Highlights
-
•
Many clinical antibiotics are natural products produced by thiotemplate-based assembly line biosynthetic pathways.
-
•
Assembly line pathways provide an opportunity for rational bioengineering to modify complex natural product structures.
-
•
New, rule-based mix and match strategies facilitate the engineering of non-ribosomal peptide assembly line synthetases.
-
•
Evolutionary guided approaches highlight new avenues for polyketide synthase assembly line reprogramming.
Abstract
Numerous important therapeutic agents, including widely-used antibiotics, anti-cancer drugs, immunosuppressants, agrochemicals and other valuable compounds, are produced by microorganisms. Many of these are biosynthesised by modular enzymatic assembly line polyketide synthases, non-ribosomal peptide synthetases, and hybrids thereof. To alter the backbone structure of these valuable but difficult to modify compounds, the respective enzymatic machineries can be engineered to create even more valuable molecules with improved properties and/or to bypass resistance mechanisms. In the past, many attempts to achieve assembly line pathway engineering failed or led to enzymes with compromised activity. Recently our understanding of assembly line structural biology, including an appreciation of the conformational changes that occur during the catalytic cycle, have improved hugely. This has proven to be a driving force for new approaches and several recent examples have demonstrated the production of new-to-nature molecules, including anti-infectives. We discuss the developments of the last few years and highlight selected, illuminating examples of assembly line engineering.
Current Opinion in Microbiology 2019, 51:88–96
This review comes from a themed issue on Antimicrobials
Edited by Mattew I Hutchings, Andrew W Truman and Barrie Wilkinson
For a complete overview see the Issue and the Editorial
Available online 16th November 2019
https://doi.org/10.1016/j.mib.2019.10.007
1369-5274/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Articles addressing antimicrobial resistance (AMR) with titles such as ‘Antibiotic apocalypse: doctors sound alarm over drug resistance’ (The Guardian), regularly hit the news headlines and are no longer limited to the scientific community. Amongst the many solutions discussed on the issue of AMR [1], a repeatedly stressed source for discovering new therapeutic agents are specialised metabolites (SM) [2,3]. SMs are obtained from living organisms and comprise structurally diverse and bioactive molecules with low molecular weight (<3000) [4] which occupy chemical property space beyond Lipinski’s rule of five [5].
Recent advances in next-generation DNA sequencing methods [6], in conjunction with genome [7] and structure mining [8, 9, 10] approaches, including mass spectrometry-based proteomics [11], have guided the identification and isolation of novel SMs with antimicrobial activity. In addition, a high-throughput screening approach for the discovery of SM antibiotics was recently reported [12], while chemoinformatic approaches [13], and enhanced methodology for the identification of antibiotics [14] and their producing strains have been developed [15]. Notably, technological advances in metagenomics [16] and in situ cultivation [17] led to the identification of two promising new classes of antibiotics, the malacidins (metagenomic acidic lipopeptide antibiotic-cidins) and teixobactins, respectively.
To become drugs, SMs are often chemically modified to fine tune their biological activities and/or improve biophysical properties [18]. Because of their complex structures, many clinical SM derivatives have been created by means of semi-synthesis, for example, azithromycin [19]. Because of technical and chemical limitations, such modifications are often confined to a few synthetically accessible functional groups and leave the backbone structure untouched. In contrast, bioengineering offers an alternative approach to alter SM backbones and access a wider range of structural diversity.
The complex chemical structures and enormous pharmaceutical potential of the non-ribosomal peptides (NRPs; e.g. teixobactin) [17] and polyketides (PKs; e.g. avermectin) [20] make these SMs key targets for bioengineering. They are synthesised on large modular non-ribosomal peptide synthetases (NRPS) [21] (Figure 1) and polyketide synthases (PKS) [22] (Figure 3) respectively, using a conserved multiple-carrier thiotemplate mechanism [23]. They use peptide bond formation (NRPS), Claisen-type condensation reactions (PKS) or combinations thereof (NRPS/PKS hybrids) to build larger molecules from simple building blocks [24,25].
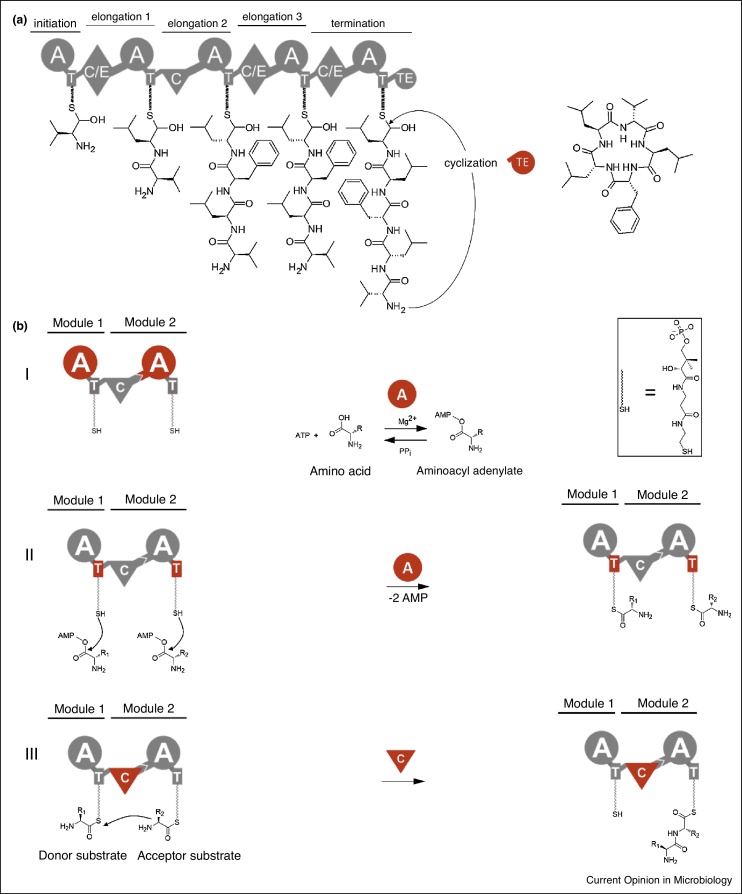
Figure 1.
