Abstract
We report the case of an 8-years-old girl with recurrent pulmonary infections and wheezing since infancy, in whom asthma and immunoglobulin-G deficiency were diagnosed at the age of 7 months. Since then, the patient was treated for asthma without any satisfactory control of the disease. Cardiomegaly was finally diagnosed radiologically that led to cardiac assessment. Echocardiography suggested left sided partial anomalous pulmonary venous return that was not confirmed at angio-computed tomography scan and cardiac magnetic resonance imaging. Instead, total absence of the pericardium with relative left lung hypoplasia and left-sided bronchiectasis was diagnosed. Immune defect was confirmed. Adequate treatment by immunoglobulin supplementation and observance of the recommended care of bronchiectasis allowed favorable evolution. This case of an unusual association between an exceptional pericardial malformation and immune deficiency causing lower respiratory tract infections complicated by leftsided bronchiectasis highlights the absolute necessity to explore further any child with insufficient asthma control.
Key words: Pericardial agenesis, child, hypogammaglobulinemia
Introduction
Total absence of the pericardium is a rare affection with an incidence of 1:10000.1,2 It is usually asymptomatic, making its diagnosis difficult to establish.3
Pericardial agenesis is due to the absence of the fusion between the pleuropericardial membrane that is the membranous fold coming from the ventral side of the common cardinal vein, and the pulmonary vascular roughing during embryogenesis at the 5th week of gestation.4 It affects boys more often than girls with a ratio of 3:1.5
Case Report
An 8-year-old girl was referred to our department following the discovery of cardiomegaly on the chest X-ray during recent exploration for recurrent lower respiratory infections.
Her personal history was uneventful until the age of 5 weeks when she was hospitalized for viral bronchiolitis. At that time, a first echocardiography was performed because of a cardiac murmur and was considered normal. Since then, she showed recurrent wheezing and - lower respiratory tract infections. Allergic asthma and a gastro- esophageal reflux were excluded. Additionally, at the age of 7 months, transient hypo-gammaglobulinemia was diagnosed that was treated for 12 weeks by immunoglobulin substitution until normalization of the immune status. Symptomatic treatment did not lead to significant improvement of the clinical condition. Instead, chronic cough and wheezing persisted as well as frequent lower respiratory tract infections requiring frequent antibiotic administrations. In the course of her chronic disease, the patient underwent at least 10 chest X-Rays in different institutions that always showed a cardiomegaly. The last one led to cardiological assessment.
At clinical examination, weight and height were on the 25th and 50-75th percentile, respectively. Blood pressure and transcutaneous oxygen saturation were normal. The precordium was hyperactive over the right ventricle and the apex beat was deviated left- and backwards. The rest of the examination was normal.
ECG showed a right axial deviation, a first-degree A-V block and an incomplete right bundle branch block.
At echocardiography there was an axial deviation of the heart to the left, an enlarged right ventricle and dilated trunk of the pulmonary artery. The right pulmonary veins appeared enlarged while the left ones could not be depicted with sufficient precision. The atrial septum was intact in all its portions. No cardinal vein could be identified.
An additional imaging with angio-computed tomography (CT) was performed to exclude a left-sided partial anomalous pulmonary venous return. Dilation of the right cardiac cavities was confirmed. Hypermobility of the heart suggested the complete absence of the pericardium. The left lung appeared relatively hypoplastic with smaller arteries and veins compared to the right-sided ones. The left lung was also partially compressed by the leftwards-displaced heart, and showed a hypoventilation of the lower lobe with left-sided posterobasal bronchiectasis (Figure 1).
This diagnosis of total absence of pericardium was confirmed by a cardiac magnetic resonance imaging (MRI) performed in supine, left- and right lateral position (Figure 2).
The immunological investigation confirmed the presence of a hypogammaglobulinemia with decreased IgG- (6.5 g/L; normal: 7.9-13.2 g/L) and IgG4 levels (0.04 g/L; normal: 0.09-1.58 g/L).
