Abstract
Objective
To determine whether insulin resistance (IR) measured by homeostasis model of insulin resistance (HOMA-IR) can further stratify diabetes risk in African Americans (AAs) beyond obesity and identify obese, low risk and non-obese, high risk individuals.
Methods
Using the Jackson Heart Study cohort, we categorized participants without diabetes into four phenotypes: non-obese/insulin-sensitive, non-obese/IR, obese/insulin-sensitive and obese/IR. Obesity was defined as BMI ≥ 30 or BMI 25–30 plus an increased waist circumference. IR was defined as HOMA-IR ≥ 2. We used modified Poisson regression models to estimate the incident risk-ratios (IRR) of diabetes across these phenotypes adjusting for potential confounders and HbA1c.
Results
Among 3219 AAs without diabetes, 14.0% were non-obese/insulin-sensitive, 24.6% non-obese/IR, 6.2% obese/insulin-sensitive, and 55.3% obese/IR. The overall crude incidence rate of diabetes was 29.91 cases/1000 person-years. In fully-adjusted models, compared to the non-obese/insulin-sensitive group, the relative risk of diabetes was highest in obese/IR (IRR = 2.35; 95% CI: 1.53, 3.60), followed by non-obese/IR (IRR = 1.59; 95% CI: 1.02, 2.46), and non-significant for the obese/insulin-sensitive (IRR = 1.70; 95% CI: 0.97, 2.99) group.
Conclusions
HOMA-IR can further stratify diabetes risk in AA adults beyond obesity, identifying non-obese high-risk and lower-risk obese individuals. However, diabetes risk should still be carefully monitored in obese populations despite insulin sensitivity.
Keywords: Overweight, Adiposity, Insulin sensitivity, Diabetes mellitus, Black race
Introduction
Type 2 diabetes mellitus (T2DM) affects approximately 30.3 million (9.4%) adults in the United States [1]. African American (AA) adults, however, are disproportionately affected by T2DM, with 12.7% of AA adults in the United States affected compared to 7.4% for non-Hispanic whites [1]. In addition, AA adults bear a disproportionate burden of morbidity and mortality associated with diabetes, with a higher rate of retinopathy, microalbuminuria, end stage renal disease, lower extremity amputation, and mortality compared with European Americans [2].
Obesity has been widely used as a risk factor for developing T2DM. However, it is uncertain whether a biomarker exists to further stratify the risk of developing T2DM beyond obesity [3], [4], [5]. As such, whether obese individuals who are of normal insulin sensitivity exist along with their respective T2DM risks are not well understood, especially in AA adults. Similarly, how non-obese individuals compare with obese individuals with similar insulin sensitivity in the risk of incident T2DM remains an important question to be answered.
In a small bi-racial cohort, Owei et al. has shown a proof of concept that the homeostasis model of insulin resistance (HOMA-IR) is a useful biomarker for insulin sensitivity in predicting incident pre-diabetes but the study was limited in power to predict diabetes [1]. Given that the time lag between pre-diabetes and T2DM is highly variable and not all individuals with pre-diabetes develop T2DM [6], a large-scale population study is needed to investigate further the interplay between obesity, HOMA-IR, and T2DM risk, especially in AAs, since early identification of high risk groups can enable prevention efforts.
The Jackson Heart Study (JHS) is the largest cohort study of AAs to date that follows participants over time for cardio-metabolic health and is ideal to answer our study question: ‘What is the relationship between obesity, as defined by BMI and waist circumference (WC), and insulin-resistance, as defined by HOMA-IR, and risk of T2DM in African-Americans?’ We hypothesized that categorization of study participants based on obesity and HOMA-IR would allow for further risk stratification and identification of the “low-risk obese” as well as “at risk non-obese” phenotypes for future development of T2DM in a common office visit or clinic setting.
2, Methods
Study population
This was a cohort study using collected data from the JHS. Details regarding the design of the JHS have been previously published [7]. In brief, JHS is a longitudinal, population-based cohort study of cardiovascular disease that recruited noninstitutionalized adult participants (N = 5301) residing in Jackson, Mississippi, who self-identified as AA [7]. The JHS was initiated in 2000 and includes participants between 21 and 94 years. Data used in this analysis were measured at baseline (2000–2004) and Visits 2 (2005–2008) and 3 (2008–2012).
The JHS was approved by the University of Mississippi Medical Center Institutional Review Board, where all study participants gave written informed consent. The current analysis of the JHS data was approved by the Brown University Institutional Review Board, who waived the requirement for informed consent for this analysis, as the data available to the authors did not contain identifiable information of the study participants.
Study covariates
All anthropometric and clinical covariates were obtained at baseline examination. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured in centimeters and was taken from an average of 2 measures of the waist at the level of the umbilicus in the upright position. Personal and family medical histories were assessed via self-report using a structured questionnaire administered by trained file center personnel. Hypertension was assessed using a combination of self-reported medical history and current medication use: self-reported history of hypertension or antihypertensive medications used in 2 weeks prior to baseline visit. Physical activity was classified as poor (0 min/week), intermediate (>0 to < 150 min/week) or ideal (≥150 min/week) based on minutes/week of moderate or vigorous physical activity. Diet was classified as poor (0–1 components), intermediate (2–3 components) or ideal (4–5 components) based on the number of diet components achieved from the following: ≥4.5 cups of fruits and vegetables/day, ≥7 oz of fish/week, <1500 mg of sodium/day, <450 calories/week of sugar-sweetened beverages, and ≥ 3 servings/day of whole grains. Smoking was defined as poor (current smoker), intermediate (quit < 12 months ago) or ideal (never smoked or quit ≥ 12 months ago) based on self-reported cigarette smoking status. Family history of diabetes was defined by presence of diabetes in any of the parents or sibling. Fasting serum total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL), and triglyceride concentrations were assessed with Roche enzymatic methods using a Cobras centrifuge analyzer (Hoffman-La Roche, Inc., Nutley, New Jersey), with the laboratory certified by the Lipid Standardization Program of the Centers for Disease Control and Prevention and the National Heart, Lung, and Blood Institute [8]. High-sensitivity C reactive protein (hsCRP) was measured using immunoturbidimetric CRP-Latex assay from Kamiya Biomedical Company. The inter-assay coefficients of variation on control samples repeated in each assay were 4.5% and 4.4% at CRP concentrations of 0.45 mg/L and 1.56 mg/L respectively. The reliability coefficient for masked quality control replicates was 0.95 for the CRP assay [8].
