Abstract
Introduction
Fine particulate matter (PM2.5) is closely associated with many neurological disorders including neurodegenerative disease, stroke, and brain tumors. However, the toxic effects of PM2.5 on neurodevelopment remain unclear. In this study, we aimed to determine the neurotoxic effects of early postnatal exposure to PM2.5 in immature and mature rats.
Methods
We exposed neonatal rats to PM2.5 (2 or 10 mg/kg body weight) through intranasal instillation from postnatal day (PND) 3–15, once a day. Emotional and cognitive development were evaluated using the elevated plus maze, forced swimming, and Morris water maze tests. Hippocampal tissue was collected and subjected to transmission electron microscopy observation and western blot analysis.
Results
Rats had lower body weight after exposure to high dose of PM2.5. The behavioral test results indicated that high‐dose PM2.5 exposure led to increased anxiety‐like symptoms in immature and mature rats, apparent depressive‐like behaviors in mature rats, and impaired spatial learning and memory abilities in immature rats, and low‐dose PM2.5 exposure increased anxiety‐like behaviors in immature rats. Further, high‐dose PM2.5 exposure contributed to fewer synapses, thinner postsynaptic density, and shorter active zone in immature and mature rats, and also decreased expressions of synaptophysin (SYP), growth associated protein‐43 (GAP43), and postsynaptic density‐95 (PSD95) in immature rats, SYP and PSD95 in mature rats. Moreover, low‐dose PM2.5 exposure diminished the expression of PSD95 in immature rats. In addition, high‐dose PM2.5 exposure reduced brain‐derived neurotrophic factor (BDNF) expression and cAMP response element binding protein (CREB) phosphorylation in both immature and mature rats, and low‐dose PM2.5 exposure lessened BDNF expression and CREB phosphorylation in immature rats.
Conclusions
Our findings indicate that PM2.5 impairs emotional and cognitive development by disrupting structural synaptic plasticity, possibly via the CREB/BDNF signaling pathway.
Keywords: BDNF, cognition, early postnatal, emotion, fine particulate matter, synaptic plasticity
Early postnatal fine particulate matter (PM2.5) exposure causes behaviour impairment. PM2.5 exposure damages structural synaptic plasticity in immature and mature rats. cAMP response element binding protein/brain‐derived neurotrophic factor signaling pathway is involved in PM2.5‐induced neurotoxicity.
![]()
1. INTRODUCTION
Fine particulate matter (PM2.5) pollution, a common type of ambient air pollution, has increased globally in recent years, especially in developing countries, and poses a substantial public health challenge (Cohen et al., 2017). PM2.5 can cause functional and pathological damage to the human body by penetrating the respiratory tract and blood and even entering the brain through the blood–brain barrier (Bondy, 2011). PM2.5 exposure increases the risk of neurological diseases, including neurodegenerative disorders, stroke, and benign brain tumors (Andersen et al., 2018; Calderón‐Garcidueñas & de la Monte, 2017; Fu, Guo, Cheung, & Yung, 2019). There is growing concern about the detrimental effects of PM2.5 on neurodevelopment, because the immature brain is more susceptible to PM2.5‐induced neurotoxicity than the mature brain is (Calderón‐Garcidueñas, González‐Maciel, et al., 2018; Ning et al., 2018). Further, a marked association between PM2.5 exposure and reduction in working memory has been found in children aged 7–10 years (Alvarez‐Pedrerol et al., 2017), and early postnatal exposure to PM2.5 induced autism spectrum disorder in children and animals (Li et al., 2018; Talbott et al., 2015), possibly due to neuroinflammation, neurotransmitter disruption, and metabolite alteration (Allen et al., 2014; Li et al., 2018; Ning et al., 2018). However, the exact mechanisms underlying PM2.5‐induced neurodevelopmental disorders have not been elucidated.
Synaptic plasticity in the hippocampus is essential to emotional and memory processes and is susceptible to environmental toxicants (Zhao et al., 2018; Vasilescu et al., 2017). Synaptic plasticity includes changes in the efficacy of synaptic transmission at preexisting synapses and structural plasticity—a term refers to structural changes through formation, modification, and elimination of existing synapses (Morris, Clark, Zinn, & Vissel, 2013). Postsynaptic density‐95 (PSD95), growth associated protein‐43 (GAP43), and synaptophysin (SYP) are often used as synaptic associated markers that represent structural plasticity (Ma et al., 2014). Structural plasticity is affected by many neuromodulatory factors, and brain‐derived neurotrophic factor (BDNF) is the most important neuronal protective factor and can enhance synaptic efficiency and structural plasticity effectively as a prime mediator of synaptic plasticity (Leal, Bramham, & Duarte, 2017; Lin, Kavalali, & Monteggia, 2018). The expression of BDNF is regulated by the second messenger cAMP response element binding protein (CREB). To be specific, phosphorylated CREB (p‐CREB), the active form of CREB, could increase BDNF expression to exert biological effects (Zhong et al., 2018).
In this study, we aimed to improve the current understanding of PM2.5‐induced neurodevelopmental defects. We established a rat model of early postnatal PM2.5 exposure, in which we evaluated emotional and cognitive behaviors, analyzed structural synaptic plasticity, and measured the hippocampal expression of BDNF, p‐CREB, and CREB proteins. The neurotoxic effects of early postnatal PM2.5 exposure were compared in immature and mature rats.
2. MATERIALS AND METHODS
2.1. PM2.5 sampling and processing
An ambient PM2.5 sample was collected onto quartz fibers (10 × 10 cm) with the use of a Thermo Anderson G‐2.5 air sampler (Model GV 2630 Series) from December 2017 to April 2018 in a busy street near Children's Hospital of Chongqing Medical University, based on a previous protocol (Cao et al., 2015). After sonicated and dried using a Thermo Scientific Power Dry LL3000 vacuum‐freeze dryer, the particles were extracted from these fibers and were stored at −20°C. Before use, these particles were weighed and diluted with 0.9% sterilized saline to the concentration of 2 and 10 mg/ml.
The daily respiratory volume of rats reaches 0.23 m3 (Li et al., 2018). According to the average annual concentrations of PM2.5 (180 µg/m3) in Beijing (Gao et al., 2018) and the measured concentration of PM2.5 (900 µg/m3) in some monitoring points in urban area during serious haze period (Long, Hong, & Sun, 2015), the equivalent dose of PM2.5 exposure would be 41.4 and 207 µg/day. Considering the body weight of neonatal rats, the PM2.5 exposure dose would be approximately 2 and 10 µg/g.b.w. We used 2 mg/kg.b.w. as a low‐dose group to exposure the actual effect of PM2.5 pollution on neurodevelopment, and chose 10 mg/kg.b.w. as a high‐dose group to study the possible neurotoxic effects of PM2.5 exposure.
2.2. Animals and treatments
Sprague Dawley (SD) rats (230–250 g) were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). All rats used in this study were kept under specific pathogen‐free conditions (12‐hr light/12‐hr dark cycle with humidity of 55 ± 5% at 25 ± 1°C) and had free access to enough food and water. Before breeding, they were acclimated to the housing room for 7 days. When vaginal plugs were found, each pregnant rat was allowed to stay in one cage for natural delivery.
To eliminate litter‐specific effects, we adjusted the litters to eight pups (males, n = 4; females, n = 4) per dam on postnatal day (PND) 2. Then we divided randomly pups in one litter into four groups: normal blank control (control), saline exposed (saline), low dose, and high dose. There were 20 male pups and 20 female pups in each group. From PND3 to PND15, a vital neurodevelopmental period, all pups in each group were taken out to the operating room, and pups in saline and experimental groups were administered saline or two doses of PM2.5 suspension (0.1 ml/100 g.b.w.), respectively, by intranasal instillation, once per day. On PND21, the pups were weaned and housed by sex (n = 4/cage), and 20 rats (10 males, 10 females) in each group were chosen randomly for further examination on PND28 or PND60 separately. Moreover, animal body weights were recorded from PND3 to PND15, PND28, and PND60 respectively.
