Abstract
Background & Aims
There is a long-standing debate regarding the biological significance of polyploidy in hepatocytes. Recent studies have provided increasing evidence that hepatocytes with different ploidy statuses behave differently in a context-dependent manner (eg, susceptibility to oncogenesis, regenerative ability after injury, and in vitro proliferative capacity). However, their overall transcriptomic differences in a physiological context is not known.
Methods
By using microarray transcriptome analysis, we investigated the heterogeneity of hepatocyte populations with different ploidy statuses. Moreover, by using single-cell quantitative reverse-transcription polymerase chain reaction (scPCR) analysis, we investigated the intrapopulational transcriptome heterogeneity of 2c and 4c hepatocytes.
Results
Microarray analysis showed that cell cycle–related genes were enriched in 8c hepatocytes, which is in line with the established notion that polyploidy is formed via cell division failure. Surprisingly, in contrast to the general consensus that 2c hepatocytes reside in the periportal region, in our bulk transcriptome and scPCR analyses, the 2c hepatocytes consistently showed pericentral hepatocyte-enriched characteristics. In addition, scPCR analysis identified a subpopulation within the 2c hepatocytes that co-express the liver progenitor cell markers Axin2, Prom1, and Lgr5, implying the potential biological relevance of this subpopulation.
Conclusions
This study provides new insights into hepatocyte heterogeneity, namely 2c hepatocytes are preferentially localized to the pericentral region, and a subpopulation of 2c hepatocytes show liver progenitor cell–like features in terms of liver progenitor cell marker expression (Axin2, Prom1, and Lgr5).
Keywords: Hepatocyte, Ploidy, Zonation, Single-Cell PCR, Transcriptome
Abbreviations used in this paper: ANOVA, analysis of variance; BEC, biliary epithelial cell; FACS, fluorescence-activated cell sorting; GO, gene ontology; LPC, liver progenitor cell; NPC, nonparenchymal cell; PCA, principal component analysis; RM, repeated-measures; scPCR, single-cell quantitative reverse-transcription polymerase chain reaction; TP, triple positive
Graphical abstract
See editorial on page 193.
Summary.
By using rat hepatocytes with different ploidy statuses, a bulk transcriptome and single-cell quantitative reverse-transcription polymerase chain reaction show that diploid hepatocytes are preferentially located in the pericentral region. Single-cell analysis further identifies a subpopulation within the 2c hepatocytes that co-express the mature hepatocyte markers and liver progenitor cell markers.
Polyploidy is a characteristic feature of hepatocytes, but its biological significance is largely unknown.1, 2, 3, 4 Hepatocytes are diploid (2c) at birth, but, after weaning, their DNA content increases. In the adult rodent liver, up to 90% of hepatocytes are polyploid under physiological conditions.5 The majority of polyploid hepatocytes are tetraploid (4c), but some are octoploid (8c) or even greater (≥16c). Although awareness of this heterogeneity is not new, whether hepatocytes with different ploidy statuses have different characteristics is still under debate.
Recent studies have provided evidence that hepatocytes with distinct ploidy statuses have different phenotypes. For example, Wang et al6 showed that pericentral Axin2+ hepatocytes are enriched with a 2c population and Axin2+ 2c hepatocytes contribute to hepatocyte turnover and the maintenance of liver homeostasis. Our group found that 2c hepatocytes, in response to growth stimuli (ie, a small molecule cocktail), acquired a higher in vitro proliferative capacity than that of their 4c and 8c counterparts.7 Similarly, Wilkinson et al8 reported that the polyploid state restricts hepatocyte proliferation and liver regeneration. On the other hand, the hepatocyte polyploidization prevents tumorigenesis by decreasing their susceptibility to genomic aberrations.8, 9 These reports suggested that ploidy status affects the phenotype of hepatocytes; however, the studies have been focused on specific processes. More comprehensive analyses are required to gain broader, more holistic insights into this phenomenon.
In this study, we performed a microarray analysis to compare the transcriptomes of 2c, 4c, and 8c rat hepatocytes. In addition, to address any potential transcriptomic heterogeneity in hepatocytes with different ploidy statuses, we performed single-cell quantitative reverse-transcription polymerase chain reaction (scPCR) using a set of hepatocyte and liver progenitor cell (LPC) marker genes. Contrary to the widely accepted notion that 2c hepatocytes reside in the periportal region,5, 10, 11, 12, 13 both our bulk transcriptome and scPCR results showed that 2c hepatocytes are preferentially located in the pericentral region. In addition, scPCR analysis showed the existence of a progenitor-like population of 2c hepatocytes.
Results
Microarray Transcriptome Analysis Identified 8c Hepatocytes as Cells With Typical Polyploid Characteristics
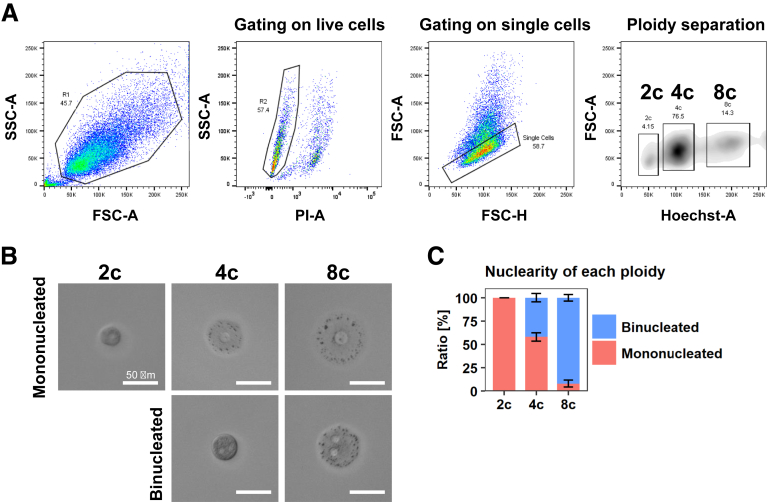
By using freshly isolated rat adult hepatocytes, we obtained 2c, 4c, and 8c hepatocytes using fluorescence-activated cell sorting (FACS) based on the fluorescence intensity of the DNA stain Hoechst 33342 (Figure 1A). We validated the accuracy of this sorting method by performing microscopic observations, which confirmed that none of the cells sorted into the 2c population were binucleated, whereas 42.0% ± 4.44% and 92.0% ± 7.10% of 4c- and 8c-sorted cells were binucleated, respectively (means ± SEM) (Figure 1B and C). Importantly, by phase-contrast imaging, we confirmed that the nucleus size of the mononucleated hepatocytes in the 4c fraction was larger than that in the 2c fraction (Figure 1B). Likewise, the nucleus size of the binucleated hepatocytes in the 8c fraction was larger than that in the 4c fraction (Figure 1B).
Figure 1.
Validation of FACS sorting of hepatocytes with different ploidy statuses. (A) Representative gating for rat hepatocytes with 2c, 4c, and 8c DNA content as assessed by Hoechst 33342 fluorescent intensity. (B) Representative images of single-sorted 2c, 4c, and 8c hepatocytes. Ploidy statuses of the sorted hepatocytes were validated via microscopy on day 1 after plating. Images were taken using the BZX-710 microscope (Keyence). (C) Microscopic validation of nuclear numbers of hepatocytes. The data indicate the means ± SEM of 4 independent experiments. FSC-A, forward scatter-area; FSC-H, forward scatter-height; PI-A, propidium iodide-area; SSC-A, side scatter-area.
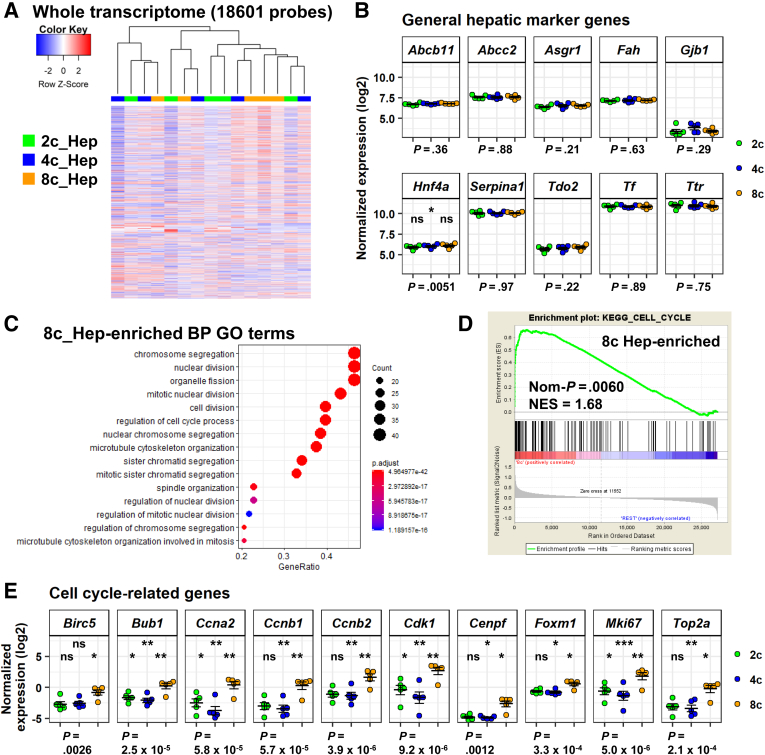
We next performed a microarray transcriptome analysis on the sorted populations of hepatocytes. After excluding probes without gene annotations and those with low expression levels (see the Materials and Methods section for details), we performed hierarchical clustering analysis at the whole-transcriptome level. As shown in Figure 2A, we did not observe a clear difference between 2c, 4c, and 8c hepatocytes. As expected, the expression levels of typical hepatocyte marker genes, including Serpina1, Tf, and Ttr, were almost the same in these 3 populations (Figure 2B), with an exception of the expression level of Hnf4a in 2c hepatocytes, which was slightly but significantly lower than the 8c counterpart.
Figure 2.
Microarray transcriptome analysis of rat hepatocytes with different ploidy statuses. (A) Heatmap with hierarchical clustering of whole-transcriptome analysis of 18,601 probes. Experiments were performed with 5 rats. (B) Expression of general hepatocyte marker genes. Data are means ± SEM (n = 5). (C) 8c hepatocyte-enriched pathways with GO terms (biological process) are shown. Dots represent term enrichment with color coding. The sizes of the dots represent the percentage of each GO term. (D) Gene signature enrichment analysis of 8c hepatocytes and 2c and 4c hepatocytes using the KEGG cell-cycle gene set. (E) Expression of cell cycle–related genes. Data are means ± SEM (n = 5). (B and E) Bottom: P values were calculated by RM 1-way ANOVA, followed by the Holm multiple comparisons test. P values for post hoc tests are presented as follows: *P < .05, **P < .01, ***P < .001. Significance symbols on the left, middle, and right in each panel indicate the comparison between 2c and 4c hepatocytes, 2c and 8c hepatocytes, and 4c and 8c hepatocytes, respectively. BP, biological process; KEGG, Kyoto Encyclopedia of Genes and Genomes; Nom, nominal.
Under physiological conditions, the polyploidization of hepatocytes is caused by cytokinesis failure during the cell cycle.3, 4 We found that most of the gene ontology (GO) bioprocess pathways enriched in 8c hepatocytes were associated with the cell cycle (Figure 2C). This observation was supported further by gene signature enrichment analysis (Figure 2D). Furthermore, we confirmed that the representative cell cycle– and cell division–associated genes were expressed at higher levels exclusively in 8c hepatocytes (Figure 2E). These results collectively show that the transcriptomes were overall similar among hepatocytes with different ploidy statuses, with the exception of the transcriptome of 8c hepatocytes. Thus, the transcriptome data are consistent with known differences between hepatocytes with different polyploidy statuses, indicating that the analysis was valid and biologically relevant.