Insulin resistant obesity phenotypes:
Obesity was defined using a combination of BMI and WC as outlined in clinical guidelines established by the National Institute of Health (NIH) [9]: BMI ≥ 30 kg/m2 or for participants with a BMI between 25 kg/m2 and 30 kg/m2, a WC > 102 cm for men and > 88 cm for women (high-risk overweight or obesity-equivalent). Insulin resistance was calculated using HOMA-IR: (fasting insulin uU/mL*fasting glucose mmol/L)/22.50 [10]. To better inform the decision of using HOMA-IR as a binary versus continuous variable, we compared the area under the receiver operating characteristic curve (AUC) in regression models that used HOMA-IR as a continuous variable versus HOMA-IR as a binary variable. Our results yielded virtually identical AUC values (AUC for binary HOMA-IR = 0.83, 95% CI: 0.80, 0.84; AUC for continuous HOMA-IR = 0.83, 95% CI: 0.81, 0.85), for which we proceeded with the more ‘clinician user friendly’ binary variable for our analyses. For the main analysis, insulin resistance was defined as a HOMA-IR value ≥ 2 [11]. Using these definitions, we categorized participants into four mutually exclusive phenotypic groups based on participant’s baseline obesity and insulin resistance status:
-
1.
Non-obese/insulin-sensitive (BMI < 25 or BMI 25–30 + normal WC, HOMA-IR < 2)
-
2.
Non-obese/insulin-resistant (BMI < 25 or BMI 25–30 + normal WC, HOMA-IR ≥ 2)
-
3.
Obese/insulin-sensitive (BMI ≥ 30 or BMI 25–30 + increased WC, HOMA-IR < 2)
-
4.
Obese/insulin-resistant (BMI ≥ 30 or BMI 25–30 + increased WC, HOMA-IR ≥ 2)
As a sensitivity analysis, we also examined population-specific thresholds using the upper quartile of BMI and gender-specific WC to define obesity and using the upper quartile of HOMA-IR to define insulin resistance:
-
1.
Non-obese/insulin-sensitive (BMI and WC < 75th percentile, HOMA-IR < 75th percentile)
-
2.
Non-obese/insulin-resistant (BMI and WC < 75th percentile, HOMA-IR ≥ 75th percentile)
-
3.
Obese/insulin-sensitive (BMI or WC ≥ 75th percentile, HOMA-IR < 75th percentile)
-
4.
Obese/insulin-resistant (BMI or WC ≥ 75th percentile, HOMA-IR ≥ 75th percentile)
Outcomes:
The primary outcome was incident T2DM. Based on the 2010 American Diabetes Association (ADA) criteria for the diagnosis of T2DM, T2DM was considered present at Visit 2 or Visit 3 follow-up exam if any of the following were present: fasting plasma glucose ≥ 126 mm/dl, hemoglobin A1c (hbA1c) ≥ 6.5%, or self-reported history of diagnosis of diabetes or anti-diabetic medication use in at least 2 weeks preceding assessment [12], [13]. Fasting blood samples were collected according to standardized procedures, and the assessments of plasma glucose and lipids were processed at the Central Laboratory (University of Minnesota).
Statistical analyses
Baseline characteristics were compared across the four phenotypic groups, chi square tests and one-way ANOVAs were used to examine differences in means and proportions, respectively. Baseline comparisons were also made within obesity and insulin-resistance subgroups, comparing those who were obese to those who were non-obese and those who were insulin-sensitive to those who were insulin-resistant using Scheffe’s test for multiple comparisons.
We first assessed the association of obesity and IR status with 10-year incident diabetes using modified Poisson regression modeling and confirmed that both variables were significantly predictive of incident diabetes in both univariate and joint analyses (all p-values < 0.01). We performed modified Poisson regression analyses to examine the association between phenotypes and incident diabetes using incident rate-ratios (IRR). All models used a robust variance estimator and employed an offset term (log(years of follow-up)) to accommodate different follow-up times for each participant [14], [15]. First, we estimated the incidence rate of T2DM over the course of follow-up across the four phenotype groups. Next, we calculated the IRR of T2DM across the four groups using the non-obese/insulin-sensitive group as the reference. Base models were adjusted for age and sex (Model 1). Subsequent models were additionally adjusted for Hemoglobin A1c [HbA1c] (Model 2), HbA1c + additional risk factors (Model 3) including parent history of diabetes, systolic blood pressure, triglycerides, HDL cholesterol, and hs-CRP; and HbA1c + additional risk factors + health behaviors (Model 4) including Ideal Cardiovascular Health metrics for physical activity, smoking, and diet. A formal test for multiplicative interaction was conducted in each model to examine the potential for effect-measure modification by gender. As a sensitivity analysis, we re-classified participants using the upper quartile of population-specific thresholds of obesity and insulin resistance to examine whether distinct thresholds of obesity or insulin resistance altered our results. A p-value < 0.05 was considered significant for all analyses with the exception of multiple comparisons at the subgroup level for which a significance level of < 0.025 was used.
