Abstract
Moringa oleifera is a medicinal plant with great therapeutic potential. The leaves of Moringa oleifera are used by Indians in herbal medicines to treat diabetes. The present study is aimed to determine the protective role of Moringa oleifera in cardiac tissues under diabetic conditions. Diabetic rats were treated orally with methanolic extract of Moringa oleifera leaves at a dose of 300 mg/Kg body weight for 60 days. The effect of extract on serum glucose, glycated hemoglobin, plasma insulin and the levels of thiobarbituric acid reactive substances (TBARS), hydroperoxides (HP), conjugated dienes (D), activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-reductase (GRD) and reduced glutathione content (GSH) were estiated. Metformin and atorvastatin were used as standard drugs. A significant increase in plasma insulin, activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-reductase (GRD) and reduced glutathione content (GSH) and a significant decrease in serum glucose, glycated hemoglobin, thiobarbituric acid reactive substances (TBARS), hydroperoxides (HP) and conjugated dienes (CD) were observed in the treated groups. This study evaluated the antioxidant potential of methanolic extract of Moringa oleifera leaves. These findings suggest the protective role of Moringa oleifera against oxidative stress in the heart of diabetic rats.
Keywords: Food Science
Antioxidants, Diabetes, Heart diseases, Moringa oleifera
1. Introduction
Diabetes mellitus is a serious metabolic disorder. An increased level of blood glucose levels in diabetes is resulting from the defects in insulin secretion and insulin action. Long term hyperglycemia can damage multiple organs and lead to severe complications especially to heart, kidneys and eyes. The occurrence of cardiovascular disease mostly accounts for the rise in mortality in type 2 diabetes (Feuvray, 2010). Oxidative stress is the main reason behind the development of cardiovascular diseases such as congestive heart failure and diabetes-associated heart dysfunction (diabetic cardiomyopathy). It was found that antioxidant therapy plays a promising function in preventing the progress of diabetic heart complications (Wold et al., 2005). Oxidative stress play an important role in the progression and complications associated with diabetes (Ustinova et al., 2000; Uemura et al., 2001). Previous studies showed hyperglycemia can induce the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and which will cause oxidative damage to multiple organs. Thus, the relationship between oxidative stress and diabetic cardiomyopathy is a major focus of current research (Cai and Kang, 2001).
Moringa oleifera is the most widely cultivated species of the genus Moringa and is native to Indian sub continent. It is also known as drumstick tree. Moringa oleifera is an edible plant. Its leaves are widely used as a vegetable in India. It is a good remedy to malnutrition and used in ayurveda for the treatment of diabetic complications. Nowadays the interest on herbal medicines increased due to the side effects associated with oral hypoglycemic drugs used in the treatment of diabetes. The present study is to evaluate the protective role of methanol extract of Moringa oleifera leaves in the heart of diabetic rats.
2. Materials and methods
2.1. Chemicals
All Chemicals for the present study were purchased from Sigma Aldrich (St. Louis, MO, USA), Merck Chemical Company (Darmstadt, Germany) and Sisco Research Laboratories (Mumbai, India).
2.2. Preparation of leaf extracts
The leaves of Moringa oleifera were collected from Trivandrum, Kerala, India. Authentication was done by Dr.G.Valsala devi, curator, Department of Botany, University of Kerala. A voucher specimen (Voucher No. KUBH 9913) has been deposited in the herbarium, Department of Botany, University of Kerala for further reference. The collected plant materials were washed with distilled water and air dried in shade at room temperature. The dried samples were milled into powder using an electric blender. Dried leaf powder (5g) was serially extracted with petroleum ether, chloroform, and methanol, using soxhlet apparatus. Methanolic extract of Moringa oleifera (MOME) leaves was used for the experimental studies.
2.3. Animals
Male albino (Sprague Dawley) rats (170–180g body weight; 36 animals in total) bred in the animal house, Department of Biochemistry were used for the study. The rats were housed in a room with temperature maintained at 23 ± 1 °C and 12 h light and dark cycles. The relative humidity of 50 ± 10% and ventilation frequency of 10–30 times per hour were maintained. The animals were acclimatized under laboratory conditions for two weeks prior to experiments. Animal care was performed per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals and the experimental protocol approved by the Institutional Animal Ethics Committee [IAEC-KU- 07/2016-17-BC-SM (38)].
2.4. Induction of experimental diabetes
Diabetes was induced in rats by a single intraperitoneal injection of freshly prepared STZ at a dose of 30 mg/kg body weight in 0.1M citrate buffer (pH 4.5) (Wang et al., 2007). The animals were allowed to drink 5 % glucose solution overnight to overcome the drug induced hypoglycemia. The animals with blood glucose levels glucose levels between 200 mg/dL and 400 mg/dL were considered as diabetic.