2c Hepatocytes Show a Zone 3–Enriched Gene Signature
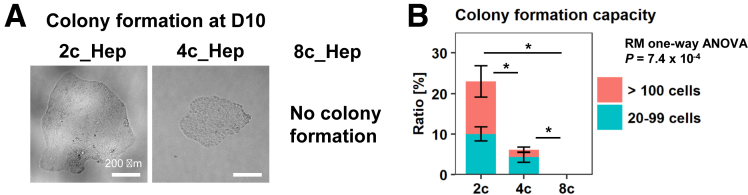
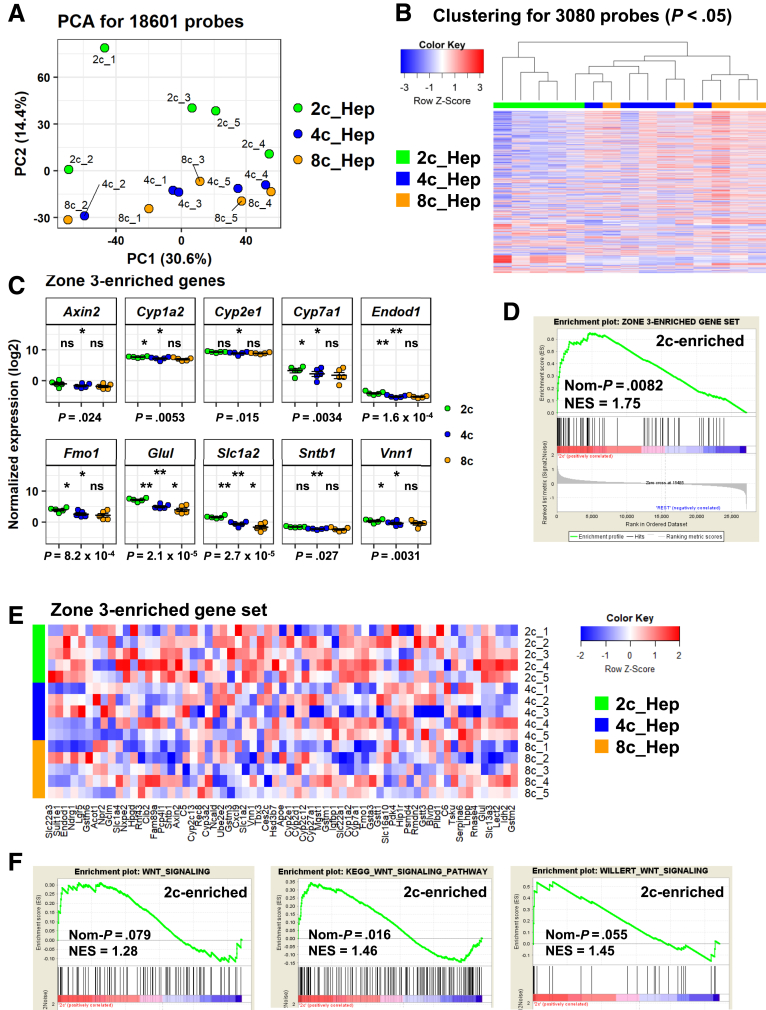
We reported previously that 2c hepatocytes and, to a lesser extent, 4c hepatocytes have in vitro colony forming ability in the presence of proliferative signals, whereas 8c hepatocytes do not7 (reproduced in this study, as shown in Figure 3A and B). This finding emphasizes that, although the overall transcriptome is similar between 2c, 4c, and 8c hepatocytes, there must still be undetected differences among these populations. Indeed, unlike the hierarchical clustering analysis, in a principal component analysis (PCA), although the first principal component (PC1) did not separate the hepatocytes with different ploidy statuses, PC2 separated 2c hepatocytes from 4c and 8c hepatocytes (Figure 4A). As indicated by the plot labels in Figure 4A, PC1 reflects the variance derived from the batch difference of microarray experiments. Given this technical issue, PC2-based information suggests that 2c hepatocytes have distinct phenotypes from 4c and 8c hepatocytes.
Figure 3.
In vitro phenotypic validation of FACS-sorted hepatocytes. (A) Colony formation assay with single-sorted 2c, 4c, and 8c hepatocytes. Images were taken using the BZX-710 microscope (Keyence). (B) Colony-forming capacity was evaluated on day 10. Colonies with 20 or more cells were counted for each fraction. The data are shown as means ± SEM (n = 4). RM 1-way ANOVA was used to determine significant differences among the 3 groups (P = 7.4 × 10-4), followed by the Tukey–Kramer post hoc test as indicated in the panel. *P < .05. D, day.
Figure 4.
Characterization of differentially expressed genes in 2c, 4c, and 8c hepatocytes. (A) PCA for whole-transcriptome analysis of 18,601 probes. Labels for each plot provide the ploidy information (2c, 4c, and 8c) with batch information of 5 experiments (_1, _2, _3, _4, and _5). (B) Heatmap with hierarchical clustering of the 3080 differentially expressed probes (defined as probes with P < .05 by RM 1-way ANOVA). (C) Expression of zone 3–enriched genes. Data are represented as means ± SEM (n = 5). P values at the bottom of each panel were calculated by RM 1-way ANOVA, followed by the Holm multiple comparisons test. P values for post hoc tests are presented as follows: *P < .05, **P < 0.01. Significance symbols on the left, middle, and right in each panel indicate the comparison between 2c and 4c hepatocytes, 2c and 8c hepatocytes, and 4c and 8c hepatocytes, respectively. (D) Gene signature enrichment analysis of 2c hepatocytes and 4c and 8c hepatocytes using a zone 3 signature gene set that was generated manually (the gene list is shown in panel E). (E) Heatmap of the zone 3–enriched gene set. (F) Gene signature enrichment analysis of 2c hepatocytes compared with 4c and 8c hepatocytes using Wnt signaling gene sets. KEGG, Kyoto Encyclopedia of Genes and Genomes; NES, Gene Set Enrichment Analysis; Nom, nominal.
We further investigated for differentially expressed genes among these 3 populations. After filtering the probes with P values less than .05, determined by repeated-measures (RM) 1-way analysis of variance (ANOVA), we identified 3080 probes that were differentially expressed among 2c, 4c, and 8c hepatocytes. By using these probes, we performed hierarchical clustering, which confirmed that the 2c hepatocytes were distinct from the other 2 fractions (Figure 4B). A GO analysis identified multiple bioprocess pathways that were enriched in 2c hepatocytes. However, despite careful analysis, we could not identify meaningful patterns within these GO terms. We did not find any GO bioprocess pathways enriched in 4c hepatocytes, suggesting that these cells share most of the features of 2c and 8c hepatocytes.
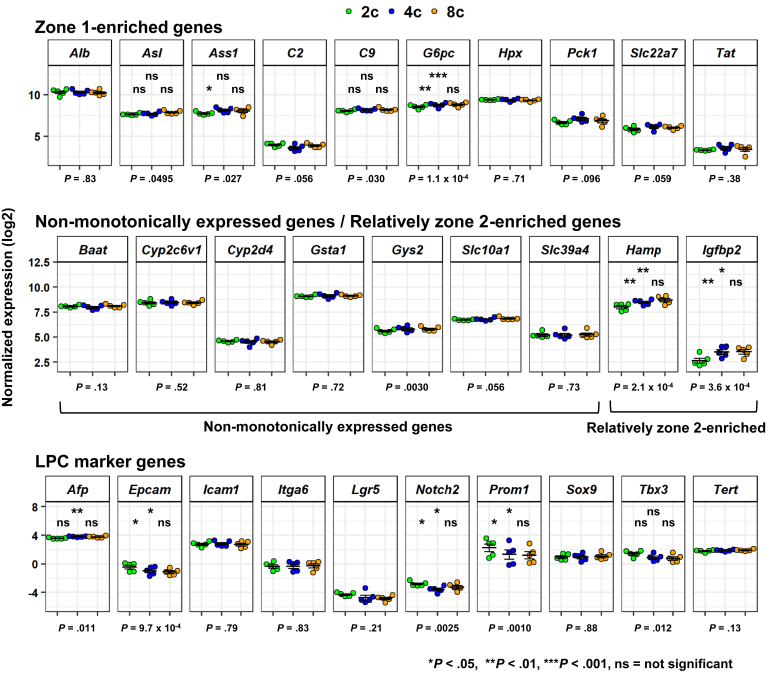
We next asked whether ploidy status is associated with hepatic zone. The structure of the liver lobule generally is divided into 3 regions: zone 1 (periportal region), zone 3 (pericentral region), and zone 2 (intermediate region between zone 1 and zone 3). This hepatic zonation is the most well-characterized factor affecting the heterogeneity of hepatocytes in terms of phenotype, such as those related to metabolic and secretory functions.14, 15, 16, 17 We examined the expression of genes that have been reported to be enriched in zone 1, zone 2, and zone 3 hepatocytes.18 We found that multiple zone 3–enriched genes were expressed at significantly higher levels in 2c hepatocytes (Figure 4C), including Glul (P = 2.1 × 10-5), Cyp7a1 (P = 3.4 × 10-3), and Slc1a2 (P = 2.7 × 10-5). We then manually prepared a “zone 3 signature gene set” by assembling 67 genes that were enriched significantly in zone 3 hepatocytes as reported by Halpern et al. Gene signature enrichment analysis showed that this zone 3 signature gene set was enriched significantly in 2c hepatocytes (nominal P = .0082) (Figure 4D and E). Consistently, 2c hepatocytes had higher Wnt signaling activity, a characteristic of the pericentral region (Figure 4F)19, 20: nominal P = .079 for Wnt Signaling (contributed by SuperArray, Frederick, MA); nominal P = .016 for Kyoto Encyclopedia of Genes and Genomes (KEGG)_Wnt_signaling_pathway; P = .055 for WILLERT_Wnt_signaling (by the comparison of 2c vs 4c and 8c). Expression analysis of representative zone 1–enriched genes showed that several genes were significantly differentially expressed between the 3 fractions of hepatocytes, including Asl (P = .0495), Ass1 (P = .027), and G6pc (P = 1.1 × 10-4) (Figure 5, top), but these differences were much smaller than those observed for zone 3–enriched genes (Figure 4C). As expected, nonmonotonic genes were expressed almost evenly throughout the 3 fractions (Figure 5, middle). By contrast, the expression levels of zone 2–enriched genes Hamp (P = 2.1 × 10-4) and Igfbp2 (P = 3.6 × 10-4) were lower in 2c hepatocytes than in the other 2 fractions (Figure 5, middle). These results strongly suggest that 2c hepatocytes are enriched in zone 3.
Figure 5.
Further characterization of FACS-sorted hepatocytes. Expression of zone 1–enriched genes (top), nonmonotonically expressed or relatively zone 2–enriched genes (middle), and LPC marker genes (bottom). Data are represented as means ± SEM (n = 5). Bottom: P values were calculated by RM 1-way ANOVA, followed by the Holm multiple comparisons test. P values for post hoc tests are presented as follows: *P < .05, **P < .01, ***P < .001. Significance symbols on the left, middle, and right in each panel indicate the comparison between 2c and 4c hepatocytes, 2c and 8c hepatocytes, and 4c and 8c hepatocytes, respectively.