Because we hypothesized that these obesity phenotypes could be a useful stratification tool in clinical practice, we next examined the ability of a minimal set of predictors (age, sex, HbA1c and obesity phenotypes) to discriminate future diabetes risk using AUCs [16], [17]. As a point of comparison, we also calculated the AUC corresponding to a model that includes the predictors from the published ARIC diabetes risk prediction model (age, height, waist circumference, HDL cholesterol, triglycerides, fasting glucose, and systolic blood pressure; race was also included in the original model but is omitted here) [18]. We further added HbA1c as a predictor to the original ARIC prediction model to reflect updated clinical guidelines [13], [19].
Results
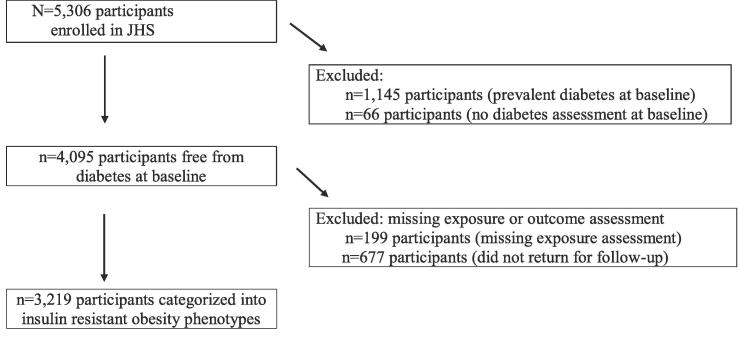
Of the 5306 participants enrolled in JHS, we excluded 1145 participants with prevalent T2DM at baseline (2000–2004) and 66 participants whose diabetes status was not assessed at baseline. We further excluded 199 participants with missing exposure assessment at baseline (BMI, waist circumference, glucose or insulin measures) and 677 participants who did not return for follow-up at Visit 2 or Visit 3 resulting in 3219 participants in the final analytic sample (Fig. 1).
Fig. 1.
Selection of the analytic cohort: study flow diagram.
Baseline characteristics
Among 3219 AA participants in the study, the mean age was 53.3 (SD12.5) years, 36% were men and 61.4% were obese based on BMI and WC. Of the participants, 14.0% were classified as non-obese/insulin-sensitive (n = 450), 24.6% were classified as non-obese/insulin-resistant (n = 791), 6.2% were classified as obese/insulin-sensitive (n = 198), and 55.3% were classified as obese/insulin-resistant (n = 1780). Baseline characteristics of participants stratified by phenotype are summarized in Table 1. Obese participants were more likely to be female (p < 0.025). Conditional on obesity status, insulin-resistant participants had a higher prevalence of hypertension and higher weight, BMI, waist circumference, HbA1c, LDL, total cholesterol and triglyceride levels (all p-values < 0.025). Conditional on insulin resistance status, obese participants had higher HbA1c and triglyceride levels, lower HDL cholesterol levels, and a higher prevalence of family history of diabetes (all p-values < 0.025).
Table 1.
Baseline characteristics of study participants by insulin resistant obesity phenotype*
| Overall | Non-obese/ insulin sensitive | Non-obese/ insulin resistant | Obese/insulin sensitive | Obese/insulin resistant | p-value** | |
|---|---|---|---|---|---|---|
| N = 3,219 | n = 450 | n = 791 | n = 198 | n = 1,780 | ||
| Age, mean (SD), years | 53.35 (12.53) | 52.32 (12.96) | 53.44 (12.98) | 52.27 (12.08) | 53.68 (12.24) | 0.12 |
| Male sex, # (%) | 1,164 (36.16) | 244 (54.22) | 424 (53.60) | 34 (17.17) | 462 (25.96) | <0.0001 |
| Height, mean (SD), cm | 169.15 (9.34) | 170.99 (9.39) | 170.83 (9.70) | 167.12 (8.67) | 168.17 (9.06) | <0.0001 |
| Weight, mean (SD), kg | 89.25 (20.86) | 71.92 (11.72) | 75.96 (11.35) | 92.05 (17.83) | 99.22 (20.12) | <0.0001 |
| Waist, mean (SD), cm | 98.60 (15.61) | 83.40 (9.16) | 87.81 (7.97) | 98.82 (11.14) | 107.21 (13.90) | <0.0001 |
| BMI, mean (SD), kg/m2 | 31.22 (7.04) | 24.53 (3.00) | 25.94 (2.48) | 32.92 (5.78) | 35.06 (6.50) | <0.0001 |
| Family history of diabetes, # (%) | 1,459 (45.92) | 185 (41.67) | 335 (42.89) | 100 (51.02) | 839 (47.78) | 0.014 |