Introduction to modular non-ribosomal peptide synthetases.
(a) The modular architecture of NRPSs, exemplified by the GameXPeptide-Synthetase (GxpS). A NRPS module is defined as the catalytic unit responsible for the incorporation of one-specific building block into the growing peptide chain and associated functional group modifications. Enzymatic activities of a canonical module reside in the adenylation (A), thiolation (T), and condensation (C) domains. (b) Firstly, amino acids (AAs) are activated through the activity of an A-domain (I). The energy derived from ATP hydrolysis is used to form an aminoacyl-adenylate intermediate. Then the AA is loaded onto the thiol of the pantetheine cofactor of the T domain (II). Consecutively activated AA on T domains are then joined by a C-domain that catalyses peptide-bond formation and transfers the upstream AA or peptide to the downstream substrate (III). Finally, most NRPS termination modules harbour a TE (thioesterase) domain, usually responsible for the release of linear (transfer to a water molecule), cyclic or branched cyclic peptides (amide or ester linkage). In addition, a cyclization (Cy) domain instead of a C-domain or a terminal condensation (Cterm) domain in place of a TE-domain can be present. Modification domains include epimerization (E), N-methylation (MT) or oxidation (Ox) domains.
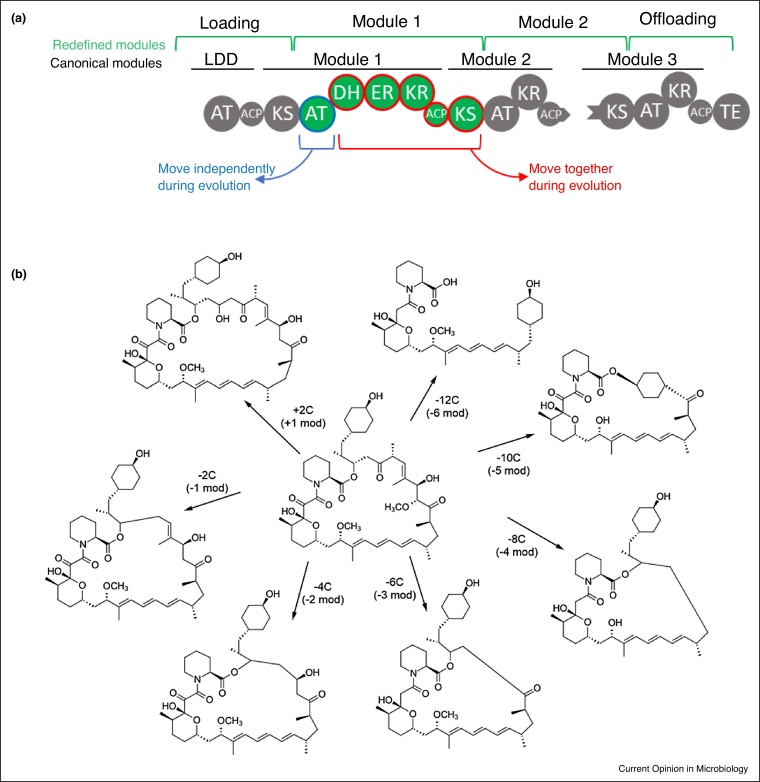
Figure 3.
Engineering polyketide synthases.
(a) Truncated representation of 6-deoxyerythronolide B synthase. Like NRPS A-domains, the PKS acyltransferase (AT) domains select malonyl-derived coenzyme A (CoA)-linked extender units. The resulting malonate derivatives are transferred to acyl carrier proteins (ACP) which function like NRPS T-domains, and ketosynthase (KS) domains act like NRPS C-domains. PKSs also have additional tailoring domains present to modify the PK-chain, that is, ketoreductase (KR), dehydratase (DH), enoylreductase (ER), and methyltransferase (MT) domains. The revised definition of modules (green) as well as co-evolving domains (blue, red) are highlighted. (b) Rapalogs, a diversity-oriented library of rapamycin derivatives gained by laboratory-scale evolution, termed ‘accelerated evolution’. Forced recombination events of the highly homologous rapamycin pathway lead to the loss of modules (mod) 1–6 or the addition of a second copy of module 13.
