Abstract
Introduction
Acute lung injury (ALI) is a severe life-threatening disease causing uncontrolled pulmonary inflammation and oxidative damage. There are still no effective therapies for this disease. The aim of this study was to evaluate the protective role of mesenchymal stem cells, moxifloxacin, sildenafil or a combination of moxifloxacin and sildenafil against hydrochloric Acid (HCl) - induced ALI.
Methods
HCl or saline was injected intra-tracheally and after 2 h, moxifloxacin, sildenafil, moxifloxacin + sildenafil or mesenchymal stem cells were injected. After 7 days, rats were sacrificed for evaluation of the blood chemistry and inflammation via determination of the level of oxidative stress markers, apoptosis and the histopathological alterations by H&E.
Results
In HCl-injected rats, there were a significant increase in total white blood cells (WBCs), lymphocytes, malondialdehyde (MDA) and caspase-3 gene expression. Also, there were a significant decrease in superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and Hemeoxygenase-1 (HO-1) gene expression in lung tissue. On the other hand, treatment of lung injured rats with mesenchymal stem cell, moxifloxacin, sildenafil or a combination of moxifloxacin and sildenafil showed a significant decrease in WBCs and lymphocytes and ameliorated the histopathological changes. MDA level in lung tissue was only significantly lowered in rats treated with moxifloxacin alone or in combination with sildenafil or MSCs. GSH was just increased in rats treated with moxifloxacin, sildenafil or with MSCs. Antioxidant parameters and gene expression of HO-1 and caspase-3 were significantly modulated in rats treated with MSCs.
Conclusion
MSCs ameliorated the toxic effects of HCl through their ability to decrease inflammation, oxidative stress, and apoptosis in acute lung injury.
Keywords: Cell biology, Biochemistry, Toxicology, Respiratory system, Pathology, Acute lung injury, Heme oxygenase-1, Sildenafil, Mesenchymal stem cells, Moxifloxacin, Caspase-3
Cell biology; Biochemistry; Toxicology; Respiratory system; Pathology; Acute lung injury; Heme oxygenase-1; Sildenafil; Mesenchymal stem cells; Moxifloxacin; Caspase-3
1. Introduction
Acute lung injury (ALI) is a serious form of lung disease. This disease has a variety of causes, including sepsis, trauma, drug toxicity, aspiration, multiple blood transfusion, ischemia, and acute pancreatitis (Standiford and Ward, 2016). ALI pathology is characterized by the destruction of the epithelium–capillary interface, the extravasation of protein-rich fluid, the release of pro-inflammatory cytokines, chemokines, and the infiltration of neutrophils. These changes lead to flooding of the alveolar spaces, which disrupts gas exchange and results in significant hypoxemia (Meng et al., 2019). Gastric aspiration is a high-risk condition for lung injury. Its effect range from subclinical pneumonitis to alveolar damage and progressive respiratory failure, according to the volume of aspirate, with fibrosis development in some patients (Ayala et al., 2018). The aspirate contents may contain low pH stomach fluid, bacteria, blood or food particles. Among the gastric contents, hydrochloric acid (HCl) has the most important impact on lung injury. Gastric aspiration often occurs in patients in the Intensive Care Unit (ICU) (Alluri et al., 2017). Aspiration also occurs in patients with altered levels of consciousness due to trauma, cerebral vascular ischemia, or metabolic encephalopathies (Mizushina et al., 2019). To date there is no effective therapy for ALI, so in this study we used sildenafil or in combination with moxifloxacin or mesencymal stem cells as therapy for the disease.
Moxifloxacin is a synthetic antibacterial agent that belongs to the fluoroquinolone family. It is well established in the treatment of pneumonia or acute exacerbation in chronic obstructive pulmonary disease (COPD) (Minov et al., 2018). The antimicrobial activity of moxifloxacin against Gram-negative and Gram-positive bacteria is based on their ability to inhibit topoisomerases (Beisswenger et al., 2014). Moreover, moxifloxacin was reported to have immunoregulatory effects via suppression of neutrophilic migration and generation of pro-inflammatory cytokine from monocytes (Lee and Chae, 2011).
Phosphodiesterase 5 PDE5 catalyzes the hydrolysis of cyclic guanosine monophosphate (cGMP). c-GMP is a second messenger and has a major role in various cellular processes, like inflammation (Gokakin et al., 2013). PDE5 inhibitors seem to be especially applicable in curing pulmonary diseases because PDE5 activities are largely increased in oxidative stress and inflammatory processes (Kosutova et al., 2018). Sildenafil is a selective inhibitor for PDE5 leading to an increase in the concentration of c-GMP and cyclic adenosine monophosphate (cAMP). Sildenafil has been demonstrated to decrease oxidative stress and the inflammatory response through the inhibition of superoxide formation (Hemmati et al., 2015).