| Hypertension, # (%) | 1,538 (47.78) | 150 (33.33) | 335 (42.35) | 79 (39.90) | 974 (54.72) | <0.0001 |
| Physical Activity | 0.003 | |||||
| Poor | 1,441 (44.79) | 186 (41.33) | 330 (41.72) | 80 (40.40) | 845 (47.53) | |
| Intermediate | 1,080 (33.57) | 147 (32.67) | 266 (33.63) | 73 (36.87) | 594 (33.41) | |
| Ideal | 696 (21.64) | 117 (26.00) | 195 (24.65) | 45 (22.73) | 339 (19.07) | |
| Diet | 0.41 | |||||
| Poor | 2,057 (63.90) | 287 (63.78) | 510 (64.48) | 116 (58.59) | 1144 (64.27) | |
| Intermediate | 1,140 (35.41) | 161 (35.78) | 272 (34.39) | 81 (40.91) | 626 (35.17) | |
| Ideal | 22 (0.68) | 2 (0.44) | 9 (1.14) | 1 (0.51) | 10 (0.56) | |
| Smoking | <0.0001 | |||||
| Poor | 364 (11.50) | 80 (18.22) | 95 (12.16) | 23 (11.79) | 166 (9.49) | |
| Intermediate | 29 (0.92) | 8 (1.82) | 8 (1.02) | 0 (0.00) | 13 (0.74) | |
| Ideal | 2,772 (87.58) | 351 (80.00) | 678 (86.81) | 172 (88.21) | 1571 (89.77) | |
| Systolic blood pressure, mean (SD), mmHg | 125.65 (16.08) | 123.60 (16.06) | 125.10 (17.15) | 124.70 (17.39) | 126.51 (15.37) | 0.003 |
| HOMA-IR, mean (SD) | 3.55 (2.30) | 1.49 (0.38) | 3.21 (2.02) | 1.56 (0.38) | 4.44 (2.32) | <0.0001 |
| Insulin, mean (SD), u/mL | 15.73 (9.27) | 7.23 (1.82) | 14.34 (8.14) | 7.58 (1.91) | 19.41 (9.21) | <0.0001 |
| Fasting plasma glucose, mean, (SD), mg/dL | 90.28 (8.92) | 83.95 (6.78) | 90.68 (8.07) | 84.23 (7.10) | 92.37 (8.89) | <0.0001 |
| HbA1c, mean (SD), % | 5.50 (0.47) | 5.29 (0.45) | 5.45 (0.47) | 5.41 (0.41) | 5.58 (0.46) | <0.0001 |
| HDL cholesterol, mean (SD), mg/dL | 52.24 (14.56) | 57.78 (16.64) | 52.38 (14.71) | 57.13 (15.32) | 50.24 (13.30) | <0.0001 |
| LDL cholesterol, mean (SD), mg/dL | 127.49 (36.30) | 120.57 (35.92) | 129.08 (36.64) | 122.36 (35.41) | 129.11 (36.11) | <0.0001 |
| Total cholesterol, mean (SD), mg/dL | 199.24 (38.89) | 193.09 (39.10) | 200.82 (39.73) | 195.55 (37.15) | 200.51 (38.51) | 0.001 |
| Triglycerides, mean (SD), mg/dL | 98.23 (56.02) | 74.22 (41.52) | 98.09 (61.56) | 80.99 (48.96) | 106.29 (55.23) | <0.0001 |
| Hs-CRP, mean (SD), mg/L | 4.68 (7.15) | 2.15 (4.29) | 2.77 (5.88) | 4.65 (5.86) | 6.17 (7.95) | <0.0001 |
Abbreviations: Body mass index (BMI ; homeostasis model of insulin resistance (HOMA-IR; hemoglobin A1c (HbA1c; high-sensitivity C-reactive protein (hs-CRP Family history of diabetes is defined as a mother, father or sibling with history of diabetes; Physical Activity is defined as Poor, Intermediate or Ideal based on minutes/week of moderate or vigorous physical activity. Poor Health (0 min/week); Intermediate Health (>0–<150 min/week); Ideal Health (≥150 min/week); Diet is defined as Poor, Intermediate or Ideal based on the number of diet components achieved. Components (based on a 2000-calorie diet): ≥4.5 cups of fruits and vegetables/day; ≥7 oz of fish/week; <1500 mg of sodium/day; <450 calories/week of sugar-sweetened beverages; ≥3 servings/day of whole grains. Poor Health (0–1 components); Intermediate Health (2–3 components); Ideal Health (4–5 components); Smoking is defined as Poor, Intermediate or Ideal based on current and former smoking status. Poor Health (Current smoker); Intermediate Health (Quit < 12 months ago); Ideal Health (Never smoked or quit ≥ 12 months ago).
* Insulin resistant obesity phenotypes were defined based on obesity and insulin resistant status at baseline. Obesity was defined based on NHLBI recommendations using a combination of BMI and gender-specific waist circumference thresholds. Insulin resistance was defined using a HOMA-IR cutoff of ≥2.