2.5. Experimental design
The animals were divided into six groups with each group comprising of six rats. Normal control rats (Group 1–2) were fed with Normal pellet diet (NPD) and High energy diet groups (Groups 3–6) with high energy diet (NPD (70%)+Sucrose (20%)+Lard oil (10%)) (Wang et al., 2007) for 60 days. Water was provided ad libitum throughout the experimental period.
After 60 days diet; diabetes was induced to groups 4 to 6 with a single intraperitoneal injection of freshly prepared STZ at a dose of 30 mg/kg body weight. MOME, metformin + atorvastatin were given orally using an intragastric tube once daily in the morning. Metformin is a first-line medication for the treatment of diabetes and atorvastatin is used to treat dyslipidemia.
Group1: Normal control rats (N)
Group 2: Normal rats treated with MOME (300 mg/Kg body weight) (N + MOME)
Group 3: High energy diet control rats (HD)
Group 4: Diabetic control rats (DM)
Group 5: Diabetic rats treated with MOME (300 mg/Kg body weight) (DM + MOME)
Group 6: Diabetic rats treated with metformin (100 mg/Kg body weight) and atorvastatin (10 mg/Kg body weight) (DM + M + A)
During the experimental period body weight and blood glucose of the rats were monitored at definite intervals. Dosage of the extract and standard drugs were maintained in accordance with the change in body weight of the rats throughout the study period. After 60 days, the rats were sacrificed and blood and heart were collected for various biochemical analyses.
2.6. Biochemical parameters
Blood samples were collected from the tail vein of the rats and blood glucose level was estimated by using one touch electronic glucometer (Lifescan, Johnson and Johnson Ltd.) (Kumar et al., 2013). Serum glucose (Agappe Diagnostics, Kerala, India) and glycated hemoglobin (HbA1c) were measured based on the ion exchange method (Nathan et al., 1984). Plasma Insulin was measured using an enzyme-linked immunosorbent assay kit (DRG Diagnostics, Marburg, Germany). The activities of antioxidant enzymes superoxide dismutase (SOD) (Kakkar et al., 1984), catalase (CAT) (Maehly and Chance, 1954), glutathione peroxidase (GPx) (Rotruck et al., 1973) and glutathione reductase (GRd) (David and Richard, 1983) were analyzed. Reduced glutathione (GSH) content (Patterson and Lazarow, 1955) in the heart was also measured. Thiobarbituric acid-reactive substances (TBARS), hydroperoxides (HP) and conjugated dienes (CD) (Okhawa et al., 1979) were also assessed.
2.7. Histopathological analysis
The histopathological studies of heart tissues were carried out according to the method of Disbrey and Rack (1970). The heart tissues were examined and photographed under a light microscope for observation of structural abnormality.
2.8. Statistical analysis
Values were expressed as mean ± standard error of the mean. Statistical analyses were performed by one-way ANOVA using SPSS version 17 (SPSS, Inc., Chicago, IL, USA). Duncan's post hoc multiple-comparison tests were used to determine significant differences among groups. p < 0.05 was considered to be significant.
2.9. Gas chromatography- mass spectrometric analysis (GCMS)
GC-MS technique was used to identify the phytochemicals present in the extract. The methanolic extract of Moringa oleifera leaves was analyzed using the Thermo Scientific GC-MS [SHIMADZU QP 2010] gas chromatograph with Software: GCMS solution ver.2.53. The gas chromatograph was interfaced to a mass spectrometer equipped with elite-1 fused silica capillary column of length: 30.0 m, diameter: 0.25mm, film thickness: 0.25 μm and composed of 100% dimethyl poly siloxane. The column oven temperature was maintained at 70 °C and injector temperature at 240 °C. The oven temperature was programmed as follows: 70 °C for 2 min rose to 300 °C for 7 min at the rate of 10°C/minute. Helium of 99.9995% purity was used as the carrier gas at 1.51ml/minute. The sample (1μl) was injected in the split mode of 10:1. The total GC running time was 32 min with ion source and interface temperatures maintained at 200 °C and 240 °C respectively. The mass spectra were taken with scan range of 40–1000 m/z of 0.5 s intervals at 70eV ionization. The relative percentage area of each component was calculated by comparing it to the total area.
2.10. Identification of components
Interpretation of mass spectrum of GC-MS was made using the databases NIST 11 and WILEY 8.
