Abstract
A novel strain of microalga Parachlorella sp. BX1.5 was isolated and its unique properties of producing lipids and extracellular polysaccharides (EPS) characterized. The cells could extracellularly produce a large amount of acidic EPS, when cultured in nitrogen-deficient BG110 medium (BG11–N) with 2 % CO2-air supply. The main component of intracellularly accumulated lipids was triacylglycerol (TAG), depending on the different cultivation conditions of BG11, BG11–N, BG11–P (phosphate depleted), and BG11–N–P (nitrogen and phosphate depleted). Fatty-methyl-esters (FAMEs), methyl-esterification of total lipids, consisted of abundant saturated C16 and unsaturated C18 fatty acids under the culture conditions. Cell spot assays on BG11 plates revealed the resistance of cells to pH 2–11, high temperatures of 50–70 °C, ultraviolet irradiation, and drought, under different culture conditions, thereby suggesting the biological significance of lipid and EPS accumulation. The prospects of BX1.5 as a dual producer has also been discussed for biorefineries.
Keywords: Biofuel, Biorefinery, Dual production, Parachlorella, Polysaccharide
1. Introduction
Microalgae are model microorganisms that have strong photosynthetic capabilities and are used as a resource in biorefineries to produce fuels, fertilizers, feed, fiber, and food [1,2]. Microalgae include prokaryotes (cyanobacteria) and eukaryotes (green algae, diatoms, stonewort, dinoflagellates, and euglena). In biofuel production, cyanobacteria are employed as producers of alkane/alkene, which is used as jet fuel, or kerosene, which is a mixture of hydrocarbons [[3], [4], [5]].
Some green algae (e.g., Chlorella, Nannochloropsis, Nephroselmis, and Chlamydomonas) have been applied in the production of triacylglycerol (TAG), a neutral lipid that may be used as biodiesel or food oil [[6], [7], [8], [9], [10], [11]]. In such cases, properties of the oil depend on the structure determined by the number of carbons and the position/number of double bonds. In lipid biosynthesis, synthesis of fatty acids is terminated once at the stage of palmitic acid (C16:0) as a saturated fatty acid, followed by the addition of sequentially synthesized acetyl-CoA to the substrates by fatty-acid synthase. Thereafter, palmitic acid is extended to stearic acid (C18:0) by fatty-acid elongation enzyme, resulting in further synthesis of C18-derivatives as oleic acid (C18:1), linoleic acid (C18:2), or α-linolenic acid (C18:3). Therefore, the productivities of C16/C18 fatty-acid derivatives in cells could be important when oil production is examined for biorefinery.
Regarding polysaccharide production, cyanobacteria and green algae have been used as good producers in biorefineries. For example, sacran or spirulan from cyanobacteria Aphanothece sacrum (called suijenji-nori as Japanese) or Arthrospira (Spirulina) sp. is an acidic extracellular polysaccharide (EPS) that can function as useful material such as water-retention reagent or additive in the fields of food/medical industries [12,13]. Rhamnan sulfate, as acidic EPS extracted from the green alga Monostroma nitidum, has also been characterized as a possible material for anti-blood clotting, antiviral infection, or anti-cancer metastasis [[14], [15], [16]]. Although useful EPS from microalgae have been reported, EPS from the green alga Parachlorella sp. has not been reported till date.
When we consider practical application of such useful materials, the cost of extraction, processing, and manufacture is critical. Therefore, effective productivities of such useful materials need to be extensively investigated. In this study, a novel green alga Parachlorella sp. strain BX1.5 was isolated and characterized as a dual producer of useful oils (lipids) and EPS that accumulate simultaneously inside and outside the cell, respectively. The usefulness of this strain and possible outdoor cultivation have also been discussed for practical biorefineries.
2. Materials and methods
2.1. Isolation of green alga BX1.5
The Parachlorella sp. BX1.5 strain was isolated from an outdoor cell culture, consisting of natural water collected from a mountainous area in Hiroshima Japan, using a mixed and diluted overlay method on BG11 agar plates [17].
2.2. Identification of the BX1.5 strain
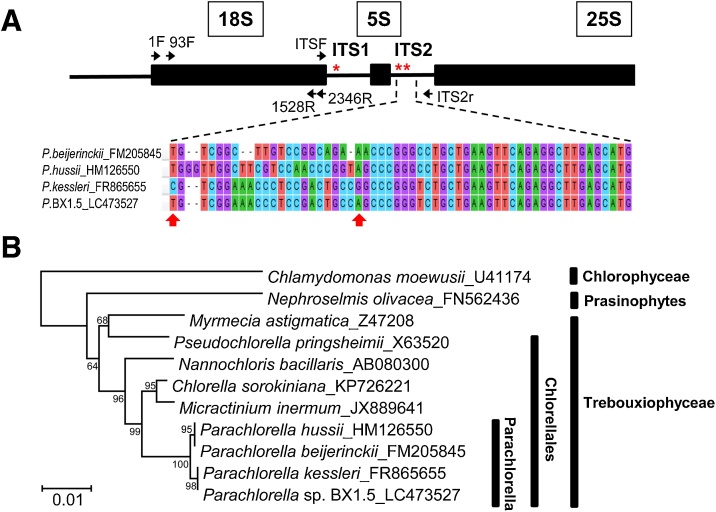
The 18S rDNA region of BX1.5 was amplified by PCR with the primers 1F (5′- AACCTGGTTGATCCTGCCAG -3′) and ITS2_r (5′- TCCTCCGCTTATTGATATGC -3′) (Fig. 1A). The amplified DNA fragment was cloned into the cloning vector pUC119B to give pBX18S. Nucleotide sequence of the 2.5-kb 18S rDNA region was verified by a dideoxy method with the universal primers 1F, 93F (5′- CTGCGAATGGCTCATTAAAWCAG-3′), 1528R (5′- CCTCTAGGTGGGAGGGTTTAATG-3′), 2346R (5′- GMAACCTTGTTACGACTT-3′), ITSF (5′- AAGTCGTAACAAGGTTTCCG -3′), and ITS2_r. The 2.5-kb 18S rDNA sequence (DDBJ accession number LC473527) was aligned by CLUSTAL W, integrated into the package MEGA7 [18], for phylogenetic analysis and cell identification [19].
Fig. 1.
Identification of isolated Parachlorella sp. BX1.5. (A) The 18S rDNA-ITS1-5.8S-ITS2 region on the genome and nucleotide-sequence alignment of the region. The region (2,505 bp, DDBJ accession number LC473527) was PCR-amplified with a set of primers, 1F and ITS2_r, cloned, sequenced, and subjected to database analysis. Asterisks show distinct nucleotide sequences from Parachlorella kessleri, which is the most homologous strain to BX1.5. (B) Phylogenetic analysis (MEGA 7 software) of the 18S rDNA sequences resulted in classification of the BX1.5 cells. Respective cases are shown with accession numbers. A bootstrap test was performed with 1,000 replicates. The 18S rDNA sequence of Chlamydomonas moewusii was utilized as an out-group. The scale bar indicates 1 % sequence divergence.
2.3. Cultivation conditions
Cells were pre-cultivated in 50 mL of BG11 in a 100-mL Erlenmeyer flask for 5 days. The flasks were placed in a CF-415 cultivation chamber (TOMY Co., Ltd., Tokyo, Japan) that supplied 2 % CO2-containing air, and were subjected to continuous reciprocating shaking (40 rpm) under continuous white light conditions at 100 μmol photons/m2 s. Five milliliters of the cell culture was collected and cells suspended in 30 mL of new BG11 [20], BG11–N (= BG110 as a nitrogen-deficient condition without NaNO3), BG11–P (as a phosphorous-deficient condition without K2HPO4), and BG11–N–P (as nitrogen- and phosphorous-deficient condition without NaNO3 and K2HPO4) media in 300-mL Erlenmeyer flasks. These flasks were placed in the same cultivation chamber to induce production of ESP and lipid. During cultivation, a part of the culture was sequentially harvested and cell turbidity measured at OD730 using MULTISKAN GO (Thermo scientific Co. Ltd., Tokyo, Japan) [17]. Cells were collected on the 6th day, during the main cultivation, for cell staining or lipid extraction. Cells were also cultured under static (standing) conditions with 0.04 % CO2-air in 50 mL BG11 in a 100-mL Erlenmeyer flask, in which pre-cultured 0.5-mL cells were used as an inoculation, for standard cultivation as a control of the above induction culture.
