Abstract
Delayed reward discounting (DRD) is a behavioral economic measure of impulsivity that has been consistently associated with addiction. It has also been identified as a promising addiction endophenotype, linking specific sources of genetic variation to individual risk. A challenge in the studies to date is that levels of DRD are often confounded with prior drug use and previous studies have also had limited genomic scope. The current investigation sought to address these issues by studying DRD in healthy young adults with low levels of substance use (N=986; 62% female, 100% European ancestry) and investigating genetic variation genome-wide. The genome-wide approach used a prioritized subset design, organizing the tests into theoretically- and empirically-informed categories and apportioning power accordingly. Three subsets were used: 1) a priori loci implicated by previous studies; 2) high-value addiction (HVA) markers from the recently developed SmokeScreen© array; and 3) an atheoretical genome-wide scan. Among a priori loci, a nominally significant association was present between rs521674 in ADRA2A. No significant HVA loci were detected. One statistically significant genome-wide association was detected (rs13395777, p = 2.8 × 10−8), albeit in an intergenic region of unknown function. These findings are generally not supportive of the previous candidate gene studies and suggest that DRD has a complex genetic architecture that will require considerably larger samples to identify genetic associations more definitively.
Keywords: Delayed reward discounting, impulsivity, decision making, genetics, genomics
INTRODUCTION
Delayed reward discounting (DRD) refers to the extent to which an individual devalues a reward based on its delay in time (Bickel and Marsch 2001; Bickel et al. 2014). It is a behavioral economic measure of impulsivity, and is also referred to as impulsive choice, intertemporal choice, and capacity to delay gratification. Typically, DRD is operationally defined by how much a person prefers smaller immediate rewards versus larger delayed rewards on experimental tasks, with more precipitous devaluation of future rewards reflecting greater impulsivity. Numerous studies have shown that individuals with addictive disorders exhibit steeper DRD than controls (Madden et al. 1997; Bickel et al. 1999; Petry 2001; Coffey et al. 2003; MacKillop et al. 2006). Significant associations have also been reported using dimensional (continuous) designs (Sweitzer et al. 2008; MacKillop et al. 2010; Murphy and Garavan 2011; MacKillop et al. 2014). In addition, meta-analyses of both case-control and dimensional studies have revealed the link between DRD and addiction is robust across studies (MacKillop et al. 2011; Amlung et al. 2016). These relationships are distinct from other measures of impulsivity, as DRD tends to be weakly correlated with other behavioral or self-report measures of impulsivity (Reynolds et al. 2006; Cyders and Coskunpinar 2011; Courtney et al. 2012; MacKillop et al. 2014; Caswell et al. 2015). This is consistent with the increasing recognition of impulsivity as a multifaceted construct comprising conceptually related domains that are quantitatively distinct (de Wit 2009; Dick et al. 2010; Jentsch et al. 2014).
There is considerable evidence that genetic factors influence addiction (Goldman et al. 2005; Agrawal and Lynskey 2008; Volkow and Baler 2014), and impulsive discounting may contribute to this risk (Mitchell 2011; MacKillop 2013). Indeed, there is accumulating evidence that DRD is genetically influenced and shares heritability with addiction phenotypes. In preclinical studies, inbred rodent strains that are isogenic within strain but differ across strains exhibit systematic differences in DRD for food or water rewards (Isles et al. 2004; Anderson and Woolverton 2005; Madden et al. 2008; Wilhelm and Mitchell 2009; Stein et al. 2012; Richards et al. 2013). Furthermore, preclinical studies indicate that rat or mouse strains exhibiting more impulsive DRD exhibit higher preference for alcohol (Wilhelm and Mitchell 2008; Oberlin and Grahame 2009; Beckwith and Czachowski 2014; Perkel et al. 2015; Linsenbardt et al. 2016). Studies in humans comparing monozygotic and dizygotic twins have similarly revealed substantial levels of heritability (Anokhin et al. 2011; Sparks et al. 2014; Isen et al. 2014; Anokhin et al. 2014). In addition, greater DRD is associated with family history of addiction (Acheson et al. 2011; Dougherty et al. 2014; VanderBroek et al. 2015). Collectively, these studies suggest that DRD is heritable and co-aggregates with addiction propensity.
Both pharmacological and molecular genetic studies with DRD suggest a role for catecholaminergic mechanisms. For example, DRD preferences in humans have been reported in relation to a locus in ANKK1 (rs1800497) (Eisenberg et al. 2007; MacKillop et al. 2015) that is proximal to the dopamine (DA) D2 receptor gene, DRD2. DRD has also been reported in association with another DA-related locus in COMT (rs4680) (Boettiger et al. 2007; Gianotti et al. 2012; MacKillop et al. 2015), which encodes catechol-O-methyl transferase, the enzyme involved in metabolizing DA in the prefrontal cortex (Tunbridge et al. 2004; Chen et al. 2004). Finally, DRD has also been reported to be associated with variants in genes contributing to serotonergic and nor-adrenergic activity (Sonuga-Barke et al. 2011; Havranek et al. 2015).
However, the existing literature on genetic correlates of DRD has a number of limitations. To start, the samples have been small, permitting mainly analyses focusing on single candidate genes or a few biologically-related loci. Further, the samples have been heterogeneous. Across studies, samples have varied from the general population to recovering alcohol users, individuals with attention deficit hyperactivity disorder, gamblers, individuals with cocaine use disorder and smokers. This heterogeneity is a problematical for several reasons. First, it means the study designs are not equivalent and not readily comparable. Second, the inclusion of individuals with addictive disorders make it difficult to determine whether the elevated DRD was a predisposing factor or a result of the users’ extended drug exposure. Although several studies suggest that impulsive DRD in humans predates addictive behavior (Audrain-McGovern et al. 2009; Fernie et al. 2013; Khurana et al. 2013; Kim-Spoon et al. 2014), there is also evidence that drug use itself induces more impulsive DRD (Simon et al. 2007; Setlow et al. 2009; Mendez et al. 2010; Mitchell et al. 2014). An unsurprising consequence of these limitations is that the current literature is both genomically narrow and inconsistent in its findings.