3. Results
3.1. Blood glucose, serum glucose, glycated haemoglobin (HbA1c) and plasma insulin
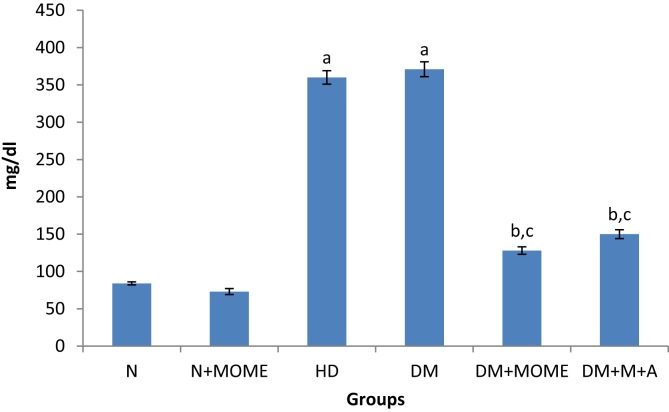
The blood glucose level was significantly increased in rats fed with high energy diet (Group 3 to 6) when compared to the rats fed with normal pellet diet (groups 1 and 2) after a period of 60 days. After 60 days of the diet, diabetes was induced to groups 4 to 6. The blood glucose levels of diabetic rats were significantly increased when compared to high energy control and normal control rats. After the 60 days of treatment the blood glucose level was significantly decreased in treatment groups (Group 5 and 6) when compared to untreated high energy control and diabetic control. However the blood glucose was significantly decreased in MOME treated rats when compared to the rats treated with the standard drugs metformin + atorvastatin (Figure 1)
Figure 1.
Blood glucose level. Values are expressed as mean ± standard error of the mean of six rats in each group. Significance determined at p < 0.05. ‘a’ Statistically significant as compared with the normal group; ‘b’ Statistically significant as compared with the high energy diet (HD) group; ‘c’ Statistically significant as compared with the diabetic group. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100 mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
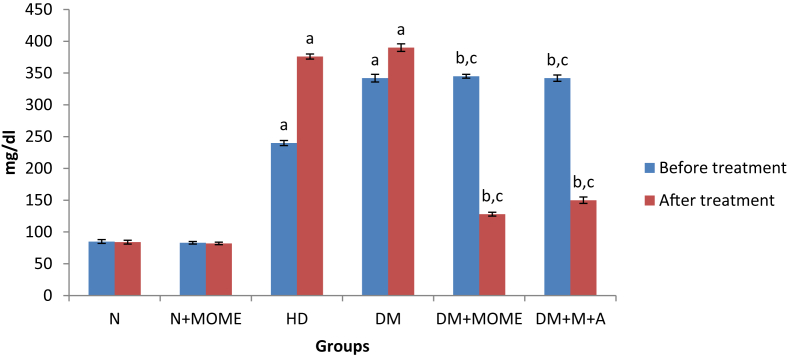
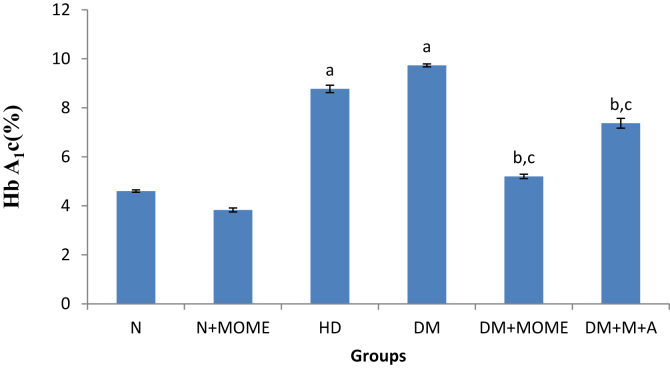
The levels of serum glucose and glycated hemoglobin (Figures 2 and 3) were significantly increased in diabetic and high energy diet control groups as compared to normal groups. The oral administration of MOME or metformin + atorvastatin significantly reduced the level of serum glucose and HbA1c in diabetic rats to the normal level. However oral administration of MOME to normal rats didn't show any significant deviation in serum glucose and HbA1c.
Figure 2.
Glycated haemoglobin Content. Values are expressed as mean ± standard error of the mean of six rats in each group. Significance determined at p < 0.05. ‘a’ Statistically significant as compared with the normal group; ‘b’ Statistically significant as compared with the high energy diet (HD) group; ‘c’ Statistically significant as compared with the diabetic group. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100 mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
Figure 3.
Serum glucose level. Values are expressed as mean ± standard error of the mean of six rats in each group. Significance determined at p < 0.05. ‘a’ Statistically significant as compared with the normal group; ‘b’ Statistically significant as compared with the high energy diet (HD) group; ‘c’ Statistically significant as compared with the diabetic group. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100 mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
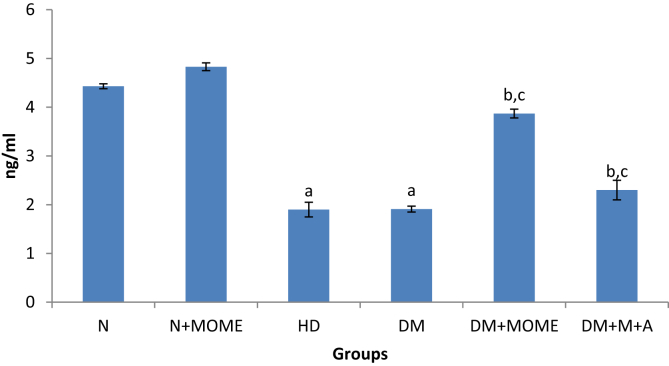
Plasma insulin (Figure 4) was significantly reduced in diabetic and high energy diet control groups. The administration of MOME or metformin + atorvastatin significantly increased plasma insulin. Oral administration of MOME to normal rats increased the level of plasma insulin.