2.4. Cell staining and microscopic observations
To detect extracellular polysaccharides, a 100-μL aliquot of the cell culture, harvested from the main cultivation, was suspended in 20 μL India ink (Daiso Co., Ltd., Japan) and observed under an optical microscope (BX53: Olympus, Tokyo, Japan) at DIC (differential interference contrast) conditions with a shutter speed of 0.48 s. Alcian blue (#015-13805: Wako Co., Ltd., Osaka, Japan) staining was conducted for detecting acidic polysaccharides [[21], [22], [23]]. PAS (#164-19705: Wako Co., Ltd.) staining was performed for detecting neutral polysaccharides [24]. Cells stained with Alcian blue or PAS were observed using BX53 (Olympus) at DIC conditions with a shutter speed of 0.48 s. Regarding intracellular lipid staining [25], a 1-mL aliquot of the cell culture was collected from the main cultivation and mixed with 20 μM of Nile red (#144-08811: Wako Co., Ltd.). Samples were observed for morphology using an optical photomicroscope at a high resolution with a Nomarski prism or using the fluorescence microscope, BX53/DP72, fitted with a special filter, U-FBW (Olympus) for excitation (460–495 nm) and emission (510 nm). The exposure time for photography was 0.2 s.
2.5. Electron microscope observations
For transmission electron microscopy (TEM), cells were collected from 20 mL cell culture, and fixed with the reagents 2 % formaldehyde (PFA) + 2 % glutaraldehyde (GA) in 0.05 M cacodylate butter (pH 7.2) for pre-fixation, and 2 % osmium tetraoxide in 0.1 M cacodylate butter for post-fixation. The resulting cells that stained with uranyl acetate and lead solution were observed under TEM (JEM-1200EX: Jeol, Tokyo, Japan) using an ultrathin sectioning method [26].
2.6. Thin-layer chromatography (TLC)
To analyze the lipids accumulating in cells, samples were prepared as follows. Cells were collected from the main cultivation and freeze-dried. Twenty milligrams of dried cells were transferred into a 15-mL glass tube, to which 2 mL of a solvent (chloroform:methanol = 2:1) was added, and lipids extracted using the UD-100 sonicator (TOMY, Tokyo, Japan: set at an output of 70 for 10 min). The resultant samples were left overnight in a cool dark place to improve extraction. Subsequently, they were centrifuged at 3,000 × g for 10 min and the supernatants were collected. To wash the samples, equal amount of 0.9 % aqueous KCl solution was added to the supernatant, mixed, and subjected to centrifugation at 2,000 × g for 5 min. Approximately 2 mL of the bottom fraction was collected as the organic layer, transferred to a 15-mL glass tube, and placed in a rotary vacuum evaporator (V-850: BUCHI, Flawil, Switzerland). The resultants were dissolved in 200 μL chloroform, and eventually used for TLC. A 4-μL aliquot of each sample was spotted onto the origin (ori) of a silica gel plate (#60F254: Merck Millipore, Darmstadt, Germany). Lipids on the plate were developed with an eluent (hexane:diethylether:acetic acid = 80:20:1) and stained with 5 % (v/w in EtOH) phosphomolybdic acid solution (#32186-00: Kanto Kagaku Co., Ltd., Tokyo, Japan).
2.7. Lipid measurements by gas chromatography/flame-ionization detection (GC/FID)
To measure the intracellular accumulation of lipids, samples were prepared as follows [27]. Ten milliliters of the cell culture harvested from the main cultivation was transferred into a 50-mL plastic tube. Cultures from the respective media were directly freeze-dried to obtain total fractions of cells with EPS. Dry-cell weight (DCW) of the fractions was measured to calculate the amount (mg) of fatty acyl methyl esters (FAMEs) in DCW (g). Dried fractions were added to 4 mL of solvent (chloroform: methanol = 2:1), and n-Eicosane (#44-2673, Sigma Aldrich, St. Louis, USA: stock solution as 10,000 ppm in ethyl acetate) was used as an internal standard up to a final concentration of 100 ppm. Lipids were extracted from the resultants, using the same procedure, with the UD-100 sonicator (see the section above). After centrifugation at 3,000 × g for 10 min, approximately 4 mL of the supernatant was collected and an equal amount of 5 % NaCl aqueous solution was added to wash the sample. After mixing, samples were centrifuged at 3,000 × g for 10 min, and the bottom fraction, as the organic layer, was transferred into a 4-mL glass tube. Samples were dried using the same method, with the rotary vacuum evaporator. Dried samples were dissolved in 1 mL of toluene, transferred to a 20-mL glass tube with a lid, and 2 mL of 3 M HCl-methanol (#33050-U: Sigma Aldrich) was added to the extract. After mixing, samples were allowed to react in a water bath at approximately 90 °C for 3 h to convert lipids to FAMEs. The resultants were transferred to a 50-mL plastic tube. After cooling to room temperature, 1 mL of sterilized water and 2 mL of hexane were added. After mixing, samples were centrifuged at 3,000 × g for 10 min, and the organic layer was transferred thereafter to a 3-mL glass tube. Samples were concentrated and dried using a rotary vacuum evaporator. The resultants were transferred into a 1.5-mL vial and dissolved in 1 mL hexane. This sample was then subjected to TLC analysis, as described above. A 1-μL aliquot of each sample was subjected to GC/FID analysis using a GC-2014 system (Shimadzu Co., Ltd., Kyoto, Japan), wherein, a capillary tube was purged with helium gas (80 kPa), hydrogen (50 kPa), and air (50 kPa), and the injector temperature was maintained at 250 °C. Temperature of the column (Stabiliwax-DA: Shimadzu GLC, Tokyo, Japan) was initially maintained at 140 °C for 5 min, increased to 220 °C at an interval of 2.5 °C per min, and maintained at 220 °C for 7 min. n-Eicosane (C20:0, #44-2673: Sigma Aldrich) as an internal standard or a C16:0/C18:0/C18:1/C18:2/C18:3 mixture (GLC-10, #1891-1AMP: Sigma Aldrich, St. Louis, USA), cis-9-hexadecenoic acid (C16:1, #373-49-9: Tokyo Chemical Industry Co., Ltd, Tokyo, Japan), cis-7, 10-hexadecadienoic acid (C16:2, #10-1622-4: Funakoshi, Tokyo, Japan), and all-cis-7, 10, 13-hexadecatrienoic acid (C16:3, #10-1403: Funakoshi) were obtained as standards from the respective companies.