The goal of the current study was to advance the investigation of genetic influences on DRD by addressing some of these issues. First, we recruited a moderately sample of healthy young adults with low levels of psychoactive drug use to minimize the extent to which DRD preferences could be interpreted as a symptom or consequence of drug misuse. Second, we tested almost 1000 individuals under controlled laboratory conditions, a sample substantially larger than most previous studies. Third, we expanded the scope of genetic associations with DRD preferences by examining genome-wide variation in single nucleotide polymorphisms (SNPs) and did so using a prioritized subset approach (Sun et al. 2006; Li et al. 2008; Lin and Lee 2010; Schork et al. 2013). Specifically, the study used three prioritized subsets: 1) a priori loci implicated by previous DRD molecular genetic studies; 2) high-value addiction (HVA) markers from the recently developed SmokeScreen© array (Baurley et al. 2016), a compilation of loci linked with diverse addiction-related phenotypes; and 3) an atheroetical genome-wide scan included to identify loci not included in the two previous categories. Collectively, these strategies were intended to provide strong tests for previously reported associations in a larger and more stringently defined design, and to expand the scope of genetic variation under consideration substantially, but doing so in a biologically-informed and principled way.
METHODS
Participants
The sample and phenotypic data collection are described in detail in a report detailing phenotypic relationships among diverse measures of impulsivity (MacKillop et al. 2016). Briefly, participants were recruited at two sites (Athens, GA and Chicago, IL). Inclusion criteria were: a) English fluency; b) age 18–30; c) self-reported European ancestry and non-Hispanic ethnicity to control for population stratification (see below for genetic verification of racial homogeneity). Exclusion criteria were: a) ≥12 on the Alcohol Use Disorders Identification Test (AUDIT; Saunders, Babor, de la Fuente, & Grant, 1993) or Drug Use Disorders Identification Test (DUDIT; Berman et al., 2005); b) addiction treatment in the last 12 months; c) self-reported presence of major depressive disorder, bipolar disorder, general anxiety disorder, social anxiety disorder, post-traumatic stress disorder, obsessive compulsive disorder, panic attacks/disorder, phobia, schizophrenia or eating disorders. Participant characteristics are provided in Table 1 and, per the study design, the sample can generally be characterized as healthy young adults reporting low levels of substance use. Other than sex, the two sites significantly differed such that the Chicago site was older, more educated, and lower income (see Table 1), reflecting a post-collegiate young adult sample. Institutional review board approval was obtained at both sites (University of Chicago #11–0549, University of Georgia, #10911, common title: “Genetic basis of impulsive behavior in humans”).
Table 1.
Participant characteristics (N = 986)
| Variable | % / Mean (SD) / Median | Site 1 (n=650) | Site 2 (n=336) |
|---|---|---|---|
| Age | 21.68 (3.31) | 22.88 (3.26) | 19.38 |
| Sex | 62.2% Female | 61.3% female | 63.6% female |
| Income | $60,000 – $89,999 | $30,000 – $59,999 | $90,000 – $119,999 |
| Education | 14.55* (2.22) | 15.36 (2.11) | 12.98 (1.45) |
| AUDIT | 4.26* (3.13) | 4.91 (2.88) | 3.00 (3.21) |
| DUDIT | 1.37* (2.2) | 1.79 (2.43) | .55 (1.34) |
| DDT | −2.370* (.80) | −2.43 (.82) | −2.25 (.76) |
| MCQ-Sa | −2.301* (.66) | −2.355 (.69) | −2.20 (.58) |
| MCQ-M | −2.131* (.69) | −2.191 (.71) | −2.01 (.62) |
| MCQ-L | −1.86* (.68) | −1.92 (.72) | −1.76 (.58) |
| PCA-DRDb | 0* (1.00) | −.09 (1.04) | .17 (.88) |
Note: AUDIT = Alcohol Use Disorders Identification Test; DUDIT= Drug Use Disorders Identification Test; MCQ = Monetary Choice Questionnaire; Site 1 = Chicago, IL; Site 2 = Athens, GA;
p<.05;
log-10 transformed;
standardized via principal components analysis
Procedure
Participants attended a single experimental session conducted in a laboratory under controlled conditions (i.e., no distractions, quiet environment, supervised task completion). They provided urine and breath samples to confirm abstinence from recent drug and alcohol use, and provided informed consent. Participants completed a computerized battery of self-report and behavioral tasks, including two measures of DRD (MacKillop et al. 2016) (Inquisit 3.0.6.0, 2012; Survey Monkey (http://surveymonkey.com; or EPrime, Psychology Software Tools, Pittsburgh, PA). The task orders were counterbalanced to minimize order effects. Participants were given two five-minute breaks for refreshments (water, snacks) and/or to use the restroom. Samples of DNA were collected via a saliva sample in an Oragene DNA kit (DNA Genotek Inc., Kanata, ON, Canada).
Measures
Demographics and Substance Use
Demographic characteristics including, sex, age, race, income and education were obtained. Alcohol use over the last year was measured using the Alcohol Use Disorders Identification Test (AUDIT), which contains 10 questions, scored from 0 to 4, pertaining to quantity, frequency, and consequences of drinking. Drug use over the last year was measured using the Drug Use Disorders Identification Test (DUDIT), which uses the same format as the AUDIT but with one additional question regarding frequency of polysubstance use.
Delayed Reward Discounting.