Figure 4.
Plasma insulin level. Values are expressed as mean ± standard error of the mean of six rats in each group. Significance determined at p < 0.05. ‘a’ Statistically significant as compared with the normal group; ‘b’ Statistically significant as compared with the high energy diet (HD) group; ‘c’ Statistically significant as compared with the diabetic group. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100 mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
3.2. Cardiac antioxidant enzymes
The activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-reductase (GRD) were significantly reduced in diabetic and high energy diet control groups as compared to normal control and MOME or metformin + atorvastatin treated groups. The oral administration of MOME or metformin + atorvastatin significantly increased the activities of antioxidant enzymes in the heart of diabetic rats. There was no significant variation in the level of antioxidant enzymes in normal rats treated with MOME (Table 1).
Table 1.
Cardiac antioxidant enzymes.
| Groups | CAT (10 −3 U/mg protein) | GPx (U/mg protein) | GRd (U/mg protein) | SOD (U/mg protein) |
|---|---|---|---|---|
| N | 6.73 ± 0.28 | 31.04 ± 0.97 | 91.94 ± 2.25 | 2.08 ± 0.03 |
| N + MOME | 8.93 ± 0.59 | 32.85 ± 3.25 | 97.64 ± 2.15 | 2.44 ± 0.32 |
| HD | 2.60 ± 0.06a | 9.53 ± 1.51a | 51.72 ± 1.16a | 0.28 ± 0.15a |
| DM | 1.98 ± 0.11a,b | 8.83 ± 1.61a,b | 45.62 ± 1.33a,b | 0.36 ± 0.04a |
| DM + MOME | 6.13 ± 0.62b,c | 28.80 ± 3.38b,c | 64.05 ± 1.44b,c | 1.83 ± 0.03b,c |
| DM + M + A | 3.93 ± 0.37b,c | 20.80 ± 1.07b,c | 82.48 ± 2.42b,c | 1.48 ± 0.03b,c |
Values are expressed as mean ± standard error of the mean of six rats in each group. Significance determined at p < 0.05. ‘a’ Statistically significant as compared with the normal group; ‘b’ Statistically significant as compared with the high energy diet (HD) group; ‘c’ Statistically significant as compared with the diabetic group. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
3.3. Lipid peroxidation products and reduced glutathione content (GSH) in heart
Thiobarbituric acid-reactive substances (TBARS), hydroperoxides (HP) and conjugated dienes (CD) were significantly increased in diabetic and high energy diet control rats as compared to normal control rats. The oral administration of MOME or metformin + atorvastatin significantly decreased lipid peroxidation products in diabetic rats. Normal rats treated with MOME didn't show any significant variations in lipid peroxidation products as compared to the normal rats. The non enzymatic antioxidant (GSH) was significantly reduced in diabetic and high energy diet control groups as compared to normal controls. MOME or metformin + atorvastatin treatment significantly increased gluthathione content in the heart of diabetic rats. There is no significant variation in the glutathione content in the normal rats treated with MOME compared to untreated normals (Table 2).