2.8. Spot assay for stress resistance
BX1.5 cells were pre-cultivated in 50 mL of BG11 medium in the 2 % CO2 gas-supplying incubator for 5 days (see the section on Cultivation Conditions in the Materials and methods). A 5-mL aliquot from a pre-inoculated cell culture was harvested, transferred into 30 mL of new BG11, BG11–N, or BG11−P medium, and cultivated thereafter under the same conditions as the main cultivation for 6 days. The cells were then collected and exposed subsequently to the respective stress conditions described. For heat stress, 0.25 mL of the cell culture was harvested, transferred into a 1.5-mL microtube, and then exposed to 50, 60, or 70 °C for 5 min. For acidic or alkaline stress, 0.25 mL of the cell culture was harvested and transferred into a 1.5-mL microtube. A 25-μL aliquot of 1 N hydrochloric acid (cell culture as pH 2) or 0.5 N sodium hydroxide (cell culture as pH 11) was added to the culture. For heat-dry (HD) stress, 1 mL of the cell culture was harvested, cell pellet collected by centrifugation at 15,000 × g, and subsequently exposed to 42 °C for a day. One milliliter of new BG11 medium was then added to the dried cell pellet and mixed well. For freeze-dry (FD) stress, 1 mL of the cell culture was harvested, cell pellet collected by centrifugation at 15,000 × g, and cells kept at a temperature -80 °C until next use. This sample was vacuum freeze-dried using V-850 (BUCHI) for 90 min under a 0.002 mbar setting. One milliliter of new BG11 medium was added to the dried cell pellet and the sample mixed well. For ultraviolet (UV) stress, 100 μL of cell culture was harvested, transferred to a 96-well plate, and UV-irradiated (100 μmol photons/m2 s, with a 260-nm light) for 5 min at a distance of 8 cm from the UV light source. Five-microliter aliquots of cell culture samples exposed to the respective stresses were spotted onto BG11 agar plates just after the stress treatment or after standing for a day following the stress treatment. HD samples were also spotted onto BG11 agar plates after the stress treatment. These cells were incubated in 0.04 % CO2-air condition with 30 μmol photons/m2 s of white-fluorescent light at 30 °C for 6 days.
3. Results and discussion
3.1. Isolation and identification of BX1.5
An aliquot of cell culture from an open pond, at a 1.5-ton scale bioreactor in Hiroshima, Japan, was harvested and the algal cells isolated. These cells were analyzed by comparing the phylogenetic relationships across 11 microalgal strains based on the 2.5-kbp nucleotide sequences of the 18S rDNA-ITS1-5.8S-ITS2 region (Fig. 1). Results revealed the BX1.5 sequence (accession number LC473527) to be homologous at 99.9 % (2,502 bp/2,505 bp × 100 %) to that (accession number FR865655) of Parachlorella kessleri. However, the cells possessed three distinct nucleotide sequences at the regions of ITS1 and ITS2 (Fig. 1A). Therefore, this strain was designated as green alga Parachlorella sp. BX1.5, a novel species (or subspecies) belonging to the order Chlorellales in the class Trebouxiophyceae, and phylum Chlorophyta (Fig. 1B).
3.2. Cultivation condition and culture profile
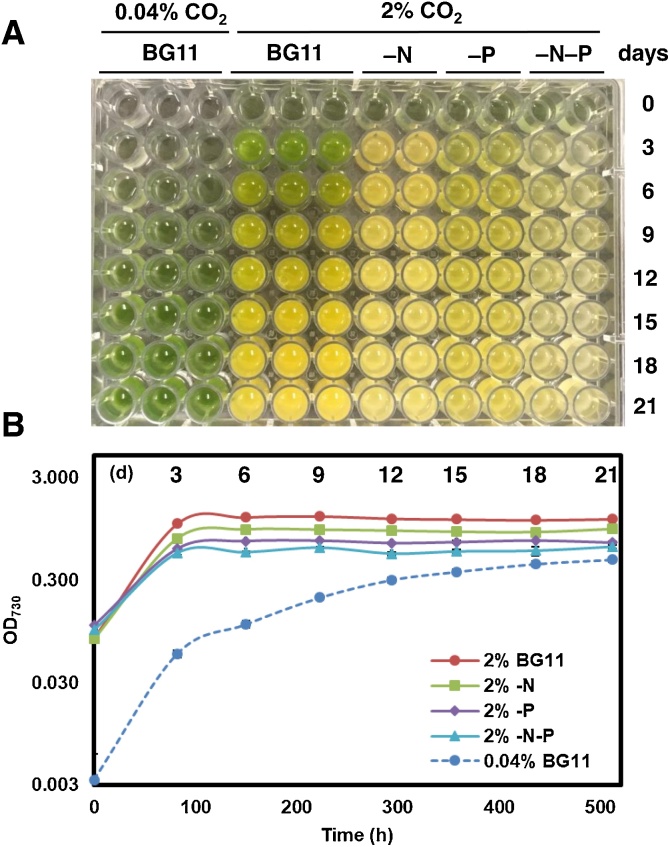
Parachlorella sp. BX1.5 was cultured in the laboratory under a variety of controlled conditions in order to examine the potential of this new alga for use in biorefinery applications. This investigation involved different concentrations of CO2 and different types of nutrient-deficient media. Especially, specific growth conditions supplying high concentration of 2 % CO2-air were efficient in prompting the induction of overproduction of microalgae for useful materials [19,28,29], compared to normal cultivation conditions supplying 0.04 % CO2-air (Materials and methods). The resulting culture color and cellular turbidity profiles are shown in Fig. 2. When cells were cultivated in BG11 media, under static conditions, with 0.04 % CO2-air, cell turbidities with green color were gradually increased, exhibiting a standard curve (Fig. 2A, B). In contrast, when the cells were cultivated in BG11 media under shaking conditions with 2 % CO2-air, a darker green color was observed and turbidity reached a maximum value by the 6th day (Fig. 2A, B and Fig. S1); it remained almost constant for 21 days although the color changed from green to yellow. Under the same conditions, a yellow color was observed for the BG11–N media. However, a greenish-yellow color was observed for the BG11−P and BG11–N−P cultivated cells. Cell turbidities of nutrient-deficient culture were relatively lower than that of BG11 culture (Fig. 2B). These results implied that cell properties are distinct in their respective cultures; both cell size and chlorophyll a (Chl a) content of cells differed significantly depending on the culture conditions (supporting information in Figs. S1 and S2). From these results, the optimal efficient period in this study was set as 6 days under induction cultivation with 2 % CO2-air (Fig. S1) for useful material production.
Fig. 2.
Culture and cell-turbidity profiles of BX1.5. Following cultivation in 2 % CO2-air for 21 days (see details in Materials and methods), an aliquot of 0.2 mL was collected from the culture, transferred into a 96-well plate (A), and cell turbidities measured as OD730 (B). Control cells were also cultivated under standard conditions with 0.04 % CO2-air, collected, and the turbidities measured. The OD730 values are shown with standard deviations (n = 3 or 2).
3.3. Acidic EPS production
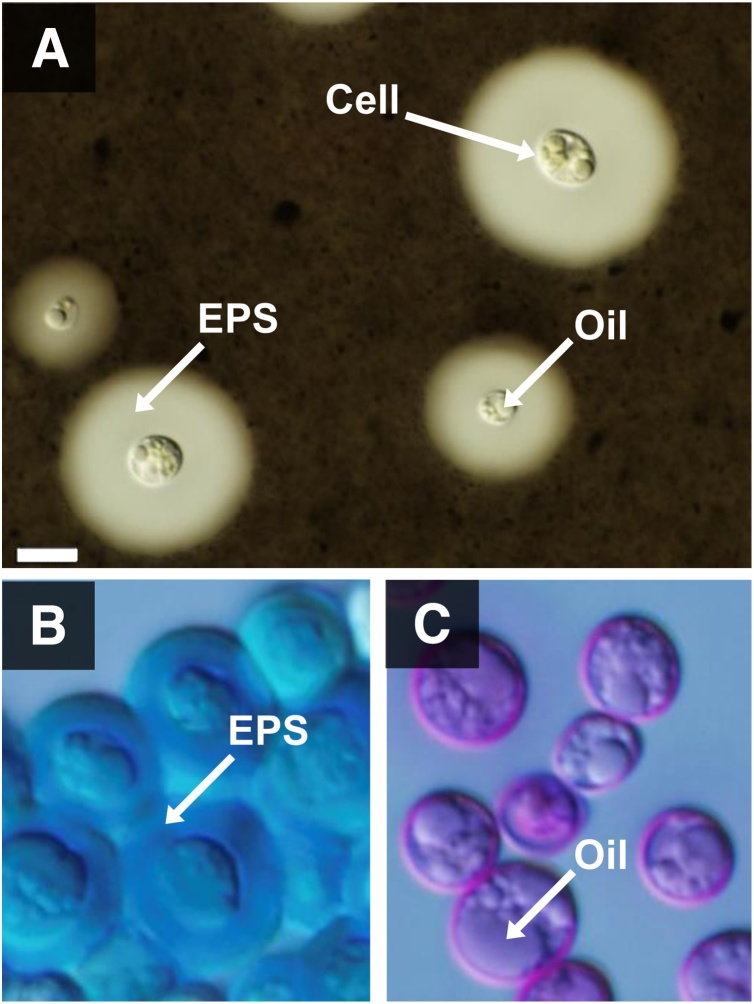
First, we confirmed the validity of India ink staining method by using several microalgae, which have been known to produce extracellular polysaccharides (EPS) (data not shown). Thereafter, the BX1.5 cells grown under different cultivation conditions, as shown in Fig. S1, were stained with India ink for EPS and observed under the microscope (Fig. S3). Large amount of EPS accumulated in the culture with 2 % CO2-air, especially in the samples from BG11–N and/or BG11–N−P medium. Therefore, the cells cultivated in the BG11–N medium were further characterized by staining with India ink for EPS, with Alcian blue for acidic polysaccharides, and with PAS for neutral polysaccharides. The results are shown in Fig. 3. It was interesting that a thick layer observed outside the cell, possibly EPS, remained unstained by India ink (Fig. 3A). Although most EPS peeled off during the course of Alcian blue staining, an acidic EPS layer that retained the blue color existed outside the cell (Fig. 3B). In contrast, the outer layer of the cell failed to stain with PAS (Fig. 3C). These results suggested that acidic EPS is selectively over-produced under BG11–N growth conditions. Carbon metabolic pathways linked to lipid synthesis are suggested to be activated under nutrient-depletion culture conditions [10,19]. Thus, there is a possibility that the same situation occurred for the overproduction of EPS under N−/P − starved conditions with 2 % CO2-air supply.