Cell-based therapy with mesenchymal stem cells (MSCs) is a potentially attractive option for treating patients with acute lung injury due to its efficacy for reduction of the magnitude of lung injury and enhance recovery from lung injury (Matthay, 2015). MSCs are multipotent stromal cells that can be isolated from bone marrow (BM), adipose tissue, umbilical cord, placenta, periodontal ligament, and dental pulp and may differentiate into adipocytes, chondrocytes, and osteoblasts (Antoniou et al., 2018). MSCs are attractive for clinical therapy due to their ability to differentiate into a variety of cell types, provide trophic support, and modulate innate immune response (Mauri et al., 2017). The aim of the current study was to evaluate the protective role of MSCs, sildenafil, and a combination of moxifloxacin and sildenafil in acute lung injury. Moxifloxacin was used as a standard drug for lung injury. In this study, the gastric acid aspiration was induced in the rats by using hydrochloric acid (El-Hamid et al., 2017).
2. Materials and methods
2.1. Reagents
HCl was purchased from Sigma® (Cat No, H1758). Moxifloxacin® was obtained from a local pharmacy as tablets (Moxiflox, 400 mg/tablet, Eva pharma©, Egypt). Moxifloxacin tablets were ground and each 100 mg of the tablet was dissolved in 100 ml of distilled H2O. Sildenafil® was purchased from Pfizer Inc. (Sildenafil citrate, 100 mg/tablet, Pfizer, Egypt) and was dissolved in 100 ml of distilled H2O.
2.2. Isolation, culture, and expansion of bone marrow mesenchymal stem cells
Isolation and culturing of mesenchymal stem cells (MSCs) was done by following the previously reported protocols (Abdel Aziz et al., 2007; Friedenstein et al., 1970). All rats were handled according to the guidelines of the Animal Ethics Committee at Zoology Department in Faculty of Science, Mansoura University, Egypt. The rats were anesthetized by halothane, then the skin was sterilized with 70% ethyl alcohol before cutting it. The femurs and tibia of male rats were carefully cut off from adherent soft tissues. Then they were put into a sterilized falcon tube containing 70% ethyl alcohol for 1–2 min. The bones were immersed in a falcon tube with Phosphate buffered saline (Cat no. BE17-516F, Lonza, USA) for washing. The bones were taken to laminar air flow for bone marrow extraction. The two ends of the bones were cut using sterile scissors. Bone marrow was flushed by Dulbecco's modified Eagles medium (DMEM) (Cat no. BE12-719F, Lonza, Belgium) supplemented with 10% fetal bovine serum (Cat no.10270, Gibco, USA) and 1% PEN-STREP (10.000 U penicillin -10.000 μg streptomycin/ml) (Cat no. DE17-602E, Lonza, USA). The marrow plugs were cultured in 20 ml complete media and incubated at 37 °C in a 5% humidified CO2 incubator (Shel lab, USA). After 24h, the old media were discarded for removing unattached cells. MSCs were differentiated from other bone marrow cells by their ability to attach to tissue culture polystyrene (flask, 75cm2, Greiner Bio-One). Cells were subcultured by using 0.25% Trypsin/ethylenediamine-tetraacetic acid (Cat no. BE17-161E, Lonza, Belgium).
2.3. Animals
Sixty male Sprague-Dawley rats (12-weeks old) weighted 180–200g. Rats were housed at regular (12 h light/dark cycle) at thermally-controlled facility. Rats received ad libitum water and food for one week prior to the experimental work. All rats were handled according to the guidelines of the Animal Ethics Committee at Zoology Department in Faculty of Science, Mansoura University, Egypt.
2.4. Experimental design
Animals were randomly divided into six groups and each group included 10 rats (Charan and Kantharia, 2013). gp1: negative control and received saline into trachea. gp2:(positive control) lung injury was induced by injected 0.1 Normality (N) HCl in trachea (2 ml/kg) (El-Hamid et al., 2017). gp3: lung injury was induced as described in gp2 and after 2 h, rats received moxifloxacin (10 mg/kg/I.P. twice daily/week) (El-Hamid et al., 2017). gp4: lung injury was induced as described in gp2 and after 2 h, rats received sildenafil (10 mg/kg/day/I.P./week) (Hemmati et al., 2015). gp5: lung injury was induced as described in gp2 and after 2 h rats received sildenafil (10 mg/kg/day/I.P./week) and moxifloxacin (10 mg/kg/I.P. twice daily/week). gp6: lung injury was induced as described in gp2 and after 2 h, rats received BM-MSCs (1 × 106) cells suspended in 0.2 ml media intravenous via penile vein, single dose (Mauri et al., 2017). At the end of the treatment period, rats were sacrificed one week after anesthesia and blood were withdrawn in the EDTA-containing tube for blood cell count (CBC) and lung tissues were taken for biochemical study and gene expression.
2.5. Biochemical study
The lung tissues were excised from each animal, and washed with 0.9 % NaCl solution, 0.2 gram of tissue was homogenized for determination of superoxide dismutase (SOD) (Cat no. SD 2521) (Nishikimi et al., 1972), catalase (CAT) (Cat no. CA 2517) (Aebi, 1984), glutathione reduced (GSH) (Cat no. GR 2511) (Beutler, 1963) and Malondialdehyde (MDA) (Cat no. MD 2529) (Ohkawa et al., 1979). The analysis was performed according to the manufacturer's instructions.