Commonly, the number of modules in a NRPS or PKS corresponds directly to the number of monomers incorporated into the associated SM, and the arrangement of the modules directly follows the SMs’ primary sequence because synthesis proceeds in a colinear fashion, enabling structural predictions [8,26]. Once discovered, their modular architecture immediately suggested the possibility for rational manipulation [27]. This was realised for the first time in 1995, for both NRPS [28] and PKS [29]. Since then the synthetic biology community has envisaged using these enzymes like a molecular toolkit to modify and create novel SMs in a tailormade fashion. Unfortunately, many attempts to achieve assembly line pathway engineering have yielded biosynthetic machineries that are either greatly impaired in their activity or non-functional [30].
Much of the fundamental biochemistry regarding mega-synth(et)ases, in addition to crystal structures of domains, didomains, and even whole modules, had been solved by the early years of the new millennium [31]. Despite this, innovation in their bioengineering slowed significantly. However, due to recent technological advances [32,33], our structural as well as conformational knowledge of these enzymes has improved significantly [34•,35]. Valuable insights into the high structural flexibility, and the importance of inter-domain communication, provided a new impetus for a series of novel strategies to engineer modular biosynthetic pathways [36, 37, 38, 39, 40, 41]. Recent publications have demonstrated the production of new-to-nature SMs, including antimicrobials, and we highlight here selected key developments from the last 2–3 years. The principles of NRPS and PKS biosynthesis as well as key enzymatic domain abbreviations are introduced in Figure 1, Figure 3, respectively.
NRPS: challenging dogma
Mega-synth(et)ase engineering involves numerous challenges, including the impact of gatekeeper domains to prevent the incorporation of incorrect extender units into the final product, or limited structural information making it difficult to predict functional protein-protein interactions. Previously, researchers have employed three general strategies to achieve this: (1) substitution of the A-domains or paired A–T domains activating an alternative substrate; (2) targeted mutagenesis of the substrate binding pocket of an A-domain; (3) substitutions that treat C–A or C–A–T domain units as inseparable pairs [30].
A key dogma, leading to strategies (1) and (2), says that A-domains represent the main gatekeeping enzymes [42]. Recent insights are challenging this view and explain why strategies (1) and (2) can only be reliably applied within initiation modules lacking an upstream starter C-domain (Cstart) [43,44]. In addition, termination domains represent often overlooked bottlenecks. For example, impaired reprogrammed NRPS assembly lines could be ‘cured’ by replacing TE-domains with ‘common internal’ C-domains to produce linear-peptides, cyclic-peptides, and depsi-peptides [45••]. Recently, Yu et al. utilised different Cterm-domains of fungal iterative NRPSs to produce new cyclic peptide derivatives of different chain length [46].
Engineering the specificity of individual A-domains (1) from initiation modules represents a proven method for creating structural diversity [47, 48, 49]. Niquille et al. presented the first high-throughput assay to selectively alter the promiscuous l-Phe activating starter A-domain from the tyrocidin NRPS (TycA) to genuinely accept the β-amino-acid residue (S)-β-Phe [50•]. A smart ‘click’-chemistry mediated fluorescent yeast cell surface display assay [51] for catalytic activity in combination with FACS allowed substrate walking from l-Phe to (S)-β-Phe via screening >1 million variants. With a (S)-β-Phe specific A-domain in-hand, the authors showed that scalable amounts of backbone-modified peptides could be produced in vitro (∼1 mmol) and in vivo (∼100 mg L−1). Although the initiation module of TycA was targeted, to prevent proofreading of upstream C-domains, the strict specificity of the TE-domain for the first amino acid resulted in hydrolytic off-loading of the modified peptide rather than cyclic dimer formation. This strategy for NRPS domain evolution is very promising and similar approaches could be leveraged to optimise biosynthetic bottlenecks.
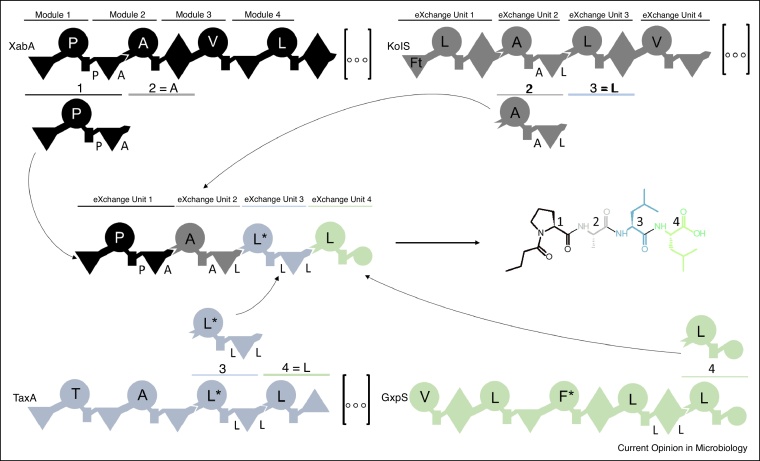
Another convention of NRPS engineering has been the inseparability of C–A domains (strategy 3). The origin of this lies in the canonical definition of a module (C–A–T). In 2008, when the first crystal structure of a full-length termination module (C–A–T–TE) was published (PDB ID: 2VSQ) [31], the convention became dogma. This outstanding work delivered valuable insights into the high structural flexibility of NRPSs and the importance of inter-domain communication that must be maintained during NRPS engineering. Yet, this snapshot of the catalytic cycle led to the premature conclusion that C–A di-domains form a stable workbench that should not be separated [34•,52]. Recently, Bozhuyuk et al. took advantage of additional structural data and challenged the convention by introducing a rule-based mix-and-match strategy (Figure 2) [45••]. The NRPS C–A linker was a prime target for swapping A–T–C units, denoted as eXchange Units (XUs). By combining XUs from 15 NRPSs the authors were able to reconstitute naturally available peptides, peptide derivatives, and generated new-to-nature peptides de novo in good yields. The most important consideration, and therefore restriction, of this strategy is the substrate specificity of the downstream C-domain, which must be respected. The Mueller group also utilised A–T–C XUs to successfully modify lipopeptide BGCs in Myxococcus xanthus, suggesting their general applicability [53], but the downstream C-domain restriction calls for a very large number of available building blocks if new-to-nature NRPs are to be designed in appreciable numbers.