Some LPC Marker Genes Are Expressed Differentially According to Ploidy Status
One question yet to be fully addressed is whether any specific subpopulation(s) of hepatocytes have stem/progenitor cell–like characteristics. Previous works have identified putative resident LPCs that are characterized by specific marker genes (eg, Afp, Epcam, and Prom1/Cd133) (reviewed by Miyajima et al21). Furthermore, several groups recently identified subsets of hepatocytes that phenotypically show LPC-like features,6, 22, 23, 24 while other groups have reported that mature hepatocytes have the capacity to be reprogrammed into LPCs.25, 26, 27, 28, 29, 30 In particular, Wang et al6 recently reported that Axin2-positive pericentral hepatocytes, which contribute to hepatocyte turnover under physiological conditions, are enriched in 2c hepatocytes. Our analysis of zone 3–enriched genes showed that 2c hepatocytes showed a slightly but significantly higher expression of Axin2 (P = .024) compared with the other hepatocyte populations (Figure 4C). In addition, other genes, including Epcam (P = 9.7 × 10-4), Notch2 (P = 2.5 × 10-3), Prom1 (P = 1.0 × 10-3), and Tbx3 (P = .012), were expressed differentially with statistical significance (Figure 5, bottom).
Dissecting 2c and 4c Hepatocyte Heterogeneity at the Single-Cell Level
Microarray transcriptome analysis provided insight into the association between ploidy status and hepatic phenotype, but we were unable to determine whether the differences observed in the bulk analysis were attributable to global (averaged) differences between these fractions or to subpopulations that behave in a very different manner relative to the majority of the population. This question is particularly important when considering the existence of LPCs, which are supposed to be a rare population.
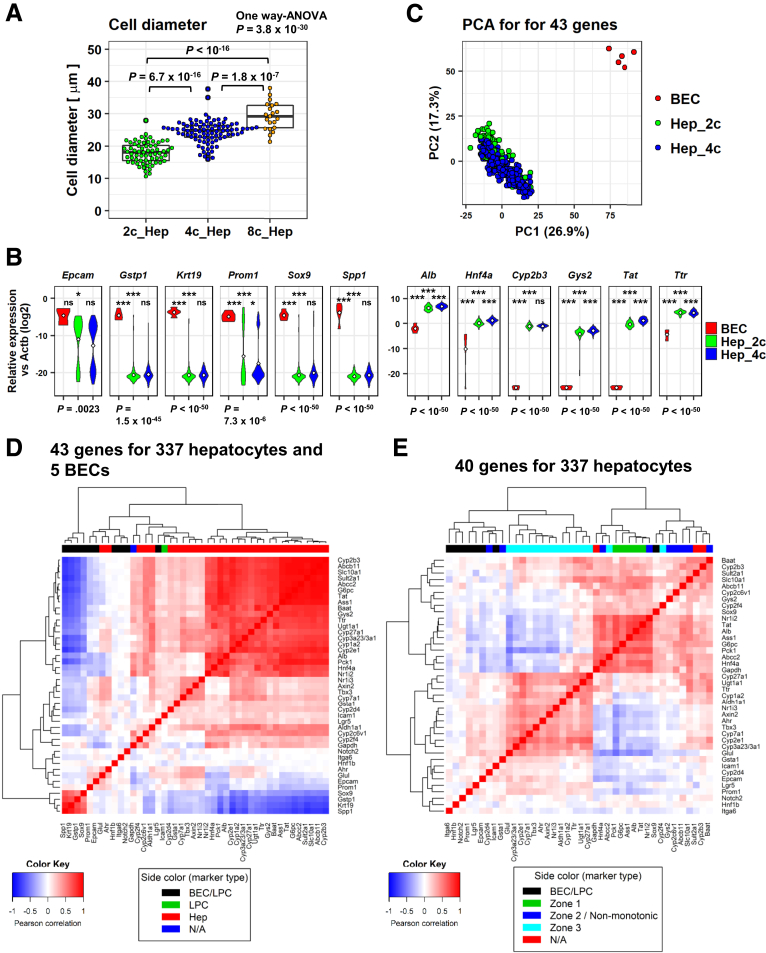
To examine the heterogeneity of hepatocytes from the point of view of different ploidy status, we performed scPCR analysis on FACS-sorted primary hepatocytes. To avoid contamination with doublet cells, we used the Fluidigm C1 system (Fluidigm, South San Francisco, CA), which is designed to capture cells with diameters ranging between 17 and 25 μm. Because the majority of 8c hepatocytes were larger than 25 μm (Figure 6A), the maximum diameter valid in the C1 platform, we excluded 8c hepatocytes from the scPCR analysis and focused on comparing 2c and 4c hepatocytes. We prepared a TaqMan probe set (Applied Biosystems, Beverly, MA) targeting 47 genes and 1 negative control (no probe added): these probes included 2 housekeeping genes, general hepatic marker genes, zone-related genes, biliary epithelial cell (BEC) marker genes, and LPC marker genes (Table 1). Because some of the LPC marker genes also are expressed by BECs, we first compared hepatocytes with the nonparenchymal cell (NPC) fraction, which contains BECs. We confirmed that 10 of 96 NPCs analyzed expressed Krt19, a definitive BEC marker, whereas only 2 of 337 hepatocytes, both of which were 2c, showed Krt19 expression, which was lower than that in Krt19+ NPCs (Figure 6B). In particular, 5 of 10 Krt19+ NPCs expressed Gstp1 and Sox9, which also are BEC markers, and thus we regarded these 5 NPCs as BECs. PCA of these 5 BECs and the 337 hepatocytes clearly discriminated the BECs from the hepatocytes (Figure 6C). Indeed, except for Krt7, which was not detected in our experiment and thus was excluded from the analysis, almost all of the BEC marker genes were expressed exclusively in BECs (Figure 6B). Accordingly, BECs showed no or much lower levels of expression of hepatocyte marker genes (Figure 6B). Importantly, the earlier-mentioned 2 Krt19-positive 2c hepatocytes were confirmed to be authentic hepatocytes because they showed robust expression of hepatic marker genes (eg, Alb, Ttr, and G6pc). A correlation heatmap for whole genes (43 genes, except Krt7, Afp, and Ncam1, which were expressed in <1% of analyzed cells) showed that the expression profiles of BEC/LPC markers and hepatocyte markers were clearly different (Figure 6D). Thus, we conclude that both the 2c and 4c hepatocytes investigated in this study were not contaminated with BECs.
Figure 6.
Validation of the results of scPCR. (A) Cell size of FACS-sorted 2c, 4c, and 8c hepatocytes. A total of 65, 88, and 21 cells were analyzed for 2c, 4c, and 8c cells, respectively. One-way ANOVA was used to determine significant differences among the 3 groups (P = 3.8 × 10-30), followed by the Tukey multiple comparisons test. (B) Violin plots of BEC marker genes (left) and hepatocyte marker genes (right). Bottom: P values were calculated by 1-way ANOVA, followed by the Tukey multiple comparisons test. P values for post hoc tests are presented as follows: *P < .05, ***P < .001. Significance symbols on the left, middle, and right in each panel indicate the comparison between BEC and 2c hepatocytes, BEC and 4c hepatocytes, and 2c and 4c hepatocytes, respectively. (C) PCA of 2c and 4c hepatocytes with 5 Krt19+Sox9+Gstp1+ BECs using 43 genes. (D) Correlation heatmap for whole genes (43 genes, except Krt7, Afp, and Ncam1, which were expressed in <1% of the analyzed cells) using 5 BECs and 337 hepatocytes. (E) Correlation heatmap of BEC/LPC makers (except Krt19, Gstp1, and Spp1, which were expressed in <1% of hepatocytes) and hepatic marker genes with zone information.
Table 1.
Gene List for scPCR Analysis
| Gene | House keeping marker | Zone 1 marker | Zone 2/nonmonotonic marker | Zone 3 marker | LPC marker | BEC marker | Description/note | Reference |
|---|---|---|---|---|---|---|---|---|
|
Actb Rn00667869_m1 |
○ | Reported to have no zonation18 Used for normalizing gene expression |
18 | |||||
|
Gapdh Rn01775763_g1 |
○ | Reported to have no zonation, but relatively more deviated than Actb18 | 18 | |||||
|
Alb Rn00592480_m1 |
○ | Hepatocyte-secreted serum protein | 15, 18 | |||||
|
Tat Rn00562011_m1 |
○ | Hepatocyte-secreted serum protein | 18 | |||||
|
Ass1 Rn00565808_g1 |
○ | Involved in the synthesis of creatine, polyamines, arginine, urea, and nitric oxide | 18 | |||||
|
G6pc Rn00689876_m1 |
○ | Functions in gluconeogenesis and glycogenolysis | 18, 53 | |||||
|
Pck1 Rn01529014_m1 |
○ | Main regulator in gluconeogenesis | 15, 18, 54 | |||||
|
Abcb11 Rn01515444_m1 |
○ | ABC transporter expressed along bile canaliculi | 18 | |||||
|
Cyp2c6v1 Rn03417171_gH |
○ | Phase I modification enzyme | 7 | |||||
|
Cyp2d4 Rn01504629_m1 |
○ | Phase I modification enzyme A rat orthologue of human CYP2D6 and mouse Cyp2d22 |
18 | |||||
|
Gsta1 Rn04223027_m1 |
○ | Phase II conjugation enzyme | 18 | |||||
|
Baat Rn00568867_m1 |
○ | Catalyzes the transfer of C24 bile acids from the acyl-CoA thioester to either glycine or taurine | 18 | |||||
| Gys2 Rn00565296_m1 |
○ | Catalyzes the rate-limiting step in the synthesis of glycogen | 18 | |||||
| Slc10a1 Rn00566894_m1 |
○ | Bile acid transporter located at the basal side of hepatocytes | 18 | |||||
| Nr1i2 Rn00583887_m1 |
○ | Detected in no more than 1% of analyzed cells, thus not included in analysis | 18 | |||||
| Sult2a1 Rn04223057_mH |
○ | Phase II conjugation enzyme | Human protein atlas: www.proteinatlas.org | |||||
| Glul Rn01483107_m1 |
○ | Catalyzes the synthesis of glutamine from glutamate and ammonia | 15, 18, 54, 55, 56 | |||||
| Sult2a1 Rn04223057_mH |
○ | ABC transporter expressed along bile canaliculi | 18 | |||||
|
Aldh1a1 Rn00755484_m1 |
○ | The next enzyme after alcohol dehydrogenase in the alcohol metabolism pathway | 18 | |||||
|
Ahr Rn00565750_m1 |
○ | Transcription factor that regulates Cytochrome P450 (CYP) enzyme expression | 15, 16, 18, 55, 56 | |||||
|
Nr1i3 Rn04339043_m1 |
○ | Nuclear receptor, also known as constitutive androstane receptor, which regulates xenobiotic and endobiotic metabolism | 15, 18, 56 | |||||
|
Cyp1a2 Rn00561082_m1 |
○ | Phase I modification enzyme | 15, 16, 18, 54, 55, 56 | |||||
|
Cyp2e1 Rn00580624_m1 |
○ | Phase I modification enzyme | 15, 16, 18, 54, 55, 56 | |||||
|
Cyp2f4 Rn00570779_m1 |
○ | Phase I modification enzyme A rat orthologue of mouse Cyp2f2 |
18, 55 | |||||
|
Cyp3a23 /3a1 Rn01412959_g1 |
○ | Phase I modification enzyme | 16 | |||||
|
Cyp7a1 Rn00564065_m1 |
○ | Phase I modification enzyme | 7, 18, 55 | |||||
|
Cyp27a1 Rn00710297_m1 |
○ | Phase I modification enzyme | 16, 18, 55 | |||||
|
Hnf4a Rn04339144_m1 |
○ | Transcription factor that plays an important role in liver development and liver functions | 18 | |||||
| Ugt1a1 Rn00754947_m1 |
○ | Phase II conjugation enzyme | 16, 18 | |||||
| Ttr Rn00562124_m1 |
○ | Hepatocyte-secreted serum protein | 18 | |||||
| Cyp2b3 Rn01476084_m1 |
Phase I modification enzyme No zonation-related information available |
N/A | ||||||
|
Axin2 Rn00577441_m1 |
○ | ○ | Plays an important role in the regulation of the stability of β-catenin | 6, 15, 18 | ||||
|
Tbx3 Rn00710902_m1 |
○ | ○ | Transcription factor involved in the regulation of various developmental processes | 18 | ||||
|
Sox9 Rn01751070_m1 |
○ | ○ | ○ | Transcription factor regulating the bile duct development of BECs Also reported to be expressed by periportal adult LPCs |
23, 32, 57 | |||
| Afp Rn00560661_m1 |
○ | A major plasma protein produced by fetal liver cells Also expressed by injury-induced rat LPCs |
21 | |||||
| Ncam1 Rn01418541_m1 |
○ | ○ | A marker expressed in developing bile ducts and LPCs | 58, 59 | ||||
| Epcam Rn01473202_m1 |
○ | ○ | A marker expressed in fetal hepatoblasts, BECs, and injury-induced adult LPCs | 21 | ||||
| Prom1 Rn00572720_m1 |
○ | ○ | A marker expressed in fetal hepatoblasts, BECs, and injury-induced adult LPCs | 21 | ||||
| Notch2 Rn01534371_m1 |
○ | A marker expressed in fetal hepatoblasts | 60 | |||||
| Icam1 Rn00564227_m1 |
○ | ○ | A marker expressed in fetal hepatoblasts, BECs, and injury-induced adult LPCs | 61 | ||||
| Itga6 Rn01512708_m1 |
○ | ○ | A marker expressed in fetal hepatoblasts, BECs, and injury-induced adult LPCs | 21 | ||||
| Lgr5 Rn01509662_m1 |
○ | ○ | A marker expressed in BECs/LPCs upon injury | 31 | ||||
| Krt19 Rn01496867_m1 |
○ | A marker of BECs | 62 | |||||
| Krt7 Rn04224249_u1 |
○ | A marker of BECs Detected in <1% of analyzed cells, thus excluded from study |
62 | |||||
| Gstp1 Rn00561378_gH |
○ | ○ | A marker of BECs and LPCs |
63, 64, 65 Human protein atlas: www.proteinatlas.org |
||||
| Hnf1b Rn00447453_m1 |
○ | A marker of BECs | 62 | |||||
|
Spp1 Rn00681031_m1 |
○ | A marker of BECs | 66 | |||||
| None | Negative control | N/A |
NOTE. For zonation information obtained from Halpern et al,18 we referred to Supplementary Table 3 in their article. We defined genes with zonation as those with a P value (Kruskal–Wallis) < .01. Genes with their expression peak in layers 1–3, 4–6, and 7–9 were defined as zone 1, zone 2, and zone 3 markers, respectively.