Behavioral experiments were performed using immature (28‐day‐old) and mature (60‐day‐old) rats to evaluate anxiety‐like symptoms (elevated plus maze [EPM]), depressive‐like behaviors (forced swimming [FS]), and learning and memory abilities (Morris water maze [MWM]; Figure 1). After adaption to behavioral test room for 3 days, 40 immature rats (five males, five females per group) and 40 mature rats (five males, five females per group) were, respectively, allowed to tested by behavioral assessments between 8:00 a.m. and 4:00 p.m. To exclude potential effects of successive behavioral tests on the synaptic ultrastructure and neuroproteins, the rats (n = 10 per group), not undergoing behavioral training, were sacrificed under deep anesthesia with 50 mg/kg sodium pentobarbital on PND28 or PND60, and hippocampus samples from three male immature rats and three male mature rats were prepared for transmission electron microscope observation and hippocampus tissues from six immature (two males, four females) and six mature rats (two males, four females) were collected for western blot analysis separately.
Figure 1.
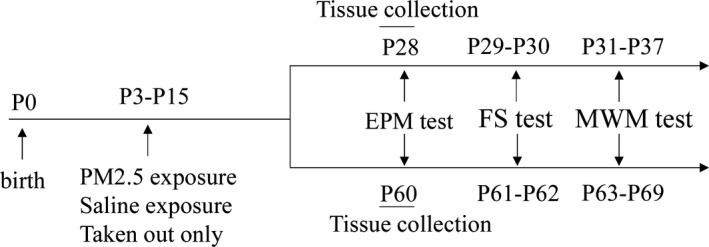
Illustration of the experimental timeline. EPM, elevated plus maze; FS, forced swimming; MWM, Morris water maze
2.3. Elevated plus maze
The EPM was consisted of two closed arms (50 × 10 × 40 cm), two open arms (50 × 10 cm), and a central platform (10 × 10 cm). Rats were placed in the center platform facing the open arm and were allowed to stay in the maze for 10 min. Between tests, the maze was thoroughly cleaned with 70% ethanol to prevent odor interference. This test is based on the natural conflict between the tendency of rats to avoid an illuminated and elevated surface (open arm), and the tendency to explore new environments. The number of entries and the length of time spent in the open and closed arms were recorded using the ANY‐Maze video tracking system (Stoelting) for 10 min. Anxiety was assessed as both the percentages of time spent in open arms and the percentages of number of entries into them, shown in the previous study (Yue et al., 2017).
2.4. Forced swimming
Rats were individually placed into a transparent cylinder (diameter 20 cm, height 60 cm) containing warm water (25 ± 1°C), and we set 40 cm as the water depth to prevent rats from supporting their body with their tails or hind limbs. Based on the previous study (Han et al., 2018), on the first day, the rats were placed into the cylinder for 5 min of adaptation, and the water in the cylinder was changed between tests. After 24 hr, the procedure was repeated for 10 min, and the session was videotaped for subsequent analysis. Immobility during this test indicated a state of despair in which the rats have learned that escape was impossible and resigned themselves to the experimental conditions and was defined as floating without struggling or performing necessary movements to keep the head above the water. The latency to immobility and the immobile time were recorded with the use of the ANY‐Maze video tracking system (Stoelting).
2.5. Morris water maze
The MWM apparatus was a circular fiberglass pool (150 cm diameter), artificially divided into four quadrants (N, E, S, and W). And the black platform (14 cm diameter) of the pool was submerged in the opaque and warm (25 ± 1°C) water. According to the procedures described previously (Song et al., 2019), on the first day, the animals were placed into the pool for 60 s to adapt to the maze. From the second day to sixth day, the acquisition test was conducted: each rat was trained in four trials per day for five successive days; during each trial, the rats were placed into the pool at one of the four quadrants and were allowed to swim freely until they found the submerged, hidden goal platform, where they remained for 3 s. If rats failed to find the platform within 1 min, they were guided to it by a stick, and stayed there for 15 s. The escape latency was recorded by the ANY‐Maze video tracking system. The probe trial was conducted on the seventh day, the platform was removed, and rats were placed in the pool for 60 s. The mean numbers of crossing the platform and the mean time spent in the target quadrant in which the platform was previously located were recorded by the ANY‐Maze video tracking system (Stoelting).
2.6. Transmission electron microscopy observation
The male rats (n = 3/group) were cardiac‐perfused with 2.5% glutaraldehyde in 0.2 M phosphate‐buffered saline (PBS). And hippocampi were separated from cerebrum, then based on the anatomical structure of the hippocampus, tissue samples (1 × 1 × 1 mm) were rapidly cut from the CA1 region of hippocampi under an anatomical microscope, fixed in 4% glutaraldehyde for 2 days at 4℃. Next, the hippocampus tissues were put in 1% osmium tetroxide in 0.1 M PBS for 2 hr to postfix, in graded ethanol to dehydrate, and then, in beam capsules to embed. The embedded tissues were cut into 70‐nm‐thick sections which were collected onto grids to air dry overnight. Finally, the grids were stained with uranyl acetate for 30 min and lead citrate for 10 min and observed under a transmission electron microscope (JEM‐1400PLUS). To analyze the synaptic ultrastructure, including synapse number, length of active zone (AZ), thickness of postsynaptic density (PSD), and width of synaptic cleft, six fields of vision were chosen based on a systematic random sampling principle and photographed at a transmission electron microscopy (TEM) magnification of 10,000× or 40,000×. Finally, synapse number, AZ length, PSD thickness, and cleft width were measured using ImageJ software.
2.7. Western blot analysis
The hippocampi of rats were rapidly collected and stored in liquid nitrogen immediately. The protein concentration was tested using a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo scientific). Samples containing 40 μg of total protein were separated in 10%–15% sodium dodecyl sulfate (SDS)‐polyacrylamide gels and then transferred to polyvinylidene difluoride (PVDF) membranes (0.22 μm; Millipore Corp). The membranes were blocked in TBST containing 5% nonfat dry milk for 90 min and incubated with the following primary antibodies overnight at 4°C: anti‐PSD95 (goat monoclonal antibody; Abcam, 1:2,000), anti‐SYP (rabbit monoclonal antibody; Abcam, 1:2,000), anti‐GAP43 (rabbit monoclonal antibody; Abcam, 1:2,000), anti‐BDNF (rabbit monoclonal antibody; Abcam, 1:2,000), anti‐p‐CREB (phosphor S133, rabbit monoclonal antibody; Abcam, 1:5,000), anti‐CREB (rabbit monoclonal antibody; Abcam, 1:2,000), and anti‐β‐actin antibody (1:500, mouse monoclonal antibody; Boster, 1:500). On the next day, the membranes were incubated with the corresponding secondary antibody (anti‐goat/rabbit/mouse IgG; ZSGB‐BIO, 1:5,000) for 90 min at room temperature. After using clarity western electrochemiluminescence (ECL) substrate (Bio‐Rad), protein bands were visualized in the Bio‐Rad Imager. The intensities of the protein bands were analyzed by densitometry using Syngene imaging systems.
2.8. Statistical analysis
Statistical analysis was performed using SPSS 17.0 statistical software. Body weight on PND28 and PND60, EPM, FS, MWM (probe trial), TEM, and densitometric western blot data were analyzed using one‐way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Body weight data from PND3 to PND15 and the data of the acquisition trials in MWM test were analyzed by two‐way repeated measures ANOVA (day × group) with the Bonferroni post hoc test. Moreover, MWM (acquisition trials) data and body weight data at each time point were analyzed using multivariate ANOVA. All data were presented as means ± SEM. Differences were considered significant if p < .05.