Figure 2.
The eXchange Unit.
Schematic representation of NRPS recombination rules using XUs from the Xenoamicin (XabA), Kolossin (KolS), Taxlllaid (TaxA), and GameXPeptide (GxpS) synthetase considering the C-domain specificities. Symbols used for domain assignment are: A, large circle; T, rectangle; C and formyl transferase (Ft), triangle; C/E, diamond; TE, C-terminal small circle. Amino acid specificities of the A-domains, C-domains and C/E-domains are indicated by capital letters using the standard one letter AA abbreviations. Promiscuous domains are indicated by ‘*’.
To access a high degree of structural diversity in good yield, a recent trend in NRPS engineering utilises evolution-guided strategies [47,54]. In the context of full-length modular NRPSs, the Abe lab successfully accomplished the manipulation of two antimycin-type cyclic depsi-peptides, both derived from a NRPS/PKS hybrid synthetase, to generate a set of derivatives in practical yields [55]. Another illuminating example was published by Meyer, Steiniger et al. [56•]. Engineering of iterative fungal cyclo-depsi-peptide pathways enabled the biosynthesis of enniatin and beauvericin derivatives by combining highly structurally and biochemically related NRPSs. The new peptide derivatives showed up to 12× increased activity against Leishmania donovani and Trypanosoma cruzi compared to the reference drugs miltefosine (Miltex™) and benznidazole (Rochagan™), respectively. Of important note was the ability to produce new-to-nature peptides at an industrially relevant scale (1.3 g L−1).
PKS engineering: from rational engineering to accelerated evolution
In general PKS engineering faces the same challenges and applies the same design principles as, NRPS engineering. Common approaches for PKS engineering have involved the exchange of whole modules or individual domains, especially AT domains [57]. Although early attempts reported mixed results, the Keasling lab comprehensively analysed interdomain linkers in PKSs to define domain boundaries and provide guidelines for replacing AT-domains [58•]. Using monomodular PKSs as a model, the new highly conserved AT-domain boundaries were utilised to great effect, producing short-chain ketones without significant loss of activity for the hybrid enzymes. More recently Kalkreuter et al. applied the newly defined AT-boundaries to enable expansion of AT substrate to include non-natural extender units and exemplified this by targeting the final two modules of the pikromycin PKS (PikAIII, PikAIV) [59]. Initially this resulted in inactive or highly impaired chimeras. By further engineering the specificity of both modules, and utilising in vitro assays of bimodular reactions, they revealed that the KR-domains and KS-domains were more substrate-permissive than respective AT-domains. To take advantage of this promiscuity, cumulative AT active site mutagenesis, guided by homology modelling and molecular dynamics simulations, resulted in robust yields of a pikromycin derivative with two non-natural extenders. The results of these two studies suggests there is, currently, no general method to engineer AT-domains, and that the reported successful attempts represent tailored, non-transferable solutions.
Although AT-domain swaps [58•] and active site mutagenesis [59] can be suitable for PKS engineering, the proofreading activity of downstream domains may limit the use of these approaches. This issue became apparent when the Keasling group introduced heterologous reductive loop swaps (KR/ER/DH) from various PKSs, as well as a TE-domain, into the borrelidin PKS module 1 to biosynthesise adipic acid [60]. Mass spectrometry-based Acyl-ACP intermediate analysis revealed an unexpected bottleneck at the dehydration step. Eventually this limitation was overcome by introducing a carboxyacyl-processing DH-domain, but the results once again highlighted that many caveats must be considered when engineering these systems, including the proof-reading activity of KR domains [61], especially in the context of full length multi-modular PKSs.
Gatekeeping domains are crucial for organisms to ensure biosynthesis of the desired SM, but such proofreading mechanisms can lead to bottlenecks of new SM production. Hansen et al. recently identified the pikromycin (Pik) AIII-TE domain to be of limited substrate flexibility and a key catalytic bottleneck in processing non-natural substrates [62]. Molecular dynamics simulations and quantum mechanical comparison revealed that introduction of a single mutation at the key active site residue (Ser148Cys) in PikAIII-TE significantly increased the substrate flexibility, enabling the production of diastereomeric macrolactones [63]. Similarly, the Leadlay group showed that a single residue change (Ala154Trp) in the KS3 of the erythromycin PKS led to an emphatic increase in turnover of a range of substrates [64]. Identifying and overcoming bottlenecks is very useful to fine tune tailored SM production, but to access a wider chemical space more disruptive bioengineering approaches are necessary.
