Abstract
Entomopathogenic nematodes have been evaluated for control of mosquito species for decades. Depending on the nematode and mosquito involved, mortality rates of larvae (L) may reach 100% in vitro. Nonetheless, nematode efficacy at oviposition sites has rarely been assessed. Heterorhabditis indica LPP35 has been shown to kill over 75% of Aedes aegypti L3/L4 in cups and bottles outdoors. To assess its efficacy in indoor oviposition sites, different types/sizes of floor drains and pot saucers, and 65 liter water barrels, were infested with L3/L4 and treated with two doses of infective juveniles (IJs). In floor drains, mortality rates varied from 45 to 82%, with better results in the smallest drains. The adjustable dose of 25 IJs/cm2 of the drain’s bottom internal surface gave better results than the fixed dose of 100 IJs/larva. Mortality rates were only 28 to 53% and 0.1 to 1.7% in pot saucers and water barrels, respectively, probably because ridges and grooves that marked the bottom internal surface of these containers hindered the encounter of larvae and IJs.
Key words: Aedes aegypti, Biological control, Dengue fever, Efficacy, Entomopathogenic nematode, Heterorhabdits indica LPP35, Mosquito
The worldwide incidence, severity, and mortality rate of diseases caused by arboviruses – particularly dengue fever – have grown dramatically in recent decades (Anonymous, 2019). One recent estimate indicates 390 million infections/year, mostly in Central and South America, Africa and Asia (Bhatt et al., 2013). In the USA, 26 states are infested by Aedes aegypti L. (Diptera: Culicidae), the main vector of dengue fever. Hawaii and Puerto Rico have faced recent outbreaks of this disease, while many locally transmitted cases have occurred in Europe (Calzolari, 2016; Hahn et al., 2016).
The main strategies to combat A. aegypti are urban habitat management, to minimize oviposition sites, and insecticide application. Insecticide resistance has increased rapidly in recent years (Moyes et al., 2017), making the search for alternatives a global priority. Larval control, in domiciliary and public spaces, is a major challenge (Roiz et al., 2018).
For larval control, greater focus has been placed on chemical and microbial larvicides, insect growth-regulators and predatory fish, copepods and Toxorhynchite larvae. Nonetheless, field trials have brought mixed reports of the prospect of these approaches to prevent or curb dengue fever outbreaks (Horstick and Runge-Ranzinger, 2018; Achee et al., 2019).
Recent reviews on biological control of mosquitoes (Benelli et al., 2016; Huang et al., 2017) have not even mentioned entomopathogenic nematodes (EPNs) as potential agents. Nonetheless, a range of Heterorhabditis and Steinernema species have been shown to kill, at different rates, larvae and pupae of several mosquito species. Early studies indicated that mosquito larval stages 3 and 4 (L3, L4) readily ingest EPN infective juveniles (IJs). Most IJs are injured by larval mouthparts, but some enter the hemocoel and overcome the insect’s defenses, causing mortality (Daad, 1971; Poinar and Kaul, 1982; Molta and Hominick, 1989).
Most studies on the efficacy of EPNs against mosquito larvae have been conducted only in the laboratory, with a variety of methodological procedures that limits generalizations (for a review see Cardoso et al., 2015). Recent assays still have not examined key parameters that may determine the efficacy and viability of EPNs for biocontrol of mosquitoes (Chaudhary et al., 2017; Ulvedal et al., 2017; Toksoz and Saruhan, 2018; Dilipkumar et al., 2019).
Heterorhabditis indica Poinar, Karunakar, and David strain LPP35 was serendipitously found during a survey of nematodes in tank-forming bromeliads, a freshwater ecosystem abundant in culicids (Robaina et al., 2015). Among different EPNs tested, H. indica LPP35 stood out with efficacy of 80 to 96% at killing L3/L4 larvae of A. aegypti. Those results were obtained with a dose as low as 100 IJs/larva (Cardoso et al., 2015).