2.6. Gene expression by real-time PCR
Real-time PCR was used to detect the gene expression of the caspase-3 and heme oxygenase-1 (HO-1). Total RNA was extracted from 0.025 g of tissues using the RNeasy® Mini Kit (Cat no. 74106) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed into cDNA using RT2SensiFAST™ cDNA Synthesis Kit (Cat. No. BIO-65053) following the manufacturer's instructions. Real-time PCR was performed using SensiFAST™ SYBR® No-ROX Kit (Cat. No. BIO-52066). The reaction mixture contained 10 μl SensiFAST SYBR® No-ROX mix (2x), 1 μl reverse primer, 1 μl forward primer (Table 1), 3 μl cDNA product and 5 μl of nuclease-free water. The amplification of the gene was performed by using the following: initial denaturation at 95 °C for 3 min, (denaturation at 94 °C for 20 s, annealing at 58 °C for 30 s and extension at 60 °C for 30 s 40 cycles). Then the samples were subjected to PIKOREAL96 Real-time thermal cycler (Thermo Fisher, USA). Target gene expression was normalized to the housekeeping gene GAPDH (Table 1). The relative expression of each target gene was calculated according to 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Primer sequence that used in real-time PCR.
| The gene | Sequences (5′-3′) | Accession No. | Product Size (bp) |
|---|---|---|---|
| GAPDH | F: TGACTTCAACAGCAACTCCCA R: AGGGCCTCTCTCTTGCTCTC |
NM_017008.4 | 211 |
| Caspase-3 | F: GGCCGACTTCCTGTATGCTT R: CGTACAGTTTCAGCATGGCG |
NM_012922.2 | 110 |
| Hemoxygenas-1(HO-1) | F: TCACCTTCCCGAGCATCGAC R: TCACCCTGTGCTTGACCTCG |
NM_012580.2 | 99 |
2.7. Histopathological analysis
Sections of lung tissues (5 μm) were prepared for hematoxylin and eosin (H&E). The stained sections were evaluated by a blind pathologist for evaluation of any histopathological abnormalities using a light microscope (Leica Microsystems, Germany).
2.8. Statistical analysis
Statistical analysis was carried out by Statistical Package for the Social Sciences SPSS program (version 19; SPSS, Chicago, Illinois, USA. All values were presented as mean ± standard error of the mean (SEM). Differences were considered to be significant at p < 0.05. One-way analysis of variance (ANOVA) and post-hoc test were used to determine differences between groups (Snedecor and Cochran, 1980).
3. Results
3.1. MSC-treated, as well as the drug-treated, decreased the number of white blood cells (WBCs) and lymphocytes
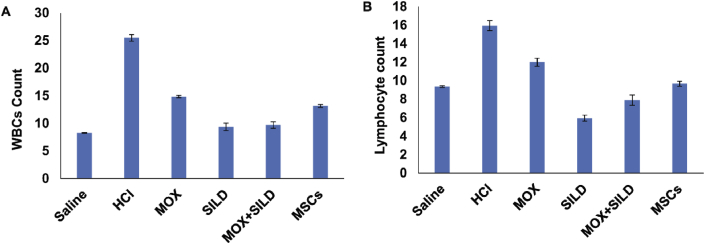
Rats injected with HCl (gp1) alone showed a significant increase in both WBCs and lymphocytes in comparison to the rats treated with any of the drugs or MSCs (p < 0.05) (Table 2) (Fig. 1).
Table 2.
White blood cells and lymphocytes in control and treated groups. MOX = moxifloxacin, SILD = Sildenafil, MSCs = Mesenchymal stem cells. Bold p values means that p < 0.05.
| Groups | gp1a | gp2b | gp3c | gp4d | gp5e | gp6f | P value |
|---|---|---|---|---|---|---|---|
| Treatment | Saline | HCl | MOX | SILD | MOX + SILD | MSCs | |
| WBCs | 8.27 ± 0.07 | 25.48 ± 0.6 | 14.85 ± 0.21 | 9.35 ± 0.66 | 9.7 ± 0.57 | 13.17 ± 0.27 | a vs b, p = 0.004 b vs c, p = 0.000 b vs d, p = 0.001 b vs e, p = 0.001 b vs f, p = 0.002 |
| Lymphocyte | 9.36 ± 0.09 | 15.94 ± 0.55 | 12 ± 0.43 | 5.95 ± 0.31 | 7.9 ± 0.55 | 9.66 ± 0.28 | a vs b, p = 0.001 b vs c, p = 0.001 b vs d, p = 0.002 b vs e, p = 0.001 b vs f, p = 0.001 |
Fig. 1.
(A) There was a significant decrease in WBCs count in different treated groups (p < 0.05) in all treated groups in comparison to HCl-injected group. (B) There was a significant decrease in lymphocyte count in different treated groups (p < 0.05) in comparison to HCl-injected group. MOX = moxifloxacin, SILD = Sildenafil, MSCs = Mesenchymal stem cells.