2.9. Ethics statement
All procedures were approved by the Animal Care Committee of Chongqing Medical University and performed according with Chongqing Science and Technology Commission guidelines for animal research. Ethical license number was SYXK2017‐0012.
3. RESULTS
3.1. Weight testing
Forty rats in each group were weighed from PND3 to PND15, and 20 rats were weighed on PND28 and PND60. The results indicated the body weight gained constantly among four groups from PND3 to PND15 (time main effects: F(12, 1,872) = 46,693.75, p < .0001; Figure 2a), but the increase in body weight in four groups at different time points differ significantly (interaction effects: F(36, 1,872) = 15.36, p < .0001; Figure 2a). According to the results of multivariate ANOVA, body weights were similar among the saline group and two doses groups at the beginning of the experiments (PND3‐PND10), and the average weight in the high‐dose group was significantly lower than that in the saline group from PND11 to PND15 on PND28 (day11: F(3, 156) = 5.794, p = .0009; day12: F(3, 156) = 7.782, p < .0001; day13: F(3, 156) = 9.663, p < .0001; day14: F(3, 156) = 12.82, p < .0001; day15: F(3, 156) = 15.26, p < .0001; day28: F(3, 76) = 10.03, p < .0001; Figure 2a,b). However, no significant difference in weight was found among four groups on PND60 (F(3, 76) = 0.1527, p = .9277; Figure 2c). There were no significant differences in body weight among the control, saline, and low‐dose groups.
Figure 2.
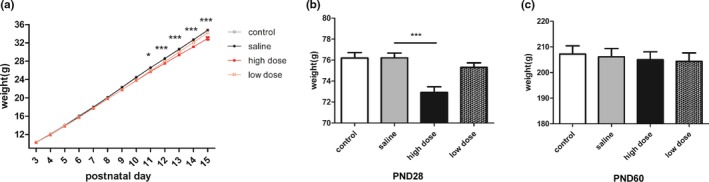
Effects of exposure to low and high doses (2 and 10 mg/kg) of PM2.5 on body weight. (a) Line graphs showing the body weight from PND3 to PND15 (n = 40). *p < .05 high‐dose group compared with saline group at the same time point, ***p < .0001 high‐dose group compared with saline group at the same time point. (b) Bar graphs showing the body weight on PND28 and (c) the body weight on PND60 (n = 20). ***p < .0001 compared with saline group
3.2. Effect of low and high doses of PM2.5 on anxiety‐ and depressive‐like behaviors in immature and mature rats
To study the effect of PM2.5 on anxiety‐like behavior in immature and mature rats, we conducted the EPM test. In immature rats, the low‐ and high‐dose groups showed a significantly lower percentage of number of entries into the open arms compared to the saline group (F(3, 36) = 5.999, p = .0020; Figure 3a), indicating that exposure to two doses of PM2.5 could lead to anxiety‐like symptom in immature rats. No significant difference in the percentage of time spent in open areas (F(3, 36) = 1.233, p = .3119; Figure 3b) was found among the saline, low‐dose group, and high‐dose group. In mature rats, the percentages of number of entries into open arms (F(3, 36) = 3.473, p = .0259; Figure 3a) and time spent in open areas (F(3, 36) = 6.114, p = .0018; Figure 3b) were significantly lower in the high‐dose group than in saline group, indicating apparent anxiety‐like behaviors in the mature rats of the high‐dose group. There was no significant difference in the percentage of number of entries into open arms and percentages of time spent in open areas between saline and low‐dose group.
Figure 3.
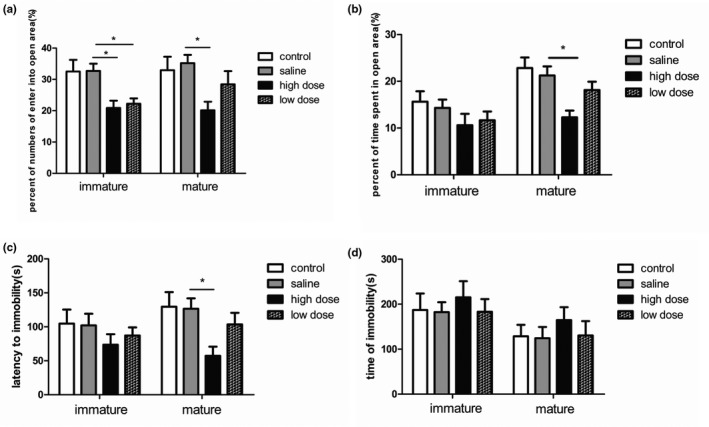
Effects of exposure to low and high doses (2 and 10 mg/kg) of PM2.5 on anxiety‐ and depressive‐like behaviors in immature and mature rats. (a) Bar graphs showing the percentage of entries into open arms during the elevated plus maze (EPM) test; (b) percentage of time spent in open arms in the EPM test; (c) latency to immobility in the forced swimming (FS) test; (d) time spent immobile during the forced swimming test. *p < .05 compared with saline group. n = 10 per group
We subsequently conducted FS tests to determine whether PM2.5 exposure contributed to depressive‐like behavior in immature and mature rats. In the immature rats, there were no significant differences in the latency to immobility (F(3, 36) = 0.7648, p = .5213; Figure 3c) and immobility time (F(3, 36) = 0.2506, p = .8604; Figure 3d) among the four groups. However, mature rats of the high‐dose group showed depressive‐like symptoms, indicated by sharply decreased latency to immobility compared with controls (F(3, 36) = 3.820, p = .0179; Figure 3c). The length of immobile time showed no significant difference among the four groups (F(3, 36) = 0.4441, p = .7230; Figure 3d).
3.3. Effects of low and high doses of PM2.5 on spatial learning and memory in immature and mature rats
We evaluated the effect of PM2.5 on spatial learning and memory in immature and mature rats by using the MWM test. The acquisition trial results showed that the escape latency of training rats decreased as training days increased (time main effects: for immature rats, F(4, 144) = 23.99, p < .0001; for mature rats, F(4, 144) = 21.12, p < .0001; Figure 4a,b), indicating that rats in each group had learning abilities. And no significant interaction between groups and days was found (interaction effects: for immature rats, F(12, 144) = 0.87, p = .5786; for mature rats, F(12, 144) = 0.32, p = .9843), suggesting that the differences among groups were dependent on the treatment. Specifically, for immature rats, the escape latency during the first 3 days of spatial training didn't differ among the four groups. However, the escape latency was much longer in the high‐dose group than in the saline group on training days 4 and 5 (day4: F(3, 36) = 4.638, p = .008; day5: F(3, 36) = 3.812 p = .018; Figure 4a), suggesting that exposure to high doses of PM2.5 could impair spatial learning in immature rats. No significant differences were found in the escape latency of mature rats among the various groups during 5 days of spatial training.
Figure 4.