Multidisciplinary care was undertaken with substitutive treatment by a subcutaneous immunoglobulin, treatment for nonallergic asthma and intensive respiratory physiotherapy.
This treatment led to a drastic improvement of the symptomatology.
The retrospective analysis of all chest X-Rays performed in our patients since the age of 5 weeks showed the typical absence of the right cardiac border and a leftward displacement of the heart (Figure 3).
Discussion
We report the case of a patient with nonallergic asthma, frequent lower respiratory infections and IgG deficiency in whom total absence of pericardium was finally diagnosed. This was associated with relative left lung hypoplasia and left postero-basal bronchiectasis.
Our case illustrates the difficulty of diagnosing total absence of the pericardium that is usually asymptomatic, in contrary to the partial form. Indeed, the partial form can manifest by chest pain related to ischemic accidents secondary to herniation of cardiac structures such as the left auricle in the pericardial orifice. However, in our patient, the absence of asthma control should have prompted to further examination. Total absence of the pericardium may be suggested by a typical image at chest XRay with leftward displacement of the heart, the absence of the right border of the heart, and the flattened and elongated contour of the left ventricle (so called Snoopy sign).6 Additionally, pulmonary tissue interposed between the pulmonary artery and the aorta may be observed. This latter is also observed in the partial form of pericardial agenesis.7 In our patient, the retrospective analysis of all chest X-rays performed since the age of 5 weeks confirmed the typical image with absence of the right heart border due the absent projection of the right atrium secondary to the leftwards rotation of the heart.
Cardiac MRI allows generally diagnosis and is superior to angio-CT. The pericardium appears normally as a curvilinear line surrounded by high signal due to epicardial and mediastinal fat but the lack of its depiction may occur in healthy patients.2,8,9 Therefore, examination in supine and right lateral position, as it was realized in our patient should be performed to confirm diagnosis.10
In the contrary to the reported literature, our patient was symptomatic with respiratory symptoms of bronchial hyper-reactivity and recurrent lower respiratory tract infections. We propose that compressive leftward deviation of the heart leading to a certain degree of hypoventilation of the left lung in the presence of hypo-gammaglobulinemia has caused secretion retention and chronic infection finally responsible for the progression to bronchiectasis. The fact that the symptomatology has improved with substitutive immunoglobulin therapy and intensive physiotherapy reinforces this view. Our case also points out that in children, asthma that does not respond to an adequately conducted treatment must be investigated further.
The association of total absence of pericardium with hypogammaglobulinemia and bronchiectasis has not been reported yet. If we can postulate that bronchiectasis is a complication of the disease, the immune deficiency is primitive. This latter is likely to be independent to the pericardial malformation.
Figure 1.
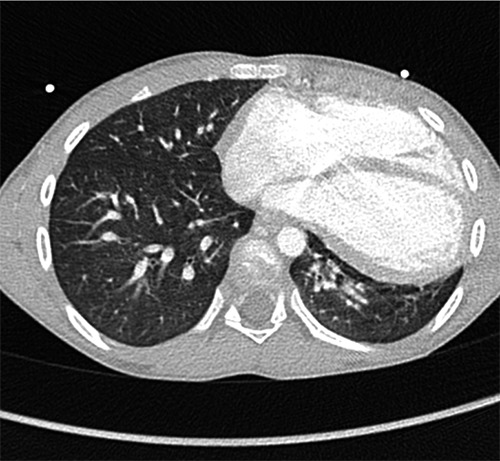
Contrast enhanced thoracic computed tomography in axial view, showing the leftward displacement of the heart and the hypoplasia of the left lower lobe. No pericardial lining is visible.
Figure 2.