Two tasks were used to measure DRD: a full iterative permuted delay discounting task and the Monetary Choice Questionnaire (MCQ; Kirby et al., 1999), which together provided four indices of DRD. Both tasks provided hyperbolic temporal discounting functions (k) (Mazur 1987). In the iterative task, a temporal discounting function is derived for each participant. Subjects are given 80 choices between smaller immediate rewards (i.e., $10.00, $20.00, $30.00, $40.00, $50.00, $60.00, $70.00, $80.00, $90.00, or $99.00) and a larger delayed reward of $100 with a delay of 1, 7, 14, 30, 60, 90, 180, or 365 days (Amlung, Sweet, Acker, Brown, & MacKillop, 2014). The amounts and delays were presented in mixed order. The MCQ (Kirby et al., 1999) consists of 27 choices between smaller immediate rewards and larger delayed rewards. The rewards range from $11 to $85 and the larger delayed rewards were available at varying intervals of delay from 1 week to 186 days (e.g., “Would you rather have $49.00 today or $60.00 in 89 days?”). The questions are presented in random order. The MCQ generates three k values for small (mean = $25), medium (mean = $55), and large (mean = $85) magnitude rewards. Ten control items provided choices between smaller versus larger rewards, both immediately available. A criterion of ≥80% correct was used to define adequate effort and attention. To maximize validity, performance on the two measures was consequated such that all participants received one in six chance to receive an outcome from their choices (value = $10-$100). Specifically, using Kirby et al.’s (1999) procedure, participants rolled a six-sided die following the tasks and those who rolled a six received the outcome for one of their choices (either immediately or at the specified delay). The actual choice received was randomly determined via the selection of one poker chip from a fishbowl containing chips pertaining to all of the items on the tasks.
SNP Genotyping and Quality Control
Genotyping was performed using the Illumina PsychArray BeadChip platform, which calls ~600,000 markers and has optimized tag SNP content from the International HapMap Project to capture the maximum amount of common variation. Quality control filtering was implemented in PLINK v1.9 (Chang et al. 2015). Autosomal SNPs were filtered for call rates < 98%, Hardy-Weinberg Equilibrium (HWE) violations of p < 1 × 10−6, MAF < 5%, and invariance. After filtering, 437,652 SNPs remained for imputation, which was performed using IMPUTE2 v.2.3.1 (Howie et al., 2009) employing the 1000 Genomes Phase 3 b37 reference panel (Delaneau et al. 2014). Imputed SNPs were excluded if they provided an information score of < .3 (Marchini and Howie 2010), MAF < 5%, HWE violations of p < 1 × 10−6, missingness > 5%, and multiallelic status. Imputed SNPs with confidence < .9 were set to missing.
Data Analysis
The DRD task was analyzed using nonlinear regression and fitting the commonly used hyperbolic temporal discounting function (Mazur 1987). To generate a single DRD index across delayed reward magnitudes and the task and MCQ, the four indices were consolidated using principal components analysis (PCA; oblique rotation [direct oblimin, δ=0]), as has been used successfully previously (Amlung and MacKillop 2014; VanderBroek et al. 2015). Thus, the primary DRD performance index in all subsequent analyses was the first principal component of the four k values. Given the established relevance of age and income to DRD (Green et al. 1994; de Wit et al. 2007), both were included as a priori covariates. Two additional candidate covariates were explored, site and sex, and were only included if significantly associated with DRD. Phenotypic analyses were conducted using SPSS, v22.0 (IBM Corp., 2011).
Genetic analyses used Genome-wide Efficient Mixed Model Association (GEMMA) software (Zhou and Stephens 2012) to conduct univariate linear mixed model associations between the loci from each subset (16 a priori loci, 12,990 HVA loci, and 4,883,968 genome-wide SNPs) and DRD performance. This approach accounts for cryptic relatedness among individuals, which is modeled out as a random effect (i.e., the genetic correlation between individuals). To maximize resolution of effects, an additive genetic effect model was used whereby participants were coded based on the number of minor alleles for each SNP (0–2). A priori loci were defined as SNPs that were previously reported in peer-reviewed publications to be significantly associated with DRD in populations of European ancestry. 2 a priori loci were excluded for excessive missing values (> 5%). Following quality control, 16 a priori loci, 12,990 of the 20,652 HVA SNPs, and 4,883,968 genome-wide SNPs were present for analysis. Type I error rate was apportioned according to the prioritized subset. For a priori tests, a nominal α≥.05 was used; for the HVA markers, a Benjamini-Hochberg (1995) false discovery rate (FDR) correction was applied; for the atheoretical genome-wide scan, the standard genome-wide significance threshold was used (p< 5 × 10−8)(Pe’er et al. 2008; Panagiotou and Ioannidis 2012). Statistical power was generated using Quanto software (Gauderman 2002) and, at power = .80, the a priori tests had a minimum detectable effect (MDE) of .8% of variance, a small-to-medium effect size, depending on conventions. For the HVA markers, formal power analysis was not conducted because FDR correction is specific to empirical p values, but the genome-wide power analysis provides a conservative estimate for the HVA marker. For the genome-wide analyses, at power = .80, the MDE was 4.0% of variance, a large effect size.
RESULTS
Preliminary Analyses
1,000 participants had valid genotyping data (call rates ≥ 98%, inbreeding coefficient absolute value ≤ .02, concordant self-reported sex and X-chromosome determined sex) and satisfied the inclusion/exclusion criteria. To verify and correct the misclassification of self-reported race, principal components analysis (PCA; Price et al., 2006) was conducted. Two population outliers were identified and removed by visual inspection of the principal components plot.
Eight participants were excluded for missing data and two participants were excluded for invalid task performance (i.e., <80% on control items). Among valid participants, consistency on the control items was very high: M = 98%; 91% = all correct responses, 8% = one error, 1% = two errors. Finally, participants were assessed for cryptic relatedness (Yang et al. 2011) and two were removed for relatedness > .05, leaving the final sample of 986 participants (Table 1).