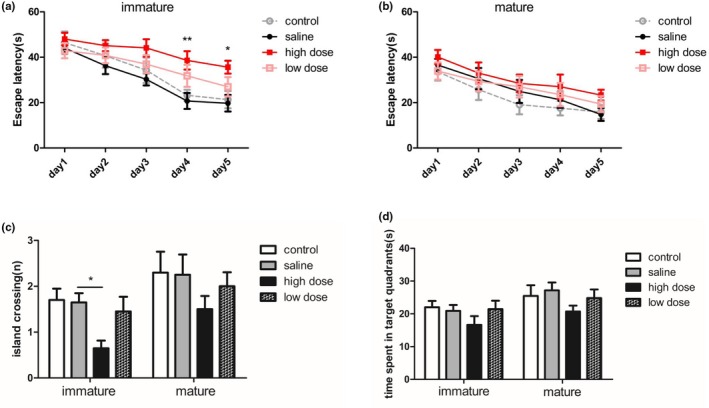
Effects of exposure to low and high doses (2 and 10 mg/kg) of PM2.5 on spatial learning and memory in immature and mature rats determined using the Morris water maze test. (a) Line graph (left) showing the escape latency of immature rats in the acquisition trial for each training day. (b) Line graph (right) showing the escape latency of mature rats during the acquisition trial for five consecutive days. *p < .05 high‐dose group compared with saline group at the same time point, **p < .01 high‐dose group compared with saline group at the same time point. (c) Bar graphs showing the number of entries into the platform zone, and (d) time spent in the target quadrants during the probe test. *p < .05 compared with saline group. n = 10 per group
To assess spatial memory retrieval, a probe test was performed 24 hr after the last training day. The results showed the number of entries into the platform zone was lower in the high‐dose group than in the saline group (F(3, 36) = 4.092, p = .0134; Figure 4c), and the rats of the high‐dose group spent less time in the target quadrant; however, there was no significant difference (F(3, 36) = 1.155, p = .3404; Figure 4d), according to the above statistical analysis, spatial memory retrieval of immature rats was impaired after early postnatal exposure to high doses of PM2.5. No significant differences were detected among the four groups in the performance of mature rats in the probe test, although the high‐dose and low‐dose groups showed fewer entries into the platform zone (F(3, 36) = 0.9234, p = .4393; Figure 4c) and shorter time in the target quadrant F(3, 36) = 1.139, p = .3464; Figure 4d) than the controls, suggesting spatial memory ability of mature rats in each group didn't differ.
3.4. Effect of low and high doses of PM2.5 on hippocampal synaptic ultrastructure in immature and mature rats
Samples obtained from immature and mature rats were used to examine the synaptic ultrastructure in the CA1 region of the hippocampus by TEM. As shown in Figure 5a–d, the changes in synaptic ultrastructure of immature and mature rats showed the same trend. The number of synapses was much lower in the high‐dose groups than in the saline groups (immature rats: F(3, 20) = 7.992, p = .0011; mature rats: F(3, 20) = 4.201, p = .0185; Figure 5e). Moreover, the thickness of PSD (immature rats: F(3, 20) = 12.91, p < .0001; mature rats: F(3, 20) = 8.807, p = .0006; Figure 5f) and length of AZ (immature rats: F(3, 20) = 4.554, p = .0137; mature rats: F(3, 20) = 4.089, p = .0204; Figure 5g) were decreased in the high‐dose group compared to the saline group, while the synaptic cleft width remained unchanged (immature rats: F(3, 20) = 2.016, p = .1440; mature rats: F(3, 20) = 0.4878, p = .6946; Figure 5h). However, statistical analysis did not show any significant differences in synaptic ultrastructure among the control, saline, and low‐dose groups.
Figure 5.
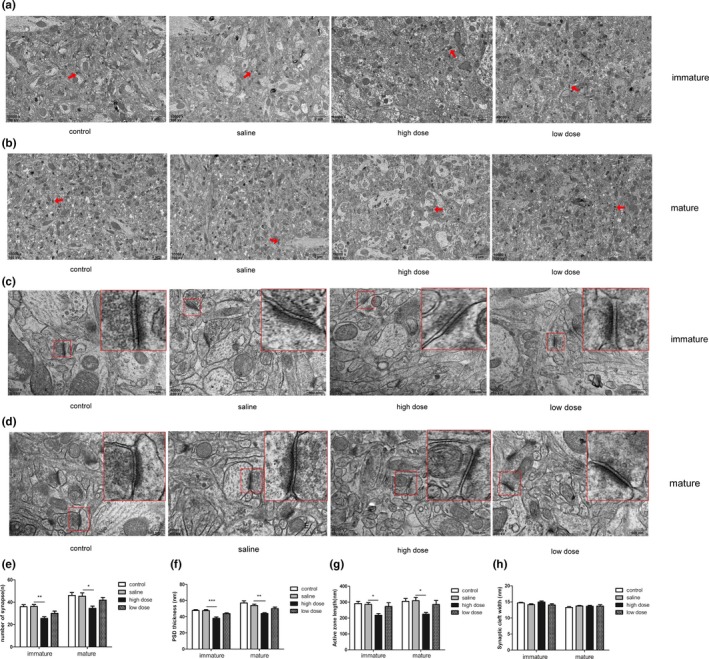
Effects of exposure to low and high doses (2 and 10 mg/kg) of PM2.5 on synaptic ultrastructure in the hippocampal CA1 region in immature and mature rats observed using transmission electron microscopy. (a–b) Representative images (×10,000) showing the difference in the number of synapses in the four groups in immature and mature rats. (Red arrows indicate a synapse), scale bars = 2 μm; (c–d) representative images (×40,000) showing the differences in micro‐ultrastructure of synapses in the four groups in immature and mature rats. (the areas enclosed by the red box were amplified to delineate the synapses clearly), scale bars = 500 nm; (e–h) data analysis of synapse number, PSD thickness, AZ length, and cleft width. *p < .05 compared with saline group, **p < .01 compared with saline group, ***p < .001 compared with saline group
3.5. Effect of low and high doses of PM2.5 on hippocampal synaptic protein expression in immature and mature rats
The expression of the synaptic proteins PSD95, GAP43, and SYP in the hippocampal specimens were studied using western blot analysis (Figure 6a). PSD95 protein expression decreased dramatically in immature rats after exposure to high and low doses of PM2.5 (F(3, 20) = 12.44, p < .0001; Figure 6b). In mature rats, the level of PSD95 was lower only in the high‐dose group, compared with the saline group (F(3, 20) = 5.001, p = .0095; Figure 6b). Moreover, when compared with saline group, lower GAP43 expression was observed in the high‐dose group on PND28 (F(3, 20) = 6.935, p = .0022; Figure 6c), but not on PND60 (F(3, 20) = 2.321, p = .1061; Figure 6c). In addition, the high‐dose groups, but not the low‐dose groups showed a significant decrease in the level of SYP expression, compared with controls, in immature (F(3, 20) = 9.100, p = .0005; Figure 6d) and mature rats (F(3, 20) = 4.760, p = .0116; Figure 6d).
Figure 6.
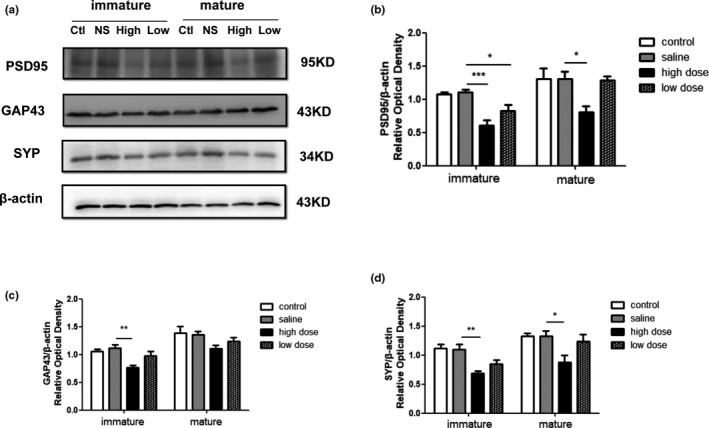
Western blot analysis showing the effects of exposure to low and high doses (2 and 10 mg/kg) of PM2.5 on hippocampal synaptic protein expression in immature and mature rats. (a) Representative immunoblots for the expression levels of PSD‐95, GAP43, and SYP in the hippocampi of immature and mature rats. Ctl, control group; High, high‐dose group; Low, low‐dose group; NS, saline group. (b–d) Quantification of PSD‐95, GAP43, and SYP normalized to β‐actin, respectively. *p < .05 compared with saline group, **p < .01 compared with saline group, ***p < .001 compared with saline group. n = 6 per group
3.6. Effects of low and high doses of PM2.5 on hippocampal BDNF expression and CREB activation in immature and mature rats
To determine whether the CREB/BDNF pathway is involved in PM2.5‐induced neurotoxicity, the expression levels of the BDNF, p‐CREB, and CREB proteins in the hippocampus were detected by western blot analysis (Figure 7a). High‐dose PM2.5 exposure significantly down‐regulated the BDNF levels in immature (F(3, 20) = 12.98, p < .0001; Figure 7b) and mature rats (F(3, 20) = 5.142, p = .0085; Figure 7b), and low‐dose PM2.5 exposure decreased BDNF expression in immature rats but not in mature rats. The level of phosphorylation of CREB was much lower in the high‐dose group than in the saline group on PND28 (F(3, 20) = 14.68, p < .0001; Figure 7c) and PND60 (F(3, 20) = 4.699, p = .0122; Figure 7c). There was a significant difference in the level of phosphorylation of CREB protein between the low‐dose and saline groups of immature but not mature rats.