In a follow-up study, the efficacy of H. indica LPP35 was assessed outdoors, in plastic cups, bottles, and buckets, which mimicked typical oviposition sites. The efficacy was above 75% in cups and bottles, but fell to 40% in buckets (Cardoso et al. 2016). The authors concluded that the key parameters for H. indica LPP35 efficacy against larval stages of A. aegypti are: (i) the dose of IJs/larva, which should be at least 80 to 100; and (ii) the dose of IJs/cm2 of the bottom internal surface of the oviposition site. This is important because mosquito larvae graze at the bottom of oviposition sites, where EPNs remain after application. Since larvae must ingest several EPNs to get infected, about 6.5 IJs/cm2 resulted in a mortality rate around 80%, while at 25 IJs/cm2 it reached 100%.
Urban populations of A. aegypti lay eggs primarily in intra- or peri-domiciliary sites, such as pot saucers, floor drains, and in neighborhoods not served by piped water, water barrels (Powell and Tabachnick, 2013). In pot saucers, the standing water may evaporate within hours, while floor drains and water barrels are perennial sites putatively favorable to EPN’s survival and action. These three kinds of sites also differ in shape and square area of the bottom internal surface. Also, floor drains have a smooth internal surface, while pot saucers and water barrels usually have grooves and ridges. These differences may interfere with the efficacy of EPNs against mosquito larvae.
Hence, the goal of this work was to assess the efficacy of H. indica LPP35 in the main domiciliary sites chosen by A. aegypti females to lay eggs – floor drains, pot saucers, and water barrels.
Material and methods
Nematode and mosquito culturing
Larvae of the wax moth (Galleria mellonela L.) were infected with H. indica LPP35 in 9 cm Petri dishes. About seven days later, the larvae were transferred to White traps for extraction of IJs. Infective juveniles were maintained in culture flasks with distilled water, in the dark, at 16°C, for no longer than seven days before being used in the assays. Eggs of A. aegypti (Rockefeller strain) were placed in trays with filtered tap water, in the dark, at 25°C, for eclosion. After hatching, a small amount of mouse feed was added to the trays to feed the larvae. Stages L3/L4 were individually picked with a Pasteur pipette and used in the assays.
Assays in floor drains
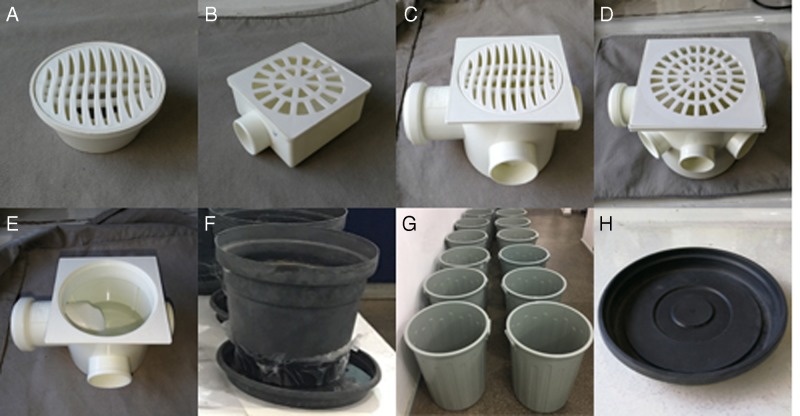
Floor drain types 1 through 4 were used (Fig. 1A–D). Type 1 have a bottom internal surface area of 34.5 cm2. In type 1, the water permanently retained in the drain’s bottom (Fig. 1E) is about 40 ml. In types 2 through 4, the bottom internal surface areas are 47.7, 83.3, and 188.5 cm², respectively, and the amounts of water retained are 110, 270, and 1,120 ml, respectively.
Figure 1:
(A–D) floor drain types (1–4); (E) water permanently retained in the bottom of all drain types; (F) medium size pot and saucer; (G) 65 liter water barrels; (H) ridges and grooves on the surface of pot saucers.
For each drain type, 10 drains received 10 L3/L4 and 1,000 IJs. The blank control consisted of 10 drains in which L3/L4 only were applied. The drains were placed in a growth chamber, in the dark, at 25°C. A voile fabric was attached to the drain to avoid releasing adult mosquitoes into the environment. The evaluation was carried out 7 to 8 d later, when adult mosquitoes had emerged in the blank control. Dead larvae and pupae were counted and inspected for the presence of nematodes in their body. This assay was repeated twice.