Nonlinear modelling provided a good fit to the DRD task (median R2 = .86). The resulting k values were positively skewed, as is typical, and were logarithmically transformed (base-10,) which substantially improved the distributions. Very high correlations among the individual magnitude k values (rs=.75-.86, ps<10−130) supported the use of PCA and the resulting eigenvalue from the first component was 3.40 (all subsequent <.30), accounting for 85% of the variance among the four discounting indices. The component loadings were uniformly high: .90-.94. With regard to covariates, age was significantly associated with DRD (r=.06, p=.05) but income was not (r = .03 p = 36), although both were included in subsequent models given their a priori status. Site differences were present (F (1,983) = 15.02, p<.001), but sex differences were not (F (1,983) = 1.17, p = .28), so only site so site was included as an additional covariate.
A Priori Loci
Of the a priori loci assessed, one locus was nominally significant (Table 2). Specifically, the minor (T) allele of rs521674 and G allele of rs1800544 in ADRA2A were significantly associated with less impulsive DRD (Bs = −.10, SEs = .05, ps = .046); identical values are because these loci were in total linkage disequilibrium (R2 = 1.0). The inverse coefficients reflect fewer minor alleles being associated with more impulsive DRD.
Table 2.
Associations between a priori loci and delayed reward discounting preferences.
| Minor | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chr | Locus | Gene | Missing | Allele | MAF | β | SE | P |
| 3 | rs3773678 | DRD3 | 41 | A | .112 | 0.002 | 0.071 | .974 |
| 3 | rs7638876 | DRD3 | 29 | C | .315 | 0.088 | 0.048 | .071 |
| 5 | rs464049 | SLC6A3 | 0 | G | .446 | −0.040 | 0.045 | .372 |
| 5 | rs12652860* | SLC6A3 | 14 | A | .279 | 0.033 | 0.050 | .505 |
| 6 | rs1360780 | FKBP5 | 14 | T | .306 | −0.070 | 0.048 | .142 |
| 7 | rs10249982a | DDC | 4 | G | .241 | 0.058 | 0.050 | .250 |
| 7 | rs10244632a | DDC | 9 | T | .257 | 0.051 | 0.049 | .307 |
| 7 | rs1466163a | DDC | 4 | A | .116 | 0.023 | 0.070 | .747 |
| 7 | rs10499696a | DDC | 10 | G | .120 | 0.031 | 0.069 | .648 |
| 10 | rs521674b | ADRA2A | 23 | T | .263 | −0.103 | 0.052 | .046 |
| 10 | rs1800544b | ADRA2A | 20 | G | .263 | −0.102 | 0.051 | .046 |
| 10 | rs602618b | ADRA2A | 39 | C | .265 | −0.076 | 0.051 | .142 |
| 10 | rs363338 | SLC18A2 | 37 | C | .299 | 0.024 | 0.050 | .631 |
| 11 | rs1800497 | ANKK1 | 0 | A | .185 | −0.028 | 0.057 | .630 |
| 11 | rs1079597 | DRD2 | 0 | T | .151 | −0.037 | 0.062 | .550 |
| 22 | rs4680 | COMT | 0 | G | .480 | −0.071 | 0.044 | .104 |
Notes. Bolding indicates nominally significant effects were identified (p < .05).
located near but not in the associated gene. Beta coefficients reflect number of minor alleles; letter superscripts reflect high linkage disequilibrium (R2>.80) among loci with common letters.
High-value Addiction Markers
Of the 12,990 HVA loci, none survived FDR correction. The top three strongest associations were for rs4986850 in breast cancer 1 (BRCA1) on chromosome 17 (p =0.000096;), rs1563119 in non-coding RNA on chromosome 2 (p = .0002), and rs10799790 in leucine zipper protein 1 (LUZP1) on chromosome 1 (p = .0004). Test statistics and other information for the top 50 most significant associations are in Supplementary Table 1.
Genome-wide Association Analysis
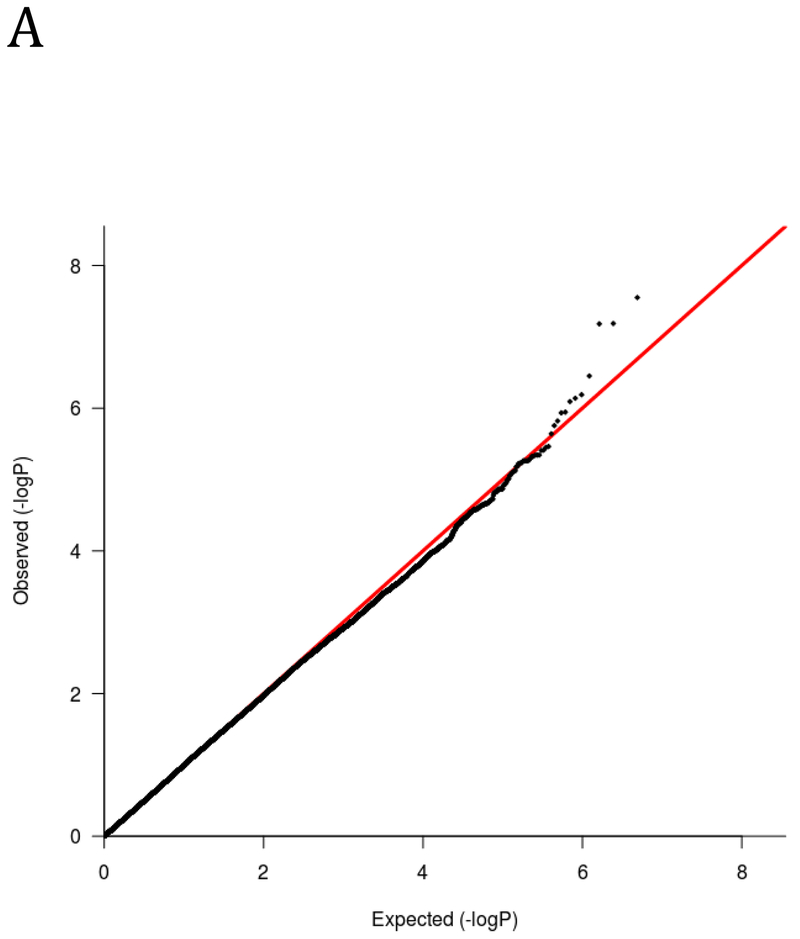
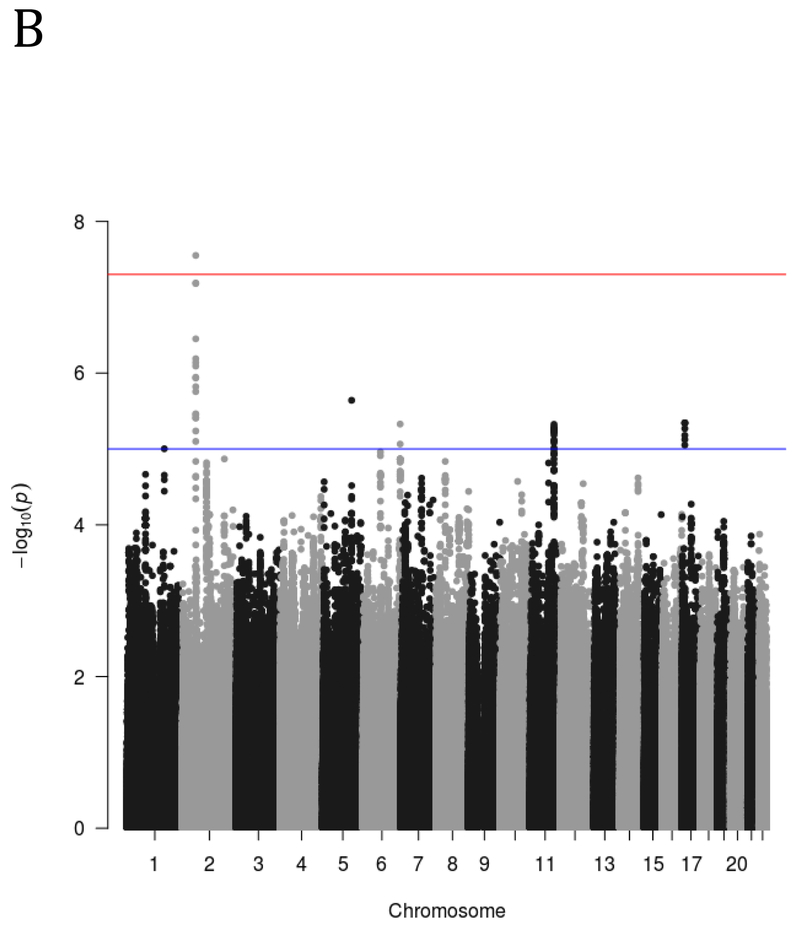