Figure 7.
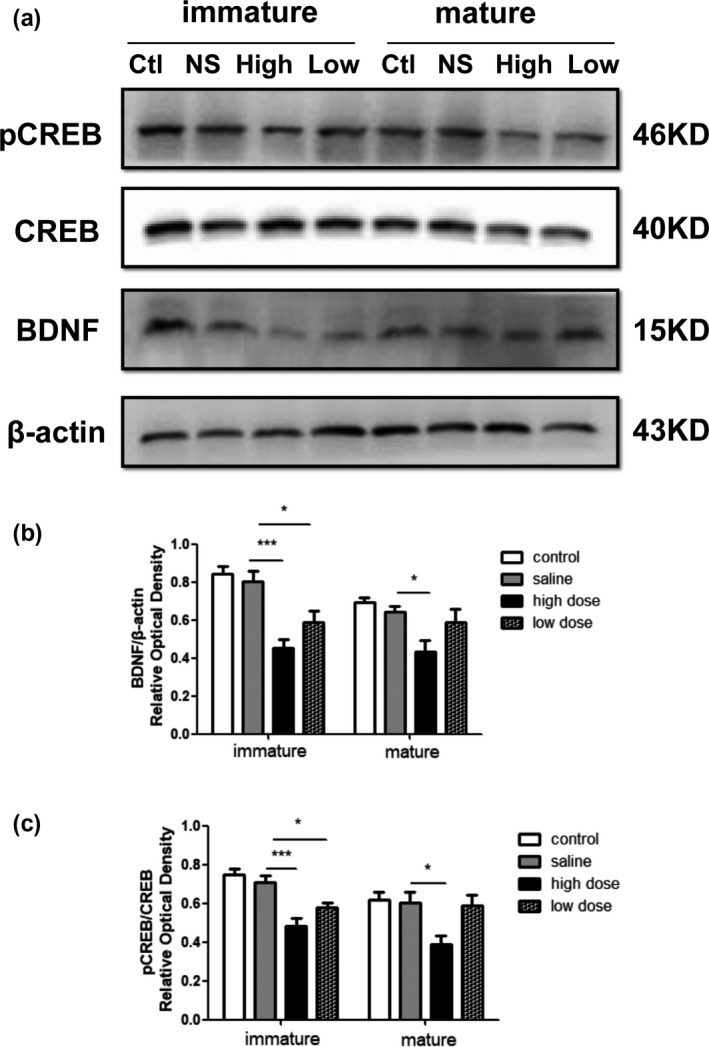
Western blot results showing effects of exposure to low and high doses (2 and 10 mg/kg) of PM2.5 on hippocampal cAMP response element binding protein (CREB) and brain‐derived neurotrophic factor (BDNF) protein expression in immature and mature rats. (a) Representative images showing the expression levels of p‐CREB, CREB, and BDNF proteins in the hippocampus of immature and mature rats. Ctl, control group; High, high‐dose group; Low, low‐dose group; NS, saline group. (b–c) Quantitative analyses of CREB phosphorylation and BDNF expression level in each group. *p < .05 compared with saline group, **p < .01 compared with saline group, ***p < .001 compared with saline group. n = 6 per group
4. DISCUSSION
Exposure to environmental toxicants during early periods of development could exert permanently deleterious effects on neurobiological and behavioral outcome (Heyer & Meredith, 2017). The PND15 rat brain is similar to the brain of a 1‐year‐old child, a PND28 rat brain is similar to the brain of a 2‐year‐old child, and a PND60 rat brain is similar to the brains of sexually mature humans (Jiang, Duong, & Lanerolle, 1999),; therefore, we analyzed PM2.5 neurotoxicity in immature (PND28) and mature rats (PND60) to determine the short‐ and long‐term effects of early postnatal PM2.5 exposure on CNS development. PM2.5 is currently a ubiquitous environmental pollutant, produced mainly by road traffic and industrial activities, and consists of diverse chemicals, including elemental carbon, minerals, metals, organic compounds, and biological compounds (Kim, Kabir, & Kabir, 2015). PM2.5 has been linked to elevated risk of respiratory and cardiovascular disease, neurological disorders, and reproductive function impairments (Calderón‐Garcidueñas, Leray, Heydarpour, Torres‐Jardón, & Reis, 2016; Sinharay et al., 2018; Xue & Zhu, 2018). Further, PM2.5 exposure during early life leads to developmental disorders. A meta‐analysis revealed low birth weight was positively associated with maternal exposure to PM2.5 pollution (Dadvand et al., 2013), and Dang et al. (2018) found PM2.5 exposure during entire pregnancy significantly declined the birth weights of offspring rats. However, the effects of PM2.5 exposure during the early postnatal period on body weight remain poorly understood. In the present study, we observed a reduction in weight gain as PM2.5 exposure time increased, until PND28. This result suggests that persistent and serious PM2.5 pollution could hinder physical growth of children, and the relative mechanisms need further research.
Fonken et al. (2011) reported that depressive‐like responses, but not anxiety‐like responses increased in male mice exposed to PM2.5 during adulthood, relative to control mice. Kulas et al. (2018) found that PM2.5 perinatal exposure in mice did not cause anxiety‐like behavior in male offspring. Our results suggested that PM2.5 exposure increased anxiety‐like behaviors in immature and mature rats, as shown by a lower percentage of number of entries into open arms or decreased percentage of time spent in open arms. Mature rats displayed depressive‐like symptoms after perinatal exposure to PM2.5, as reflected by shorter latency to immobility during the FS test. Since immobility time, an important index of depressive‐like state, was unchanged significantly in each group, tail suspension test or sucrose preference test would be conducted to reconfirm this PM2.5‐induced behavioral alteration in further study. Taken together, these discrepancies between different studies could be due to differences in PM2.5 exposure time and dosage. Moreover, evidences have shown exposure to PM2.5 in adulthood is an important risk factor for depression and anxiety symptoms in old adults and pregnant women (Pun, Manjourides, & Suh, 2019; Sheffield et al., 2018). However, the relationship between early postnatal exposure to PM2.5 and anxiety or depression phenotype in children and adults remains unknown at present; our findings provide experimental basis for relative epidemiological investigation.
PM2.5 exposure decreased improvements in cognitive development in children and caused deterioration of spatial learning and memory in young mice (Ning et al., 2018). Our data indicated that early PM2.5 exposure significantly increased the latency to find the platform and decreased the number of entries into the platform zone in immature rats during the MWM test, indicating that PM2.5 induced a deficit of learning and memory in young rats, and this finding is consistent with previous observations showing that PM2.5 exposure decreased improvements in cognitive development in children (Alvarez‐Pedrerol et al., 2017). Considering the detrimental effects of PM2.5 exposure on cognitive function in adults (Salinas‐Rodríguez et al., 2018), we speculated PM2.5 exposure during any period of life could be a harmful environmental factor for human and animal cognition. Our data also showed the negative effect of PM2.5 on spatial learning and memory in mature rats was absent, suggesting after a long period of separation from PM2.5 exposure, cognitive abilities may be restored to some extent. Overall, PM2.5 could impair cognitive and neuropsychiatric development. Sex‐based differences in our model should be further studied, since some behavioral defects were dependent on sex in other disease model (McCarthy, 2016; de Melo, David Antoniazzi, Hossain, & Kolb, 2018), although we selected the exposure group randomly to match the quantity and sex ratio of the control group.