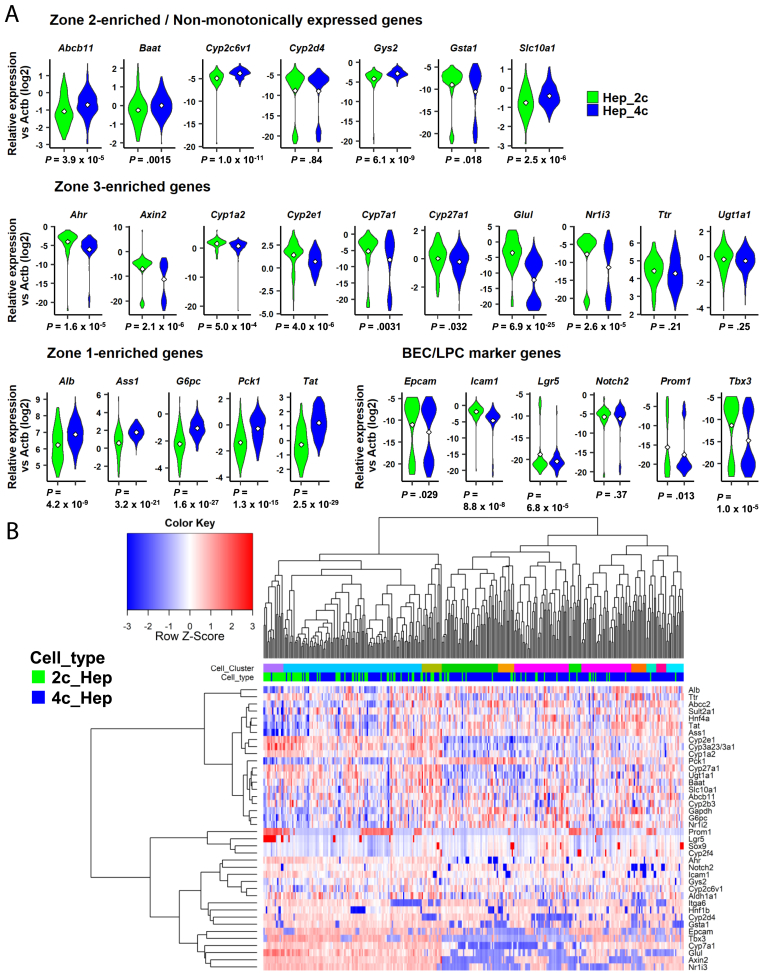
By using 92 2c hepatocytes and 245 4c hepatocytes, we compared the expression levels of zone-related genes. A correlation heatmap of BEC/LPC makers (except Krt19, Gstp1, and Spp1, which were expressed in <1% of hepatocytes) and hepatic marker genes with zone information showed that hepatic genes were clustered in a zone-dependent manner (Figure 6E). Consistent with the observations from the bulk transcriptome, we confirmed that 2c hepatocytes showed higher expression levels for most of the zone 3–enriched genes (Figure 7A, middle).18 Specifically, 94.6% of 2c hepatocytes expressed Glul, whose expression is sharply localized in proximity to the central vein, whereas only 58.8% of 4c cells expressed this gene. Accordingly, the zone 1–enriched genes Alb (P = 4.2 × 10-9), Ass1 (P = 3.2 × 10-21), G6pc (P = 1.6 × 10-27), Pck1 (P = 1.3 × 10-15), and Tat (P = 2.5 × 10-29) were expressed consistently at lower levels in 2c hepatocytes (Figure 7A, bottom left). We also confirmed that nonmonotonic genes were expressed at relatively similar levels in 2c and 4c hepatocytes (Figure 7A, bottom left). We also investigated the expression of LPC marker genes, including Epcam, Icam1, Lgr5, Notch2, Prom1, and Tbx3. Consistent with the bulk transcriptome results, Epcam (P = .029), Prom1 (P = .013), Tbx3 (P = 1.0 × 10-5), and Axin2 (P = 2.1 × 10-6) were expressed at higher levels in 2c than in 4c hepatocytes (Figure 7A, bottom right, see middle for Axin2). In addition, we found that a small number of cells expressed Lgr5, whose expression levels were very low in the bulk microarray (Figure 5, bottom), presumably because the majority of hepatocytes, irrespective of their ploidy status, do not express this gene.31 Hierarchical clustering of the 40 genes expressed in at least 1% of the total cells analyzed (≥4 cells) clustered the 2c and 4c hepatocytes into distinct groups (Figure 7B, lower column side bar). As highlighted in the upper column side bar (see the purple-colored cluster on the far left), the 2c hepatocyte-rich subpopulation showed higher expression of multiple LPC markers including Axin2, Prom1, and Lgr5 (Figure 7B, upper column side bar).
Figure 7.
scPCR-based expression profile of hepatocyte and LPC markers in 2c and 4c hepatocytes. (A) Violin plots of nonmonotonically expressed hepatic genes (top), zone 3–enriched genes (middle), zone 1–enriched genes (bottom left), and LPC marker genes (bottom right). Expression level for each gene was normalized with that of Actb. White diamonds indicate mean values. P values were calculated using the Welch t test (n = 92 and 245 for 2c and 4c hepatocytes, respectively). (B) Heatmap with hierarchical clustering of 92 2c and 245 4c hepatocytes using 40 genes. The upper and lower column side color bars, designated as “Cell_Cluster” and “Cell_type”, indicate 12 cell clusters and hepatocyte ploidy, respectively.
PCA of Single Cells Using Zonation and LPC Signature
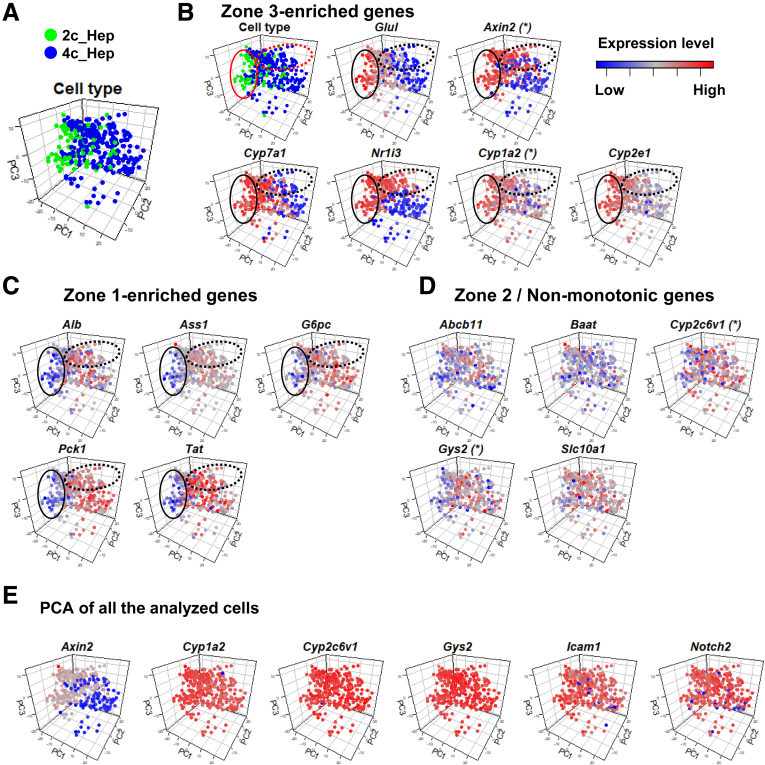
We next visualized each of the analyzed cells by PCA and confirmed that the 2c and 4c hepatocytes form distinct populations (Figure 8A). Loading these PCA plots with the expression levels of zone-associated genes indicated that 2c hepatocytes have a pericentral expression signature (Figure 8B–D). Some of the 4c hepatocytes expressed zone 3 marker genes, including Axin2, Cyp7a1, and Nr1i3 (Figure 8B, dotted ellipses), showing that not only 2c but also 4c hepatocytes distribute to zone 3. However, the highest expression levels of Glul, Cyp1a2, and Cyp2e1 were almost restricted to the 2c cells (Figure 8B, solid ellipses). Indeed, the top 10% of cells ranked according to Glul expression level (34 of 337 cells) were 2c hepatocytes. Convincingly, cells with the highest expression of Glul showed lower expression levels of zone 1–enriched genes such as Alb, Ass1, G6pc, Pck1, and Tat (Figure 8C, solid ellipses). On the other hand, the subpopulation of 4c hepatocytes with partial expression of zone 3 marker genes also exhibited high expression levels of zone 1–enriched genes (Figure 8C, dotted ellipses). Compared with zone 1– and zone 3–enriched genes, we confirmed that 2c-enriched/nonmonotonic genes showed similar levels of expression among the analyzed cells (Figure 8D).