To overcome PKS reprogramming issues an understanding of biosynthetic gene cluster evolution might provide a rationale for reprogramming assembly line machinery. For example, Zhang et al. challenged a long-standing paradigm in modular PKS, the definition of a module (Figure 3a). They suggested that for module swapping, the canonical module KS-AT-(DH/KR/ER)-ACP should be altered to AT-(DH/KR/ER)-ACP-KS [65••]. Bioinformatic analysis of highly homologous but functionally diverse domains from four giant aminopolyol producing PKSs demonstrated evolutionary relationships between equivalent domains. As also suggested previously [66], they proposed that AT domains move independently during assembly line evolution, whereas the β-processing domains, ACPs, and downstream KS-domains should be considered as a unit, and swaps that conserve the (DH‐ER‐KR)‐ACPn‐KSn+1 relationship would facilitate rational modular PKS engineering. Similar intermodular relationships can also be observed in the evolutionary analysis of several trans‐AT PKSs [67,68], suggesting the generality of this mechanism during the evolution of PKS genes through recombination.
Stochastic approaches to PKS engineering can also lead to new SMs [69]. An illuminating example utilises a homologous recombination-based method to mimic evolutionary pressure, resulting in the accelerated evolution of modular PKS genes in which multiple modules were deleted, added, or replaced leading to a diversity orientated library of new rapamycin derivatives (rapalogs) with altered biological activities in high yields (Figure 3b) [70••]. In contrast to rapamycin, several of the rapalogs were devoid of immunosuppressive activity but retained potent inhibition of FKBP12 like enzymes including macrophage infectivity potentiator proteins (MIPs), validated virulence factors of Gram-negative pathogens. One rapalog was shown to increase the survival of human macrophages infection by the pathogen Burkholderia pseudomallei. Detailed sequence analysis of the recombinant genes revealed junction sites representing non-canonical module boundaries, and recombination ‘hot-spots’ were identified within KS and AT domains and in the linker upstream of ACP domains. ‘Non-rational’ strategies like this in combination with in depth comparative sequence analysis may lead to new design principles that eventually support novel rational engineering strategies. Further development of this method in combination with high throughput screening (e.g. fluorescence-activated cell sorting; FACS) could be used to generate structurally diverse SM-like libraries to identify novel antimicrobial entities.
Concluding remarks
Although landmark achievements in chemical and structural biology [34•,35] have advanced our understanding of modular SM pathway mechanisms, nature’s proven ability to ‘engineer’ assembly-line enzymes remains difficult to recapitulate in the lab. However, the ever-increasing amount of genomic data, as well as the development of non-rational [70••] and high-throughput methods [50•], have enabled the development of novel strategies, especially when highly similar and iterative NRPS, PKS, or hybrids thereof are combined [45••,55,56•].
The ability to rationally swap modules and domains in assembly-line pathways has long been a ‘holy grail’; with the redefinition of PKS modules, experiments based on evolutionary principles, and the identification of catalytically and structurally exchangeable units in NRPSs, this goal may soon be achievable [45••,65••]. Nevertheless, caution is advised, and these disruptive new insights must be extensively evaluated, as future developments can be easily hampered when premature assumptions become dogma. To avoid this, we advocate the publication of unsuccessful or negative results, and an annual review on this subject would prove invaluable in evaluating the generality of recent developments. Insights gained could be used to delineate future guidelines, develop scoring methods to predict the capability of building blocks, and to identify and clarify bottlenecks. The authors call upon colleagues for concerted action in this direction, possibly publishing their negative data in an annual ‘inglorious’ review of assembly-line chemistry and biology. We learn valuable lessons from mistakes, and failure only serves to make us stronger.
Conflict of interest statement
BW is a board member and shareholder of Isomerase Ltd. (Cambridge UK).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
B.W. acknowledges support from the Biotechnology and Biological Sciences Research Council (BBSRC) via Institute Strategic Program Project BBS/E/J/000PR9790 to the John Innes Centre. K.A.J.B. is supported by BBSRC/Innovate UK Biocatalyst Grant BB/N02351/1.