Fig. 3.
Acidic polysaccharide accumulation on the cell exterior and oil production in the interior. The BX1.5 cells were cultivated under BG11−N conditions shown in Fig. S1. An aliquot of 100 μL culture was collected and subjected to staining with India ink (A), Alcian blue (B), or PAS (C) (see details in Materials and methods). The cells were then subjected to microscopic observation. Bar, 10 μm.
Acidic EPS is referred to as rhamnan sulfate when produced from the green alga Monostroma nitidum. Rhamnan sulfate has been reported to confer a blood-clotting effect as well as antiviral effect [[14], [15], [16]]. Acidic EPS is also known as sacran (acidic sugar) when extracted from the cyanobacterium Aphanothece sacrum [30]. This cyanobacterium is edible and marketed as Suijenji-nori in Japan. Sacran is also utilized for product development (e.g., metal absorber, absorbent polymer, etc.) due to high water retentivity. We had recently reported a novel filamentous cyanobacterium Limnothrix sp. SK1-2-1 that can co-produce pentadecane and acidic EPS, thereby contributing to cell flocculation [19]. Therefore, microalgal EPS might contribute to many fields such as medical, food, and industry. Although it is very interested in a mechanism for production and a molecular structure of BX1.5 EPS, they are research agenda for elucidation.
3.4. Oil production in cells
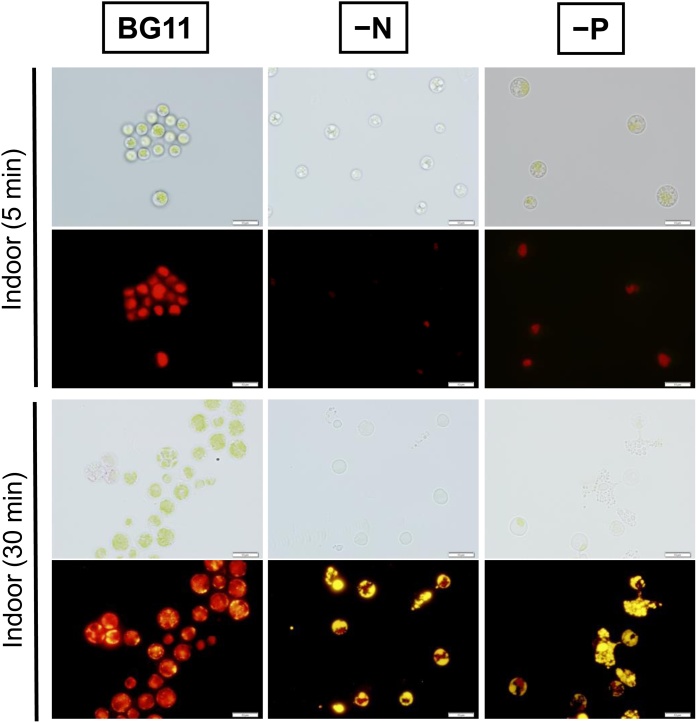
BX1.5 cells were next utilized to simulate oil production within them. Nile red, which is a common reagent used for lipid-staining in microalgae, was utilized to stain the cells [8,31,32]. Cells cultivated in BG11 medium with 2 % CO2-air were stained for 5 min on glass slides and then observed under the microscope. Perhaps due to oil drops existing in the cells, almost all the cells with a diameter of 5.23 ± 0.04 μm (Fig. S2A) were insufficiently stained with Nile red. However, when these cells were stained for 30 min on glass slides, larger cell size was observed, as the color changed from red (by autofluorescence with photosynthesis pigments) to yellow (by oil production) in some parts of the cells (Fig. 4, left). Further, when cells were cultivated in the BG11−N medium with 2 % CO2 and stained for 5min on glass slides, microscopic observation revealed oil drops in the cells, with significantly smaller cells with an average diameter of 4.42±0.03 μm (Fig. S2A), which also failed to stain sufficiently, indicative of possible inhibition of EPS accumulation. Following 30-min staining, the sample again resulted in larger cells, changing color from thin red (by autofluorescence with fewer photosynthesis pigments) to strong yellow (by significant oil production) either in the cells or outside the lysed cells (Fig. 4, center). This phenomenon might have occurred due to cellular desiccation, causing damage to the cells and oozing of oil, gradually over time, on the glass. A similar tendency in staining was observed when cells were cultivated in the BG11−P medium with 2 % CO2 (Fig. 4, right). The cell size increased, producing larger cells, with a diameter of 6.45±0.06μm (Fig. S2A), which were significantly larger than that of the BG11 medium. In this situation, cells from the BG11−P culture contained significant oil and a certain amount of photosynthetic pigment corresponding to the yellow-green color of cells. Thus, we further investigated this reaction by measuring and evaluating the amount of chlorophyll a present in cells harvested from BG11, BG11–N, and BG11−P. Chlorophyll a levels (μg/mL of cell culture) were observed as mean values: 2.7 ± 0.07, 0.09 ± 0.009, or 0.23 ± 0.02 for BG11, BG11–N, or BG11−P, respectively (Fig. S2B). These values seemed to support the photosynthesis-induced cell color observed in this study, such as green, yellow, or yellow-green, as shown in Figs. 2, S1, and 4 (top panels).
Fig. 4.
Oil production in the cell interior. BX1.5 cells were cultivated under respective conditions shown in Fig. S1. Following this, an aliquot of 1 mL was collected from the culture, transferred into a new microtube, and Nile red was added to stain the lipids accumulated in the cells. Cell morphology was observed using the optical photomicroscope, at a high resolution, with a Nomarski prism (Top: optics, differential interference contrast) or with a fluorescence microscope (Bottom). The respective times of 5 or 30 min are the sequential times when the sample was put on a glass slide. Bar, 10 μm.