3.2. Antioxidant and oxidative stress parameters were improved in treated groups
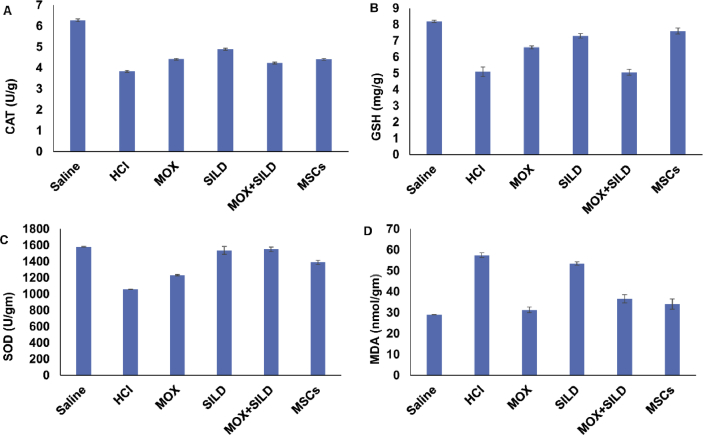
In a moxifloxacin-treated rats (gp3), the activity of catalase (CAT), level of reduced glutathione (GSH) and activity of superoxide dismutase (SOD) were significantly increased (p < 0.05) in comparison to HCl-injected group (gp2). Sildenafil treatment of lung injury led to a significant increase in the activities of SOD (p = 0.001), CAT (p = 0.001) and the content of reduced glutathione GSH (p = 0.001) (Table 3) while no significant change in the level of MDA (p = 0.5).
Table 3.
CAT, GSH, SOD and MDA levels in lung tissues in control and different treated groups. MOX = moxifloxacin, SILD = Sildenafil, MSCs = Mesenchymal stem cells. Bold p values means that p < 0.05.
| Groups | gp1a | gp2b | gp3c | gp4d | gp5e | gp6f | P value |
|---|---|---|---|---|---|---|---|
| Treatment | Saline | HCl | MOX | SILD | MOX + SILD | MSCs | |
| CAT (U/g) | 6.28 ± 0.07 | 3.84 ± 0.05 | 4.42 ± 0.04 | 4.9 ± 0.05 | 4.24 ± 0.041 | 4.41 ± 0.042 | a vs b, p = 0.001 b vs c, p = 0.002 b vs d, p = 0.001 b vs e, p = 0.003 b vs f, p = 0.001 |
| GSH (mg/g) | 8.2 ± 0.07 | 5.09 ± 0.29 | 6.6 ± 0.09 | 7.3 ± 0.14 | 5.06 ± 0.18 | 7.6 ± 0.19 | a vs b, p = 0.001 b vs c, p = 0.004 b vs d, p = 0.001 b vs e, p = 1.000 b vs f, p = 0.001 |
| SOD (U/gm) | 1581.7 ± 3.1 | 1058.4 ± 2.2 | 1232 ± 8.5 | 1536.6 ± 49.48 | 1549.9 ± 29.5 | 1388.3 ± 27.4 | a vs b, p = 0.001 b vs c, p = 0.002 b vs d, p = 0.001 b vs e, p = 0.002 b vs f, p = 0.001 |
| MDA (nmol/gm) | 28.9 ± 0.11 | 57.5 ± 1.13 | 31.3 ± 1.35 | 53.44 ± 0.72 | 36.57 ± 1.95 | 34.05 ± 2.41 | a vs b, p = 0.002 b vs c, p = 0.001 b vs d, p = 0.5 b vs e, p = 0.001 b vs f, p = 0.001 |
The SOD and CAT activities were attenuated in the rats treated with both Sildenafil and Moxifloxacin (gp5) (Table 3) (p = 0.002, p = 0.003). Moreover, the combination treatment decreased the level of MDA (p = 0.001) without a significant change in the level of GSH. The rats that were treated with MSCs (gp6) showed a significant amelioration for the negative effect caused by HCl injection via the increase in the activities of both CAT and SOD with a significant increase in the level of GSH and a decrease in the level of MDA in lung tissue (p = 0.001) (Fig. 2).
Fig. 2.
(A) There was a significant increase in CAT activity in different treated group in comparison to HCl-injected group (p < 0.05). (B) There was a significant increase in GSH in moxifloxacin, sildenafil and MSCs treated groups (p < 0.05) in comparison to HCl-treated group but there was no significant change in combination (moxifloxacin + sildenafil) treated group (p = 1.000). (C) SOD activity was significantly increased in different treated group in comparison to HCl-injected group (p < 0.05). (D) The level of MDA was significantly decreased in moxifloxacin, combination (moxifloxacin + sildenafil) and MSCs treated groups but there was no significant change in sildenafil treated group (P = 0.5). MOX = moxifloxacin, SILD = Sildenafil, MSCs = Mesenchymal stem cells.
3.3. MSCs induced the expression of HO-1 and Caspase-3 genes
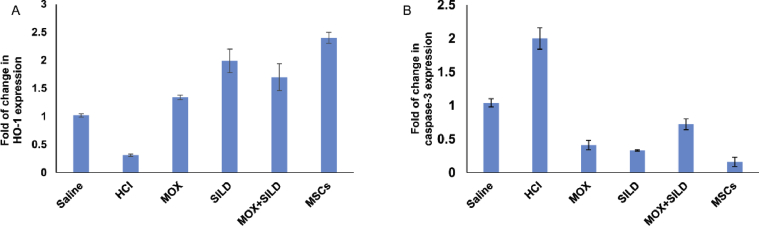
The relative expression of hemeoxygease-1 (HO-1) was significantly increased in Moxifloxacin (gp3, p = 0.002), sildenafil (gp4, p = 0.001), in combination treatment (gp5, p = 0.002) as well as in the MSCs treated rats (gp6, p = 0.001). The relative expression of caspase-3 was significantly decreased in Moxifloxacin (gp3, p = 0.001), sildenafil (gp4, p = 0.002), in combination treatment (gp5, p = 0.001) as well as in the MSCs treated rats (gp6, p = 0.001) (Table 4) (Fig. 3).