References
- 1.van Belkum A. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol. 2019;17:51–62. doi: 10.1038/s41579-018-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moloney M.G. Natural products as a source for novel antibiotics. Trends Pharmacol Sci. 2016;37:689–701. doi: 10.1016/j.tips.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Rossiter S.E., Fletcher M.H., Wuest W.M. Natural products as platforms to overcome antibiotic resistance. Chem Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies J. Specialized microbial metabolites: functions and origins. J Antibiot (Tokyo) 2013;66:361–364. doi: 10.1038/ja.2013.61. [DOI] [PubMed] [Google Scholar]
- 5.Shultz M.D. Two decades under the influence of the rule of five and the changing properties of approved oral drugs. J Med Chem. 2018;62:1701–1714. doi: 10.1021/acs.jmedchem.8b00686. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Escribano J.P., Alt S., Bibb M.J. Next generation sequencing of Actinobacteria for the discovery of novel natural products. Mar Drugs. 2016;14:78. doi: 10.3390/md14040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremblay N. The use of ClusterMine360 for the analysis of polyketide and nonribosomal peptide biosynthetic pathways. Methods Mol Biol. 2016;1401:233–252. doi: 10.1007/978-1-4939-3375-4_15. [DOI] [PubMed] [Google Scholar]
- 8.Blin K. antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017;45:W36–W41. doi: 10.1093/nar/gkx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khater S. SBSPKSv2: structure-based sequence analysis of polyketide synthases and non-ribosomal peptide synthetases. Nucleic Acids Res. 2017;45:W72–W79. doi: 10.1093/nar/gkx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medema M.H. Minimum information about a biosynthetic gene cluster. Nat Chem Biol. 2015;11:625–631. doi: 10.1038/nchembio.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohimani H. Dereplication of peptidic natural products through database search of mass spectra. Nat Chem Biol. 2017;13:30–37. doi: 10.1038/nchembio.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scanlon T.C., Dostal S.M., Griswold K.E. A high-throughput screen for antibiotic drug discovery. Biotechnol Bioeng. 2014;111:232–243. doi: 10.1002/bit.25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira F., Latino D.A., Gaudencio S.P. A chemoinformatics approach to the discovery of lead-like molecules from marine and microbial sources en route to antitumor and antibiotic drugs. Mar Drugs. 2014;12:757–778. doi: 10.3390/md12020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurita K.L., Linington R.G. Connecting phenotype and chemotype: high-content discovery strategies for natural products research. J Nat Prod. 2015;78:587–596. doi: 10.1021/acs.jnatprod.5b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaker M.N., Waglechner N., Wright G.D. Antibiotic resistance-mediated isolation of scaffold-specific natural product producers. Nat Protoc. 2014;9:1469–1479. doi: 10.1038/nprot.2014.093. [DOI] [PubMed] [Google Scholar]
- 16.Hover B.M. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant gram-positive pathogens. Nat Microbiol. 2018;3:415–422. doi: 10.1038/s41564-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling L.L. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright P.M., Seiple I.B., Myers A.G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew Chem Int Ed Engl. 2014;53:8840–8869. doi: 10.1002/anie.201310843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutak S. Azalides from azithromycin to new azalide derivatives. J Antibiot (Tokyo) 2007;60:85–122. doi: 10.1038/ja.2007.10. [DOI] [PubMed] [Google Scholar]
- 20.Burg R.W. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother. 1979;15:361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.G. Purification of the polyenzymes responsible for tyrocidine synthesis and their dissociation into subunits. Biochemistry. 1973;12:398–405. doi: 10.1021/bi00727a006. [DOI] [PubMed] [Google Scholar]
- 22.Donadio S. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252:675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 23.Stein T. The multiple carrier model of nonribosomal peptide biosynthesis at modular multienzymatic templates. J Biol Chem. 1996;271:15428–15435. doi: 10.1074/jbc.271.26.15428. [DOI] [PubMed] [Google Scholar]
- 24.Klaus M., Grininger M. Engineering strategies for rational polyketide synthase design. Nat Prod Rep. 2018;35:1070–1081. doi: 10.1039/c8np00030a. [DOI] [PubMed] [Google Scholar]
- 25.Sussmuth R.D., Mainz A. Nonribosomal peptide synthesis-principles and prospects. Angew Chem Int Ed Engl. 2017;56:3770–3821. doi: 10.1002/anie.201609079. [DOI] [PubMed] [Google Scholar]
- 26.Mootz H.D., Schwarzer D., Marahiel M.A. Ways of assembling complex natural products on modular nonribosomal peptide synthetases. Chembiochem. 2002;3:490–504. doi: 10.1002/1439-7633(20020603)3:6<490::AID-CBIC490>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Stachelhaus T., Marahiel M.A. Modular structure of peptide synthetases revealed by dissection of the multifunctional enzyme GrsA. J Biol Chem. 1995;270:6163–6169. doi: 10.1074/jbc.270.11.6163. [DOI] [PubMed] [Google Scholar]
- 28.Stachelhaus T., Schneider A., Marahiel M.A. Rational design of peptide antibiotics by targeted replacement of bacterial and fungal domains. Science. 1995;269:69–72. doi: 10.1126/science.7604280. [DOI] [PubMed] [Google Scholar]
- 29.Cortes J. Repositioning of a domain in a modular polyketide synthase to promote specific chain cleavage. Science. 1995;268:1487–1489. doi: 10.1126/science.7770773. [DOI] [PubMed] [Google Scholar]
- 30.Calcott M.J., Ackerley D.F. Genetic manipulation of non-ribosomal peptide synthetases to generate novel bioactive peptide products. Biotechnol Lett. 2014;36:2407–2416. doi: 10.1007/s10529-014-1642-y. [DOI] [PubMed] [Google Scholar]
- 31.Tanovic A. Crystal structure of the termination module of a nonribosomal peptide synthetase. Science. 2008;321:659–663. doi: 10.1126/science.1159850. [DOI] [PubMed] [Google Scholar]
- 32.Bonomi M., Vendruscolo M. Determination of protein structural ensembles using cryo-electron microscopy. Curr Opin Struct Biol. 2018;56:37–45. doi: 10.1016/j.sbi.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Murata K., Wolf M. Cryo-electron microscopy for structural analysis of dynamic biological macromolecules. Biochim Biophys Acta Gen Subj. 2018;1862:324–334. doi: 10.1016/j.bbagen.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 34•.Drake E.J. Structures of two distinct conformations of holo-non-ribosomal peptide synthetases. Nature. 2016;529:235–238. doi: 10.1038/nature16163. [DOI] [PMC free article] [PubMed] [Google Scholar]; Drake et al. describe the structures of two different holo-NRPSs modules, each revealing a distinct step in the catalytic cycle. These structures and single-particle electron microscopy analysis not only demonstrate a highly dynamic domain architecture but demonstrate that the C-A domain platform is more dynamic then previously proposed. This outstanding work provides the foundation for understanding the structural mechanisms to enable engineering novel NRPSs and therefore is a must read for all who intend to engineer NRPSs.