** p-value for heterogeneity among phenotypes.
Obesity phenotypes and T2DM
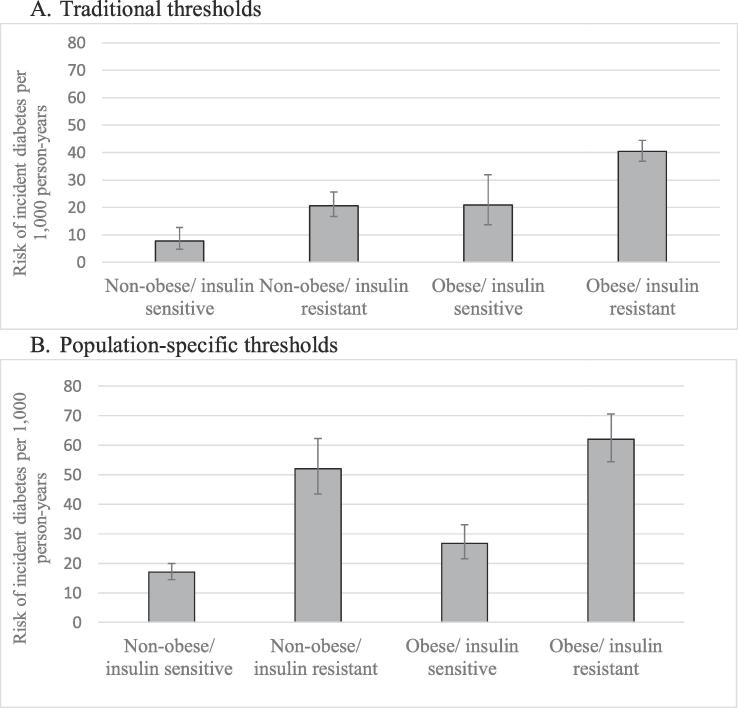
A total of 559 participants developed T2DM over the course of follow-up (17.37% of the analytic sample). The overall crude incidence rate of T2DM was 29.91 cases/1000 person-years (95% CI: 25.94, 34.61). Fig. 2 displays the incidence rates of diabetes across the four phenotypes. Rates were the lowest in non-obese/insulin-sensitive group (7.83 cases/1000 person-years; 97.5% CI: 4.81, 12.75) and highest in the obese/insulin-resistant group (40.41 cases/person-years; 97.5% CI: 36.78, 44.41), and comparable amongst the non-obese/insulin-resistant versus the obese/insulin-sensitive groups. Rates of T2DM were higher among insulin-resistant participants (conditional on obesity status) and among obese participants (conditional on insulin resistance status), suggesting that both obesity and insulin resistance further sub-classified individuals for T2DM risk (both p-values < 0.025). In fully-adjusted regression models (Table 2), compared to the non-obese/insulin-sensitive group, diabetes risk was significantly higher in both the non-obese/insulin-resistant group (Incidence rate ratio (IRR) = 1.59; 95% CI: 1.02, 2.46) and the obese/insulin resistant group (IRR = 2.35; 95% CI: 1.53, 3.60); in the obese/insulin-sensitive group, the risk of developing diabetes did not differ significantly from the non-obese/insulin sensitive group (IRR = 1.70; 95% CI: 0.97, 2.99). Interaction tests for gender were non-significant for all phenotypic groups irrespective of the cut-offs for obesity and insulin-resistance utilized (all p > 0.20). Findings were similar in sensitivity analyses utilizing population-specific thresholds of obesity and insulin-resistance with one main exception: under these new thresholds, the non-obese/insulin-resistant group had a significantly higher rate of T2DM (51.98 cases/1,000 person-years; 97.5% CI: 43.42, 62.24) compared to the obese/insulin-sensitive group (26.73 cases/1,000 person-years; 97.5% CI: 21.61, 33.06; Fig. 2). However, the risk of developing diabetes did not differ significantly in the obese/insulin-sensitive group compared to the non-obese/insulin-sensitive group (IRR = 1.25; 95% CI: 0.99, 1.59) in fully-adjusted regression models (Table 3).
Fig. 2.
Incidence rates of type 2 diabetes mellitus per 1000 person-years by insulin resistant obesity phenotype. Incidence rates are presented for each group along with error bars representing the 97.5% confidence intervals around the rate. Fig. 2A presents the incidence rates using conventional cutoffs. Fig. 2B presents the incidence rates using population-specific cut-offs. Conventional cut-offs were defined as: Non-obese/insulin-sensitive (BMI < 25 or BMI 25–30 + normal waist circumference, HOMA-IR < 2); non-obese/insulin-resistant (BMI < 25 or BMI 25–30 + normal waist circumference, HOMA-IR ≥ 2); obese/insulin-sensitive (BMI ≥ 30 or BMI 25–30 + increased waist circumference, HOMA-IR < 2); obese/insulin-resistant (BMI ≥ 30 or BMI 25–30 + increased waist circumference, HOMA-IR ≥ 2). Population-specific cut-offs were defined using the upper quartile of BMI and gender-specific waist circumference to define obesity and using the upper quartile of HOMA-IR to define insulin resistance: non-obese/insulin-sensitive (BMI and waist circumference < 75th percentile, HOMA-IR < 75th percentile); non-obese/insulin-resistant (BMI and waist circumference < 75th percentile, HOMA-IR ≥ 75th percentile); obese/insulin-sensitive (BMI or waist circumference ≥ 75th percentile, HOMA-IR < 75th percentile); obese/insulin-resistant (BMI or waist circumference ≥ 75th percentile, HOMA-IR ≥ 75th percentile).
Table 2.
Association between insulin resistant obesity phenotypes* and incident diabetes.
| Non-obese/insulin sensitive n = 450 |
Non-obese/insulin resistant n = 791 |
Obese/insulin sensitive n = 198 |
Obese/insulin resistant n = 1,780 |
|
|---|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | |
| Model 1 | ||||
| Adjusted for phenotypes, age and sex | REF | 2.60 (1.64, 4.13) | 2.99 (1.70, 5.25) | 5.56 (3.61, 8.56) |
| Model 2 | ||||
| Adjusted for phenotypes, age, sex + HbA1c | REF | 1.79 (1.15, 2.77) | 1.94 (1.11, 3.39) | 2.98 (1.96, 4.54) |
| Model 3 | ||||
| Adjusted for phenotypes, age, sex, HbA1c + risk factors** | REF | 1.66 (1.07, 2.57) | 1.73 (0.99, 3.04) | 2.44 (1.59, 3.73) |
| Model 4 | ||||
| Adjusted for phenotypes, age, sex, HbA1c + risk factors + health behaviors*** | REF | 1.59 (1.02, 2.46)) | 1.70 (0.97, 2.99) | 2.35 (1.53, 3.60) |
Abbreviations: Incidence rate ratio (IRR); Confidence interval (CI).
*Insulin resistant obesity phenotypes were defined based on obesity and insulin resistant status at baseline. Obesity was defined based on NHLBI recommendations using a combination of BMI and gender-specific waist circumference thresholds. Insulin resistance was defined using a HOMA-IR cutoff of ≥ 2.
**Risk factors include: family history of diabetes, hypertension, HDL cholesterol, triglycerides and hs-CRP.
***Health behaviors include Ideal Cardiovascular Health metrics for physical activity, smoking and diet.
Table 3.