Figure 8.
Characterization of 2c and 4c hepatocytes by PCA mapping. (A) PCA mapping of 92 2c (green) and 245 4c (blue) hepatocytes. (B–D) Each cell is colored according to the designated gene expression level as scaled with the color key. (B) Solid ellipses and dotted ellipses indicate a Glul-high 2c cell-rich population and zone 3–oriented 4c cell-rich population, respectively. In panels with asterisks, some cells were excluded from the analysis because their inclusion reduced the resolution of the expression profiles for the designated genes. Complete mapping of these genes is shown in panel E. (E) Complete PCA mapping of 337 hepatocytes for Axin2, Cyp1a2, Cyp2c6v1, Gys2, Icam1, and Notch2.
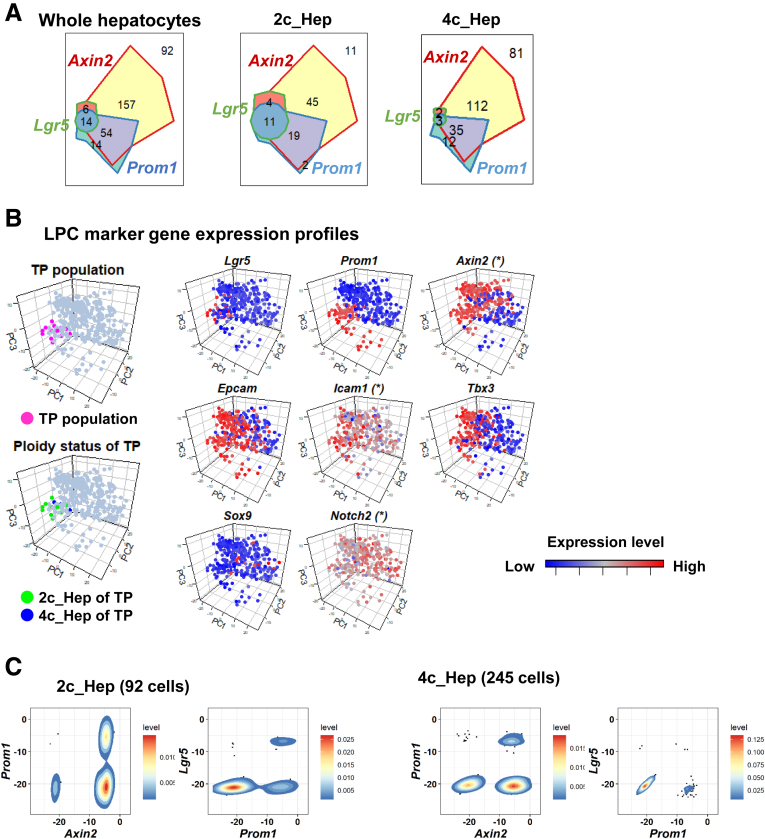
As described earlier, hierarchical clustering showed a 2c hepatocyte-rich subpopulation that was enriched with cells expressing Axin2, Prom1, and Lgr5 (Figure 7B). A Venn diagram of the whole hepatocyte population indicated that 100% (20 of 20) and 70.0% (14 of 20) of Lgr5+ hepatocytes expressed Axin2 and Prom1, respectively, and that 82.9% (68 of 82) of Prom1-positive hepatocytes expressed Axin2 (Figure 9A). These results were suggestive of a population hierarchy among LPC marker–expressing hepatocytes in which a population positive for all 3 LPC markers (hereafter designated as triple positive [TP] population) resided at the top. The TP population comprised 4.15% of the entire hepatocyte pool (14 of 337 hepatocytes). The abundancy of TP cells was higher in 2c hepatocytes (12.0%; 11 of 92 cells) than in 4c hepatocytes (1.2%; 3 of 245 cells) (Figure 9B, see also Figure 9C for detailed co-expression profiles), confirming that the TP population is highly enriched among 2c hepatocytes.
Figure 9.
Characterization of LPC-like subpopulation in 2c hepatocytes. (A) Venn diagram describing the co-expression profile of 3 LPC marker genes: Axin2, Prom1, and Lgr5. (B) Cells of Axin2+Prom1+Lgr5+ TP population are highlighted in magenta (upper left). The ploidy status of TP population cells is indicated in green (2c) and blue (4c) (lower left). Expression levels for all of the analyzed LPC marker genes are shown (right). In panels with asterisks, some cells were excluded from the analysis because their inclusion reduced the resolution of the expression profiles for the designated genes. Complete mapping of these genes is shown in Figure 8E. (C) Scatter plot with density estimations for Axin2 vs Prom1 and Prom1 vs Lgr5. Expression levels for each gene are normalized to Actb. (D) Scatter plot with density estimations for Axin2 vs Prom1 and Prom1 vs Lgr5. Expression levels for each gene are normalized to Actb.
We further explored the expression profiles of all of the LPC markers, including Lgr5, Axin2, Prom1, Epcam, Icam1, Sox9, and Notch2 in the TP population. We found that the TP population also was enriched for Epcam, Icam1, and Tbx3 (Figure 9B). By contrast, these cells did not express Sox9 (Figure 9B). Sox9 expression is reported to be limited to a subpopulation of zone 1 hepatocytes called “hybrid hepatocytes.”23, 32 Thus, the TP population can be regarded as a population distinct from hybrid hepatocytes. We also confirmed that all of the TP population cells (14 of 14) expressed Glul, strongly suggesting that these cells are localized to the pericentral region. Indeed, Planas-Paz et al33 recently reported that, in mice, Lgr5 messenger RNA expression was observed preferentially in pericentral hepatocytes.
Mononucleated 4c Hepatocytes Are More Zone 3–Enriched Than Binucleated 4c Hepatocytes
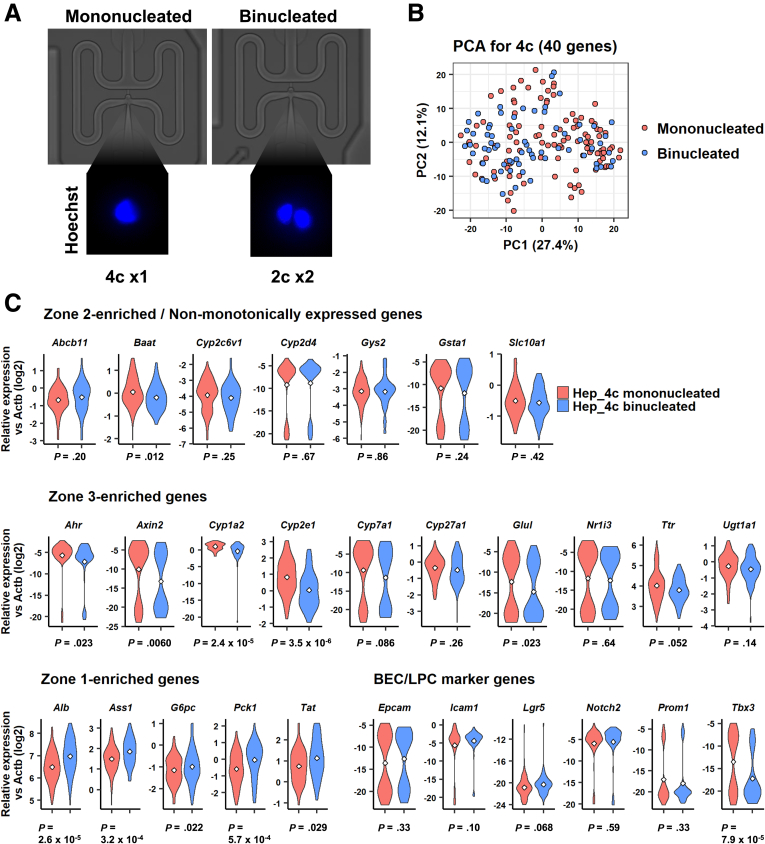
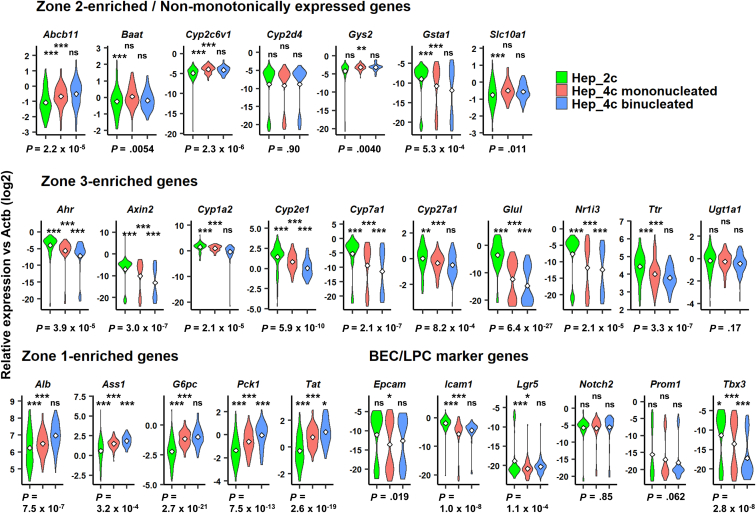
Finally, we investigated whether nuclearity affects the gene expression profiles of 4c hepatocytes. A recent study examined the difference in metabolic capacity between mononucleated and binucleated hepatocytes.34 In the present study, we asked whether zonation varies between mononucleated and binucleated 4c hepatocytes. Taking advantage of the single-cell–capturing ability of the C1 platform, we annotated 175 4c hepatocytes with mononuclear or binuclear states (Figure 10A). PCA mapping of 106 mononucleated and 69 binucleated hepatocytes did not show a clear separation (Figure 10B). However, individual comparison of the expression levels of zonation-related genes showed that mononucleated 4c hepatocytes expressed zone 3–enriched (Figure 10C, middle) and zone 1–enriched (Figure 5C, bottom left) genes at higher and lower levels, respectively, than binucleated hepatocytes, while nonmonotonic genes were mostly expressed evenly (Figure 10C, top row). Except for Tbx3 (P = 7.9 × 10-5), most LPC marker genes were not expressed differentially between mononucleated and binucleated 4c hepatocytes (Figure 10C, bottom right). Tbx3 is a downstream gene in the Wnt signaling pathway similar to Axin2, and therefore its expression supports the idea that mononucleated 4c hepatocytes are enriched in zone 3.35 Despite this finding, the zonal signature is much more clearly distinguishable between 2c and 4c hepatocytes (Figure 11).
Figure 10.
Nuclearity-based dissection of 4c hepatocytes. (A) Microscopic identification of the nuclearity of 4c hepatocytes using the C1 microfluidics platform. A total of 106 mononucleated and 69 binucleated hepatocytes were included in this analysis. Images were taken using the BZX-710 microscope (Keyence). (B) PCA for mononucleated and binucleated 4c hepatocytes using 40 genes. (C) Violin plots of nonmonotonically expressed hepatic genes (top), zone 3–enriched genes (middle), zone 1–enriched genes (bottom left), and LPC marker genes (bottom right). P values were calculated using the Welch t test.