To elucidate the possible relationship between PM2.5 exposure and neurodevelopment, we exposed rats to PM2.5 from PND3 to PND15, which is a critical period of synaptogenesis and plasticity (Jensen, 2009). Structural plasticity, including synaptic formation, ultrastructure, and changes in synaptic associated protein, plays a crucial role in cognitive and emotional development, and defects in structural plasticity could cause neuropsychiatric illnesses, cognitive decline, and neurodegenerative disease (Huntley, 2012; Phillips & Pozzo‐Miller, 2015). PSD95 is the most important scaffold protein on the postsynaptic membrane and is a marker of dendrite branching and remodeling. GAP43 is closely related to axonal growth and is a vital factor for synapse formation. SYP is a presynaptic vesicle protein and is often used to represent synaptic density (Ma et al., 2014; Shi et al., 2018). Reports on the influence of PM2.5 exposure on structural synaptic plasticity remain contradictory and insufficient. Kulas et al. (2018) found that prenatal exposure to PM2.5 in mice led to elevated SYP expression and unchanged PSD95 expression in the hippocampus of male offspring. Zhang et al. (2018) reported that prenatal PM2.5 exposure in mice decreased postsynaptic densities of membranes and the number of synaptic vesicles in the cerebral cortex of offspring, and an in vitro study suggested that PM2.5 exposure reduced PSD95 expression in cortical neurons (Chen, Li, & Sang, 2017). These results suggested that PM2.5 exposure during early life might change synaptic structural plasticity. The CA1 region is a key part of hippocampus and is vulnerable to ischemia, hypoxia, seizure and other insults (Ou & Weber, 2018), and myelin changes in neurons in the CA1 area, not in the dentate gyrus region, were discovered in adult mice after exposure to traffic‐related air pollution for 10 weeks (Woodward et al., 2017), so CA1 region, as representative hippocampal area, was selected for synaptic ultrastructure examination by TEM.
We observed impaired synaptic ultrastructure in the hippocampal CA1 area of PM2.5‐exposed immature and mature rats, as demonstrated by smaller number of synapses, thinner PSD, and shorter AZ. Further, PM2.5 exposure induced a significant decrease in the levels of SYP, GAP43, and PSD95 in immature rats, showing that the deleterious effect of PM2.5 on synaptic structure was closely related to the diminished expression of SYP, GAP43, and PSD95. Early postnatal exposure to PM2.5 reduced the expression of SYP and PSD95 in mature rats, suggesting what impairment of structural plasticity in mature rats could be alleviated to a certain extent compared to that in immature rats. Our data revealed that early postnatal PM2.5 exposure could induce long‐term damage to structural plasticity, characterized by synaptic loss and impaired synaptic ultrastructure, which implied PM2.5 exposure may contribute to poor abilities of neurons to deal with the synaptic signaling transmission between the presynaptic and the postsynaptic membranes and cause synaptic dysfunction. In addition, synaptic plasticity is commonly acknowledged to be the vital biological basis of cognition at the cellular level, and damaged hippocampal synaptic plasticity could also participate in the process of anxiety and depression (Bannerman et al., 2014; Lu et al., 2019). From the current results, we considered that the decreasing of structural plasticity appears to be a possible mechanism of emotional disorder and spatial learning and memory deficits induced by early PM2.5 exposure. However, considering the inconsistency between reduced structural plasticity and normal spatial learning and memory in mature rats, recognition memory and working memory need to be evaluated by novel object recognition test and rewarded T‐maze alternation test to verify the effect of early postnatal PM2.5 exposure on cognitive ability in mature rats.
The mechanism underlying the toxic effects of PM2.5 exposure on synaptic development remains unclear. BDNF is essential to brain signaling and synaptic plasticity and triggers various signaling pathways, including the mitogen‐activated protein kinase (MAPK), phosphoinositide 3‐kinase (PI3K), and phospholipase C‐γ (PLC‐γ) pathways to regulate synaptic structure and function (Kowiański et al., 2018). BDNF concentrations in cerebrospinal fluid were lower in children exposed to PM2.5 pollution than in normal children (Calderón‐Garcidueñas, Mukherjee, et al., 2018). The inactive form of CREB seems to bind BDNF promoter, after which CREB phosphorylation recruits transcription factor components to the promoter and initiates gene transcription of BDNF. p‐CREB is therefore a vital upstream factor of BDNF expression (Sasi, Vignoli, Canossa, & Blum, 2017). We measured the levels of BDNF expression and CREB phosphorylation to elucidate the possible molecular mechanism and found that early postnatal exposure of PM2.5 reduced BDNF expression levels in the hippocampus in immature and mature rats. Consistent with these changes in BDNF expression, p‐CREB protein levels were significantly lower in immature and mature PM2.5‐exposed rats. Our data suggested that the down‐regulated CREB/BDNF signaling pathway in the hippocampus could be responsible for PM2.5‐induced disruption of synaptic development, and further study is needed to identify other essential molecules involved in the CREB/BDNF signaling pathway and explore the relationships between PM2.5 exposure and such molecules by using specific signal pathway inhibitors in vitro. The illustration of the molecular mechanism by which PM2.5 exposure damages neurodevelopment may provide more selectable strategies for better cognitive and neuropsychiatric outcomes in children exposed to serious PM2.5 pollution in the future.
A dose‐dependent trend was observed between PM2.5 exposure and its neurotoxic effects. Compared with control groups, the high‐dose PM2.5 groups of immature and mature rats exhibited apparent behavioral defects, but the low‐dose PM2.5 group of immature rats only showed significant anxiety‐like symptoms. Synaptic structural plasticity damage and disrupted CREB/BDNF signaling pathway in immature rats in low‐ and high‐dose PM2.5 groups showed a gradual increase in statistically significant differences, indicating that the toxicity of PM2.5 increases with the increase of the dose. In adulthood, after the cessation of PM2.5 exposure for 45 days, the neuro‐impairments were detected in the high‐dose group, but were alleviated in the low‐dose group, suggesting that self‐repair mechanisms may exist following PM2.5‐induced injury in the hippocampus. Mechanisms of neuroprotection against PM2.5 exposure require further investigation. Overall, the present study provides basic evidence for better understanding the neurotoxic effects of PM2.5 on immature and mature brains.
5. CONCLUSION
In summary, the present study demonstrated that early postnatal PM2.5 exposure could affect weight gain of immature rats and impair emotional and cognitive development by damaging synaptic structural plasticity and down‐regulating the CREB/BDNF signaling pathway. The neurotoxic effects of PM2.5 appeared to be dose‐dependent; high‐dose PM2.5 exposure would exert significant and persistent influences on neurodevelopment. Future investigations are warranted to explore deep molecular mechanisms involved in PM2.5 neurotoxicity.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC Grant 81371452).