The genome-wide analysis yielded one significant association on chromosome 2. Specifically, the minor (T) allele of rs13395777 was significantly positively associated with more impulsive DRD (β = .27, SE = .05, p = 2.8 × 10−8; MAF = .33). Figure 1 shows the results of the GWAS using both quantile-quantile (Q-Q) plots and Manhattan plots. The significant locus is intergenic and closest to RNA5SP94 and XR_940133.1. To inform future studies, the top 50 most significant genome-wide associations are reported in Supplementary Table 2.
Figure 1.
A) Q-Q and B) Manhattan plot of genome-wide associations for DRD performance. Significance values were –log10 transformed in order to display the smaller p-values as larger in the figures. The Q-Q plot depicts the observed and expected p-values. The Manhattan plot displays level of significance for each SNP, organized by chromosomal position from chromosomes 1–22. The blue line indicates suggestive significance (p<10−5) and the redline indicated genome-wide significance (p<5×10−8).
DISCUSSION
This study examined genetic influences on impulsive DRD, a psychological phenotype robustly associated with addiction, using a genome-wide prioritized subset approach. First, we examined a priori selected loci based on previously reported significant associations. Most of the previous associations were not replicated, although rs521674 and rs1800544, in ADRA2A, were nominally significant (the association would not have survived Type I error rate correction). ADRA2A encodes the α2 adrenoreceptor and this locus has previously been implicated in impulsive DRD among individuals with cocaine use disorders (Havranek et al., 2013). This association is consistent with preclinical pharmacological studies that found the noradrenaline-specific reuptake inhibitor atomoxetine reduces DRD (Robinson et al. 2008; Sun et al. 2012). However, it is notable that the pattern of association in healthy individuals was opposite the pattern in individuals with cocaine addiction, where possession of more minor alleles was associated with steeper discounting. With the potential exceptions of these loci, the current findings do not confirm previously reported associations. We cannot reach any firm conclusions about whether the previous findings were false positives or whether that those associations were moderated by methodological differences (e.g., differences between clinical groups and a healthy normative sample).
The second prioritized subset comprised a moderately sized set of markers specifically related to addiction. Although we predicted that this enriched marker set would reveal associations with DRD, no loci survived the type I error correction and none showed promising trends given the number of tests conducted. Interestingly, the genome-wide scan detected one significant association that exceeded the conventional threshold of 5×10−8 (Pe’er et al. 2008; Panagiotou and Ioannidis 2012). However, this locus is intergenic and nearby genes have no known relationships to the brain or behavior, making its functional role unclear. It is possible that this locus influences the regulation of distant genes (Krijger and de Laat 2016), but, absent any evidence that this is the case, caution should be applied to this finding. Furthermore, the study did not include a replication sample to provide independent corroboration of the association. Further evidence in support of this association is needed.