3.5. TEM observation of the Parachlorella sp. BX1.5 cells
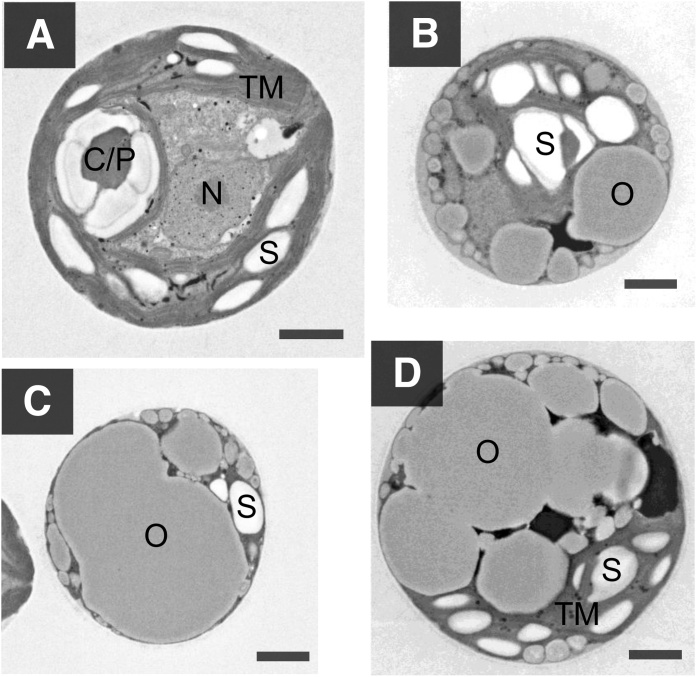
Starch and oil production in BX1.5 cells was examined via TEM observations (Fig. 5), in order to visualize the cell contents of this novel alga. Cells were observed following cultivation under controlled conditions in the BG11 medium with 0.04 % CO2 for 13 days (Figs. 2B and 5 A). A characteristic feature of this alga is that thylakoid membranes are spread throughout the cytoplasm when the cells are cultivated under standard culture conditions (Fig. 5A). Another of its characteristics was the absence of clear chloroplasts, and the cell profile seemed to be similar to those of cyanobacteria or green algae from Chlamydomonas. Starch had visibly accumulated along multiple layers of thylakoid membranes, a pyrenoid body for storage, and starch formation was also observed. Moreover, the cells also produced starch (white particles) and oil (gray particles) when they were cultivated under variable conditions in BG11 media at 2 % CO2-air (Fig. 5B). This might indicate that supplementing CO2 into the environment effectively conferred oil production ability to the cells. Cultivation in nitrogen-deficient BG11–N medium with 2 % CO2-air resulted in mass production of oil in the cell, which occurred due to the degeneration of thylakoid membranes (Fig. 5C). Oil storage accounted for over 70 % of the cross-sectional area of a cell. Such overproduction of oils was also observed in the hypertrophic cells cultivated under phosphorus-deficient conditions in BG11−P medium with 2 % CO2-air (Fig. 5D). Interestingly, although oil storage accounted for over 55–65 % of the cross-sectional area in a cell, the area of thylakoid membranes involved with starch production was more prominently visible in the representative cells (Fig. 5D). These observations were consistent with the test results in Figs. 2, S1, and 4. The results from Fig. 5 also indicated that starch or oil production and cell morphology depend significantly on cell culture condition, including CO2-gas concentration and type of nutrient depletion in the medium.
Fig. 5.
Electron microscopic observation of the BX1.5 cells. Cells were cultivated under static conditions in BG11 medium with 0.04 % CO2-air for 13 days (A) or under shaking conditions with either BG11 (B), BG11−N (C), or BG11−P (D) media and 2 % CO2-air for 6 days (Fig. S1), as shown in Fig. 2. Resulting cells were harvested, treated with reagents, and then observed with a transmission electron microscope (TEM). C/P, carboxysome in pyrenoid; N, nucleus; O, oil body; S, starch; TM, thylakoid membrane. Bar, 1 μm.
Overproduction of oil was observed to be inversely correlated with the reduction in the accumulated amount of starch in the thylakoid membranes of cells cultivated under 2 % CO2 conditions (Fig. 5B–D). In contrast, starch accumulated, as did clear thylakoid membranes, in the cells cultivated at 0.04 % CO2 conditions in BG11 medium (Fig. 5A). In the cell, pyrenoid was confirmed to occur, which can in turn confer an ability of CO2 fixation for generating and maintaining a rich environment of CO2 concentration in combination with the photosynthetic enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) found in the carboxysome of cyanobacteria. Therefore, carbon flow from starch might supply a part of a precursor for synthesizing lipids or EPS. In green alga, Chlamydomonas, starch and lipids also accumulated along the spaces of thylakoid membranes in the cells, and the content profiles appeared to be similar to those seen in the BX1.5 cells [7,33].
3.6. TLC analysis for the lipids
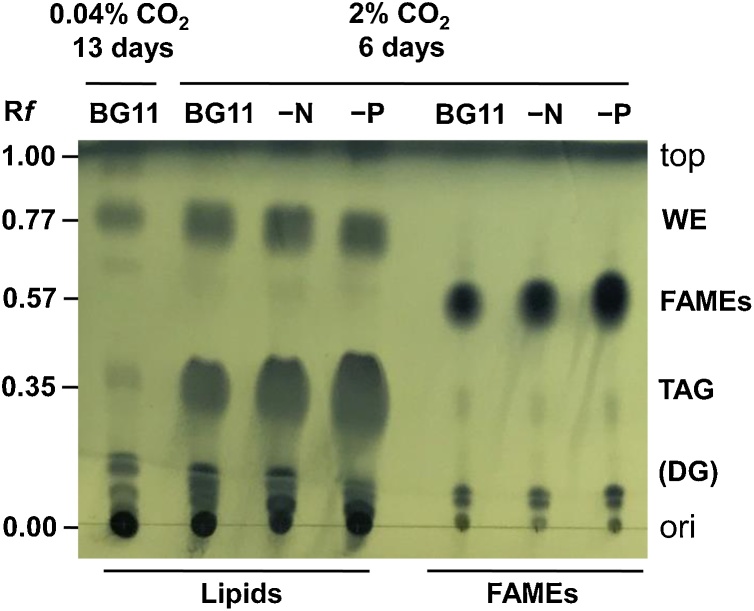
Thin Layer Chromatography (TLC) revealed the lipid composition of cells, and the results are depicted in Fig. 6. Samples were prepared from cells shown in Fig. 2. Remarkable accumulation of TAG, whose Rf value was 0.35 on a silica plate [7,10], was observed from the unit per dry-cell weight (DCW), cultivated in BG11, BG11–N, and BG11–P media at 2 % CO2-air. The order corresponding to the accumulation rate of TAG in cells in each medium was as follows: BG11–P>BG11–N>BG11. Apparent accumulation of wax ester (WE), whose Rf value was 0.77 on the silica plate [7,10], was also observed in the cells. In contrast, only a minute amount of accumulated TAG was observed in cells cultivated in the BG11 medium at 0.04 % CO2-air. We also confirmed that lipids (Fig. 6, left) were totally converted to fatty-acid methyl esters (FAMEs: Fig. 6, right), Rf value being 0.57 on the silica plate for GC/FID analysis.
Fig. 6.
TLC analysis of lipids. Cells were harvested following cultivation described in Fig. S1, and then freeze-dried. Lipid fractions were extracted and prepared from the unit per dry-cell weight (DCW) (see details in Materials and methods). An aliquot of the 4-μL extract was spotted onto origin (ori) in 4 lanes at the left of a silica gel plate. FAMEs were also prepared from the lipid fractions (Materials and methods). An aliquot of the 4-μL extract was also spotted onto origin in 3 lanes at the right of the silica gel plate. This plate was subjected to thin-layer chromatography (TLC) with a developer eluent, and then stained with phosphomolybdic acid solution. Positions referring to respective lipids are shown as wax ester (WE), triacylglycerol (TAG), possible diacylglycerol (DG), and fatty acid methyl esters (FAMEs), on the right. The Rf values are shown on the left.