Liu J, Yang C, Yang J, et al. Effects of early postnatal exposure to fine particulate matter on emotional and cognitive development and structural synaptic plasticity in immature and mature rats. Brain Behav. 2019;9:e01453 10.1002/brb3.1453
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1453.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCE
- Allen, J. L. , Liu, X. , Pelkowski, S. , Palmer, B. , Conrad, K. , Oberdörster, G. , … Cory‐Slechta, D. A. (2014). Early postnatal exposure to ultrafine particulate matter air pollution: Persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environmental Health Perspectives, 122(9), 939–945. 10.1289/ehp.1307984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Pedrerol, M. , Rivas, I. , López‐Vicente, M. , Suades‐González, E. , Donaire‐Gonzalez, D. , Cirach, M. , … Sunyer, J. (2017). Impact of commuting exposure to traffic‐related air pollution on cognitive development in children walking to school. Environmental Pollution, 231, 837–844. 10.1016/j.envpol.2017.08.075 [DOI] [PubMed] [Google Scholar]
- Andersen, Z. J. , Pedersen, M. , Weinmayr, G. , Stafoggia, M. , Galassi, C. , Jørgensen, J. T. , … Raaschou‐Nielsen, O. (2018). Long‐term exposure to ambient air pollution and incidence of brain tumor: The European Study of Cohorts for Air Pollution Effects (ESCAPE). Neuro‐Oncology, 20(3), 420–432. 10.1093/neuonc/nox163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman, D. M. , Sprengel, R. , Sanderson, D. J. , McHugh, S. B. , Rawlins, J. N. , Monyer, H. , & Seeburg, P. H. (2014). Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews Neuroscience, 15(3), 181–192. 10.1038/nrn3677 [DOI] [PubMed] [Google Scholar]
- Bondy, S. C. (2011). Nanoparticles and colloids as contributing factors in neurodegenerative disease. International Journal of Environmental Research and Public Health, 8(6), 2200–2211. 10.3390/ijerph8062200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón‐Garcidueñas, L. , & de la Monte, S. M. (2017). Apolipoprotein E4, gender, body mass index, inflammation, insulin resistance, and air pollution interactions: Recipe for Alzheimer's disease development in Mexico City young females. Journal of Alzheimer's Disease, 58(3), 613–630. 10.3233/JAD-161299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón‐Garcidueñas, L. , González‐Maciel, A. , Reynoso‐Robles, R. , Kulesza, R. J. , Mukherjee, P. S. , Torres‐Jardón, R. , … Doty, R. L. (2018). Alzheimer's disease and alpha‐synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤ 40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environmental Research, 166, 348–362. 10.1016/j.envres.2018.06.027 [DOI] [PubMed] [Google Scholar]
- Calderón‐Garcidueñas, L. , Leray, E. , Heydarpour, P. , Torres‐Jardón, R. , & Reis, J. (2016). Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Revue Neurologique, 172(1), 69–80. 10.1016/j.neurol.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Calderón‐Garcidueñas, L. , Mukherjee, P. S. , Waniek, K. , Holzer, M. , Chao, C. K. , Thompson, C. , … Lachmann, I. (2018). Non‐phosphorylated tau in cerebrospinal fluid is a marker of Alzheimer's disease continuum in young urbanites exposed to air pollution. Journal of Alzheimer's Disease, 66(4), 1437–1451. 10.3233/JAD-180853 [DOI] [PubMed] [Google Scholar]
- Cao, X. N. , Yan, C. , Liu, D. Y. , Peng, J. P. , Chen, J. J. , Zhou, Y. , … Wei, G. H. (2015). Fine particulate matter leads to reproductive impairment in male rats by overexpressing phosphatidylinositol 3‐kinase (PI3K)/protein kinase B (Akt) signaling pathway. Toxicology Letters, 237(3), 181–190. 10.1016/j.toxlet.2015.06.015 [DOI] [PubMed] [Google Scholar]
- Chen, M. , Li, B. , & Sang, N. (2017). Particulate matter (PM2.5) exposure season‐dependently induces neuronal apoptosis and synaptic injuries. Journal of Environmental Sciences China, 54, 336–345. 10.1016/j.jes.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Cohen, A. J. , Brauer, M. , Burnett, R. , Anderson, H. R. , Frostad, J. , Estep, K. , … Forouzanfar, M. H. (2017). Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. The Lancet, 389(10082), 1907–1918. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand, P. , Parker, J. , Bell, M. L. , Bonzini, M. , Brauer, M. , Darrow, L. A. , … Woodruff, T. J. (2013). Maternal exposure to particulate air pollution and term birth weight: A multi‐country evaluation of effect and heterogeneity. Environmental Health Perspectives, 121(3), 267–373. 10.1289/ehp.1205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, S. , Ding, D. , Lu, Y. , Su, Q. , Lin, T. , Zhang, X. , … Li, H. (2018). PM2.5 exposure during pregnancy induces hypermethylation of estrogen receptor promoter region in rat uterus and declines offspring birth weights. Environmental Pollution, 243, 851–861. 10.1016/j.envpol.2018.09.065 [DOI] [PubMed] [Google Scholar]
- de Melo, S. R. , de David Antoniazzi, C. T. , Hossain, S. , & Kolb, B. (2018). Neonatal stress has a long‐lasting sex‐dependent effect on anxiety‐like behavior and neuronal morphology in the prefrontal cortex and hippocampus. Developmental Neuroscience, 40(2), 93–103. 10.1159/000486619 [DOI] [PubMed] [Google Scholar]
- Fonken, L. K. , Xu, X. , Weil, Z. M. , Chen, G. , Sun, Q. , Rajagopalan, S. , & Nelson, R. J. (2011). Air pollution impairs cognition, provokes depressive‐like behaviors and alters hippocampal cytokine expression and morphology. Molecular Psychiatry, 16(10), 987–995. 10.3390/ijerph8062200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, P. , Guo, X. , Cheung, F. M. H. , & Yung, K. K. L. (2019). The association between PM2.5 exposure and neurological disorders: A systematic review and meta‐analysis. Science of the Total Environment, 655, 1240–1248. 10.1016/j.scitotenv.2018.11.218 [DOI] [PubMed] [Google Scholar]
- Gao, J. , Wang, K. , Wang, Y. , Liu, S. , Zhu, C. , Hao, J. , … Tian, H. (2018). Temporal‐spatial characteristics and source apportionment of PM2.5 as well as its associated chemical species in the Beijing‐Tianjin‐Hebei region of China. Environmental Pollution, 233, 714–724. 10.1016/j.envpol.2017.10.123 [DOI] [PubMed] [Google Scholar]
- Han, W. , Dong, X. , Song, X. , Cheng, L. , Xie, L. , Chen, H. , & Jiang, L. (2018). Effects of advanced maternal age on cognitive and emotional development in offspring rats. Behavioral Brain Research, 353, 218–226. 10.1016/j.bbr.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Heyer, D. B. , & Meredith, R. M. (2017). Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology, 58, 23–41. 10.1016/j.neuro.2016.10.017 [DOI] [PubMed] [Google Scholar]
- Huntley, G. W. (2012). Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nature Reviews Neuroscience, 13(11), 743–757. 10.1038/nrn3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, F. E. (2009). Neonatal seizures: An update on mechanisms and management. Clinics in Perinatology, 36(4), 881–900. 10.1016/j.clp.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W. , Duong, T. M. , & de Lanerolle, N. C. (1999). The neuropathology of hyperthermic seizures in the rat. Epilepsia, 40(1), 5–19. 10.1111/j.1528-1157.1999.tb01982.x [DOI] [PubMed] [Google Scholar]