Table 4.
Fold of change in Hemeoxygenase-1 and Caspase-3 expression in the different treated groups. MOX = moxifloxacin, SILD = sildenafil, MSCs = mesenchymal stem cells. Bold p values means that p<0.05.
| Groups | gp1anormal | gp2b | gp3c | Gp4d | Gp5e | Gp6f | P value |
|---|---|---|---|---|---|---|---|
| Treatment | Saline | HCl | MOX | SILD | MOX + SILD | MSCs | |
| HO-1 | 1.02 ± 0.03 | 0.31 ± 0.02 | 1.34 ± 0.04 | 1.99 ± 0.21 | 1.7 ± 0.24 | 2.4 ± 0.1 | a vs b, p = 0.004 b vs c, p = 0.002 b vs d, p = 0.001 b vs e, p = 0.002 b vs f, p = 0.001 |
| Caspase-3 | 1.04 ± 0.06 | 2 ± 0.16 | 0.41 ± 0.07 | 0.33 ± 0.01 | 0.72 ± 0.08 | 0.16 ± 0.07 | a vs b, p = 0.003 b vs c, p = 0.001 b vs d, p = 0.002 b vs e, p = 0.001 b vs f, p = 0.001 |
Fig. 3.
(A) The relative expression of (HO-1) was significantly increased in different treated group (p < 0.05). (B) The relative expression of caspase-3 was significantly decreased in different treated group in comparison to HCl-injected group (p < 0.05).
3.4. Histopathological findings in the lung tissues
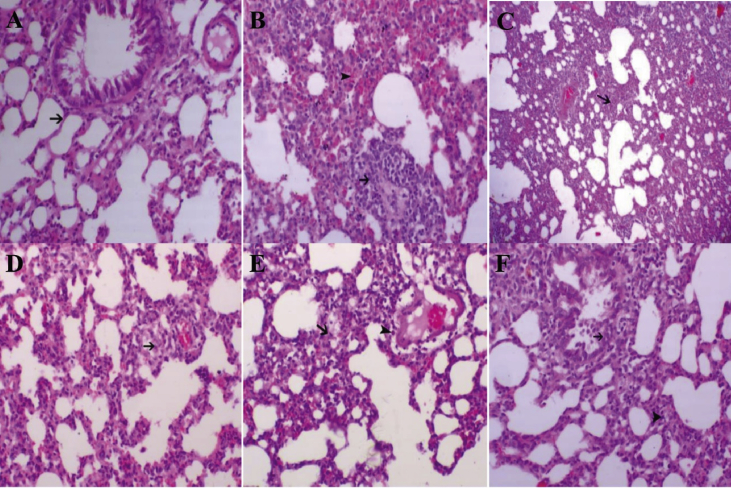
In our present study, the histological examination of the lung tissue in control group showed normal lung tissues without hemorrhage or inflammation (Fig. 4A) the positive control group (HCl-injected rats) showed presence of vasculitis (black arrow) and hemorrhage was noticed within the alveoli or within the perivascular areas (arrowhead) (Fig. 4B) in comparison to the healthy rats (Fig. 4A). On the other hand, moxifloxacin led to an increase in the alveolar spaces and a moderate decrease of interstitial thickening (black arrow) (Fig. 4C).
Fig. 4.
Morphologic changes of rat lung. (A) Control showing normal lung tissue (HE, 200x). (B) HCl-injected rats showing vasculitis (black arrow) and alveolar hemorrhage (arrowhead) (HE, 200x). (C) rats treated with moxifloxacin standard drug after induction of lung injury with HCl showed an increase in the alveolar spaces and a moderate decrease of interstitial thickening (black arrow) (HE, 40x). (D) rats treated with sildenafil standard drug after induction of lung injury with HCl showed a marked decrease in inter-alveolar thickening with minute hemorrhage (black arrow) (HE, 200x). (E) rats treated with a combination of moxifloxacin and sildenafil after induction of lung injury with HCl showed a decrease in inter-alveolar thickening (black arrow) and mild perivascular inflammatory cell infiltration (Arrowhead) (HE, 200x). (F) rats treated with MSCs after induction of lung injury with HCl showed a marked decrease inter-alveolar thickening (arrowhead) and marked bronchial lining epithelium hyperplasia (black Arrow) (HE, 200x).
Our results in sildenafil-treated group showed a marked decrease in inter-alveolar thickening with minute hemorrhage (black arrow (Fig. 4D). The mixed treated group showed a marked decrease in necrotic, vascular and infiltrative lesions and a marked increase in alveolar spaces (Fig. 4E). In MSCs-treated group showed a marked increase in the ventilation spaces and a decrease in the interstitial fibrosis, inflammatory cells infiltration (Fig. 4F).