Figure 11.
Comparison between mononucleated and binucleated 4c hepatocytes and 2c hepatocytes. Violin plots of nonmonotonically expressed hepatic genes (top), zone 3–enriched genes (middle), zone 1–enriched genes (bottom left), and LPC marker genes (bottom right). A total of 92 2c hepatocytes, 106 mononucleated 4c hepatocytes, and 69 binucleated 4c hepatocytes were used. P values at the bottom of each panel were calculated by 1-way ANOVA, followed by the Tukey multiple comparisons test. P values for post hoc tests are presented as follows: *P < .05, **P < .01, ***P < .001. Significance symbols on the left, middle, and right in each panel indicate the comparison between 2c and mononucleated 4c hepatocytes, 2c and binonucleated 4c hepatocytes, and mononucleated and binucleated 4c hepatocytes, respectively.
Discussion
This study provides new insights into hepatocyte heterogeneity, namely 2c hepatocytes are preferentially localized to the pericentral region; and a subpopulation of 2c hepatocytes show LPC-like features in terms of LPC marker expression (Axin2, Prom1, and Lgr5).
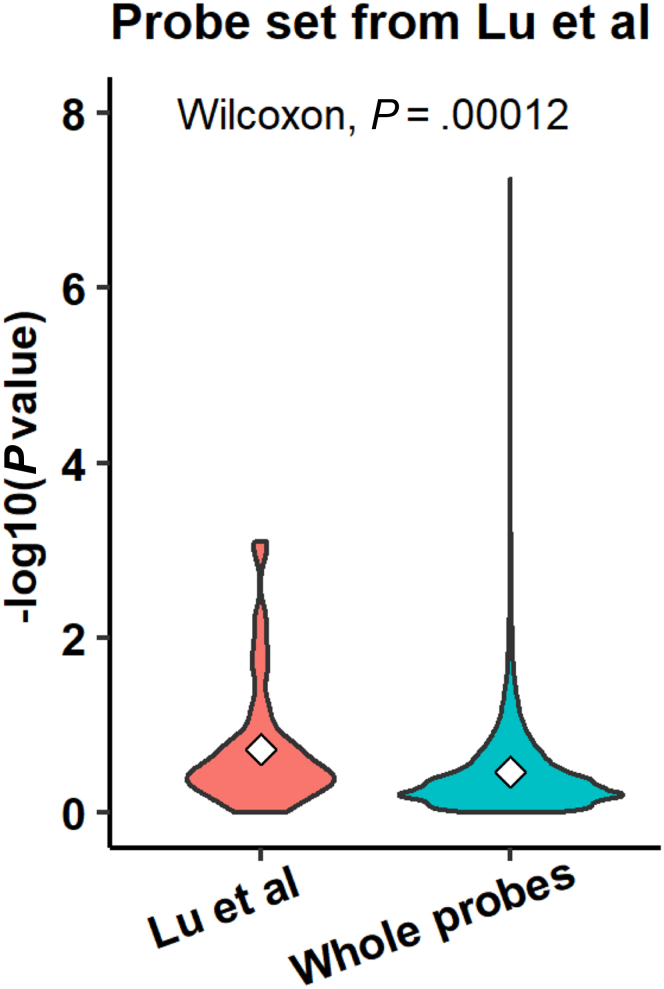
In a previous study, Lu et al36 reported no major differences in the gene expression patterns between mouse hepatocytes of different ploidy. Inconsistencies between their observations and ours might derive from differences in, first, the analyzed probe numbers: approximately 12,000 probes (designed based on the information available in 2002) were included in the Lu et al36 study, whereas approximately 45,000 probes were used in our study; and, second, the microarray platforms: Lu et al36 used the Affymetrix (Santa Clara, CA) platform, whereas we used the Agilent platform. However, we confirmed that the differentially expressed genes reported by Lu et al36 also were expressed differentially in our study. Eleven of the 75 probes that correspond to the 56 genes identified as being expressed differentially by Lu et al36 also were identified as expressed differentially in the present study (P < .05, RM 1-way ANOVA). The P values for these 75 probes were significantly lower than those of the whole 35,852 probes (P = 1.2 × 10-4 by the Wilcoxon test) (Figure 12), confirming, at least partly, consistency between the observations of Lu et al36 and those described here. In addition, we confirmed the enrichment of cell cycle–related genes in 8c hepatocytes, which is consistent with the established notion that polyploidy is formed via cell division failure. Thus, we believe that our analysis successfully identified characteristic features of hepatocytes with different ploidy statuses.
Figure 12.
Analysis of genes that were reported to be expressed differentially among hepatocytes with different ploidy status.P values were calculated by RM 1-way ANOVA using 2c, 4c, and 8c hepatocytes (n = 5 experiments) for 75 probes, which correspond to the 56 differentially expressed genes reported by Lu et al36 and compared with the P values for whole probes (n = 75 probes for Lu et al36; n = 18,021 probes for whole probes).
Zonal localization of hepatocytes with different ploidy statuses is an unresolved issue in the field. Our bulk transcriptome and scPCR analysis consistently showed that 2c hepatocytes are localized preferentially to the pericentral region. Specifically, scPCR showed that the hepatocytes with the highest expression levels of Glul, one of the most stringent pericentral marker genes, were almost exclusively 2c. The proposition of Wang et al6 that pericentral hepatocytes are enriched among 2c hepatocytes is consistent with ours. However, their finding does not necessarily indicate that 2c hepatocytes are located in the pericentral region, whereas the present study directly showed that 2c hepatocytes are localized preferentially to the pericentral region. At the same time, the finding that 2c hepatocytes show zone 3–enriched features was surprising because it is widely accepted that diploid hepatocytes are enriched in the periportal region and polyploid hepatocytes are enriched in the pericentral region.5, 10, 11, 12, 13 At present, we cannot resolve this discrepancy, but one possible explanation is the methodology-dependent bias of isolated hepatocytes. In the present study, to avoid contamination with BECs, we used a stringent hepatocyte enrichment protocol in which digested whole-liver cells were subjected to sequential centrifugation steps (57 × g for 1 min, 2 cycles) and then separated further using Percoll density gradient centrifugation (see the Materials and Methods section). Our method highly enriches mature hepatocytes but, in turn, possibly loses a minor fraction of smaller hepatocytes, the so-called “small hepatocytes,” which are found in both parenchymal and nonparenchymal fractions.37 Indeed, in this study, the 2c fraction accounted for approximately 5.4% ± 2.2% (means ± SD, n = 16 rats aged between 5 and 14 weeks) of total hepatocytes. This is lower than the widely accepted percentage of 2c hepatocytes: it generally is accepted that approximately 10%–15% of hepatocytes are diploid in rats and mice.3, 5 We cannot rule out the possibility that, in our experiments, some of the 2c hepatocytes were lost. Nonetheless, it still is noteworthy that a substantial number of 2c hepatocytes showed zone 3–enriched characteristics. Moreover, unlike previous studies in which conclusions were drawn based on histologic analyses, this study provides novel insights from the transcriptomic perspective.
This study also provides novel insights into LPC biology. It remains controversial whether the liver has resident stem/progenitor cells. Our scPCR study does not provide direct evidence for their existence but shows instead that 2c hepatocytes are more enriched in well-established LPC markers than 4c hepatocytes. In particular, our data suggest that these progenitor-like cells have a hierarchy in which Lgr5+Prom1+Axin2+ TP cells reside at the top (at least according to the gene set used here). On the other hand, because the sample size in this study was small, in particular for the TP population (11 of 92 and 3 of 245 cells for 2c and 4c populations, respectively), we have to be careful about the conclusion. In addition, our single-cell analysis was based on a limited panel of genes, thereby leaving the possibility that we have missed other LPC markers that would further dissect the hepatocyte population. Further exploration will require single-cell RNA sequencing with a larger sample scale. Nonetheless, we propose that this study provides a wealth of transcriptomic data and provides some novel insights about localization and function of the heterogeneous population of hepatocytes, which can be tested in the future.
Finally, we clarify the limitation of the present study. First, given the interspecies difference in ploidy profile of hepatocytes, the relevance of our findings here is not applicable simply to human liver biology. The degree of liver polyploidization varies between mammals: although 80%–97%9, 34, 38, 39, 40 and 70%–95%41, 42, 43, 44 of adult mouse and rat hepatocytes are polyploid, respectively, only 10%–40% of adult human hepatocytes are polyploid.45, 46, 47 Strikingly, although polyploid hepatocytes become the majority within a week after weaning in rodents, polyploid cells generally do not exceed 15% in 20-year-old human beings. These reports collectively suggest that the role division that might be provided by the ploidy heterogeneity is different between rodents and human beings. Further study with the use of freshly isolated human hepatocytes will be required.
Materials and Methods
Rats
Animal experiments in this study were performed in compliance with the guidelines of the Institute for Laboratory Animal Research of the National Cancer Center Research Institute. The protocol was approved by the Committee on the Ethics of Animal Experiments of the National Cancer Center Research Institute. Rats were housed in specific pathogen-free facilities on a 12-hour light/dark cycle and were given food and water ad libitum. All animals used in this study were female Wistar rats aged between 5 and 14 weeks (CLEA Japan, Shizuoka, Japan). Details regarding the animals used in the study are described for each part of the experiment in the Materials and Methods section.
Isolation of Rat Adult Hepatocytes
Adult rat hepatocytes were isolated from using the procedure described by Seglen48 with minor modifications (for details, see Katsuda et al49). Briefly, after preperfusion with Ca2+-free Hank’s/ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid solution through the portal vein, the liver was perfused with approximately 400 mL Hank’s solution containing 0.05% collagenase at 25–30 mL/min. The extracted liver was mechanically digested with a surgical knife and then enzymatically digested in a mixture of 0.05% collagenase solution and 20 mL E-MEM (Sigma, St. Louis, MO) at 37°C for 15 minutes. The digested liver then was filtered twice through a sterilized cotton mesh (single-folded and then double-folded). The cell suspension was aliquoted into two 50-mL tubes, which then were filled with E-MEM, and the cells were collected via centrifugation at 57 × g for 1 minute. After resuspension in a 50 mL/tube E-MEM, large-cell aggregates were eliminated by filtering the cell suspension through a 60-μm stainless double-mesh cell strainer (Ikemoto Scientific Technology Co, Ltd, Tokyo, Japan) and the cells were collected by centrifuging the filtrate at 57 × g for 1 minute. Next, the cells were resuspended in 24.5 mL/tube complete Percoll medium (25 mL L-15 medium [Life Technologies, Carlsbad, CA] supplemented with 0.429 g/L HEPES [Sigma], 2 g/L bovine serum albumin [Sigma], 1 × 10-7 mol/L insulin [Sigma], 2.4 mL 10× Hank’s balanced salt solution(-) [Life Technologies], and 21.6 mL Percoll [GE Healthcare, Chicago, IL]), and dead cells were removed via centrifugation at 57 × g for 10 minutes. Finally, the cells were washed in 50 mL/tube E-MEM twice via centrifugation at 57 × g for 2 minutes. The purified hepatocytes were used for the downstream experiments.