Table 2.
Lipid peroxidation products and reduced glutathione content (GSH) in heart.
| Groups | TBARS (mM/100 g tissue) | CD (mM/100 g tissue) | HP (mM/100 g tissue) | GSH content (mM/100 g tissue) |
|---|---|---|---|---|
| N | 033 ± .01 | 2.72 ± 0.54 | 8.41 ± 0.23 | 77.46 ± 0.66 |
| N + MOME | 0.28 ± .01 | 2.43 ± 0.07 | 8.53 ± 0.25 | 79.79 ± 0.75 |
| HD | 0.76 ± .02a | 8.58 ± 0.13a | 24.36 ± 0.67a | 36.33 ± 0.69a |
| DM | 0.96 ± .02a,b | 8.62 ± 0.24a | 37.27 ± 1.02a,b | 26.42 ± 0.18a,b |
| DM + MOME | 0.43 ± .01b,c | 4.64 ± 0.38b,c | 14.80 ± 0.41b,c | 53.08 ± 0.81b,c |
| DM + M + A | 0.60 ± .01b,c | 5.72 ± 0.16b,c | 18.06 ± 0.50b,c | 43.79 ± 0.34b,c |
Values are expressed as mean ± standard error of the mean of six rats in each group. Significance determined at p < 0.05. ‘a’ Statistically significant as compared with the normal group; ‘b’ Statistically significant as compared with the high energy diet (HD) group; ‘c’ Statistically significant as compared with the diabetic group. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
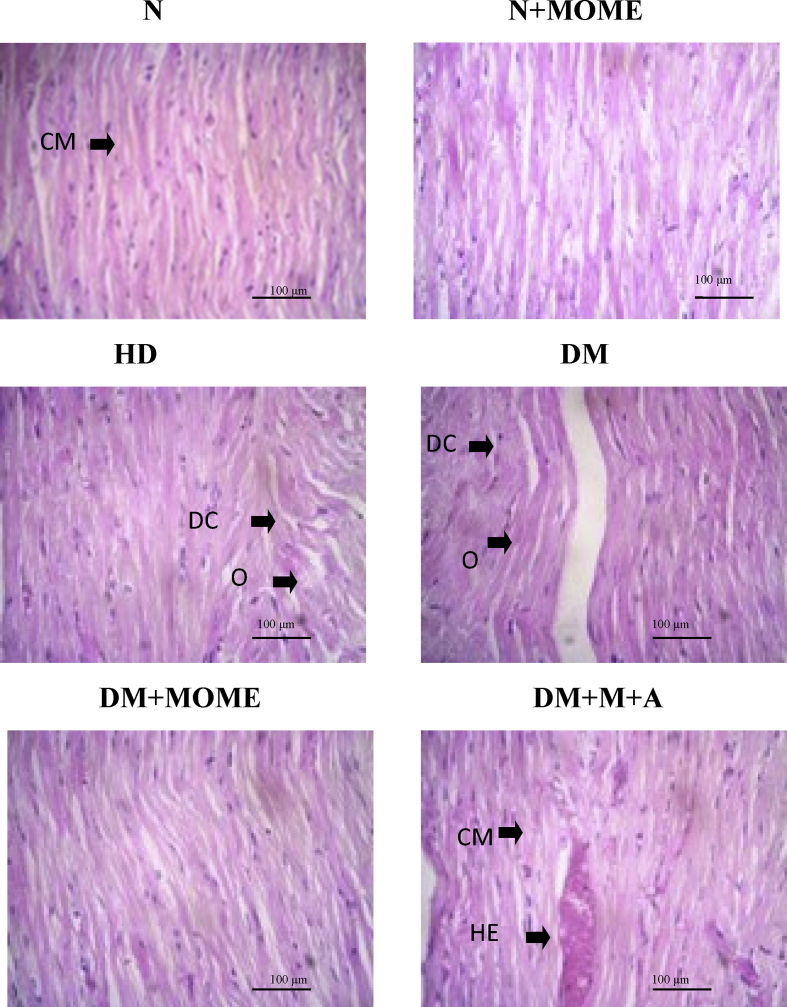
3.4. Histopathological analysis
In diabetic and high energy control groups, myocyte became hyaline, striations were blurred, myocytosis and moderate intestinal edema were also observed. Some fibres became swollen, vacuolated and showed dense focal fatty infiltration into the myocardial cells. However, MOME, and M + A supplementation was able to improve the histopathology of the diabetic heart compared to untreated diabetic group. Necrotic, inflammatory nor hemorrhagic changes were visualized in the heart of MOME administrated group. Further, normal rats supplemented with MOME showed normal heart histology. The results are shown in Figure 5.
Figure 5.
Histopathological analysis of heart. Light micrographs (20X) of heart paraffin sections stained with H&E. CM- Cardiac myocytes, DC-Degenerative changes in myocytes, O-Oedema, HE- Hemorrhage. N = 6 Rats. N- Normal control rats, N + MOME- Normal rats treated with MOME (300 mg/Kg body weight), HD- High energy diet control rats, DM- Diabetic control rats, DM + MOME- Diabetic rats treated with MOME (300 mg/Kg body weight), DM + M + A-Diabetic rats treated with metformin (100mg/Kg body weight) and atorvastatin (10 mg/Kg body weight).
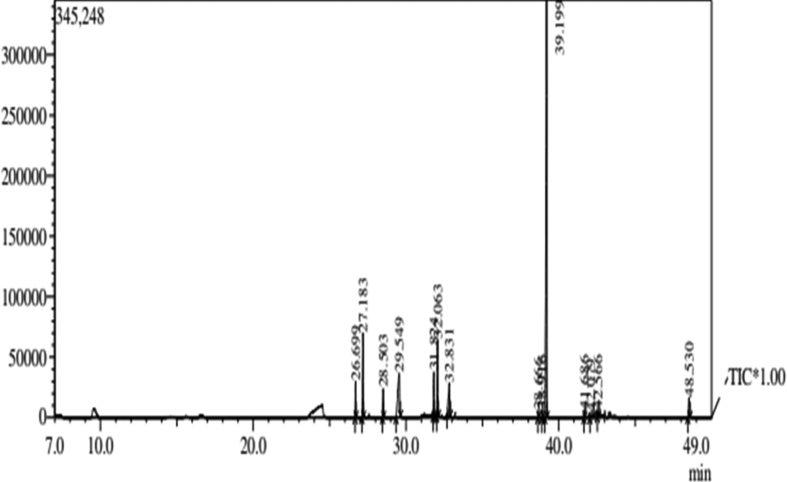
3.5. GC–MS profiling of MOME
GC-MS profiling of MOME (Figure 6) revealed the presence of 14 compounds (Table 3). Out of the 14 compounds found; 9 compounds were reported to have various biological activities. The antioxidant compounds present in MOME include, 2-benzenedicarboxylic acid, heptadecanoic acid, hexadecanoic acid, Dl-.alpha.-Tocopherol. 11, 14, 17-eicosatrienoic acid and 9, 12, 15-octadecatrienal were the compounds present in MOME with anti arthritic and anti coronary activities. So the activity connected with MOME may be due to the presence of these compounds.