4. Discussion
Acute lung injury is characterized by severe inflammatory response leading to deterioration of gas exchange and till now there is no effective therapy for ALI. The main model to induce acute lung injury in animals is the administration of hydrochloric acid by intra-tracheal route according to Sahin et al. (2011). In our study, the lung injury was induced by applying the HCl model followed by administration of mesenchymal stem cells and compared their activities to the standard drugs for ALI treatment. There was an increase in WBCs count in positive group (HCl-injected) compared to other treated groups. The results are in agreement with the study of Imam et al. and (Imam et al., 2015) this increase may be due to the acute inflammation leading to leukocytes migration and damaged tissues. In moxifloxacin treated group, WBCs count decreased and this is due to the modulation of the immune system in addition to its antimicrobial properties. The same findings were reported by Müller et al. (Müller-Redetzky et al., 2014). On the other hand, the most significant decrease in WBCs was found to be in sildenafil-treated group. The result is due to the inhibitory effect of sildenafil through the elevation of cyclic-guanosine monophosphate (cGMP) level. Moreover, Wang et al. showed that sildenafil was able to activate cGMP in acrolein-induced airway inflammation rat model (Wang et al., 2009).
In the mixed treated group WBCs count decreased due to the dual action of moxifloxacin and sildenafil. In MSCs-treated group, the level of WBCs decreased. It has been reported that bone marrow mesenchymal stem cells have an anti-inflammatory activity by reducing the influx of inflammatory cells in the injured tissue as reported in an endotoxin-induced ALI model in mice (Hao et al., 2015). Lymphocytes play a necessary role in the regulation of inflammation in different lung diseases. Our results showed that HCl-injected group increased in the count of lymphocytes due to pulmonary injury and dysfunction. While the different treatment groups showed a decrease. Our results are in agreement with Imam et al. (2015) but in the same time are in contrary to El-Hamid et al. (2017). The decrease may be due to inhibition of lymphocytes infiltration in inflamed lung tissues in different treatment groups.
Antioxidant proteins (enzymatic (SOD and CAT) or non-enzymatic (GSH)) decrease the level of reactive oxygen species (ROS) (Pejic et al., 2006). Different reports confirmed the correlation between oxidative stress and the development of lung injury (Choi and Alam, 1996). In this study, HCl induced lung injury via the decrease in the level of SOD, CAT, and GSH. Moreover, HCl increased lipid peroxidation product (MDA). Sun et al., 2017 showed that lung injury was induced by decreasing the antioxidant parameters and increasing the MDA level in the sepsis model (Sun et al., 2017). On the other hand, the tested standard drugs in this study, as well as MSCs, ameliorated the injury induced by HCl via the attenuation of the level of antioxidant parameters and MDA. These findings may be due to the ability of the standard drugs and MSCs to modulate the level of free radicals as described by other researchers (El-Hamid et al., 2017; Gokakin et al., 2013; Shen et al., 2018). Furthermore, MSCs were reported to improve the level of cysteine and GSH (Iyer et al., 2010). Sildenafil, as a standard drug for ALI treatment, decreases the oxidative stress and inhibits the inflammation (Gokakin et al., 2013). Moxifloxacin has immunoregulatory effects, including suppression of neutrophilic migration and generation of a proinflammatory cytokine from monocytes (El-Hamid et al., 2017).
Hemeoxygenase-1 (HO-1) is a key player in the cellular antioxidant system (Choi and Alam, 1996). HO-1 is the rate-limiting enzyme in the heme degradation pathway and at the same time, it was found that its expression can be induced in response to oxidative stress. Here in this study, HCl induced the lung injury by decreasing the gene expression of HO-1 while its effect was ameliorated by MSCs as well as the standard drugs either alone or in combination. Zhang et al., 2017 showed that BM-MSCs upregulate the level of HO-1 leading to a decrease in ROS production (Z. H. Zhang et al., 2017). Zhang et al., 2019 explained the indirect mechanism of MSCs that trigger overexpression of Nrf2 gene and over expression of the antioxidant HO-1 (L. Zhang et al., 2019). Additionally, MSCs and standard drugs were able to decrease the lung injury via the downregulation of caspase-3 expression leading to the protection of alveolar epithelial cells from apoptosis (Kadry and Abdel-Megeed, 2017; Liu et al., 2018). Our result showed that the most significant decrease of caspase-3 in MSCs-treated group and this due to secretion of growth factors by MSCs, including Hepatocyte growth factor (HGF). HGF is a multifunctional factor that promotes angiogenesis and reduces apoptosis by improvement of B-cell lymphoma 2 (Bcl-2) expression and reduction of caspase-3 expression (Jiang et al., 2015).
In our present study, the histological examination of the lung tissue showed and confirmed the effectiveness of the MSCs and the standard drugs in improving the lung pathology. HCl induced lung pathological alteration via the recruitment of polymorphonuclear neutrophils (PMNs) (El-Hamid et al., 2017) while moxifloxacin improved the pathological alteration via its immunomodulatory activity and preventing the infiltration and migration of neutrophils (El-Hamid et al., 2017). It has been reported that sildenafil modulates the immune cell response via the regulation cGMP pathway and protein kinase G (Shih et al., 2013). The improvement in lung pathology after MSCs treatment could be due to the ability of MSCs to migrate into lung tissue and to promote tissue repair (Zheng et al., 2016). In conclusion, the present study suggests that BM-MSCs could limit HCl-induced acute lung injury by modulation inflammation, oxidative stress and apoptosis compared with other drugs.