Isolation of BEC-Enriched NPCs
NPCs were isolated from 7-week-old rats as described previously.50, 51 Briefly, we harvested bile duct containing remnant tissue after 2-step collagenase perfusion of an adult rat liver as described earlier. Then, the remnant tissue was digested by shaking at 37°C for 30 minutes in Leibovitz L-15 medium (Invitrogen, Carlsbad, CA) supplemented with 400 U/mL collagenase (Wako, Tokyo, Japan), 700 U/mL hyaluronidase (Sigma-Aldrich), 10-7 mol/L insulin, and 10-7 mol/L dexamethasone. After filtration with a 40-mm cell strainer (BD Biosciences, Franklin Lakes, NJ), the cells were collected by centrifugation at 800 × g for 10 minutes. Then the pellet was resuspended in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and centrifuged twice at 800 × g for 5 minutes. Purified NPCs were captured by the C1 Auto Prep system and analyzed by scPCR.
FACS of Hepatocytes With Different Ploidy Statuses
To fractionate the 2c, 4c, and 8c hepatocytes, FACS-based single-cell isolation was performed according to a previously described protocol.39 Briefly, rat hepatocytes harvested from 5- to 14-week old rats were suspended in 10% fetal bovine serum–Dulbecco’s modified Eagle medium supplemented with 15 mg/mL Hoechst 33342 and 5 μmol/L reserpine and incubated at 37°C for 10 minutes. After the addition of 5 μg/mL propidium iodide (BD Biosciences), the cells were sorted using a FACSAria II or FACSAria III (BD Biosciences).
Colony Formation Assay of Single 2c, 4c, and 8c Rat Hepatocytes
Hepatocytes harvested from 5- to 8-week-old rats were sorted into 96-well collagen-coated plates filled with 100 μL/well of Dulbecco’s modified Eagle medium/F12 (Life Technologies) containing 2.4 g/L NaHCO3 and l-glutamine, which was supplemented with 5 mmol/L HEPES (Sigma), 30 mg/L L-proline (Sigma), 0.05% bovine serum albumin (Sigma), 10 ng/mL epidermal growth factor (Sigma), insulin-transferrin-serine-X (Life Technologies), 10-7 mol/L dexamethasone (Sigma), 10 mmol/L nicotinamide (Sigma), 1 mmol/L ascorbic acid-2 phosphate (Wako), an antibiotic/antimycotic solution (Life Technologies), and 3 small molecules: 10 µmol/L Y-27632 (Wako), 0.5 µmol/L A-83-01 (Wako), and 3 µmol/L CHIR99021 (Axon Medchem, Reston, VA). On day 1, the cell and nuclei number for each well was determined, and wells that contained dead cells and, in the 8c plates, cells greater than doublets were excluded from further analysis. Ten days after seeding, cells from each of the formed colonies were counted manually.
Microarray Analysis
Hepatocytes isolated from 5 rats aged between 5 and 14 weeks were used. A 1-color microarray-based gene expression analysis system (Agilent Technologies, Santa Clara, CA) using the SurePrint G3 Rat GE 8 × 60 K Kit (G4853A) was used following the manufacturer’s instructions. Normalization was performed using Agilent GeneSpring version 12.6.1 (per chip: normalization to 75th percentile shift; per gene: normalization to the median of all samples). Probes with signal values lower than 5 in average (75 of total signal value for the 15 samples) were excluded from further analysis. The intensity values were log2-transformed, and RM 1-way ANOVA was performed using the lmne R package to identify differentially expressed genes. Probes with P values less than .05 were regarded as differentially expressed. Unsupervised clustering was performed using the heatmap.2 or heatmap.3 R package. PCA was performed using the prcomp R package, and mapped with the ggplot2 R package. Pathway analysis was performed using the clusterProfiler R package.52
Measurement of Cell Diameter of 2c, 4c, and 8c Hepatocytes
FACS-sorted hepatocytes isolated from a 9-week-old rat were sparsely loaded into a hemocytometer and imaged with a Keyence BZX-710 camera (Keyence, Osaka, Japan). Cell area was calculated using the Hybrid Cell Count program (Keyence). Cell diameter was determined using the following equation: .
Capturing Single Cells
Single-cell capture, lysis, and RT pre-amplification experiments were performed using the C1 Auto Prep System (Fluidigm, South San Francisco, CA) according to the manufacturer’s instructions. Isolated hepatocytes and NPCs were loaded into the C1 integrated fluidic circuit at a concentration of 300 cells/μL in phosphate-buffered saline containing 5% fetal bovine serum and 5 mmol/L of EDTA (Nacalai Tesque, San Diego, CA). Once cell capture was archived, cells were assessed for viability stain using Calcein-AM and ethidium homodimer 1 (Live/Dead Kit; Life Technologies). Chips were imaged using a BZ-X710 and chambers containing 2 or more, none, or dead cells were removed from further analysis. After lysis, reverse transcription (25°C for 10 min, 42°C for 60 min, 85°C for 5 min) and pre-amplification for 18 cycles (each cycle: 95°C for 15 sec, 60°C for 4 min), single-cell complementary DNA was harvested, transferred to a 96-well plate, and diluted 6 times with complementary DNA dilution buffer.
Single-Cell scPCR
For this study, hepatocytes isolated from 5- to 7-week-old rats were used. Single-cell gene-expression experiments were performed using Fluidigm’s 96.96 or 48.48 quantitative PCR DynamicArray microfluidic chips (Fluidigm) according to the manufacturer’s instructions. Individual probe assay mixes were generated by loading 2.5 μL of 2× Assay Loading Reagent (Fluidigm) and 2.5 μL 20× TaqMan gene expression assay (Applied Biosystems, Beverly, MA). Sample mixes were generated by 2.5 μL 2× TaqMan Universal PCR Master Mix (Applied Biosystems), 0.25 μL of 20× GE Sample Loading Reagent (Fluidigm), and 2.25 μL of diluted complementary DNA. Plates were vortexed and centrifuged to homogenize the solutions. Before loading probe assays and sample mixes into 96.96 or 48.48 DynamicArray microfluidic chips, chips were primed in a HX integrated fluidic circuit controller (Fluidigm) machine. After priming, 5 μL of both sample and probe mix were loaded individually in the same machine. Then, chips were transferred into the BioMarkHD real-time quantitative PCR (Fluidigm) and run according to the manufacturer’s instructions. Expression levels were normalized with Actb.
Statistics
When comparing 2 means, P values were calculated by the Welch t test unless otherwise mentioned. Paired comparisons for more than 2 means was conducted using RM 1-way ANOVA, followed by the Holm multiple comparisons test. Unpaired comparisons for more than 2 means were conducted using 1-way ANOVA, followed by the Tukey multiple comparisons test.
Accession Numbers
The microarray data are deposited in GEO under accession number GSE132409. The raw data of scPCR (cycle threshold values) are deposited in GEO under accession number GSE132459.
Acknowledgments
The authors thank Ryou-u Takahashi and Hirokazu Ohata for assistance with FACS experiments, Ayako Inoue for technical assistance, and Luc Gailhouste for constructive discussion. The authors also thank Hideki Nishikawa (Keyence Corporation) for assistance with the microscopic analyses.
Takeshi Katsuda is currently at Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104.
Footnotes
Author contributions Takahiro Ochiya supervised the project; Takeshi Katsuda and Kazunori Hosaka designed the experiments; Kazunori Hosaka performed the majority of the experiments with support from Takeshi Katsuda, Juntaro Matsuzaki, Wataru Usuba, Marta Prieto-Vila, and Tomoko Yamaguchi; Takeshi Katsuda analyzed the data with assistance from Kazunori Hosaka; Atsunori Tsuchiya and Shuji Terai assisted with data interpretation and provided helpful discussions; Takeshi Katsuda wrote the manuscript with support from Kazunori Hosaka, Juntaro Matsuzaki, and Takahiro Ochiya; and Takahiro Ochiya and Takeshi Katsuda obtained funding for the study.
Conflicts of interest The authors disclose no conflicts.
Funding Supported by Grants-in-Aid from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development (AMED: 16fk0310512h0005 and 17fk0310101h0001 to T.O.) and Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists B (16K16643 to T.K.).
References
- 1.Gentric G., Celton-Morizur S., Desdouets C. Polyploidy and liver proliferation. Clin Res Hepatol Gastroenterol. 2012;36:29–34. doi: 10.1016/j.clinre.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Wang M.J., Chen F., Lau J.T.Y., Hu Y.P. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentric G., Desdouets C. Polyploidization in liver tissue. Am J Pathol. 2014;184:322–331. doi: 10.1016/j.ajpath.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Pandit S.K., Westendorp B., De Bruin A. Physiological significance of polyploidization in mammalian cells. Trends Cell Biol. 2013;23:556–566. doi: 10.1016/j.tcb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Duncan A.W. Aneuploidy, polyploidy and ploidy reversal in the liver. Semin Cell Dev Biol. 2013;24:347–356. doi: 10.1016/j.semcdb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Wang B., Zhao L., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsuda T., Kawamata M., Hagiwara K., Takahashi R.U., Yamamoto Y., Camargo F.D., Ochiya T. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 2017;20:41–55. doi: 10.1016/j.stem.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson P.D., Delgado E.R., Alencastro F., Leek M.P., Roy N., Weirich M.P., Stahl E.C., Otero P.A., Chen M.I., Brown W.K., Duncan A.W. The polyploid state restricts hepatocyte proliferation and liver regeneration. Hepatology. 2019;69:1242–1258. doi: 10.1002/hep.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S., Zhou K., Luo X., Li L., Tu H.C., Sehgal A., Nguyen L.H., Zhang Y., Gopal P., Tarlow B.D., Siegwart D.J., Zhu H. The polyploid state plays a tumor-suppressive role in the liver. Dev Cell. 2018;44:447–459.e5. doi: 10.1016/j.devcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanami S., Ben-Moshe S., Elkayam A., Mayo A., Bahar Halpern K., Itzkovitz S. Dynamic zonation of liver polyploidy. Cell Tissue Res. 2017;368:405–410. doi: 10.1007/s00441-016-2427-5. [DOI] [PubMed] [Google Scholar]
- 11.Schmucker D.L. Hepatocyte fine structure during maturation and senescence. J Electron Microsc Tech. 1990;14:106–125. doi: 10.1002/jemt.1060140205. [DOI] [PubMed] [Google Scholar]
- 12.Chao H.W., Doi M., Fustin J.M., Chen H., Murase K., Maeda Y., Hayashi H., Tanaka R., Sugawa M., Mizukuchi N., Yamaguchi Y., Yasunaga J.I., Matsuoka M., Sakai M., Matsumoto M., Hamada S., Okamura H. Circadian clock regulates hepatic polyploidy by modulating Mkp1-Erk1/2 signaling pathway. Nat Commun. 2017;8:1–14. doi: 10.1038/s41467-017-02207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asahina K., Shiokawa M., Ueki T., Yamasaki C., Aratani A., Tateno C., Yoshizato K. Multiplicative mononuclear small hepatocytes in adult rat liver: their isolation as a homogeneous population and localization to periportal zone. Biochem Biophys Res Commun. 2006;342:1160–1167. doi: 10.1016/j.bbrc.2006.02.076. [DOI] [PubMed] [Google Scholar]
- 14.Gebhardt R., Matz-Soja M. Liver zonation: novel aspects of its regulation and its impact on homeostasis. World J Gastroenterol. 2014;20:8491–8504. doi: 10.3748/wjg.v20.i26.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre C., Perret C., Colnot S. Molecular determinants of liver zonation. Prog Mol Biol Transl Sci. 2010;97:127–150. doi: 10.1016/B978-0-12-385233-5.00005-2. [DOI] [PubMed] [Google Scholar]