Association between population-specific insulin resistant obesity phenotypes* and incident diabetes.
| Non-obese/insulin sensitive n = 1770 | Non-obese/insulin resistant n = 350 | Obese/insulin sensitive n = 584 | Obese/insulin resistant n = 515 | |
|---|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | |
| Model 1 | ||||
| Adjusted for age and sex | REF | 3.08 (2.50, 3.80) | 1.61 (1.27, 2.03) | 3.76 (3.13, 4.50) |
| Model 2 | ||||
| Adjusted for population-specific phenotypes, age, sex + HbA1c | REF | 2.00 (1.62, 2.47) | 1.31 (1.05, 1.65) | 2.27 (1.88, 2.74) |
| Model 3 | ||||
| Adjusted for phenotypes, age, sex, HbA1c + risk factors** | REF | 1.82 (1.46, 2.26) | 1.22 (0.97, 1.54) | 2.00 (1.64, 2.45) |
| Model 4 | ||||
| Adjusted for phenotypes, age, sex, HbA1c + risk factors + health behaviors*** | REF | 1.86 (1.49, 2.33) | 1.25 (0.99, 1.59) | 2.04 (1.65, 2.51) |
Abbreviations: Incidence rate ratio (IRR); Confidence interval (CI).
*Population-specific cut-offs were determined using the upper quartile of BMI, waist circumference (sex-specific distribution) and HOMA-IR; obese = BMI ≥ 34.4030 or waist circumference ≥ 106 cm for males or 107 cm for females; insulin resistant = HOMA-IR ≥ 4.20.
**Risk factors include: family history of diabetes, hypertension, HDL cholesterol, triglycerides and hs-CRP.
***Health behaviors include Ideal Cardiovascular Health metrics for physical activity, smoking and diet.
Discrimination of obesity phenotypes in predicting T2DM
AUC analyses revealed that an obesity phenotype-based model with age, sex, and HbA1c achieved very good discrimination (AUC = 0.83). As a comparison, the set of nine predictors from the ARIC diabetes risk prediction model (age, parent history of diabetes, waist circumference, height, HDL cholesterol, triglycerides, fasting glucose and systolic blood pressure) achieved an AUC of 0.78 in this sample. When we further add HbA1c to the ARIC model, the AUC from this full set of 9 predictors plus HbA1c achieved a comparable discrimination (AUC = 0.84).
Discussion
In the largest AA population-based cohort to date, close to a quarter of the cohort was non-obese but insulin-resistant, whereas, among the obese participants, only 6% were insulin-sensitive using standard cutoffs for obesity and insulin resistance. HOMA-IR was able to identify high-risk individuals amongst the non-obese as well as lower-risk individuals amongst the obese for future development of T2DM, and these findings held true despite adjusting for confounding risk factors such as HbA1c, age, sex, family history of diabetes, hypertension, HDL cholesterol, triglycerides, hs-CRP, physical activity, smoking, and diet.
While studies on metabolic health and obesity phenotypes abound, only a handful of studies have attempted to understand the relationship among obesity, insulin-resistance phenotypes, and incident T2DM in the AA population [1], [20], [21]. Among the studies addressing the question of interest, our study is by far the largest population-cohort study to date. Cross-sectional studies by Cherqaoui et al. [13] (n = 126) and Gaillard et al. [14] (n = 196) found that metabolically healthy obese AAs had lower levels of fasting glucose [13], [14] and less metabolic syndrome [14]. A recent study by Owei et al. (n = 176 AAs) showed that IR, defined by insulin sensitivity based on hyperinsulinemic euglycemic clamp and/or by HOMA-IR, could predict a combined end-point of incident pre-diabetes and diabetes in obese AAs, but had limited statistical power for diabetes because only ten participants developed T2DM. Our study resolved the limitations of study design, small sample size of AAs, and length of follow-up from the previous reports, showing that a single measure of HOMA-IR can further risk stratify future T2DM risk beyond obesity and HbA1c in a large AA cohort.
To the best of our knowledge, the combined effects of both HOMA-IR and obesity have not been investigated in a population-based cohort. We were able to reclassify individual’s risk of diabetes beyond obesity status. We were able to incorporate these metrics into a diabetes risk prediction index comprised of other easily observable patient characteristics such as sex and age, which achieved excellent prediction (AUC 0.83) over known, more complex, 9-variable diabetes risk prediction models in the literature, such as the ARIC model (AUC 0.78). The clinical advantage we envision aside from ease of use is that a patient who appears to have a normal BMI may still warrant a HOMA-IR check despite normal A1c levels to enable early detection of high-risk patients. Conversely, amongst the seemingly high T2DM risk obese individuals, an insulin-sensitive phenotype based on HOMA-IR levels reclassify this group at a comparable diabetes risk to the non-obese insulin sensitive counterparts. This is a group that warrant further study to understand the key biological or behavioral differences, if any, when compared to the standard obese and insulin resistant group to enable T2DM prevention. HOMA-IR is also a useful tool in assessing IR because obtaining laboratory values for HOMA-IR requires little training and constitutes a much lower cost per subject (one fasting blood draw) compared to the euglycemic hyperinsulinemic clamp or the frequently sampled insulin glucose tolerance test [22]. But HOMA-IR thresholds are not universal, with clinically relevant threshold of HOMA-IR different across populations [11]. Thus, our analysis included both the standard threshold of 2, but also the JHS population-specific 75th percentile threshold (≥4.20), so clinicians and public health officials can be informed of their uses either for active intervention versus population screening, respectively.