Declarations
Author contribution statement
R. El-Demerdash, S. El-Metwaly, F. El-Senduny and A.F. Abdel-Aziz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdel Aziz M.T., Atta H.M., Mahfouz S., Fouad H.H., Roshdy N.K., Ahmed H.H., Hasan N.M. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem. 2007;40(12):893–899. doi: 10.1016/j.clinbiochem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase assay. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alluri R., Kutscher H.L., Mullan B.A., Davidson B.A., Knight P.R. Open tracheostomy gastric acid aspiration murine model of acute lung injury results in maximal acute nonlethal lung injury. J. Vis. Exp. 2017;(120) doi: 10.3791/54700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou Katerina M., Karagiannis Konstantinos, Tsitoura Eliza, Bibaki Eleni, Lasithiotaki Ismini, Proklou Athanasia, Tzanakis Nikos. Clinical applications of mesenchymal stem cells in chronic lung diseases. Biomed. Rep. 2018;8(4):314–318. doi: 10.3892/br.2018.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala Pedro, Vivar Raúl, Montalva Rebeca, Olmos Pablo, Meneses Manuel, Borzone Gisella R. Elastin degradation products in acute lung injury induced by gastric contents aspiration. Respir. Res. 2018;19(1):165. doi: 10.1186/s12931-018-0873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger C., Honecker A., Kamyschnikow A., Bischoff M., Tschernig T., Bals R. Moxifloxacin modulates inflammation during murine pneumonia. Respir. Res. 2014;15(1):82. doi: 10.1186/1465-9921-15-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler Ernest. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Charan Jaykaran, Kantharia N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013;4(4):303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A.M., Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996;15(1):9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- El-Hamid SM Abd, El-Demerdash R.S., Arafat H.F.H., Sadeek S.A. Spectroscopic studies and thermal analysis of mononuclear metal complexes with moxifloxacin and 2, 2′-bipyridine and their effects on acute lung injury induced by hydrochloric acid in rats. J. Mol. Struct. 2017;1149:613–625. [Google Scholar]
- Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell Prolif. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Gokakin Ali Kagan, Deveci Koksal, Kurt Atilla, Karakus Boran Cihat, Duger Cevdet, Tuzcu Mehmet, Topcu Omer. The protective effects of sildenafil in acute lung injury in a rat model of severe scald burn: a biochemical and histopathological study. Burns. 2013;39(6):1193–1199. doi: 10.1016/j.burns.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Hao Q., Zhu Y.G., Monsel A., Gennai S., Lee T., Xu F., Lee J.W. Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cell. Transl. Med. 2015;4(7):832–840. doi: 10.5966/sctm.2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati Ali-Asghar, Kazemi Mohsen, Pashmforoosh Marziyeh, Rajabi-Vardanjani Hossein, Shabib Somayeh, Nili-Ahmadabadi Amir, Larki Amir. 2015. Sildenafil, a Selective Inhibitor of Type 5 Phosphodiesterase, Attenuates Bleomycin-Induced Lung Fibrosis in Rat. Paper Presented at the Biological Forum. [Google Scholar]
- Imam Faisal, Al-Harbi Naif O., Al-Harbi Mohammed M., Ansari Mushtaq Ahmad, Zoheir Khairy MA., Iqbal Muzaffar, Ahmad Sheikh Fayaz. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015;102:1–11. doi: 10.1016/j.phrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Iyer S.S., Torres-Gonzalez E., Neujahr D.C., Kwon M., Brigham K.L., Jones D.P., Rojas M. Effect of bone marrow-derived mesenchymal stem cells on endotoxin-induced oxidation of plasma cysteine and glutathione in mice. Stem Cell. Int. 2010;2010:868076. doi: 10.4061/2010/868076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Xinping, Jiang Xin, Qu Chao, Chang Pengyu, Zhang Chu, Qu Yaqin, Liu Yongjun. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy. 2015;17(5):560–570. doi: 10.1016/j.jcyt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Kadry Mai O., Abdel-Megeed Rehab M. Bone marrow-derived mesenchymal stem cells mitigate caspase-3 and 8-hydroxy proline induced via β-adrenergic agonist in pulmonary injured rats. J. Biochem. Mol. Toxicol. 2017;31(8):e21913. doi: 10.1002/jbt.21913. [DOI] [PubMed] [Google Scholar]
- Kosutova P., Mikolka P., Balentova S., Kolomaznik M., Adamkov M., Mokry J., Mokra D. Effects of phosphodiesterase 5 inhibitor sildenafil on the respiratory parameters, inflammation and apoptosis in a saline lavage-induced model of acute lung injury. JPP. 2018;(5):15. doi: 10.26402/jpp.2018.5.15. [DOI] [PubMed] [Google Scholar]
- Lee Young-Man, Chae Whi-Gun. Ameliorating effects of moxifloxacin on endotoxin-induced acute lung injury in rats. J. Life Sci. 2011;21(8):1100–1108. [Google Scholar]