A second set of floor drain assays was conducted as described before, with the exception of using the adjustable dose of 25 IJs/cm2 of drain bottom internal surface. This assay was repeated once.
Assays in pot saucers
Small, medium (Fig. 1F), and large pots with saucers were used. When tap water was poured in the pot, it leaked through the bottom holes and created the saucer’s standing water. For the small, medium, and large saucers, the water volumes were 20, 65, and 368 ml, respectively. These volumes were retained mainly around the pot, in areas of 13.3, 87.9, and 245 cm², respectively.
For each saucer size, the assay procedures were as described before, except that the pots and saucers were maintained on laboratory benches, at 25°C. The assays, using either 1,000 IJs/saucer or the adjustable IJ dose, were conducted twice.
Assays in water barrels
65 liter barrels were used, with a bottom internal surface of 961 cm2. Sixty liters of tap water was added, with the water column reaching 50 cm high. Ten barrels received 10 L3/L4 and 25 IJs/cm2 (24,000 IJs), and 10 barrels served as blank controls, receiving 10 L3/L4 only. The assay procedures were as described before, except that the barrels were kept closed with their own lids on the laboratory floor, at 25°C. This assay was repeated once.
Data availability and analysis
The assays’ raw data are publicly available at: https://figshare.com/articles/Silva_et_al_Raw_Data/8320595.
For all assays, the data on the dead mosquito larvae were tested for homogeneity of variances (Cochran and Bartlett tests) and for normality of errors (Lilliefors test), at 5% probability (Ribeiro, 2001). Since the assumptions were satisfied, ANOVA was conducted considering time as one of the factors. No statistical significance was found for the floor drain assays, so their data were pooled. Time was a significant factor for assays in pot saucers and water barrels, so the data were analyzed separately for each assay. The treatments’ mean numbers of dead larvae were compared through the Tukey test at 5% probability, and expressed in the tables as mortality rate (%).
Results and discussion
Heterorhabditis indica LPP35 is a promising agent for A. aegypti larval control in small, cryptic domiciliary environments. When floor drains were treated with 1,000 IJs, the mean mortality rate reached 74% in the smallest drain type, and progressively fell to 45% in the largest one (Table 1). Most nematodes were seen in the larval thorax, but occasionally also in the head (Fig. 2). In a second set of assays using the IJ dose suggested by Cardoso et al. (2016) – 25 IJs/cm2 – the mortality rate peaked at 82%. The nematode efficacy gradually declined to 56% in the largest drains.
Table 1.
Mortality rate (%) of Aedes aegypti L3/L4 in four types of floor drains, seven days after treatment with Heterorhabditis indica LPP35 infective juveniles (IJs), at two distinct doses.
| Types (bottom internal surface area, in cm2) | ||||
|---|---|---|---|---|
| Treatments | 1 (34.5) | 2 (47.7) | 3 (83.3) | 4 (188.5) |
| 100 IJs/larva | ||||
| Treated | 74a | 67a | 64a | 45a |
| Control | 23b | 23b | 10b | 0b |
| 25 IJs/cm 2 | ||||
| Treated | 82a | 78a | 76a | 56a |
| Control | 3.5b | 3.5b | 5b | 1b |
Notes: Values are means of three assays, each with 10 drains/type, and 10 larvae/drain. In the columns, values followed by different letters are statistically different according to the Tukey test at 5%.
Figure 2:

Parthenogenetic female of Heterorhabditis indica LPP35 inside the head of an Aedes aegypti larvae.
This suggests that each mosquito larva browses a certain bottom area of the oviposition site, and that at 25 IJs/cm2 most larvae ingest enough nematodes to get infected and killed. Nonetheless, it remains unclear why nematode efficacy declined in the largest drains, since the IJ dose was the same in all sizes. It is possible that with more nematodes applied in the largest drains, they formed more and larger clumps, as typically occur with some Heterorhabditis species when maintained in water. This would make encounters between the larvae and nematodes less likely.