While obese, the insulin sensitive group’s adjusted T2DM risk did not reach significance when compared to the non-obese insulin sensitive group. These results differed with other studies conducted in other ethnicity and settings. A large Japanese cohort (N = 8090) by Heianza et al. who reported 5-year incidence of T2DM to be higher in the metabolically healthy obese than in metabolically healthy normal weight, but did not use insulin resistance to define metabolic health [23]. A meta-analysis from 7 studies by Bell et al. reported that metabolically healthy obese (defined by BMI and normal cardiometabolic clustering, insulin risk profile or risk score) adults carried an adjusted, pooled relative risk of 4.03 (95% CI: 2.66–6.09) for developing T2DM compared to metabolically healthy, non-obese adults [24]; however, a number of the studies included in this meta-analysis did not use HOMA-IR in the definition of metabolic health and ethnic compositions of the cohorts were not reported. A large Israeli cohort study (N = 33,939) by Twig et al. reported that among metabolically healthy young adults, diabetes risk increased monotonically for the overweight (HR = 1.89 [95% CI: 1.25–2.86]) and obese (HR = 3.88 [95% CI: 1.94–7.77]) [25], suggesting obesity alone is not benign with regard to diabetes risk. Therefore, despite the findings of a lower T2DM risk in insulin-sensitive obese AAs, clinicians should continue to monitor these patients carefully.
Our study has limitations. First, we could not distinguish T2DM from type 1 diabetes mellitus with high clinical certainty in a population study such as the JHS. Given the age and BMI of the study population, it is reasonable to assume the majority of incident diabetes to be T2DM. While we have controlled for several clinical variables that may confound our results, given the observational research setting, the possibility of residual confounding still exists. Also, we only used obesity and IR measures at baseline, and did not consider trajectories of these measures over time. Though the JHS is the largest AA cohort study to date, it is still localized in the Jackson, MS; therefore, results may not be generalizable to AAs in other regions of the country. While insulin resistance measured in our study is an important determinant of T2DM risk [26], studies have shown that insulin secretion is also another important factor, where the hyperbolic relationship between both factors is accounted for, in the disposition index, is likely a better determinant of future diabetes risk than insulin resistance alone [27] Finally, based on available data in the Jackson Heart Study, we were not able to include the oral glucose tolerance test in our diagnostic criteria for identifying cases of diabetes which may have resulted in misclassification given racial differences in the blood-glucose versus HbA1c relationship [28], [29]. This may be especially important given the consistent higher HbA1c for comparable average blood glucose values seen in AA populations in general compared to whites [29]. Based on the above, it is possible that we might potentially have a misclassification bias that lowered our power to detect smaller differences between the IR obesity phenotypes. Despite the limitations, our study represents the largest AA cohort to date that provided relevant clinical and epidemiological evidence to support HOMA-IR as a useful biomarker that can further classify patients on their T2DM risk beyond obesity alone.
In conclusion, HOMA-IR as a marker of IR can further stratify future T2DM risk in AA adults beyond obesity by identifying the non-obese yet high risk and the obese but lower risk subgroups. The risk of T2DM in the insulin-sensitive obese should also be carefully monitored despite favorable insulin profiles. Future studies should explore additional mechanisms of T2DM development in obesity beyond insulin resistance.
Disclosure
The authors declared no conflict of interest.
Author contributions
Acquisition of data: Lacy, Correa, Wu
Statistical analysis: Lacy
Interpretation of results: Lee, Lacy, Jankowich, Correa, Wu
Drafting of manuscript: Lee
Critical Revision of the manuscript: Lacy, Jankowich, Correa, Wu
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the staff and participants of the Jackson Heart Study for their contributions. The funders of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript or the decision to submit for publication.
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The authors thank the participants and data collection staff of the Jackson Heart Study.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services or the Department of Veterans Affairs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2019.100210.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Owei I., Umekwe N., Provo C., Wan J., Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabet Res Care. 2017;5(1) doi: 10.1136/bmjdrc-2017-000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall M.C., Jr. Diabetes in African Americans. Postgrad Med J. 2005;81(962):734–740. doi: 10.1136/pgmj.2004.028274. Epub 2005/12/14., PubMed PMID: 16344294; PubMed Central PMCID: PMCPMC1743410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluher M. The distinction of metabolically 'healthy' from 'unhealthy' obese individuals. Current opinion in lipidology. 2010;21(1):38–43. doi: 10.1097/MOL.0b013e3283346ccc. Epub 2009/11/17. PubMed PMID: 19915462. [DOI] [PubMed] [Google Scholar]
- 4.Chen Lei, Magliano Dianna J., Zimmet Paul Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 5.Wildman Rachel P. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168(15):1617. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 6.Tabak A.G., Herder C., Rathmann W., Brunner E.J., Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. Epub 2012/06/12. PubMed PMID: 22683128; PubMed Central PMCID: PMCPMC3891203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor H.A., Wilson J.G., Jones D.W., Sarpong D.F., Srinivasan A., Garrison R.J. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6) S6-4-1Epub 2005/12/02. PubMed PMID: 16320381. [PubMed] [Google Scholar]