- 35.Dutta S. Structure of a modular polyketide synthase. Nature. 2014;510:512–517. doi: 10.1038/nature13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown A.S. Structural, functional and evolutionary perspectives on effective re-engineering of non-ribosomal peptide synthetase assembly lines. Nat Prod Rep. 2018;35:1210–1228. doi: 10.1039/c8np00036k. [DOI] [PubMed] [Google Scholar]
- 37.Dodge G.J., Maloney F.P., Smith J.L. Protein-protein interactions in "cis-AT" polyketide synthases. Nat Prod Rep. 2018;35:1082–1096. doi: 10.1039/c8np00058a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izore T., Cryle M.J. The many faces and important roles of protein-protein interactions during non-ribosomal peptide synthesis. Nat Prod Rep. 2018;35:1120–1139. doi: 10.1039/c8np00038g. [DOI] [PubMed] [Google Scholar]
- 39.Kosol S. Protein-protein interactions in trans-AT polyketide synthases. Nat Prod Rep. 2018;35:1097–1109. doi: 10.1039/c8np00066b. [DOI] [PubMed] [Google Scholar]
- 40.Miyanaga A., Kudo F., Eguchi T. Protein-protein interactions in polyketide synthase-nonribosomal peptide synthetase hybrid assembly lines. Nat Prod Rep. 2018;35:1185–1209. doi: 10.1039/c8np00022k. [DOI] [PubMed] [Google Scholar]
- 41.Whicher J.R. Structural rearrangements of a polyketide synthase module during its catalytic cycle. Nature. 2014;510:560–564. doi: 10.1038/nature13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stachelhaus T., Mootz H.D., Marahiel M.A. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol. 1999;6:493–505. doi: 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 43.Li R., Oliver R.A., Townsend C.A. Identification and characterization of the sulfazecin monobactam biosynthetic gene cluster. Cell Chem Biol. 2017;24:24–34. doi: 10.1016/j.chembiol.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer S. Biochemical dissection of the natural diversification of microcystin provides lessons for synthetic biology of NRPS. Cell Chem Biol. 2016;23:462–471. doi: 10.1016/j.chembiol.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 45••.Bozhuyuk K.A.J. De novo design and engineering of non-ribosomal peptide synthetases. Nat Chem. 2018;10:275–281. doi: 10.1038/nchem.2890. [DOI] [PubMed] [Google Scholar]; The authors report a strategy for rational manipulation of NRPSs centered on the use of newly identified exchange units (XUs), as opposed to the traditionally-defined modules. They were able to reconstitute naturally available peptides, peptide derivatives, and generated new-to-nature peptides de novo. The XU approach represents a promising method for creating 'designer' NRPS assembly lines.
- 46.Yu D. Decoding and reprogramming fungal iterative nonribosomal peptide synthetases. Nat Commun. 2017;8 doi: 10.1038/ncomms15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kries H., Niquille D.L., Hilvert D. A subdomain swap strategy for reengineering nonribosomal peptides. Chem Biol. 2015;22:640–648. doi: 10.1016/j.chembiol.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Kries H. Reprogramming nonribosomal peptide synthetases for "clickable" amino acids. Angew Chem Int Ed Engl. 2014;53:10105–10108. doi: 10.1002/anie.201405281. [DOI] [PubMed] [Google Scholar]
- 49.Villiers B., Hollfelder F. Directed evolution of a gatekeeper domain in nonribosomal peptide synthesis. Chem Biol. 2011;18:1290–1299. doi: 10.1016/j.chembiol.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 50•.Niquille D.L. Nonribosomal biosynthesis of backbone-modified peptides. Nat Chem. 2018;10:282–287. doi: 10.1038/nchem.2891. [DOI] [PubMed] [Google Scholar]; Niquille et al. developed and utilized a high-throughput assay coupled with a 'substrate-walking' strategy to selectively alter an l-Phe-specific A-domain to accept a backbone-modified (S)-β-Phe, β-amino-acid residue.