These findings should be considered in the context of the study’s strengths and weaknesses. In terms of strengths, the study had much wider genomic scope than most prior studies and the prioritized subset design provided a principled framework for doing so. In addition, the study used a high-resolution latent DRD phenotype comprising four temporal discounting functions and incentivized procedure that maximized participant engagement and salience of the reward. Evidence in support of the characterization of the phenotype was present in terms of the very high performance on control items. However, the study had a number of limitations also. Although it was arguably the largest human laboratory study on DRD decision making to date, the sample size was modest in terms of genome-wide studies. Beyond the a priori tests, the study was only powered to detect relatively large effects. As such, an important corollary of these findings is that the study cannot speak to smaller effect size associations. Necessarily, if, as this study would suggest, the genetic architecture of DRD is in fact a function of numerous alleles with small effects, substantially larger sample sizes will be necessary for sufficient power to detect them reliably. This is proving to be the case for clinical addiction phenotypes (e.g., Thorgeirsson et al. 2008) and other complex phenotypes (e.g., Okbay et al. 2016). Larger sample sizes would also permit the application of additional genome-wide methods (e.g., Yang et al. 2010). In addition, independent of sample size, a limitation to the current findings is that that they may not be generalizable beyond individuals of European ancestry. This has also been the case for virtually all of the previous studies in this area, albeit a small number, but it is no less a limitation because of it.
A feature of the current study that was both a strength and a potential limitation its inclusion criteria in terms of substance use. All participants reported relatively low levels of substance use during the prior year by self-report and were verified to have not recently used drugs or alcohol (with varying detection windows by test). This permitted the study to rule out recent drug involvement as a substantial determinant of DRD, either as a symptom or a neurocognitive consequences of heavy use. On the other hand, however, low substance use may have truncated the observed variability in the larger population. Moreover, epidemiologically, substance use and misuse peak in the age group recruited for the study (Slutske 2005; Courtney and Polich 2009), so low substance use may make this sample even less representative of the general population. Whether the exclusive focus on individuals with low levels of substance use increased or decreased resolution in this study cannot be addressed directly, but it is an important consideration for contextualizing the current findings.
At a broader level, it is worth considering the optimal experimental designs for investigating DRD (and other processes) as an endophenotype for addiction. Cases can be made for studies focusing on samples that either exclusively do not or do have heavy levels of substance use, but case-control or dimensional studies that are sufficiently powered and fully span the spectrum of severity (i.e., absence of problems to severe addiction) may maximize the capacity to detect differentially predictive loci. Furthermore, all the molecular genetic studies on DRD to date, including the current one, have been cross-sectional. This substantially limits the capacity for inferring causality (MacKillop and Munafò 2016). Longitudinal designs that permit disentangling the interrelationships between genetic variation, DRD decision making, and addiction will be necessary to test causal pathways more definitively.
In sum, the current study investigated genetic associations with DRD preferences in three different domains. We found little support for previously reported associations, with the possible exception of ADRA2A, or an addiction-enriched marker set, but found one significant genome-wide association, warranting further study. The findings advance the understanding of genetic influences on DRD, both via the associations positively detected and the implication of DRD’s genetic complexity and, in turn, the need for larger samples to identify its genetic underpinnings more conclusively.
Supplementary Material
PUBLIC HEALTH SIGNIFICANCE.
Steep discounting of future rewards has been substantial associated with addiction and other psychiatric disorders. Increasing evidence implicates genetic influences, but most previous studies have had relatively small sample sizes, limited genomic scope, and potentially confounding levels of previous substance use. This study addresses these issues and finds further evidence for one previously reported genetic association and a novel genome-wide association, further elucidating genetic contributions to this form of impulsivity.
REFERENCES
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR (2011) Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res 35:1607–13. doi: 10.1111/j.1530-0277.2011.01507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT (2008) Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction 103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x [DOI] [PubMed] [Google Scholar]
- Amlung M, MacKillop J (2014) Clarifying the relationship between impulsive delay discounting and nicotine dependence. Psychol Addict Behav 28:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Sweet LH, Acker J, et al. (2013) Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addict Biol. doi: 10.1111/adb.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, et al. (2016) Steep Delay Discounting and Addictive Behavior: A Meta-Analysis of Continuous Associations. Addiction. doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL (2005) Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav 80:387–393. doi: 10.1016/j.pbb.2004.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC (2011) Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet 41:175–183. doi: 10.1007/s10519-010-9384-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Grant JD, Mulligan RC, Heath AC (2014) The Genetics of Impulsivity: Evidence for the Heritability of Delay Discounting. Biol Psychiatry 77:887–94. doi: 10.1016/j.biopsych.2014.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, et al. (2009) Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend 103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurley JW, Edlund CK, Pardamean CI, et al. (2016) Smokescreen: a targeted genotyping array for addiction research. BMC Genomics 17:145. doi: 10.1186/s12864-016-2495-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith SW, Czachowski CL (2014) Increased Delay Discounting Tracks with a High Ethanol-Seeking Phenotype and Subsequent Ethanol Seeking But Not Consumption. Alcohol Clin Exp Res 38:2607–2614. doi: 10.1111/acer.12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Statistical Methodol 57:289–300. [Google Scholar]
- Berman AH, Bergman H, Palmstierna T, Schlyter F (2005) Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res 11:22–31. doi: 10.1159/000081413 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, et al. (2014) The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol 10:641–77. doi: 10.1146/annurev-clinpsy-032813-153724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA (2001) Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96:73–86. doi: 10.1080/09652140020016978 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ (1999) Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacol 146:447–454. doi: 91460447.213 [pii] [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, et al. (2007) Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci 27:14383–14391. doi: 27/52/14383 [pii] 10.1523/JNEUROSCI.2551-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell AJ, Bond R, Duka T, Morgan MJ (2015) Further evidence of the heterogeneous nature of impulsivity. Pers Individ Dif 76:68–74. doi: 10.1016/j.paid.2014.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, et al. (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. [DOI] [PMC free article] [PubMed]
- Chen J, Lipska BK, Halim N, et al. (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75:807–21. doi: 10.1086/425589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003) Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol 11:18–25. [DOI] [PubMed] [Google Scholar]
- Corp I (2011) IBM SPSS Statistics for Windows, Version 20.0.