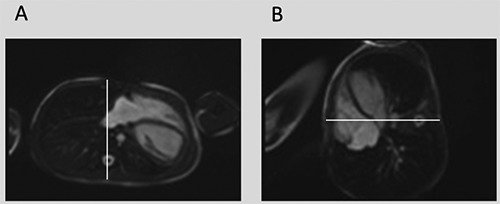
Cardiac Magnetic Resonance Imaging performed in dorsal decubitus (A) and in right lateral decubitus (B). The sequence is acquired in steady-state free precession. The white line represents the anterior-posterior axis of the thorax. It allows the estimation of the rightward cardiac displacement in right lateral position.
Figure 3.
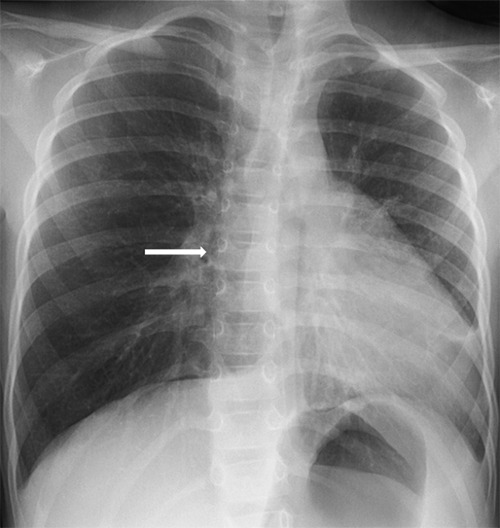
Chest X-Ray, frontal view. It documents the leftward shift of the heart and the loss of the right cardiac border (arrow).
Conclusions
Our case illustrates a not yet reported association of a total absence of the pericardium causing compression of the left lung, and hypo-gammaglobulinemia complicated by bronchiectasis. The clinical manifestations were those of bronchial hyper-reactivity with recurrent lower respiratory tract infections. Typical signs of total absence of the pericardium on chest X-Ray were present early in life. Their recognition had probably allowed early diagnosis and prophylactic treatment that, together with immunoglobulin substitution might have avoided bronchiectasis development. This case points out the necessity to consider extra-pulmonary abnormalities in case of uncontrolled asthma.
Funding Statement
Funding: None.
References
- 1.Nasser WK. Congenital absence of the left pericardium. Am J Cardiol 1970;26: 466-70. [DOI] [PubMed] [Google Scholar]
- 2.Shah AB, Kronzon I. Congenital defects of the pericardium: A review. Eur Heart J Cardiovasc Imag 2015;16: 821-7. [DOI] [PubMed] [Google Scholar]
- 3.Van Son JA, Danielson GK, Schaff HV, et al. Congenital Partial and Complete Absence of the Pericardium. Mayo Clin Proc 1993;68:743-7. [DOI] [PubMed] [Google Scholar]
- 4.Sunderland S. Congenital pericardial defects. Br Heart J 1944;6:167-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaurasia BD. Congenital pericardial defects. Teratology 1973;8:55-67. [DOI] [PubMed] [Google Scholar]
- 6.von Bernuth G, Nissen H, Lang D, Hofstetter R. [Congenital defects of the left-sided pericardium]. Z Kardiol 1976;65:1022-32. [PubMed] [Google Scholar]
- 7.Gatzoulis MA, Munk MD, Merchant N, et al. Isolated congenital absence of the pericardium: clinical presentation, diagnosis, and management. Ann Thorac Surg 2000;69:1209-15. [DOI] [PubMed] [Google Scholar]
- 8.Rajiah P. Cardiac MRI: Part 2, Pericardial Diseases. Am J Roentgenol 2011;197:W621-34. [DOI] [PubMed] [Google Scholar]
- 9.Valero E, Ferrero JA, López-Lereu MP, Chorro FJ. Symptomatic Partial Congenital Absence of the Pericardium Revealed Using Cardiac Magnetic Resonance. Can J Cardiol 2015;31:e5- e7. [DOI] [PubMed] [Google Scholar]
- 10.Connolly HM, Click RL, Schattenberg TT, et al. Congenital absence of the pericardium: Echocardiography as a diagnostic tool. J Am Soc Echocardiogr 1995;8:87-92. [DOI] [PubMed] [Google Scholar]