In pot saucers, applying 10 IJs/mosquito larva resulted in mortality rates of 46 to 53% in the smallest saucers, decreasing to 35% in the largest ones (Table 2). The IJ dose of 25 IJs/cm2 did not improve the efficacy, even when the IJs/mosquito larva rate was about 600. The bottom internal surface of pot saucers, marked with grooves and ridges (Fig. 1H), may have hindered the encounter of mosquito larvae and nematodes. Finney and Harding (1981) reported a steep decline in the efficacy of S. carpocapsae in causing mortality of A. aegypti in containers filled at the bottom with sand or leaves, which also hindered the encounter of larvae and nematodes.
Table 2.
Mortality rate (%) of Aedes aegypti L3/L4 in pot saucers, seven days after treatment with Heterorhabditis indica LPP35 infective juveniles (IJs), at two distinct doses.
| Sizes (bottom internal surface area, in cm2) | |||
|---|---|---|---|
| Treatments | Small (13.3) | Medium (87.9) | Large (245) |
| 100 IJs/larva | |||
| Assay 1 | |||
| Treated | 46a | 29a | 35a |
| Control | 3b | 11b | 14b |
| Assay 2 | |||
| Treated | 53a | 53a | 35a |
| Control | 18b | 13b | 8b |
| 25 IJs/cm 2 | |||
| Assay 1 | |||
| Treated | 31a | 44a | 38a |
| Control | 0b | 0b | 0b |
| Assay 2 | |||
| Treated | 28a | 37a | 30a |
| Control | 0b | 0b | 0b |
Notes: Values are means of 10 pot saucers/size, and 10 larvae/pot saucer. In the columns, values followed by different letters are statistically different according to the Tukey test at 5%.
Grooves and ridges were also present in the bottom internal surface of the water barrels. The dose of 25 IJs/cm2 resulted in 24,000 IJs applied/barrel, but the efficacy was unacceptably low: mortality rates of 0.1 and 1.7% in assays 1 and 2, respectively. This clearly suggests that a much higher dose would be needed to treat water barrels, and that EPNs would not be feasible to treat abandoned swimming pools, which are also important domiciliary oviposition sites.
Since Welch (1960) (cited by Finney and Harding, 1981), EPNs have been investigated for biocontrol of various mosquito species. In some in vitro assays, mortality rates were high, with LC99 being reached with doses as low as 170 IJs/larva (e.g. Poinar and Kaul, 1982; Zohdy et al., 2013; Chaudhary et al., 2017). Nonetheless, few studies have tested EPNs in semi-field or field conditions, or investigated micro-environmental factors that might affect EPNs’ efficacy. For instance, although larvae of the black fly (Simulium vittatum) were readily killed by S. carpocapsae in vitro, this was ineffective when applied in streams (Gaugler et al., 1983). In bromeliads, Cardoso et al. (2016) reported a mortality rate of A. aegypti larvae of only 23%, probably because most IJs fell into the plant leaf axils, away from grazing larvae. Reports such as these should not come as a surprise, since a range of abiotic factors have been shown to affect EPN efficacy, at least in agricultural settings (Shapiro-Ilan and Dolinski, 2015).
In this study, H. indica LPP35 was particularly efficient in floor drains, with an indication that doses above 25 IJs/cm2 should be applied. Peri-domiciliary oviposition sites, such as pot saucers and large water containers, seem not to be appropriate for EPNs. These sites are more prone to sun exposure, heating of the water retained, and desiccation.
Although EPNs seem promising to control A. aegypti in just one subset of oviposition sites, this should not discourage further studies, particularly on the matters of doses and application technology. The control of A. aegypti has become a daunting task worldwide, since it requires efficient methods, good local governance, and community engagement. A new concept of mosquito control has emerged, which involves the development of approaches that are complementary, niche strategies, rather than expecting one approach to become the default intervention across a wide range of settings (Achee et al., 2019). Further studies may lead EPNs to become one more option for larval control of A. aegypti in domiciliary cryptic oviposition sites.