- Courtney KE, Arellano R, Barkley-Levenson E, et al. (2012) The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach. Alcohol Clin Exp Res 36:923–31. doi: 10.1111/j.1530-0277.2011.01635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J (2009) Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull 135:142–56. doi: 10.1037/a0014414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean J, Richards JB, De Wit H (2002) Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav Brain Res 136:349–357. doi: 10.1016/S0166-4328(02)00132-8 [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A (2011) Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev 31:965–982. doi: 10.1016/j.cpr.2011.06.001 [DOI] [PubMed] [Google Scholar]
- de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31. doi: ADB129 [pii] 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Flory JD, Acheson A, et al. (2007) IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Pers Individ Dif 42:111–121. doi: 10.1016/j.paid.2006.06.026 [DOI] [Google Scholar]
- Delaneau O, Marchini J, 1000 Genomes Project Consortium, 1000 Genomes Project Consortium (2014) Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat Commun 5:3934. doi: 10.1038/ncomms4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, et al. (2010) Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol 15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Mathias CW, et al. (2014) Delay discounting differentiates pre-adolescents at high and low risk for substance use disorders based on family history. Drug Alcohol Depend 143:105–111. doi: 10.1016/j.drugalcdep.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, et al. (2007) Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct 3:2. doi: 1744-9081-3-2 [pii] 10.1186/1744-9081-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, et al. (2013) Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction 108:1916–1923. doi: 10.1111/add.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ (2002) Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol 155:478–484. [DOI] [PubMed] [Google Scholar]
- Gianotti LRR, Figner B, Ebstein RP, Knoch D (2012) Why Some People Discount More than Others: Baseline Activation in the Dorsal PFC Mediates the Link between COMT Genotype and Impatient Choice. Front Neurosci 6:54. doi: 10.3389/fnins.2012.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6:521–532. doi: 10.1038/nrg1635 [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J (1994) DISCOUNTING OF DELAYED REWARDS:. A Life-Span Comparison. Psychol Sci 5:33–36. doi: 10.1111/j.1467-9280.1994.tb00610.x [DOI] [Google Scholar]
- Havranek MM, Hulka LM, Tasiudi E, et al. (2015) α2A -Adrenergic receptor polymorphisms and mRNA expression levels are associated with delay discounting in cocaine users. Addict Biol. doi: 10.1111/adb.12324 [DOI] [PubMed] [Google Scholar]
- Isen JD, Sparks JC, Iacono WG (2014) Predictive validity of delay discounting behavior in adolescence: a longitudinal twin study. Exp Clin Psychopharmacol 22:434–43. doi: 10.1037/a0037340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS (2004) Common genetic effects on variation in impulsivity and activity in mice. J Neurosci 24:6733–6740. doi: 10.1523/JNEUROSCI.1650-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, et al. (2014) Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci 1327:1–26. doi: 10.1111/nyas.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, et al. (2013) Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction 108:506–15. doi: 10.1111/add.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, McCullough ME, Bickel WK, et al. (2014) Longitudinal Associations Among Religiousness, Delay Discounting, and Substance Use Initiation in Early Adolescence. J Res Adolesc n/a-n/a. doi: 10.1111/jora.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128:78–87. doi: 10.1037/0096-3445.128.1.78 [DOI] [PubMed] [Google Scholar]
- Krijger PHL, de Laat W (2016) Regulation of disease-associated gene expression in the 3D genome. Nat Rev Mol Cell Biol 17:771–782. doi: 10.1038/nrm.2016.138 [DOI] [PubMed] [Google Scholar]
- Li C, Li M, Lange EM, Watanabe RM (2008) Prioritized subset analysis: improving power in genome-wide association studies. Hum Hered 65:129–41. doi: 10.1159/000109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W-Y, Lee W-C (2010) Incorporating prior knowledge to facilitate discoveries in a genome-wide association study on age-related macular degeneration. BMC Res Notes 3:26. doi: 10.1186/1756-0500-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Smoker MP, Janetsian-Fritz SS, Lapish CC (2016) Impulsivity in rodents with a genetic predisposition for excessive alcohol consumption is associated with a lack of a prospective strategy. Cogn Affect Behav Neurosci. doi: 10.3758/s13415-016-0475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J (2013) Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav 99:14–31. doi: 10.1002/jeab.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung M, Few L, et al. (2011) Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 216:305–321. doi: 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Anderson EJ, Castelda BA, et al. (2006) Divergent validity of measures of cognitive distortions, impulsivity, and time perspective in pathological gambling. J Gambl Stud 22:339–354. doi: 10.1007/s10899-006-9021-9 [DOI] [PubMed] [Google Scholar]
- MacKillop J, Gray JC, Bidwell LC, et al. (2015) Genetic influences on delay discounting in smokers: examination of a priori candidates and exploration of dopamine-related haplotypes. Psychopharmacology (Berl) 232:3731–9. doi: 10.1007/s00213-015-4029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Miller JD, Fortune E, et al. (2014) Multidimensional examination of impulsivity in relation to disordered gambling. Exp Clin Psychopharmacol 22:176–85. doi: 10.1037/a0035874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti PM, et al. (2010) Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol 119:106–14. doi: 10.1037/a0017513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Munafò MR (2016) Commentary: Delay discounting and smoking: robust correlation, but uncertain causation. Int J Epidemiol dyw303. doi: 10.1093/ije/dyw303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Weafer J, Gray JC, et al. (2016) The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacol 233:3361–3370. doi: 10.1007/s00213-016-4372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK (1997) Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256–262. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, et al. (2008) Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J Exp Anal Behav 90:333–344. doi: 10.1901/jeab.2008.90-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini J, Howie B (2010) Genotype imputation for genome-wide association studies. Nat Rev Genet 11:499–511. doi: 10.1038/nrg2796 [DOI] [PubMed] [Google Scholar]
