Holleran WM, Elias PM, Uchida Y (2014) The dietary ingredient, genistein, stimulates cathelicidin antimicrobial peptide expression through a novel S1P-dependent mechanism. J Nutr Biochem 25:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schwaderer AL, Wang H, Kim S, Kline JM, Liang D, Brophy PD, McHugh KM, Tseng GC, Saxena V, Barr-Beare E, Pierce KR, Shaikh N, Manak JR, Cohen DM, Becknell B, Spencer JD, Baker PB, Yu CY, Hains DS (2016) Polymorphisms in alpha-Defensin-Encoding DEFA1A3 Associate with Urinary Tract Infection Risk in Children with Vesicoureteral Reflux. J Am Soc Nephrol 27:3175–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Watson JR, Hains DS, Cohen DM, Spencer JD, Kline JM, Yin H, Schwaderer AL (2016) Evaluation of novel urinary tract infection biomarkers in children. Pediatr Res 79:934–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Forster CS, Johnson K, Patel V, Wax R, Rodig N, Barasch J, Bachur R, Lee RS (2017) Urinary NGAL deficiency in recurrent urinary tract infections. Pediatr Nephrol 32:1077–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lubell TR, Barasch JM, Xu K, Ieni M, Cabrera KI, Dayan PS (2017) Urinary Neutrophil Gelatinase-Associated Lipocalin for the Diagnosis of Urinary Tract Infections. Pediatrics 140:e20171090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Spencer JD, Schwaderer AL, Becknell B, Watson J, Hains DS (2014) The innate immune response during urinary tract infection and pyelonephritis. Pediatr Nephrol 29:1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu Y, Memet S, Saban R, Kong X, Aprikian P, Sokurenko E, Sun TT, Wu XR (2015) Dual ligand/receptor interactions activate urothelial defenses against uropathogenic E. coli. Sci Rep 5:16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Choi HW, Bowen SE, Miao Y, Chan CY, Miao EA, Abrink M, Moeser AJ, Abraham SN (2016) Loss of Bladder Epithelium Induced by Cytolytic Mast Cell Granules. Immunity 45:1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN (2009) TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U SA 106:14966–14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hains DS, Chen X, Saxena V, Barr-Beare E, Flemming W, Easterling R, Becknell B, Schwartz GJ, Schwaderer AL (2014) Carbonic anhydrase 2 deficiency leads to increased pyelonephritis susceptibility. Am J Physiol Renal Physiol 307:F869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, Francis KP, Ray S, Vijayakumar S, Seward S, Bovino ME, Xu K, Takabe Y, Amaral FE, Mohan S, Wax R, Corbin K, Sanna-Cherchi S, Mori K, Johnson L, Nickolas T, D’Agati V, Lin CS, Qiu A, Al-Awgati Q, Ratner AJ, Barasch J (2014) alpha-intercalated cells defend the urinary system from bacterial infection. J Clin Invest 124:2963–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Murtha MJ, Eichler T, Bender K, Metheny J, Li B, Schwaderer AL, Mosquera C, James C, Schwartz L, Becknell B, Spencer JD (2018) Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J Clin Invest 128:5634–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Eichler TE, Becknell B, Easterling RS, Ingraham SE, Cohen DM, Schwaderer AL, Hains DS, Li B, Cohen A, Metheny J, Tridandapani S, Spencer JD (2016) Insulin and the phosphatidylinositol 3-kinase signaling pathway regulate Ribonuclease 7 expression in the human urinary tract. Kidney Int 90:568–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, Dennison TW, Kuper C, Neuhofer W, Clatworthy MR (2017) Renal Sodium Gradient Orchestrates a Dynamic Antibacterial Defense Zone. Cell 170:860–874 e19 [DOI] [PubMed] [Google Scholar]
- [58].Haraoka M, Hang L, Frendeus B, Godaly G, Burdick M, Strieter R, Svanborg C (1999) Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis 180:1220–1229 [DOI] [PubMed] [Google Scholar]
- [59].Hannan TJ, Roberts PL, Riehl TE, van der Post S, Binkley JM, Schwartz DJ, Miyoshi H, Mack M, Schwendener RA, Hooton TM, Stappenbeck TS, Hansson GC, Stenson WF, Colonna M, Stapleton AE, Hultgren SJ (2014) Inhibition of Cyclooxygenase-2 Prevents Chronic and Recurrent Cystitis. EBioMedicine 1:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Grone HJ, Garbi N, Kastenmuller W, Knolle PA, Kurts C, Engel DR (2014) Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156:456–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Minagawa S, Ohyama C, Hatakeyama S, Tsuchiya N, Kato T, Habuchi T (2005) Activation of natural killer T cells by alpha-galactosylceramide mediates clearance of bacteria in murine urinary tract infection. J Urol 173:2171–2174 [DOI] [PubMed] [Google Scholar]
- [62].Gur C, Coppenhagen-Glazer S, Rosenberg S, Yamin R, Enk J, Glasner A, Bar-On Y, Fleissig O, Naor R, Abed J, Mevorach D, Granot Z, Bachrach G, Mandelboim O (2013) Natural killer cell-mediated host defense against uropathogenic E. coli is counteracted by bacterial hemolysinA-dependent killing of NK cells. Cell Host Microbe 14:664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Isaacson B, Hadad T, Glasner A, Gur C, Granot Z, Bachrach G, Mandelboim O (2017) Stromal Cell-Derived Factor 1 Mediates Immune Cell Attraction upon Urinary Tract Infection. Cell Rep 20:40–47 [DOI] [PubMed] [Google Scholar]
- [64].Malaviya R, Ikeda T, Ross E, Abraham SN (1996) Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77–80 [DOI] [PubMed] [Google Scholar]
- [65].Malaviya R, Ikeda T, Abraham SN, Malaviya R (2004) Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol Lett 91:103–111 [DOI] [PubMed] [Google Scholar]
- [66].Morrison G, Kilanowski F, Davidson D, Dorin J (2003) Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun 70:3053–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A (2006) The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12:636–641 [DOI] [PubMed] [Google Scholar]
- [68].Spencer JD, Schwaderer AL, Dirosario JD, McHugh KM, McGillivary G, Justice SS, Carpenter AR, Baker PB, Harder J, Hains DS (2011) Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int 80:174–180 [DOI] [PubMed] [Google Scholar]
- [69].Steigedal M, Marstad A, Haug M, Damas JK, Strong RK, Roberts PL, Himpsl SD, Stapleton A, Hooton TM, Mobley HL, Hawn TR, Flo TH (2014) Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J Immunol 193:6081–6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Houamel D, Ducrot N, Lefebvre T, Daher R, Moulouel B, Sari MA, Letteron P, Lyoumi S, Millot S, Tourret J, Bouvet O, Vaulont S, Vandewalle A, Denamur E, Puy H, Beaumont C, Gouya L, Karim Z (2016) Hepcidin as a Major Component of Renal Antibacterial Defenses against Uropathogenic Escherichia coli. J Am Soc Nephrol 27:835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, Cetin Y, Stremmel W (2005) The iron-regulatory peptide hormone hepcidin: expression and cellular localization in the mammalian kidney. J Endocrinol 184:361–370 [DOI] [PubMed] [Google Scholar]
- [72].Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S (2004) Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65:791–797 [DOI] [PubMed] [Google Scholar]
- [73].Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ (2009) Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5:463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]