Acknowledgments
The authors are indebted to the crowdfunding platform Experiment (https://experiment.com/) for supporting this study, and to Professor Francisco J. A. Lemos and Lab Technician Rivea Rodrigues (UENF/LBT) for providing mosquito eggs.
References
- Achee N. L., Grieco J. P., Vatandoost H., Seixas G., Pinto J., Ching-NG L., Martins A. J., Juntarajumnong W., Corbel V., Gouagna C., David J.-P., Logan J. G., Orsborne J., Marois E., Devine G. J. and Vontas J.. 2019. Alternative strategies for mosquito-borne arbovirus control. PLoS Neglected Tropical Diseases 13(1), available at: 10.1371/journal.pntd.0006822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous 2019. Dengue and severe dengue. World Health Organization, Geneva, available at: www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue. [Google Scholar]
- Benelli G., Jeffries C. L. and Walker T.. 2016. Biological control of mosquito vectors: past, present, and future. Insects 7:52, doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., Drake J. M., Brownstein J. S., Hoen A. G., Sankoh O., Myers M. F., George D. B., Jaenisch T., Wint G. R. W., Simmons C. P., Scott T. W., Farrar J. J. and Hay S. I.. 2013. The global distribution and burden of dengue. Nature. 49:504–7, doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari M. 2016. Mosquito-borne diseases in Europe: an emerging public threat. Reports in parasitology 5:1–12, available at: 10.2147/RIP.S56780 [DOI] [Google Scholar]
- Cardoso D. O., Gomes V. M., Dolinski C. and Souza R. M.. 2015. Potential of entomopathogenic nematodes as biocontrol agents of immature stages of Aedes aegypti Nematoda Vol. 2 available at: 10.4322/nematoda.09015 [DOI] [Google Scholar]
- Cardoso D. O., Gomes V. M., Dolinski C., Souza R. M. and Idalino W. S. S.. 2016. Efficacy of Heterorhabditis indica LPP35 against Aedes aegypti larvae in human-generated containers and bromeliads. Nematoda Vol. 3 available at: 10.4322/nematoda.01715 [DOI] [Google Scholar]
- Chaudhary M. Z., Majeed M., Tayyib M., Javed N., Farzand A., Moosa A., Shehzad M. and Mushtaq F.. 2017. Antagonistic potential of Steinernema kraussei and Heterorhabditis bacteriophora against dengue fever mosquito Aedes aegypti. Journal of Entomology and Zoology Studies 5:865–9. [Google Scholar]
- Daad R. H. 1971. Size limitations on the infectibility of mosquito larvae by nematodes during filter-feeding. Journal of Invertebrate Pathology 18:246–71. [DOI] [PubMed] [Google Scholar]
- Dilipkumar A., Ramalingam K. R., Chinnaperumal K., Govindasamy B., Paramasivam D., Dhayalan A. and Pachiappan P.. 2019. Isolation and growth inhibition potential of entomopathogenic nematodes against three public health important mosquito vectors. Experimental Parasitology 197:76–84, available at: 10.1016/j.exppara.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Finney J. R. and Harding J. B.. 1981. Some factors affecting the use of Neoaplectana sp. for mosquito control. Mosquito News 41:798–800. [Google Scholar]
- Gaugler R., Kaplan B., Alvarado C., Montoya J. and Ortega M.. 1983. Assessment of Bacillus thuringiensis serotype 14 and Steinernema feltiae (Nematoda: Steinernematidae) for control of the Simulium vectors of onchocercasis in Mexico. Entomophaga 28:309–15. [Google Scholar]
- Hahn M. B., Eisen R. J., Eisen L., Boegler K. A., Moore C. G., McAllister J., Savage H. M. and Mutebi J.-P.. 2016. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae). Journal of Medical Entomology 53:1169–75, available at: 10.1093/jme/tjw072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstick O. and Runge-Ranzinger S.. 2018. Protection of the house against chagas disease, dengue, leishmaniasis, and lymphatic filariasis: a systematic review. The Lancet Infectious Diseases 18:e147–e158, available at: http://dx.doi.org/10.1016/S1473-3099(17)30422-X [DOI] [PubMed] [Google Scholar]