- Mazur JE (1987) An adjusting procedure for studying delayed reinforcement In: Commons ML, Mazur JE, Nevin JA (eds) The Effect of Delay and of Intervening Event on Reinforcement Value. Quantitative Analyses of Behavior. Lawrence Erlbaum Associates Inc., Hillsdale, NJ, pp 55–73 [Google Scholar]
- Mendez IA, Simon NW, Hart N, et al. (2010) Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci 124:470–7. doi: 10.1037/a0020458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Ouimet DJ, et al. (2014) Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci 128:419–29. doi: 10.1037/a0036742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH (2011) The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav Processes 87:10–7. doi: 10.1016/j.beproc.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P, Garavan H (2011) Cognitive predictors of problem drinking and AUDIT scores among college students. Drug Alcohol Depend 115:94–100. doi: 10.1016/j.drugalcdep.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ (2009) High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res 33:1294–303. doi: 10.1111/j.1530-0277.2009.00955.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, et al. (2016) Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533:539–542. doi: 10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotou OA, Ioannidis JPA (2012) What should the genome-wide significance threshold be? Empirical replication of borderline genetic associations. Int J Epidemiol 41:273–86. doi: 10.1093/ije/dyr178 [DOI] [PubMed] [Google Scholar]
- Pe’er I, Yelensky R, Altshuler D, Daly MJ (2008) Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 32:381–385. doi: 10.1002/gepi.20303 [DOI] [PubMed] [Google Scholar]
- Perkel JK, Bentzley BS, Andrzejewski ME, Martinetti MP (2015) Delay discounting for sucrose in alcohol-preferring and nonpreferring rats using a sipper tube within-sessions task. Alcohol Clin Exp Res 39:232–8. doi: 10.1111/acer.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 154:243–250. doi: 10.1007/s002130000638 [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR (2002) Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol 63:83–90. [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904–9. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H (2006) Dimensions of impulsive behavior: Personality and behavioral measures. Pers Individ Dif 40:305–315. doi: 10.1016/j.paid.2005.03.024 [DOI] [Google Scholar]
- Richards JB, Lloyd DR, Kuehlewind B, et al. (2013) Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav 12:490–502. doi: 10.1111/gbb.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Mar AC, et al. (2008) Similar Effects of the Selective Noradrenaline Reuptake Inhibitor Atomoxetine on Three Distinct Forms of Impulsivity in the Rat. Neuropsychopharmacology 33:1028–1037. doi: 10.1038/sj.npp.1301487 [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, et al. (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Schork AJ, Thompson WK, Pham P, et al. (2013) All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet 9:e1003449. doi: 10.1371/journal.pgen.1003449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Mendez IA, Mitchell MR, Simon NW (2009) Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav Pharmacol 20:380–389. doi: 10.1097/FBP.0b013e3283305eb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci 121:543–9. doi: 10.1037/0735-7044.121.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS (2005) Alcohol use disorders among US college students and their non-college-attending peers. Arch Gen Psychiatry 62:321–7. doi: 10.1001/archpsyc.62.3.321 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Kumsta R, Schlotz W, et al. (2011) A Functional Variant of the Serotonin Transporter Gene (SLC6A4) Moderates Impulsive Choice in Attention-Deficit/Hyperactivity Disorder Boys and Siblings. Biol Psychiatry 70:230–236. doi: 10.1016/j.biopsych.2011.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JC, Isen JD, Iacono WG (2014) Preference on cash-choice task predicts externalizing outcomes in 17-year-olds. Behav Genet 44:102–12. doi: 10.1007/s10519-013-9638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Pinkston JW, Brewer AT, et al. (2012) Delay discounting in Lewis and Fischer 344 rats: steady-state and rapid-determination adjusting-amount procedures. J Exp Anal Behav 97:305–21. doi: 10.1901/jeab.2012.97-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA (2012) Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology (Berl) 219:285–301. doi: 10.1007/s00213-011-2419-9 [DOI] [PubMed] [Google Scholar]
- Sun L, Craiu RV, Paterson AD, Bull SB (2006) Stratified false discovery control for large-scale hypothesis testing with application to genome-wide association studies. Genet Epidemiol 30:519–30. doi: 10.1002/gepi.20164 [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, et al. (2008) Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob Res 10:1571–1575. doi: 904706261 [pii] 10.1080/14622200802323274 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, et al. (2008) A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452:638–642. doi: nature06846 [pii] 10.1038/nature06846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004) Catechol-O-Methyltransferase Inhibition Improves Set-Shifting Performance and Elevates Stimulated Dopamine Release in the Rat Prefrontal Cortex. J Neurosci 24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderBroek L, Acker J, Palmer AA, et al. (2015) Interrelationships among parental family history of substance misuse, delay discounting, and personal substance use. Psychopharmacology (Berl). doi: 10.1007/s00213-015-4074-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD (2014) Addiction science: Uncovering neurobiological complexity. Neuropharmacology 76 Pt B:235–49. doi: 10.1016/j.neuropharm.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH (2009) Strain differences in delay discounting using inbred rats. Genes Brain Behav 8:426–34. doi: 10.1111/j.1601-183X.2009.00484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH (2008) Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav 7:705–13. doi: 10.1111/j.1601-183X.2008.00406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Benyamin B, McEvoy BP, et al. (2010) Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42:565–9. doi: 10.1038/ng.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM (2011) GCTA: A Tool for Genome-wide Complex Trait Analysis. [DOI] [PMC free article] [PubMed]
- Zhou X, Stephens M (2012) Genome-wide efficient mixed-model analysis for association studies. Nat Genet 44:821–4. doi: 10.1038/ng.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.