- 16.Oinonen T., Lindros K.O. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem J. 1998;329:17–35. doi: 10.1042/bj3290017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungermann K., Keitzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr. 1996;16:179–203. doi: 10.1146/annurev.nu.16.070196.001143. [DOI] [PubMed] [Google Scholar]
- 18.Halpern K.B., Shenhav R., Matcovitch-Natan O., Tóth B., Lemze D., Golan M., Massasa E.E., Baydatch S., Landen S., Moor A.E., Brandis A., Giladi A., Stokar-Avihail A., David E., Amit I., Itzkovitz S. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352–356. doi: 10.1038/nature21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behari J. The Wnt/β-catenin signaling pathway in liver biology and disease. Expert Rev Gastroenterol Hepatol. 2010;4:745–756. doi: 10.1586/egh.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell J.O., Monga S.P. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol. 2018;13:351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyajima A., Tanaka M., Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Lin S., Nascimento E.M., Gajera C.R., Chen L., Neuhöfer P., Garbuzov A., Wang S., Artandi S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244–248. doi: 10.1038/s41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A., Taniguchi K., Nakagawa H., Valasek M.A., Ye L., Kopp J.L., Sander M., Carter H., Deisseroth K., Verma I.M., Karin M. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanimizu N., Ichinohe N., Yamamoto M., Akiyama H., Nishikawa Y., Mitaka T. Progressive induction of hepatocyte progenitor cells in chronically injured liver. Sci Rep. 2017;7:39990. doi: 10.1038/srep39990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yimlamai D., Christodoulou C., Galli G.G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B.Z., Camargo F.D. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanger K., Zong Y., Maggs L.R., Shapira S.N., Maddipati R., Aiello N.M., Thung S.N., Wells R.G., Greenbaum L.E., Stanger B.Z. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarlow B.D.D., Pelz C., Naugler W.E.E., Wakefield L., Wilson E.M., Finegold M.J., Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanimizu N., Nishikawa Y., Ichinohe N., Akiyama H., Mitaka T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J Biol Chem. 2014;289:7589–7598. doi: 10.1074/jbc.M113.517243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yovchev M.I., Locker J., Oertel M. Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes. J Hepatol. 2016;64:1348–1357. doi: 10.1016/j.jhep.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaub J.R., Huppert K.A., Kurial S.N.T., Hsu B.Y., Cast A.E., Donnelly B., Karns R.A., Chen F., Rezvani M., Luu H.Y., Mattis A.N., Rougemont A.L., Rosenthal P., Huppert S.S., Willenbring H. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature. 2018;557:247–251. doi: 10.1038/s41586-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., Haft A., Vries R.G., Grompe M., Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han X., Wang Y., Pu W., Huang X., Qiu L., Li Y., Yu W., Zhao H., Liu X., He L., Zhang L., Ji Y., Lu J., Lui K.O., Zhou B. Lineage tracing reveals the bipotency of SOX9+ hepatocytes during liver regeneration. Stem Cell Reports. 2019;12:624–638. doi: 10.1016/j.stemcr.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planas-Paz L., Orsini V., Boulter L., Calabrese D., Pikiolek M., Nigsch F., Xie Y., Roma G., Donovan A., Marti P., Beckmann N., Dill M.T., Carbone W., Bergling S., Isken A., Mueller M., Kinzel B., Yang Y., Mao X., Nicholson T.B., Zamponi R., Capodieci P., Valdez R., Rivera D., Loew A., Ukomadu C., Terracciano L.M., Bouwmeester T., Cong F., Heim M.H., Forbes S.J., Ruffner H., Tchorz J.S. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18:467–479. doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 34.Kreutz C., MacNelly S., Follo M., Wäldin A., Binninger-Lacour P., Timmer J., Bartolomé-Rodríguez M.M. Hepatocyte ploidy is a diversity factor for liver homeostasis. Front Physiol. 2017;8:1–15. doi: 10.3389/fphys.2017.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renard C.A., Labalette C., Armengol C., Cougot D., Wei Y., Cairo S., Pineau P., Neuveut C., de Reyniès A., Dejean A., Perret C., Buendia M.A. Tbx3 is a downstream target of the Wnt/β-catenin pathway and a critical mediator of β-catenin survival functions in liver cancer. Cancer Res. 2007;67:901–910. doi: 10.1158/0008-5472.CAN-06-2344. [DOI] [PubMed] [Google Scholar]
- 36.Lu P., Prost S., Caldwell H., Tugwood J.D., Betton G.R., Harrison D.J. Microarray analysis of gene expression of mouse hepatocytes of different ploidy. Mamm Genome. 2007;18:617–626. doi: 10.1007/s00335-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 37.Mitaka T. The current status of primary hepatocyte culture. Int J Exp Pathol. 1998;79:393–409. doi: 10.1046/j.1365-2613.1998.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu S.H., Delgado E.R., Otero P.A., Teng K.Y., Kutay H., Meehan K.M., Moroney J.B., Monga J.K., Hand N.J., Friedman J.R., Ghoshal K., Duncan A.W. MicroRNA-122 regulates polyploidization in the murine liver. Hepatology. 2016;64:599–615. doi: 10.1002/hep.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan A.W., Taylor M.H., Hickey R.D., Hanlon Newell A.E., Lenzi M.L., Olson S.B., Finegold M.J., Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M.J., Chen F., Li J.X., Liu C.C., Zhang H.B., Xia Y., Yu B., You P., Xiang D., Lu L., Yao H., Borjigin U., Yang G.S., Wangensteen K.J., He Z.Y., Wang X., Hu Y.P. Reversal of hepatocyte senescence after continuous in vivo cell proliferation. Hepatology. 2014;60:349–361. doi: 10.1002/hep.27094. [DOI] [PubMed] [Google Scholar]
- 41.Gandillet A., Alexandre E., Holl V., Royer C., Bischoff P., Cinqualbre J., Wolf P., Jaeck D., Richert L. Hepatocyte ploidy in normal young rat. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:665–673. doi: 10.1016/s1095-6433(02)00374-4. [DOI] [PubMed] [Google Scholar]
- 42.Gorla G.R., Malhi H., Gupta S., Gupta S. Polyploidy associated with oxidative injury attenuates proliferative potential of cells. J Cell Sci. 2001;114:2943–2951. doi: 10.1242/jcs.114.16.2943. [DOI] [PubMed] [Google Scholar]
- 43.Lamas E., Chassoux D., Decaux J., Brechot C., Debey P. Quantitative fluorescence imaging approach for the study of polyploidization in hepatocytes. J Histochem Cytochem. 2009;51:1–12. doi: 10.1177/002215540305100307. [DOI] [PubMed] [Google Scholar]
- 44.Schwarze P.E., Pettersen E.O., Shoaib M.C., Seglen P.O. Emergence of a population of small, diploid hepatocytes during hepatocarcinogenesis. Carcinogenesis. 1984;5:1267–1275. doi: 10.1093/carcin/5.10.1267. [DOI] [PubMed] [Google Scholar]
- 45.Bou-Nader M., Caruso S., Donne R., Celton-Morizur S., Calderaro J., Gentric G., Cadoux M., L'Hermitte A., Klein C., Guilbert T., Albuquerque M., Couchy G., Paradis V., Couty J.P., Zucman-Rossi J., Desdouets C. Polyploidy spectrum: a new marker in HCC classification. Gut. 2019 doi: 10.1136/gutjnl-2018-318021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyoda H., Bregerie O., Vallet A., Nalpas B., Pivert G., Brechot C., Desdouets C. Changes to hepatocyte ploidy and binuclearity profiles during human chronic viral hepatitis. Gut. 2005;54:297–302. doi: 10.1136/gut.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudryavtsev B.N., Kudryavtseva M.V., Sakuta G.A., Stein G.I. Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:387–393. doi: 10.1007/BF02915139. [DOI] [PubMed] [Google Scholar]
- 48.Seglen P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 49.Katsuda T., Hosaka K., Ochiya T. Generation of chemically induced liver progenitors (CLiPs) from rat adult hepatocytes. Bioprotocol. 2018;7:1–26. doi: 10.21769/BioProtoc.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsuda T., Ochiya T., Sakai Y. Generation of hepatic organoids with biliary structures. Methods Mol Biol. 2019;1905:175–185. doi: 10.1007/978-1-4939-8961-4_16. [DOI] [PubMed] [Google Scholar]
- 51.Katsuda T., Kojima N., Ochiya T., Sakai Y. Biliary epithelial cells play an essential role in the reconstruction of hepatic tissue with a functional bile ductular network. Tissue Eng Part A. 2013;19:2402–2411. doi: 10.1089/ten.TEA.2013.0021. [DOI] [PubMed] [Google Scholar]
- 52.Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Septer S., Edwards G., Gunewardena S., Wolfe A., Li H., Daniel J., Apte U. Yes-associated protein is involved in proliferation and differentiation during postnatal liver development. AJP Gastrointest Liver Physiol. 2012;302:G493–G503. doi: 10.1152/ajpgi.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jungermann K., Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 55.Braeuning A., Ittrich C., Köhle C., Hailfinger S., Bonin M., Buchmann A., Schwarz M. Differential gene expression in periportal and perivenous mouse hepatocytes. FEBS J. 2006;273:5051–5061. doi: 10.1111/j.1742-4658.2006.05503.x. [DOI] [PubMed] [Google Scholar]
- 56.Maronpot R.R., Yoshizawa K., Nyska A., Harada T., Flake G., Mueller G., Singh B., Ward J.M. Liver enlargement–STP regulatory policy papers: hepatic enzyme induction: histopathology. Toxicol Pathol. 2010;38:776–795. doi: 10.1177/0192623310373778. [DOI] [PubMed] [Google Scholar]
- 57.Antoniou A., Raynaud P., Cordi S., Zong Y., Tronche F., Stanger B.Z., Jacquemin P., Pierreux C.E., Clotman F., Lemaigre F.P. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strazzabosco M., Fabris L. Neural cell adhesion molecule and polysialic acid in ductular reaction: the puzzle is far from completed, but the picture is becoming more clear. Hepatology. 2014;60:1469–1472. doi: 10.1002/hep.27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmelzer E., Zhang L., Bruce A., Wauthier E., Ludlow J., Yao H.L., Moss N., Melhem A., McClelland R., Turner W., Kulik M., Sherwood S., Tallheden T., Cheng N., Furth M.E., Reid L.M. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spee B., Carpino G., Schotanus B.A., Katoonizadeh A., Vander Borght S., Gaudio E., Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2009;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- 61.Tanimizu N., Ichinohe N., Ishii M., Kino J., Mizuguchi T., Hirata K., Mitaka T. Liver progenitors isolated from adult healthy mouse liver efficiently differentiate to functional hepatocytes in vitro and repopulate liver tissue. Stem Cells. 2016;34:2889–2901. doi: 10.1002/stem.2457. [DOI] [PubMed] [Google Scholar]
- 62.Tanimizu N., Miyajima A. Molecular mechanism of liver development and regeneration. Int Rev Cytol. 2007;259:1–48. doi: 10.1016/S0074-7696(06)59001-1. [DOI] [PubMed] [Google Scholar]
- 63.Mitaka T., Sato F., Mizuguchi T., Yokono T., Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29:111–125. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- 64.Lowes K.N., Brennan B.A., Yeoh G.C., Olynyk J.K. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sell S. Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology. 1998;27:317–331. doi: 10.1002/hep.510270202. [DOI] [PubMed] [Google Scholar]
- 66.Español-Suñer R., Carpentier R., Van Hul N., Legry V., Achouri Y., Cordi S., Jacquemin P., Lemaigre F., Leclercq I.A. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575.e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]