Figure 6.
GCMS chromatogram of MOME. GC-MS chromatogram of MOME showing various antioxidant compounds.
Table 3.
MOME GCMS analysis.
| Peak | R.Time | Area | Area% | Height | Height% | Name | Base m/z |
|---|---|---|---|---|---|---|---|
| 1 | 26.699 | 59482 | 2.71 | 30093 | 4.46 | 16-HEPTADECENAL | 68.05 |
| 2 | 27.183 | 165243 | 7.54 | 70203 | 10.39 | 1,2-BENZENE DICARBOXYLIC ACID, BIS(2-METHYLPROPYL) ESTER | 149.00 |
| 3 | 28.503 | 55721 | 2.54 | 24213 | 3.58 | HEPTADECANOIC ACID, METHYL ESTER | 74.05 |
| 4 | 29.549 | 271109 | 12.37 | 36078 | 5.34 | HEXADECANOIC ACID | 73.05 |
| 5 | 31.824 | 86511 | 3.95 | 36832 | 5.45 | 11,14,17-EICOSATRIENOIC ACID, METHYL ESTER | 79.05 |
| 6 | 32.063 | 157663 | 7.19 | 63384 | 9.38 | PHYTOL | 71.05 |
| 7 | 32.831 | 141128 | 6.44 | 26985 | 4.00 | 9,12,15-OCTADECATRIENAL | 79.05 |
| 8 | 38.666 | 8889 | 0.41 | 4969 | 0.74 | DODECANE, 1,1-DIFLUORO- | 57.05 |
| 9 | 38.916 | 13074 | 0.60 | 6543 | 0.97 | OCTADECANOIC ACID, 2-HYDROXY-1-(HYDROXYMETHYL)ETHYL ESTER | 57.10 |
| 10 | 39.199 | 1119119 | 51.05 | 345248 | 51.12 | 1,2-BENZENE DICARBOXYLIC ACID | 149.05 |
| 11 | 41.686 | 13624 | 0.62 | 6138 | 0.91 | (S)-4-IODO-1,2-EPOXYBUTANE | 57.05 |
| 12 | 42.079 | 22001 | 1.00 | 3795 | 0.56 | 6,6-DIMETHYL-9-METHYLENE BICYCLO[3.3.1]NONAN-3-ONE | 149.00 |
| 13 | 42.566 | 12171 | 0.56 | 4645 | 0.69 | 4-ISOXAZOLAMINE, 5-(1-METHYLETHYL)-N-[(1-METHYLETHYL)CARBONIMIDOYL]- | 71.10 |
| 14 | 48.530 | 66420 | 3.03 | 16292 | 2.41 | DL-.ALPHA.-TOCOPHEROL | 165.05 |
| 2192155 | 100.00 | 675418 | 100.00 |
4. Discussion
The reactive oxygen species can cause oxidative damage in tissues. Oxidative stress plays a significant role in the progress of vascular complications in type 2 diabetes. The previous epidemiological studies showed that, the mortalities associated with diabetes can be explained notably by an increase in vascular diseases other than hyperglycemia (Pham-Huy et al., 2008). Due to the variations in the antioxidant enzyme level, the tissue became more susceptible to oxidative stress and leads to the progress of diabetic complications (Lipinski, 2001). The previous in vivo studies showed that hyperglycemia play a major role in the development of oxidative stress which leads to endothelial dysfunction in blood vessels of diabetic patients (Ceriello, 2006). Oxidative stress and diabetes are connected to each other. Previous studies showed that there is a link between DNA damage biomarkers and lipid peroxidation products (Asmat et al., 2016).
Type 2 diabetes is a common disorder of carbohydrate and lipid metabolism (Nisoli et al., 2000). The long term existence of type 2 diabetes increase the level of reactive oxygen species that induces oxidative stress and that will damage all parts of our body; especially heart that shows increased signs of oxidative stress (Baynes, and Thorpe, 1999).
Cardiovascular complications are the most important cause of morbidity and mortality associated with diabetes. Hyperglycemia results in the production of free radicals and which leads to oxidative stress. Oxidative stress leads to various complications associated with diabetes. Overproduction and insufficient removal of free radicals result in vascular dysfunction, damage to cellular proteins, membrane lipids and nucleic acids (Johansen et al., 2005).