- 51.Zhang K. Engineering the substrate specificity of the DhbE adenylation domain by yeast cell surface display. Chem Biol. 2013;20:92–101. doi: 10.1016/j.chembiol.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller B.R. Structures of a nonribosomal peptide synthetase module bound to MbtH-like proteins support a highly dynamic domain architecture. J Biol Chem. 2016;291:22559–22571. doi: 10.1074/jbc.M116.746297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan F. Synthetic biology approaches and combinatorial biosynthesis towards heterologous lipopeptide production. Chem Sci. 2018;9:7510–7519. doi: 10.1039/c8sc02046a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson M.C., Piel J. Metagenomic approaches for exploiting uncultivated bacteria as a resource for novel biosynthetic enzymology. Chem Biol. 2013;20:636–647. doi: 10.1016/j.chembiol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Awakawa T. Reprogramming of the antimycin NRPS-PKS assembly lines inspired by gene evolution. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Steiniger C. Harnessing fungal nonribosomal cyclodepsipeptide synthetases for mechanistic insights and tailored engineering. Chem Sci. 2017;8:7834–7843. doi: 10.1039/c7sc03093b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using Aspergillus niger as a heterologous expression host, the authors were able to engineer iterative fungal non-ribosomal cyclo-depsipeptide synthetases. By applying smart combinatorial swapping among mechanistically and evolutionary related BGCs they obtained enniatin, beauvericin and bassianolide derivatives with improved bioactivity at high titre.
- 57.Barajas J.F. Engineered polyketides: synergy between protein and host level engineering. Synth Syst Biotechnol. 2017;2:147–166. doi: 10.1016/j.synbio.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Yuzawa S. Comprehensive in vitro analysis of acyltransferase domain exchanges in modular polyketide synthases and its application for short-chain ketone production. ACS Synth Biol. 2017;6:139–147. doi: 10.1021/acssynbio.6b00176. [DOI] [PubMed] [Google Scholar]; Yuzawa et al. systematically analysed AT-domain segments and interdomain linkers in PKSs to determine recombination points for swapping AT-domains. The authors identified unreported boundaries within a module that can be used to exchange AT-domains while maintaining protein stability and enzyme activity. They reported high-efficiency AT-exchanges from a variety of systems that led to production of industrially important short-chain ketones, in vitro and in vivo.
- 59.Kalkreuter E. Engineering the substrate specificity of a modular polyketide synthase for installation of consecutive non-natural extender units. J Am Chem Soc. 2019;141:1961–1969. doi: 10.1021/jacs.8b10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagen A. Engineering a polyketide synthase for in vitro production of adipic acid. ACS Synth Biol. 2016;5:21–27. doi: 10.1021/acssynbio.5b00153. [DOI] [PubMed] [Google Scholar]
- 61.Zheng J., Piasecki S.K., Keatinge-Clay A.T. Structural studies of an A2-type modular polyketide synthase ketoreductase reveal features controlling alpha-substituent stereochemistry. ACS Chem Biol. 2013;8:1964–1971. doi: 10.1021/cb400161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen D.A., Koch A.A., Sherman D.H. Identification of a thioesterase bottleneck in the pikromycin pathway through full-module processing of unnatural pentaketides. J Am Chem Soc. 2017;139:13450–13455. doi: 10.1021/jacs.7b06432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koch A.A. A single active site mutation in the pikromycin thioesterase generates a more effective macrocyclization catalyst. J Am Chem Soc. 2017;139:13456–13465. doi: 10.1021/jacs.7b06436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy A.C. Broadening substrate specificity of a chain-extending ketosynthase through a single active-site mutation. Chem Commun (Camb) 2016;52:8373–8376. doi: 10.1039/c6cc03501a. [DOI] [PubMed] [Google Scholar]
- 65••.Zhang L. Characterization of giant modular PKSs provides insight into genetic mechanism for structural diversification of aminopolyol polyketides. Angew Chem Int Ed Engl. 2017;56:1740–1745. doi: 10.1002/anie.201611371. [DOI] [PubMed] [Google Scholar]; Zhang et al. report the characterization of giant modular polyketide synthases (PKSs) responsible for production of aminopolyol polyketides. But more important, herein a long-standing paradigm in modular polyketide synthase enzymology, namely the definition of a module, has been challenged.
- 66.Jenke-Kodama H., Borner T., Dittmann E. Natural biocombinatorics in the polyketide synthase genes of the actinobacterium Streptomyces avermitilis. PLoS Comput Biol. 2006;2 doi: 10.1371/journal.pcbi.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fisch K.M. Polyketide assembly lines of uncultivated sponge symbionts from structure-based gene targeting. Nat Chem Biol. 2009;5:494–501. doi: 10.1038/nchembio.176. [DOI] [PubMed] [Google Scholar]
- 68.Ueoka R. Metabolic and evolutionary origin of actin-binding polyketides from diverse organisms. Nat Chem Biol. 2015;11:705–712. doi: 10.1038/nchembio.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chemler J.A. Evolution of efficient modular polyketide synthases by homologous recombination. J Am Chem Soc. 2015;137:10603–10609. doi: 10.1021/jacs.5b04842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70••.Wlodek A. Diversity oriented biosynthesis via accelerated evolution of modular gene clusters. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; A new method for laboratory-scale evolution of modular gene clusters was reported, termed ‘accelerated evolution’. Forced recombination events of the highly homologous rapamycin pathway, along with precursor-directed biosynthesis, led to the production of over one hundred new rapalogs.