- Liu Y.E., Tong C.C., Zhang Y.B., Cong P.F., Shi X.Y., Liu Y., Hou M.X. Chitosan oligosaccharide ameliorates acute lung injury induced by blast injury through the DDAH1/ADMA pathway. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak Kenneth J., Schmittgen Thomas D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matthay Michael A. Therapeutic potential of mesenchymal stromal cells for acute respiratory distress syndrome. Ann. Am. Thorac. Soc. 2015;12(Supplement 1):S54–S57. doi: 10.1513/AnnalsATS.201406-254MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri T., Zambelli V., Cappuzzello C., Bellani G., Dander E., Sironi M., Pesenti A. Intraperitoneal adoptive transfer of mesenchymal stem cells enhances recovery from acid aspiration acute lung injury in mice. Intens. Care Med. Exp. 2017;5(1):13. doi: 10.1186/s40635-017-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Xiangli, Hu Lin, Li Wenqiang. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedeberg's Arch. Pharmacol. 2019 doi: 10.1007/s00210-019-01680-9. [DOI] [PubMed] [Google Scholar]
- Minov Jordan, Stoleski Sasho, Petrova Tatjana, Vasilevska Kristin, Mijakoski Dragan, Bislimovska-Karadzhinska Jovanka. Moxifloxacin in the outpatient treatment of moderate exacerbations of chronic obstructive pulmonary disease. Open Access Macedonian J. Med. Sci. 2018;6(11):2017–2022. doi: 10.3889/oamjms.2018.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushina Yoshiko, Karasawa Tadayoshi, Aizawa Kenichi, Kimura Hiroaki, Watanabe Sachiko, Kamata Ryo, Koyama Shinichiro. Inflammasome-independent and atypical processing of IL-1β contributes to acid aspiration–induced acute lung injury. J. Immunol. 2019;203(1):236–246. doi: 10.4049/jimmunol.1900168. [DOI] [PubMed] [Google Scholar]
- Müller-Redetzky H.C., Wienhold S.M., Berg J., Hocke A.C., Hippenstiel S., Hellwig K., Rückert J. Moxifloxacin is not anti-inflammatory in experimental pneumococcal pneumonia. J. Antimicrob. Chemother. 2014;70(3):830–840. doi: 10.1093/jac/dku446. [DOI] [PubMed] [Google Scholar]
- Nishikimi M., Appaji N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa Hiroshi, Ohishi Nobuko, Yagi Kunio. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pejic S., Kasapovic J., Todorovic A., Stojiljkovic V., Pajovic S.B. Lipid peroxidation and antioxidant status in blood of patients with uterine myoma, endometrial polypus, hyperplastic and malignant endometrium. Biol. Res. 2006;39(4):619–629. [PubMed] [Google Scholar]
- Sahin S.H., Kanter M., Ayvaz S., Colak A., Aksu B., Guzel A., Ozcan A. The effect of hyperbaric oxygen treatment on aspiration pneumonia. J. Mol. Histol. 2011;42(4):301–310. doi: 10.1007/s10735-011-9334-6. [DOI] [PubMed] [Google Scholar]
- Shen Y., Jiang X., Meng L., Xia C., Zhang L., Xin Y. Transplantation of bone marrow mesenchymal stem cells prevents radiation-induced artery injury by suppressing oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2018;2018:5942916. doi: 10.1155/2018/5942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Pin-Keng, Cheng Chih-Mei, Li Hsien-Pin, Huang Meei-Feng, Chiu Chia-Wei, Chen Jian-Xun, Chou Shah-Hwa. Pretreatment with sildenafil alleviates early lung ischemia-reperfusion injury in a rat model. J. Surg. Res. 2013;185(2):e77–e83. doi: 10.1016/j.jss.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Snedecor George W., Cochran William G. seventhedition. Iowa State University Press, Ames, IA; Ames: 1980. Statistical Methods. [Google Scholar]
- Standiford Theodore J., Ward Peter A. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl. Res. : J. Lab. Clin. Med. 2016;167(1):183–191. doi: 10.1016/j.trsl.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Wu Q., Zhang X., He Q., Zhao H. Mechanistic evaluation of the protective effect of carnosine on acute lung injury in sepsis rats. Pharmacology. 2017;100(5-6):292–300. doi: 10.1159/000479879. [DOI] [PubMed] [Google Scholar]
- Wang T., Liu Y., Chen L., Wang X., Hu X.R., Feng Y.L., Wen F.Q. Effect of sildenafil on acrolein-induced airway inflammation and mucus production in rats. Eur. Respir. J. 2009;33(5):1122–1132. doi: 10.1183/09031936.00055908. [DOI] [PubMed] [Google Scholar]
- Zhang Lichun, Li Qiuhe, Liu Wei, Liu Zhenning, Shen Haitao, Zhao Min. Mesenchymal stem cells alleviate acute lung injury and inflammatory responses induced by paraquat poisoning. Med. Sci. Monit. : Int. Med. J. Exp. Clin. Res. 2019;25:2623–2632. doi: 10.12659/MSM.915804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.H., Zhu W., Ren H.Z., Zhao X., Wang S., Ma H.C., Shi X.L. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res. Ther. 2017;8(1):70. doi: 10.1186/s13287-017-0524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.L., Cai W.W., Zhou S.G., Xu L.M., Jiang C.X. Protective effect of bone marrow derived mesenchymal stem cells in lipopolysaccharide-induced acute lung injury mediated by claudin-4 in a rat model. Am. J. Tourism Res. 2016;8(9):3769–3779. [PMC free article] [PubMed] [Google Scholar]