In the present study we used high energy diet as a source to make the rats insulin resistant. Previous studies showed that a combination of low dose streptozotocin and high energy diet can effectively induce type 2 diabetes in rat model. Insulin resistance, cells failure to respond to the internal insulin, always occurs in the early stage of type 2 diabetes. Beta cell decline and lipid metabolism confusion are the other risks associated with long term insulin resistance. Insulin resistance is closely linked with behavioral factors such as food habits and exercise (Tuomilehto et al., 2001). Disturbance in the carbohydrate and lipid metabolisms generates free radicals and this in turn will damage various organs.
In the present study diabetic and high energy diet control rats showed severe hyperglycemia interrelated with reduced insulin secretion and release. Here serum glucose and glycated hemoglobin were significantly elevated in diabetic and high energy diet control groups. The treatment with methanol extract of Moringa oleifera leaves effectively reduced hyperglycemia in diabetic rats. It is evident from the glycated haemoglobin and plasma insulin levels in the treated groups. The antidiabetic effect of the plant may be through the release of insulin from the pancreatic cells of treated groups which is clearly evident from the increasing level of plasma insulin. The effect of Moringa oleifera leaves is superior to that of metformin. Previous studies showed that increase in the levels of glucose and insulin along with dyslipidemia in patients suffering from diabetes develops macro angiopathies that cause oxidative stress leading to atherosclerosis (Giugliano et al., 1995).
Antioxidant defense mechanisms include both enzymatic and non enzymatic. Non enzymatic antioxidants include vitamin A, C, E and glutathione and enzymatic antioxidants include super oxide dismutase, catalase, glutathione peroxidase and glutathione reductase (Maritim et al., 2003). The antioxidant therapy is gaining popularity due to its high potential against oxidative stress induced diseases especially diabetes mellitus. The lipid peroxidation products (Thiobarbituric acid-reactive substances (TBARS), hydroperoxides (HP) and conjugated dienes (CD) were increased in diabetic as well as high energy controlled groups. This may be due to decreased levels of enzymatic antioxidants (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-reductase (GRD) and non enzymatic anti oxidant glutathione (GSH) in their body which promotes the free radical generation and lipid peroxidation. The oral administration of MOME or metformin + atorvastatin significantly reduced the lipid peroxidation products and increased the enzymatic as well as non enzymatic antioxidants in the diabetic rats. There are no significant differences in the levels of lipid peroxidation products, antioxidant enzymes and glutathione content between normal rats and normal rats treated with MOME.
Previous studies showed that diabetes has a negative effect on cardiovascular functions. The diabetes associated oxidative stress causes structural and functional cardiomyopathic changes (Aksakal et al., 2011). In the present study we observed marked cardiac damage in the form of myocytosis and intestinal edema. But the administration of MOME and (metformin + atorvastatin) regains the normal histological architecture of the heart.
GC-MS profiling of MOME revealed the presence of various biologically active compounds. 1, 2-benzene dicarboxylic acid; used in the preparation of perfumes and cosmetics (Roy et al., 2011) is the major compound found in the methanol extract of Moringa oleifera leaves. Hexadecanoic acid is a strong antioxidant and hypocholesterolomic compound. Phytol is an antimicrobial, anticancer, anti-inflammatory and diuretic compound. Dl-.alpha.-Tocopherol (vitamin E) is an anti ageing, analgesic, anti diabetic, anti inflammatory, antioxidant, anti dermatitic, anti leukemic, anticancer, hepatoprotective compound present in MOME (Kumar et al., 2010). Other compounds present in MOME include heptadecanoic acid methyl ester, is an antioxidant compound (Zayed et al., 2014); 11,14,17-eicosatrienoic acid methyl ester is an anti arthritic, anti coronary, anti-inflammatory compound (Rajeswari et al., 2012); 9,12,15-Octadecatrienal is a cancer preventive, hypo-cholesterolemic, anti coronary compound; Octadecanoic acid, 2-hydroxy-1-(hydroxyl methyl) ethyl ester anti-inflammatory, hypo-cholesterolemic, cancer preventive, hepato-protective compound (Karthika et al., 2016). Most of the compounds present in the MOME were antioxidants. The antioxidant potential of MOME may due to the presence of these compounds.
5. Conclusion
The results of the present study point out that the treatment of MOME at a dose of 300 mg/kg body weight can effectively reduce hyperglycemia and associated oxidative stress in the heart of diabetic rats. The anti diabetic and anti oxidative potential of MOME is due to the presence of various biologically active compounds present in it. These findings suggest a possible protective role of Moringa oleifera against oxidative stress in the heart of diabetic rats. Further extensive studies are in progress to clarify the exact mechanism by which the MOME elicits its modulatory effect.
Declarations
Author contribution statement
S Mini: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
R Rajalakshmi: Conceived and designed the experiments; Analyzed and interpreted the data.