- Kim, K. H. , Kabir, E. , & Kabir, S. (2015). A review on the human health impact of airborne particulate matter. Environment International, 74, 136–143. 10.1016/j.envint.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Kowiański, P. , Lietzau, G. , Czuba, E. , Waśkow, M. , Steliga, A. , & Moryś, J. (2018). BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 38(3), 579–593. 10.1007/s10571-017-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulas, J. A. , Hettwer, J. V. , Sohrabi, M. , Melvin, J. E. , Manocha, G. D. , Puig, K. L. , … Combs, C. K. (2018). In utero exposure to fine particulate matter results in an altered neuroimmune phenotype in adult mice. Environmental Pollution, 241, 279–288. 10.1016/j.envpol.2018.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal, G. , Bramham, C. R. , & Duarte, C. B. (2017). BDNF and hippocampal synaptic plasticity. Vitamins and Hormones, 104, 153–195. 10.1016/bs.vh.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Li, K. , Li, L. , Cui, B. , Gai, Z. , Li, Q. , Wang, S. , … Xi, Z. (2018). Early postnatal exposure to airborne fine particulate matter induces autism‐like phenotypes in male rats. Toxicological Sciences, 162(1), 189–199. 10.1093/toxsci/kfx240 [DOI] [PubMed] [Google Scholar]
- Lin, P. Y. , Kavalali, E. T. , & Monteggia, L. M. (2018). Genetic dissection of presynaptic and postsynaptic BDNF‐TrkB signaling in synaptic efficacy of CA3‐CA1 synapses. Cell Reports, 24(6), 1550–1561. 10.1016/j.celrep.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Y. , Hong, X. R. , & Sun, Q. H. (2015). Da Qi Ke Li Wu Dui Ren Shen He Tai Er Fa Yu De Ying Xiang [Effects of atmospheric particulate matter on pregnancy and fetal development]. Chinese Journal of Perinatal Medicine, 18(1), 57–60. 10.3760/cma.j.issn.1007-9408.2015.01.015 [in Chinese] [DOI] [Google Scholar]
- Lu, K. , Jing, X. B. , Xue, Q. , Song, X. J. , Wei, M. D. , & Wang, A. G. (2019). Impaired fear memory extinction during adolescence is accompanied by the depressive‐like behaviors. Neuroscience Letters, 699, 8–15. 10.1016/j.neulet.2019.01.039 [DOI] [PubMed] [Google Scholar]
- Ma, J. , Zhang, Z. , Kang, L. , Geng, D. , Wang, Y. , Wang, M. , & Cui, H. (2014). Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Experimental Gerontology, 58, 256–268. 10.1016/j.exger.2014.08.011 [DOI] [PubMed] [Google Scholar]
- McCarthy, M. M. (2016). Sex differences in the developing brain as a source of inherent risk. Dialogues in Clinical Neuroscience, 18(4), 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, G. P. , Clark, I. A. , Zinn, R. , & Vissel, B. (2013). Microglia: A new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiology of Learning and Memory, 105, 40–53. 10.1016/j.nlm.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Ning, X. , Li, B. , Ku, T. , Guo, L. , Li, G. , & Sang, N. (2018). Comprehensive hippocampal metabolite responses to PM2.5 in young mice. Ecotoxicology and Environmental Safety, 165, 36–43. 10.1016/j.ecoenv.2018.08.080 [DOI] [PubMed] [Google Scholar]
- Ou, Y. , & Weber, S. G. (2018). Higher aminopeptidase activity determined by electroosmotic push‐pull perfusion contributes to selective vulnerability of the hippocampal CA1 region to oxygen glucose deprivation. ACS Chemical Neuroscience, 9(3), 535–544. 10.1021/acschemneuro.7b00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. , & Pozzo‐Miller, L. (2015). Dendritic spine dysgenesis in autism related disorders. Neuroscience Letters, 601, 30–40. 10.1016/j.neulet.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun, V. C. , Manjourides, J. , & Suh, H. H. (2019). Close proximity to roadway and urbanicity associated with mental ill‐health in older adults. Science of the Total Environment, 658, 854–860. 10.1016/j.scitotenv.2018.12.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas‐Rodríguez, A. , Fernández‐Niño, J. A. , Manrique‐Espinoza, B. , Moreno‐Banda, G. L. , Sosa‐Ortiz, A. L. , Qian, Z. M. , & Lin, H. (2018). Exposure to ambient PM2.5 concentrations and cognitive function among older Mexican adults. Environment International, 117, 1–9. 10.1016/j.envint.2018.0o.033 [DOI] [PubMed] [Google Scholar]
- Sasi, M. , Vignoli, B. , Canossa, M. , & Blum, R. (2017). Neurobiology of local and intercellular BDNF signaling. Pflugers Archiv ‐ European Journal of Physiology, 469, 593–610. 10.1007/s00424-017-1964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, P. E. , Speranza, R. , Chiu, Y. M. , Hsu, H. L. , Curtin, P. C. , Renzetti, S. , … Wright, R. J. (2018). Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS One, 13(4), e0195267 10.1371/journal.pone.0195267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Nan, C. , Yan, Z. , Liu, L. , Zhou, J. , Zhao, Z. , & Li, D. (2018). Synaptic plasticity of human umbilical cord mesenchymal stem cell differentiating into neuron‐like cells in vitro induced by edaravone. Stem Cells International, 2018, 5304279 10.1155/2018/5304279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinharay, R. , Gong, J. , Barratt, B. , Ohman‐Strickland, P. , Ernst, S. , Kelly, F. J. , … Chung, K. F. (2018). Respiratory and cardiovascular responses to walking down a traffic‐polluted road compared with walking in a traffic‐free area in participants aged 60 years and older with chronic lung or heart disease and age‐matched healthy controls: A randomised, crossover study. The Lancet, 391(10118), 339–349. 10.1016/S0140-6736(17)32643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X. , He, R. , Han, W. , Li, T. , Xie, L. , Cheng, L. , … Jiang, L. (2019). Protective effects of the ROCK inhibitor fasudil against cognitive dysfunction following status epilepticus in male rats. Journal of Neuroscience Research, 97(4), 506–519. 10.1002/jnr.24355 [DOI] [PubMed] [Google Scholar]
- Talbott, E. O. , Arena, V. C. , Rager, J. R. , Clougherty, J. E. , Michanowicz, D. R. , Sharma, R. K. , & Stacy, S. L. (2015). Fine particulate matter and the risk of autism spectrum disorder. Environmental Research, 140, 414–420. 10.1016/j.envres.2015.04.021 [DOI] [PubMed] [Google Scholar]
- Vasilescu, A. N. , Schweinfurth, N. , Borgwardt, S. , Gass, P. , Lang, U. E. , Inta, D. , & Eckart, S. (2017). Modulation of the activity of N‐methyl‐d‐aspartate receptors as a novel treatment option for depression: Current clinical evidence and therapeutic potential of rapastinel (GLYX‐13). Neuropsychiatric Disease and Treatment, 13, 973–980. 10.2147/NDT.S119004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, N. C. , Pakbin, P. , Saffari, A. , Shirmohammadi, F. , Haghani, A. , Sioutas, C. , … Finch, C. E. (2017). Traffic‐related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiology of Aging, 53, 48–58. 10.1016/j.neurobiolaging.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, T. , & Zhu, T. (2018). Increment of ambient exposure to fine particles and the reduced human fertility rate in China, 2000–2010. Science of the Total Environment, 642, 497–504. 10.1016/j.scitotenv.2018.06.075 [DOI] [PubMed] [Google Scholar]
- Yue, N. , Huang, H. , Zhu, X. , Han, Q. , Wang, Y. , Li, B. , … Yu, J. (2017). Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress‐induced depressive‐like behaviors. Journal of Neuroinflammation, 14(1), 102–117. 10.1186/s12974-017-0865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Zheng, X. , Wang, X. , Zhao, H. , Wang, T. , Zhang, H. , … Yu, L. (2018). Maternal exposure to PM2.5 during pregnancy induces impaired development of cerebral cortex in mice offspring. International Journal of Molecular Sciences, 19(1), E257 10.3390/ijms19010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. H. , Zheng, G. , Wang, T. , Du, K. J. , Han, X. , Luo, W. J. , … Chen, J. Y. (2018). Low‐level gestational lead exposure alters dendritic spine plasticity in the hippocampus and reduces learning and memory in rats. Scientific Reports, 8, 3533–3543. 10.1038/s41598-018-21521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Y. , Chen, J. , Li, L. , Qin, Y. , Wei, Y. , Pan, S. , … Xie, Y. (2018). PKA‐CREB‐BDNF signaling pathway mediates propofol‐induced long‐term learning and memory impairment in hippocampus of rats. Brain Research, 1691, 64–74. 10.1016/j.brainres.2018.04.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