3.7. FAME content in the cells
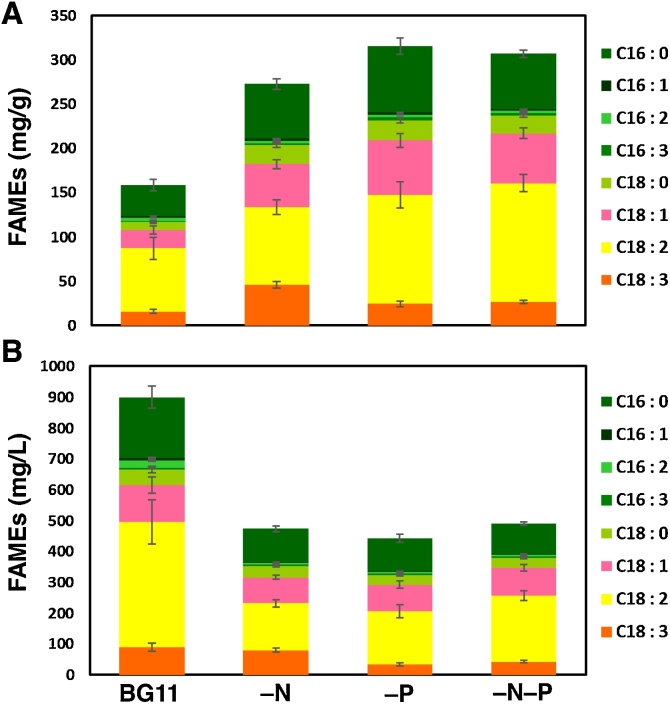
The results for C16 and C18 FAMEs are shown in Fig. 7. The total amount of BX1.5 dry-cell weight (DCW) harvested from 1 L of cell culture grown on BG11, BG11–N, BG11−P, and BG11–N−P media was approximately 5.70, 1.77, 1.40, and 1.60 g, respectively. This indicates that the order of biomass production per unit of cell culture is BG11 > BG11–N > BG11−N−P > BG11−P. Total amounts of FAME (lipids) were 16 %, 27 %, 32 %, or 31 % in DCW of BG11, BG11−N, BG11–P, or BG11−N−P, respectively, in media (Fig. 7A). Lipid (oils) composition and FAMEs were also evaluated and listed in Supplementary Table S1. Interestingly, the range of proportionally integrated unsaturated fatty acids for C18:1 + C18:2 + C18:3 was 68 %, 67 %, 66 %, or 71 % in the FAMEs from cells cultivated in BG11, BG11−N, BG11−P, or BG11−N−P media, respectively, indicating that BX1.5 could significantly accumulate useful lipids utilized as food oils. The ability of lipid production was converted and shown per unit of cell-culture (Fig. 7B). The total productivities of FAMEs were 900, 473, 441, or 491 mg from one-liter of cell culture grown on BG11, BG11−N, BG11−P, or BG11−N−P media, respectively. These results indicated that abundant unsaturated C18 FAMEs, which are useful food oils, could be obtained from BX1.5 cultivation in BG11 medium.
Fig. 7.
FAME contents in the BX1.5 cells. Samples were prepared, as described in Materials and methods, from 10 mL culture of the cells, as shown in Fig. S1, and then subjected to GC/FID analysis in which eicosanoic acid (C20: n-0) methyl-ester was used as an internal standard substance. (A) The amount (mg) of FAMEs in dry-cell weight (g) are shown. (B) Content (mg) of FAMEs from 1 L of cell-culture is also shown. Data with standard deviations (n = 3) were obtained from independent triplicate experiments.
TAG production by the green alga Parachlorella kessleri is usually characterized by its growth on sulfur-resource-deficient liquid medium [9,34,35]. The production range of total lipid values was reported to be approximately 0.25–1.9 g/L or 30–55% in dry-cell weight, whose biomass was given as 0.04–2 g/L of medium per day. In this report, the values are approximately 0.44 to 0.90-g/L-medium or 16 (27) to 32 % in dry-cell weight whose biomass was given as 0.23 to 0.95 g/L per day from BX1.5 at cultivation (Fig. 7, Table S1). The production values of 27 or 32 % in DCW did not match the extensive oil accumulation found in BG11−N or BG11−P that accounted for 70 % or 65 %, respectively, of the cross-sectional area of cells during cultivation (Fig. 5). DCW involving EPS eventually resulted in increased biomass, correlating with lower values of lipid production rate per DCW. Therefore, the lipid production value per liter of culture may be appropriate for evaluating the BX1.5 case. In fact, 0.9-g lipid production from one-liter of BX1.5 cell-culture in the BG11 medium could be high among all Parachlorella cases (Fig. 7, Table S1).
C16 and C18 FAMEs derived from TAG are generally known to be detected in green algae [8,9,31,35]. The ingested α-linolenic acid (C18:3) is further converted to n-3 (ω3)-essential fatty acids as EPA (C20:5) and DHA (C22:6) for useful oils. At this time, no clear accumulation of unsaturated polyvalent fatty acid C20:5 or C22:6 FAMEs, derived from the BX1.5 cells, was observed (data not shown). Further analysis of long-chain unsaturated fatty acid accumulation is still awaited. Till date, oil production potential of green algae has mainly been discussed as a good example of biodiesel fuel production due to the high productivity and abundant renewable oil content of algae [6,36]. However, the BX1.5 strain could be a candidate for food production as well, since it produces useful food oil and EPS acidic sugar, thus suggesting a novel characteristic, never seen before, in Parachlorella spp. strains.
We have utilized BG11 medium for both cyanobacteria and green algae. Therefore, it was remarkable to discover that this medium, when utilized for the cultivation of the new alga, was capable of producing several useful materials by simply altering the growth conditions. During this study, the conditions of 2 % CO2-air, nutrient (N/P)-depletion, and high-light irradiation at 100 μmol photons/m2 s resulted in overproduction of oil and EPS. A dilution of liquid culture, resulting in cell density, or air-drying of cells might be important strategies for future research [8,10,32]. In addition, trials using other media or reinforcing BG11 medium by adding sodium acetate might be advantageous [37,38]. Moreover, addition of carbon-source supplements or sugars created by heterotrophic cultivation [39,40], may also help further in effective production. Use of genetically modified BX1.5 strains may also create an opportunity for increased production potential for the future since genes related to TAG production have been reported [7,31,33,41,42].