- Huang Y.-J. S., Higgs S. and Vanlandingham D. L.. 2017. Biological control strategies for mosquito vectors of arboviruses. Insects 8:21, doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molta N. B. and Hominick W. M.. 1989. Dose- and time-response assessments of Heterorhabitis heliothidis and Steinernema feltiae (Nem.: Rhabitida) against Aedes aegpytii larvae. Entomophaga 34:485–93. [Google Scholar]
- Moyes C. L., Vontas J., Martins A. J., Ng L. C., Koou S. Y., Dusfour I., Raghavendra K., Pinto J., Corbel V., David J.-P. and Weetman D.. 2017. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negleted Tropical Diseases 11(7), available at: 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar G. O. Jr and Kaul H. N.. 1982. Parasitism of the mosquito Culex pipiens by the nematode Heterorhabditis bacteriophora. Journal of Invertebrate Pathology 39:382–7. [Google Scholar]
- Powell J. R. and Tabachnick W. J.. 2013. History of domestication and spread of Aedes aegypti – a review. Memórias do Instituto Oswaldo Cruz 108:11–17, doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. I., Jr 2001. Análises estatísticas no SAEG (Sistema para análises estatísticas Universidade Federal de Viçosa, Viçosa. [Google Scholar]
- Robaina R. R., Souza R. M., Gomes V. M., Cardoso D. O. and Almeida A. M.. 2015. Nematode trophic structure in phytotelmata of Canistropsis billbergioides and Nidularium procerum (Bromeliaceae) in the Atlantic Forest – variability in relation to climate variables and plant architecture. Nematoda Vol. 2 available at: 10.4322/nematoda.01615 [DOI] [Google Scholar]
- Roiz D., Wilson A. L., Scott T. W., Fonseca D. M., Jourdain F., Müller P., Velayudhan R. and Corbel V.. 2018. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Neglected Tropical Diseases 12(12), available at: 10.1371/journal.pntd.0006845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan D. and Dolinski C.. 2015. Entomopathogenic nematode application technology Pp. 231–254 in Campos-Herrera Rachel (Ed). Nematode Pathogenesis of Insects and Other Pests. Heidelberg, Springer International Publishing. [Google Scholar]
- Toksoz S. and Saruhan I.. 2018. Efficacy of entomopathogenic nematode isolates from Turkey and Kyrgyzstan against the larvae of the mosquito species Culex pipiens L. (Diptera: Culicidae) under laboratory conditions. Egyptian Journal of Biological Pest Control 28:84 available at: 10.1186/s41938-018-0088-2 [DOI] [Google Scholar]
- Ulvedal C., Bertolotti M. A., Cagnolo S. R. and Almirón W. A.. 2017. Ensayos de sensibilidad de larvas de Aedes aegypti y Culex quinquefasciatus frente al nematodo Heterorhabditis bacteriophora en condiciones de laboratório. Biomédica 37:67–76, available at: 10.7705/biomedica.v34i2.3470 [DOI] [PubMed] [Google Scholar]
- Zohdy N. M., Shamseldean M. M., Abd-El-Samie E. M. and Hamama H. M.. 2013. Efficacy of the steinernematid and heterorhabditid nematodes for controlling the mosquito, Culex quinquefasciatus (Diptera: Culicidae). Journal of Mosquito Research 3:33–44, doi: 105376/jmr.2013.03.0005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The assays’ raw data are publicly available at: https://figshare.com/articles/Silva_et_al_Raw_Data/8320595.
For all assays, the data on the dead mosquito larvae were tested for homogeneity of variances (Cochran and Bartlett tests) and for normality of errors (Lilliefors test), at 5% probability (Ribeiro, 2001). Since the assumptions were satisfied, ANOVA was conducted considering time as one of the factors. No statistical significance was found for the floor drain assays, so their data were pooled. Time was a significant factor for assays in pot saucers and water barrels, so the data were analyzed separately for each assay. The treatments’ mean numbers of dead larvae were compared through the Tukey test at 5% probability, and expressed in the tables as mortality rate (%).