- 8.Carpenter M.A., Crow R., Steffes M., Rock W., Heilbraun J., Evans G. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. PubMed PMID: 15367870. [DOI] [PubMed] [Google Scholar]
- 9.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults – The Evidence Report. National Institutes of Health. Obesity research. 1998;6 Suppl 2:51S-209S. PubMed PMID: 9813653. [PubMed]
- 10.Jennings C.L., Lambert E.V., Collins M., Joffe Y., Levitt N.S., Goedecke J.H. Determinants of insulin-resistant phenotypes in normal-weight and obese Black African women. Obesity (Silver Spring) 2008;16(7):1602–1609. doi: 10.1038/oby.2008.233. Epub 2008/04/19. PubMed PMID: 18421268. [DOI] [PubMed] [Google Scholar]
- 11.Gayoso-Diz P., Otero-Gonzalez A., Rodriguez-Alvarez M.X., Gude F., Garcia F., De Francisco A. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13:47. doi: 10.1186/1472-6823-13-47. Epub 2013/10/18. PubMed PMID: 24131857; PubMed Central PMCID: PMCPMC4016563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zitkus B.S. Update on the American Diabetes Association standards of medical care. Nurse Practitioner. 2014;39(8):22–32. doi: 10.1097/01.NPR.0000451880.48790.50. PubMed PMID: 24979246. [DOI] [PubMed] [Google Scholar]
- 13.Lacy M.E., Wellenius G.A., Carnethon M.R., Loucks E.B., Carson A.P., Luo X. Racial differences in the performance of existing risk prediction models for incident type 2 diabetes: the CARDIA study. Diabetes Care. 2016;39(2):285–291. doi: 10.2337/dc15-0509. Epub 2015/12/03. PubMed PMID: 26628420; PubMed Central PMCID: PMCPMC4722943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon D.R., Doron-LaMarca S., Bell M., O’Farrell T.J., Taft C.T. Poisson regression for modeling count and frequency outcomes in trauma research. J Trauma Stress. 2008;21(5):448–454. doi: 10.1002/jts.20359. Epub 2008/10/29. PubMed PMID: 18956443. [DOI] [PubMed] [Google Scholar]
- 15.Zou G.Y., Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–670. doi: 10.1177/0962280211427759. Epub 2011/11/11. PubMed PMID: 22072596. [DOI] [PubMed] [Google Scholar]
- 16.Hanley J.A., McNeil B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. Epub 1982/04/01. PubMed PMID: 7063747. [DOI] [PubMed] [Google Scholar]
- 17.Gönen M, SAS Institute. Analyzing receiver operating characteristic curves with SAS. Cary, NC: SAS Pub.; 2007. x, 134 p. p.
- 18.Schmidt M.I., Duncan B.B., Bang H., Pankow J.S., Ballantyne C.M., Golden S.H. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(8):2013–2018. doi: 10.2337/diacare.28.8.2013. Epub 2005/07/27 PubMed PMID: 16043747. [DOI] [PubMed] [Google Scholar]
- 19.Introduction: Standards of Medical Care in Diabetes-20Diabetes Care. 2019;42(Suppl 1):S1-S2. Epub 2018/12/doi: 10.2337/dc19-Sint01. PubMed PMID: 30559224. [DOI] [PubMed]
- 20.Cherqaoui R., Kassim T.A., Kwagyan J., Freeman C., Nunlee-Bland G., Ketete M. The metabolically healthy but obese phenotype in African Americans. J Clin Hypertens (Greenwich) 2012;14(2):92–96. doi: 10.1111/j.1751-7176.2011.00565.x. Epub 2012/01/27. PubMed PMID: 22277141; PubMed Central PMCID: PMCPMC3270369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillard T.R., Schuster D., Osei K. Characterization of metabolically unhealthy overweight/obese African American women: significance of insulin-sensitive and insulin-resistant phenotypes. J Natl Med Assoc. 2012;104(3–4):164–171. doi: 10.1016/s0027-9684(15)30141-3. Epub 2012/07/11 PubMed PMID: 22774383. [DOI] [PubMed] [Google Scholar]
- 22.Trout K.K., Homko C., Tkacs N.C. Methods of measuring insulin sensitivity. Biol Res Nurs. 2007;8(4):305–318. doi: 10.1177/1099800406298775. Epub 2007/04/26. PubMed PMID: 17456592. [DOI] [PubMed] [Google Scholar]
- 23.Heianza Y., Arase Y., Tsuji H., Fujihara K., Saito K., Hsieh S.D. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20) J Clin Endocrinol Metab. 2014;99(8):2952–2960. doi: 10.1210/jc.2013-4427. Epub 2014/05/16. PubMed PMID: 24823457. [DOI] [PubMed] [Google Scholar]
- 24.Bell J.A., Kivimaki M., Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014;15(6):504–515. doi: 10.1111/obr.12157. Epub 2014/03/26. PubMed PMID: 24661566; PubMed Central PMCID: PMCPMC4309497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twig G., Afek A., Derazne E., Tzur D., Cukierman-Yaffe T., Gerstein H.C. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care. 2014;37(11):2989–2995. doi: 10.2337/dc14-0869. Epub 2014/08/21. PubMed PMID: 25139886. [DOI] [PubMed] [Google Scholar]
- 26.DeFronzo R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The claude bernard lecture 2009. Diabetologia. 2010;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. Epub 2010/04/03. PubMed PMID: 20361178; PubMed Central PMCID: PMCPMC2877338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman R.N. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes. 2007;56(6):1489–1501. doi: 10.2337/db07-9903. Epub 2007/05/29. PubMed PMID: 17526912. [DOI] [PubMed] [Google Scholar]
- 28.Briker S.M., Aduwo J.Y., Mugeni R., Horlyck-Romanovsky M.F., DuBose C.W., Mabundo L.S. A1C underperforms as a diagnostic test in Africans even in the absence of nutritional deficiencies, anemia and hemoglobinopathies: insight From the Africans in America study. Front Endocrinol (Lausanne) 2019;10:533. doi: 10.3389/fendo.2019.00533. Epub 2019/08/27. PubMed PMID: 31447780; PubMed Central PMCID: PMCPMC6692432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergenstal R.M., Gal R.L., Connor C.G., Gubitosi-Klug R., Kruger D., Olson B.A. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95–102. doi: 10.7326/M16-2596. Epub 2017/06/13. PubMed PMID: 28605777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.