B.Y. Aju: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by DST/INSPIRE, New Delhi, India (DST/INSPIRE Fellowship/2014/205).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aksakal E., Akaras N., Kurt M., Tanboga I.H., Halici Z., Odabasoglu F., Bakirci E.M., Unal B. The role of oxidative stress in diabetic cardiomyopathy: an experimental study. Eur. Rev. Med. Pharmacol. Sci. 2011;15(11):1241–1246. [PubMed] [Google Scholar]
- Asmat U., Abad K., Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm. J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Cai L., Kang Y.J. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc. Toxicol. 2001;1:181–194. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 2006;12(l):60–62. doi: 10.4158/EP.12.S1.60. [DOI] [PubMed] [Google Scholar]
- David M., Richard J.S. Glutathione reductase. In: Bermeyer H.U., editor. Methods of Enzymatic Analysis. Academic Press; New York: 1983. p. 258e65. [Google Scholar]
- Disbrey Brenda D., Rack John Horsman. 1970. Histological Laboratory Methods. [Google Scholar]
- Feuvray D. Cardiac metabolism in the diabetic patient. Heart Metab. 2010;46:11–15. [Google Scholar]
- Giugliano D., Ceriello A., Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995;44(3):363–368. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Johansen J.S., Harris A.K., Rychly D.J., Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc. Diabetol. 2005 Dec;4(1):5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthika C., Yogeshwari G., Muruganantham K., Manivannan S. Phytochemical analysis of ruellia patula using gas chromatography-mass spectrometry. Phytochem. Anal. 2016;9(2) [Google Scholar]
- Kakkar P., Dos B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130e2. [PubMed] [Google Scholar]
- Kumar A., Ilavarasan R., Deecaraman M., Aravindan P., Padmanabhan N., Krishan M.R.V. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. J. Med. Plants Res. 2013;2(9):246–249. [Google Scholar]
- Kumar P.P., Kumaravel S., Lalitha C. n.d. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010 Jul 31;4(7):191–195. [Google Scholar]
- Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Compl. 2001;15(4):203–210. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- Maehly A.C., Chance B. The assay of catalases and peroxidases. In: Glick D., editor. Vol. 1. Inter Science; New York: 1954. p. 357e424. (Methods of Biochemical Analysis). [DOI] [PubMed] [Google Scholar]
- Maritim A.C., Sanders A., Watkins J.3. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003 Jan 1;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Nathan D.M., Singer D.E., Hurxthal K., Goodson J.D. The clinical information value of the glycosylated hemoglobin assay. N. Engl. J. Med. 1984;310:341e6. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- Nisoli E., Carruba M.O., Tonello C., Macor C., Federspil G., Vettor R. Induction of fatty acid translocase/CD36 peroxisome proliferator-activated receptor-γ2, leptin, uncoupling proteins 2 and 3, and tumor necrosis factor-α gene expression in human subsutaneous fat by lipid infusion. Diabetes. 2000;49:319–325. doi: 10.2337/diabetes.49.3.319. [DOI] [PubMed] [Google Scholar]
- Okhawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal. Biochem. 1979;95:351e8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Patterson J.W., Lazarow A. Determination of glutathione. In: Glick D., editor. Methods of Biochemical Analysis. Inter Science; New York: 1955. p. 259. [DOI] [PubMed] [Google Scholar]
- Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. IJBS. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- Rajeswari G., Murugan M., Mohan V.R. GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae) Res. J. Pharm. Biol. Chem. Sci. 2012;3:16. [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E. Selenium: biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588e90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Roy P., Amdekar S., Kumar A., Singh V. Preliminary study of the antioxidant properties of flowers and roots of Pyrostegia venusta (Ker Gawl) Miers. BMC Complement Altern. Med. 2011;11 doi: 10.1186/1472-6882-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., Salminen V., Unsitupa M. Prevention of type 2 mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;334:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Uemura S., Matsushita H., Li W., Glassford A.J., Asagami T., Lee K.H. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ. Res. 2001;88:1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- Ustinova E.E., Barrett C.J., Sun S.Y., Schultz H.D. Oxidative stress impairs cardiac chemoreflexes in diabetic rats. Am. J. Physiol. 2000;279:2176–2187. doi: 10.1152/ajpheart.2000.279.5.H2176. [DOI] [PubMed] [Google Scholar]
- Wang H.J., Jin Y.X., Shen W., Neng J., Wu T., Li Y.J., Fu Z.W. n.d. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression 6. Asia Pac. J. Clin. Nutr. 2007;16(Suppl 1):412–417. [PubMed] [Google Scholar]
- Wold L.E., Ceylan-isik A.F., Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol. Sin. 2005;26(8):908–917. doi: 10.1111/j.1745-7254.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Zayed M.Z., Ahmad F.B., Ho W.S., Pang S.L. GC-MS analysis of phytochemical constituents in leaf extracts of neolamarckia cadamba (rubiaceae) from Malaysia 6. Int. J. Pharm. Pharm. Sci. 2014;6(9):123–127. [Google Scholar]