3.8. Spot assay for cell resistance to stress
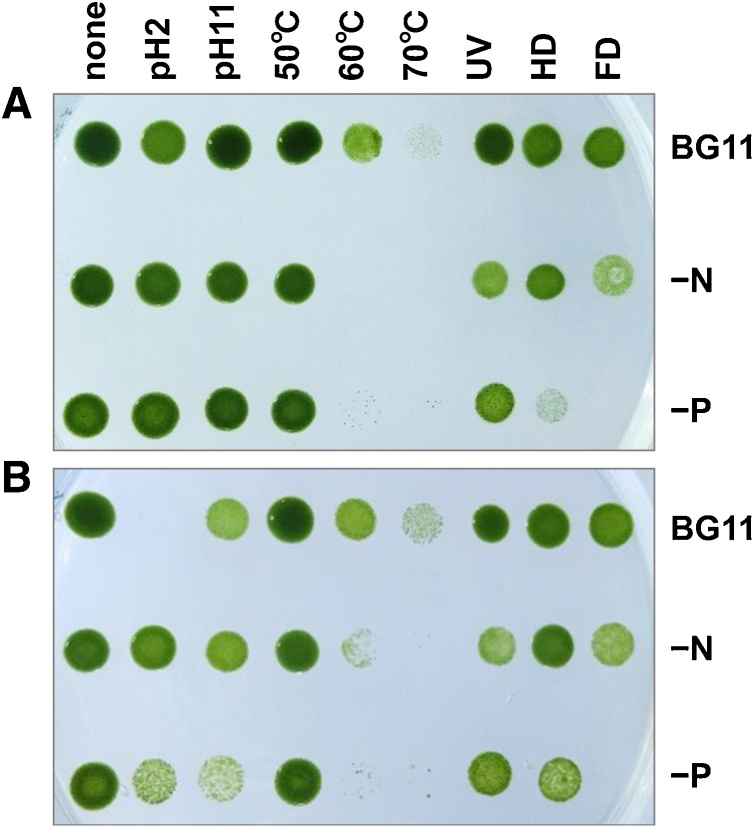
Stress tolerance of the cell is an important factor to consider while developing large-scale cultivation, particularly in an outdoor biorefinery. Therefore, we trialed resistance to environmental stress factors using BX1.5 cells, and the results are shown in Fig. 8. Cell samples were spotted onto the plate once immediately following stress tests (Fig. 8A) and again 24 h later (Fig. 8B). Following the stress treatments, photographs of cell profiles were taken after 6-days of cultivation. Signal intensities of respective cell spots on the plate were quantified and presented here as a Supplementary figure (Fig S4). The data relating BX1.5-cell resistance to stress are characterized by pH resistance; the BG11-cultivated cells exhibited tolerance over a wide range of pH, from pH 2 to pH 11. Notably, the instability of BG11-cultivated cell on the acidic side was reinforced by utilizing the cells cultivated in BG11−N/−P medium. Cells overproducing oil and acidic EPS may become resistant to acidic conditions. Interestingly, these cells also exhibited a relatively strong tolerance to alkaline-stress conditions at pH 11. This knowledge is advantageous to the cultivator since they can facilitate the penetration of CO2 gas into an alkaline liquid growth medium and utilize this method to suppress contamination by other microorganisms in field culture. Similarly, cyanobacterium Spirulina tolerated highly alkaline growth SOT medium with a pH of 10–11 and withstood higher levels of sodium bicarbonate (NaHCO3) during outdoor cultivation [43]. pH of the medium at the start of BG11 culture was about 6.5; the supply of 0.04 % CO2-air increased the pH of liquid culture to 8–10 for 6 days until the conclusion of the trial (Fig. S2C). This tendency resulted in pH 7–8 under 2 % CO2-air supply in the culture, indicating that uptake of a high concentration of CO2 gas will lead to a shift in pH from alkaline to the acidic side (Fig. S2C).
Fig. 8.
Spot assay for stress resistance. Cultured cells were harvested, exposed to stress factors, and 5 μL aliquots were spotted onto a BG11 plate (see details in Materials and methods). The cells were incubated for 6 days to observe stress resistance. (A) Samples were spotted onto the plate immediately after the stress treatments. (B) Samples were spotted onto the plate 24 h after the stress treatments. UV, ultraviolet irradiation; HD, heat dry; FD, freeze dry.
The BG11-cultivated cells exhibited tolerance at high temperatures of 50–70 °C. It would favor the sterilization of other contaminants in cultivation or processing of BX1.5 cells to utilize them as health supplements or as functional foods. UV-irradiation resistance testing of cells found the tolerance effect to be BG11 > BG11−P> BG11−N, correlating well with the accumulation rates of chlorophyll a (Fig. S2B). We considered, based on the results in Figs. 2, 8, S1, and S2, that the extent of UV tolerance might not contradict the chlorophyll a (and EPS) amounts accumulated in (and outside) the cells. Most types of stress involving photolesions (e.g., high-light or UV irradiation) have been postulated to inhibit the fixation of CO2 within the resultant ROS (reactive oxygen species), thereby inactivating the photochemical reaction center of PSII, consisting of photosynthesis pigments [44]. For dry resistance, the BG11, BG11−N, or BG11−P cultivated cells exhibited tolerance to heat-dry (HD) and freeze-dried (FD) stress factors. Regarding drought resistance, although the cells survived the HD and FD stresses, instability of the BG11−P cell was observed in case of FD stress (Figs. 8 and S4). Phosphate is known to be an essential element for cells. Its depletion in the BG11 medium could cause reduced strength of biological membranes since phospholipids are a necessary component of the cell membrane, resulting in enlarged and lysed cells during microscopic observation (Fig. 4, Fig. 5). The nature of these altered cells, despite being more vulnerable, allowed for easy extraction of sugars and oils, hence resulting in lower extraction cost. Moreover, the altered BX1.5 cells may be effectively absorbed into the digestive system in vivo when they are eaten. These results together suggested that the Parachlorella sp. BX1.5 cells possess high resistance to environmental stress, exhibiting characteristics that facilitate its use in an outdoor cultivation for biorefineries.
4. Conclusions
A novel green alga Parachlorella sp. BX1.5 can produce high yields of both lipids and extracellular polysaccharides, without conflicting with the internal space required for other processes, even under cultivation conditions utilizing the BG11 liquid media. The BX1.5 cells also showed significant stress tolerance, and their characteristics drastically changed with cultivation conditions, which regulated the amount and content of potential useful finished products or materials, thus promising outdoor cultivation for biorefineries.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This work was partially supported by a propulsive project from Ibaraki University (to MA), Ministry of Agriculture, Forestry and Fisheries, Japan (to DS and MA), Japan Society for Bioscience, Biotechnology, and Agrochemistry (to MA and DS: Academic-Industrial R&D Support for Small-to-Medium-sized Enterprises, SME-3), and Japan Society for the Promotion of Science (to MA and DS: A-STEP, JPMJTM19BT; OPERA).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00392.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Brennan L., Owende P. Biofuels from microalgae–a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;14:557–577. [Google Scholar]
- 2.Caporgno M.P., Mathys A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018;5:58. doi: 10.3389/fnut.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates R.C., Podell S., Korobeynikov A., Lapidus A., Pevzner P., Sherman D.H., Allen E.E., Gerwick L., Gerwick W.H. Characterization of cyanobacterial hydrocarbon composition and distribution of biosynthetic pathways. PLoS One. 2014;9:e85140. doi: 10.1371/journal.pone.0085140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintana N., Kooy F.V., Rhee M.D., Voshol G.P., Verpoorte R. Renewable energy from Cyanobacteria: energy production optimization by metabolic pathway engineering. Appl. Microbiol. Biotechnol. 2011;91:471–490. doi: 10.1007/s00253-011-3394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirmer A., Mathew A.R., Li X., Popova E., Cardayre S.B. Microbial biosynthesis of alkanes. Science. 2010;329:559–562. doi: 10.1126/science.1187936. [DOI] [PubMed] [Google Scholar]
- 6.Chisti Y. Biodiesel from microalga. Biotech Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Fan J., Andre C., Wu C. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett. 2011;585:1985–1991. doi: 10.1016/j.febslet.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Přibyl P., Bišová K., Kawano S., Cepák V., Zachleder V., Čížková M., Brányiková I., Vítová M. The microalga Parachlorella kessleri––a novel highly efficient lipid producer. Biotechnol. Bioeng. 2013;110:97–107. doi: 10.1002/bit.24595. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno Y., Sato A., Watanabe K., Hirata A., Takeshita T., Ota S., Sato N., Zachleder V., Tsuzuki M., Kawano S. Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour. Technol. 2013;129:150–155. doi: 10.1016/j.biortech.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Shiratake T., Sato A., Minoda A., Tsuzuki M., Sato N. Air-drying of cells, the novel conditions for stimulated synthesis of triacylglycerol in a green alga, Chlorella kessleri. PLoS One. 2013;8:e79630. doi: 10.1371/journal.pone.0079630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue Z., Wan F., Yu W., Liu J., Zhang Z., Kou X. Edible oil production from microalgae. Eur. J. Lipid Sci. Technol. 2018;120:6. [Google Scholar]
- 12.Choi J.H., Kim S., Kim S.J. Spirulan from blue-green algae inhibits fibrin and blood clots: its potent antithrombotic effects. J. Biochem. Mol. Toxicol. 2015;29:240–248. doi: 10.1002/jbt.21690. [DOI] [PubMed] [Google Scholar]
- 13.Ngatu N.R., Okajima M.K., Yokogawa M., Hirota R., Eitoku M., Muzembo B.A., Dumavibhat N., Takaishi M., Sano S., Kaneko T., Tanaka T., Nakamura H., Suganuma N. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene-induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012;108:117–122. doi: 10.1016/j.anai.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Karnjanapratum S., You S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2011;48 doi: 10.1016/j.ijbiomac.2010.12.002. 311–308. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.B., Koizumi S., Hayashi K., Hayashi T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010;81:572–577. [Google Scholar]
- 16.Mao W.J., Fang F., Li H.Y., Qi X.H., Sun H.H., Chen Y., Guo S.D. Heparinoid-active two sulfated polysaccharides isolated from marine green algae Monostroma nitidum. Carbohydr. Polym. 2008;74:834–839. [Google Scholar]
- 17.Nishizawa T., Hanami T., Hirano E., Miura T., Watanabe Y., Takanezawa A., Komatsuzaki M., Ohta H., Shirai M., Asayama M. Isolation and molecular characterization of a multicellular cyanobacterium, Limnothrix/Pseudanabaena sp. strain ABRG5-3. Biosci. Biotechnol. Biochem. 2010;74:1827–1835. doi: 10.1271/bbb.100216. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara T., Chinzei M., Numano S., Kitazaki C., Asayama M. Flocculation and pentadecane production of a novel filamentous cyanobacterium Limnothrix sp. strain SK1-2-1. Biotechnol. Lett. 2018;40:829–836. doi: 10.1007/s10529-018-2525-4. [DOI] [PubMed] [Google Scholar]
- 20.Rippka R. Isolation and purification of cyanobacteria. Methods Enzymol. 1989;167:3–27. doi: 10.1016/0076-6879(88)67004-2. [DOI] [PubMed] [Google Scholar]
- 21.Tuffery A.A. Light and Electron microscopy of the sheath of a blue-green alga. J. Gen. Microbiol. 1969;57:41–50. [Google Scholar]
- 22.Shepherd V.A., Beilby M.J. The effect of an extracellular mucilage on the response to osmotic shock in the charophyte alga Lamprothamnium papulosum. J. Membr. Biol. 1999;170:229–242. doi: 10.1007/s002329900552. [DOI] [PubMed] [Google Scholar]
- 23.Whiteman P. Quantitative measurements of Alcian Blue-glycosaminoglycan complexes. Biochem. J. 1973;131:343–350. doi: 10.1042/bj1310343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMANUS J.F. Histological demonstration of mucin after periodic acid. Nature. 1946;158:202. doi: 10.1038/158202a0. [DOI] [PubMed] [Google Scholar]
- 25.Greenspan P., Mayer E.P., Fowler S.D. Nile red: a selective fluorescent strain for intracellular lipid droplets. J. Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitazaki C., Numano S., Takanezawa A., Nishizawa T., Shirai M., Asayama M. Characterization of lysis of the multicellular cyanobacterium Limnothrix/Pseudanabaena sp. strain ABRG5-3. Biosci. Biotechnol. Biochem. 2013;77:2339–2347. doi: 10.1271/bbb.130409. [DOI] [PubMed] [Google Scholar]
- 27.Breuer G., Lamers P.P., Martens D.E., Draaisma R.B., Wijffels R.H. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour. Technol. 2012;124:217–226. doi: 10.1016/j.biortech.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Hondo S., Takahashi M., Osanai T., Matsuda M., Hasunuma T., Tazuke A., Nakahira Y., Chohnan S., Hasegawa M., Asayama M. Genetic engineering and metabolite profiling for overproduction of polyhydroxybutyrate in cyanobacteria. J. Biosci. Bioeng. 2015;120:510–517. doi: 10.1016/j.jbiosc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S., Takahashi M., Ikeda A., Fukuda H., Kitazaki C., Asayama M. Overproduction and easy recovery of biofuels from engineered cyanobacteria, autolyzing multicellular cells. J. Biochem. 2015;157:519–527. doi: 10.1093/jb/mvv011. [DOI] [PubMed] [Google Scholar]
- 30.Okajima M.K., Bamba T., Kaneso Y., Hirata K., Fukusaki E., Kajiyama S., Kaneko T. Supergiant ampholytic sugar chains with imbalanced charge ratio form saline ultra-absorbent hydrogels. Macromolecules. 2008;41:4061–4064. [Google Scholar]
- 31.Sato A., Matsumura R., Hoshino N., Tsuzuki M., Sato N. Responsibility of regulatory gene expression and repressed protein synthesis for triacylglycerol accumulation on sulfur-starvation in Chlamydomonas reinhardtii. Front. Plant Sci. 2014;5(444):1–12. doi: 10.3389/fpls.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirai K., Hayashi T., Hasegawa Y., Sato A., Tsuzuki M., Sato N. Hyperosmosis and its combination with nutrient-limitation are novel environmental stressors for induction of triacylglycerol accumulation in cells of Chlorella kessleri. Sci. Rep. 2016;6(25825):1–12. doi: 10.1038/srep25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung C.H., Kanehara K., Nakamura Y. Isolation and characterization of a mutant defective in triacylglycerol accumulation in nitrogen-starved Chlamydomonas reinhardtii. Biochim. Biophys. Acta. 2016;186:1282–1293. doi: 10.1016/j.bbalip.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Ota S., Oshima K., Yamazaki T., Kim S., Yu Z., Yoshihara M., Takeda K., Takeshita T., Hirata A., Bišová K., Zachleder V., Hattori M., Kawano S. Highly efficient lipid production in the green alga Parachlorella kessleri: draft genome and transcriptome endorsed by whole-cell 3D ultrastructure. Biotechnol. Boifuels. 2016;9:13. doi: 10.1186/s13068-016-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeshita T., Ota S., Yamazaki T., Hirata A., Zachleder V., Kawano S. Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour. Technol. 2014;158:127–134. doi: 10.1016/j.biortech.2014.01.135. [DOI] [PubMed] [Google Scholar]
- 36.Stansell G.R., Gray V.M., Sym S.D. Microalgal fatty acid composition: implications for biodiesel quality. J. Appl. Phycol. 2012;24:791–801. [Google Scholar]
- 37.Lee S.J., Yoon B.D., Oh H.M. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Techniq. 1998;12:553–556. [Google Scholar]
- 38.Tanoi T., Kawachi M., Watanabe M.M. Effect of carbon source on growth and morphology of Botoryococcus brauni. J. Appl. Phycol. 2011;23:25–33. [Google Scholar]
- 39.Marudhupandi T., Sathishkumar R., Kumar T.T.A. Heterotrophic cultivation of Nannochloropsis salina for enhancing biomass and lipid production. Biotechnol. Rep. 2016;10:8–16. doi: 10.1016/j.btre.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piasecka A., Krzemińska I., Tys J. Enrichment of Parachlorella kessleri biomass with bioproducts: oil and protein by utilization of beet molasses. J. Appl. Phycol. 2017;29:1735–1743. doi: 10.1007/s10811-017-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daboussi F., Leduc S., Maréchal A., Dubois G., Guyot V., Perez-Michaut C., Amato A., Falciatore A., Juillerat A., Beurdeley M., Voytas D.F., Cavarec L., Duchateau P. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014;29:3831–3837. doi: 10.1038/ncomms4831. [DOI] [PubMed] [Google Scholar]
- 42.Hung C.H., Ho M.Y., Kanehara K., Nakamura Y. Functional study of diacylglycerol acyltransferase type 2 family in Chlamydomonas reinhardtii. FEBS Lett. 2013;587:2364–2370. doi: 10.1016/j.febslet.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Vonshak A., Boussiba S., Abeliovich A., Richmond A. Production of Spirulina biomass: maintenance of monoalgal culture outdoors. Biotechnol. Bioeng. 1983;25:341–349. doi: 10.1002/bit.260250204. [DOI] [PubMed] [Google Scholar]
- 44.Murata N., Takahashi S., Nishiyama Y., Allakhverdiev S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta. 2007;1767:414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.