Abstract
Indonesian cassia (Cinnamomum burmannii Blume) is commonly used as a condiment. It reportedly contains a number of major phytochemical constituents such as trans-cinnamaldehyde and coumarin. Sappan wood (Caesalpinia sappan) is a native plant of Southeast Asia that contains brazilin, a widely known red pigment. This study aimed to determine the optimal extraction conditions using a choline chloride-glycerol (ChCl-glycerol)-based natural deep eutectic solvent (NADES) to obtain greater trans-cinnamaldehyde and brazilin levels from Indonesian cassia and sappan wood. The powders of Indonesian cassia and sappan wood were extracted using ChCl-glycerol-based NADES varied at three different levels: ratio of ChCl to glycerol, ratio of powder to NADES, and the amount of water in NADES. All variables were designed using the Box-Behnken design of response surface methodology to provide 15 extraction conditions. The extraction was performed using ultrasonication-assisted extraction for 30 and 50 min for Indonesian cassia and sappan wood, respectively. Determination of the active compound contents was performed using a high-performance liquid chromatography system equipped with a UV-VIS detector at λmax = 280 nm. The optimization results revealed that the highest levels of trans-cinnamaldehyde, coumarin, and brazilin in NADES extracts were 1907.32, 1735.68, and 368.67 μg/ml, respectively, whereas the lowest levels of these compounds were 453.59, 616.76, and 74.21 μg/ml, respectively. The maximal levels exceeded those obtained using a conventional extraction method, in which 5000 μg/ml Indonesian cassia reflux extract contained only 108.45 μg/ml trans-cinnamaldehyde. Similarly, 1000 μg/ml sappan wood contained only 124.64 μg/ml brazilin. ChCl-glycerol-based NADES was suitable for extracting active compounds from Indonesian cassia and sappan wood; moreover, this solvent is more effective than organic ethanolic coventional solvent.
Keywords: Chromatography, Natural product chemistry, Green chemistry, Pharmaceutical chemistry, Natural product, Indonesian cassia, Sappan wood, Trans-cinnamaldehyde, Coumarin, Brazilin, Response surface methodology, HPLC
Chromatography; Natural product chemistry; Green chemistry; Pharmaceutical chemistry; Natural product; Indonesian cassia, Sappan wood, Trans-cinnamaldehyde, Coumarin, Brazilin, Response surface methodology, HPLC.
1. Introduction
Indonesian cassia (Cinnamomum burmannii) and sappan wood (Caesalpinia sappan) are ingredients of various traditional health drinks that are widely consumed in Indonesia (Emilda, 2018). Moreover, Indonesia is the largest source of Indonesian cassia, as this commodity supplies 19.5% of all cinnamon requirements worldwide (Setyowati et al., 2018). Cinnamon bark can be found on the market in a dry roll form that is used as a condiment and flavoring agent (Al-Dhubiab, 2012). Cinnamon has a number of benefits against type 2 diabetes mellitus (T2DM) through various mechanisms including the activation of glucose transporter type 4 (GLUT4) (Cao et al., 2007) and inhibitory activity against α-glycosidase (Kim et al., 2006). Furthermore, a double-blind randomized controlled trial performed by Anderson et al. (2016) revealed that standardized cinnamon extract decreased both fasting and postprandial blood sugar levels.
Indonesian cassia contains a number of phytochemical constituents such as burmanol, coumarin, and vanillin (Li et al., 2012). The most common essential oil constituent from cinnamon is trans-cinnamaldehyde (Rao and Gan, 2014). This bioactive compound has been used for various purposes in the food industry such as flavoring and essence (Zhu et al., 2017). This compound is an aldehyde featuring a phenyl ring substitution. Trans-cinnamaldehyde is an acrolein derivative. An aldehyde transition to acrolein causes a shift to the visible wavelength region to produce a colored compound (Ashakirin et al., 2017). Coumarin, a type of benzopyrone, consists of an α-pyrone ring bound to a benzene ring. It was first isolated from Tonka beans (Dipteryx odorata). Initially, coumarin was mistakenly considered a benzoic acid compound. After being isolated by Guibourt, it was realized that the compound was not benzoic acid, and it was recognized as coumarine. The term coumarin was used when the compound was successfully synthesized for the first time (Srikrishna et al., 2018). It was reported that coumarin compounds are present in approximately 150 plant species from more than 30 different genera (Venugopala et al., 2013).
Sappan wood is a native plant in Southeast Asia. The most frequently used part of sappan is the heartwood, which contains water-soluble dyes such as brazilin, protosappanin, sappan chalcone, and hematoxylin. The dyes can be easily obtained by extracting heartwood chips using boiling water (Bechtold, 2009). Homoisoflavonoid compounds such as brazilin represent the major phytochemical components of sappan wood. Additionally, sappan wood also contains dibenzoxin derivatives such as protosappanin and its isomers (Wagner et al., 2016). Similarly, as cinnamon, sappan wood has beneficial effects against T2DM. It was reported that brazilin has inhibitory activity against hepatic gluconeogenesis (You et al., 2005). In vitro studies found that sappan wood extract has inhibitory activity against α-glucosidase (IC50 = 300.52 ± 1.467 μg/ml) (Tulin et al., 2017) and dipeptidyl peptidase-4 (Setyaningsih et al., 2019).
To obtain beneficial effects from natural products, a strategy for separating active compounds from the natural product matrix via extraction is needed. Approaches involving conventional extraction methods usually require a large amount of solvents, including organic solvents, and a long extraction process (Zhang et al., 2018). In addition, various organic solvents carry risks to human health and the environment. These issues facilitated the discovery of “green solvents” to replace organic solvents, especially those originating from fossil fuels (Häckl and Kunz, 2018). Ionic liquid (IL) and deep eutectic solvent (DES) are the main types of green solvents. Both IL and DES contain ionic salts featuring high salvation power, high stability, and low volatility, resulting in low environmental emissions (Clarke et al., 2018). In particular, DES exhibits deep eutectic behavior (decrease in melting point) after mixing the ionic salt with other components. Therefore, it can be prepared on an “ephemeral” basis when needed (Häckl and Kunz, 2018). Natural deep eutectic solvent (NADES) can be produced by replacing components in DES that act as hydrogen bond donors (HBDs) with natural products to improve the safety of the green solvent (Panić et al., 2019).
A number of studies illustrated that NADES can extract active constituents from Morus alba (Alishlah et al., 2019), Coffea canephora (Yuniarti et al., 2019), and Catharanthus roseus (Dai et al., 2016). The advantages of NADES include its easy application, relatively inexpensive costs, ready-to-use formulation, and biodegradable nature. Moreover, it can increase the amounts of active compounds extracted (Liu et al., 2018). NADES can thus serve as the basis of extremely efficient extraction methods; however, optimization is required because these methods have a number of process variables that influence the final results obtained. This study is performed to optimize the NADES for extraction of bioactive substances from Cinnamomum burmannii barks and Caesalpinia sappan woods.
2. Materials and methods
2.1. Materials
Indonesian cassia bark was obtained from a local market in Padang, West Sumatra, Indonesia, whereas sappan wood was originally obtained from Magelang, Central Java, Indonesia, each sample obtained was 2 kg. Both samples were authenticated by Herbarium Bogoriense. The samples were cleaned, impurities were removed, and the samples were then powdered using an electric blender. The obtained powders then were filtered through sieve mesh No. 40 and stored in desiccator cabinets at room temperature until further analysis.
2.2. NADES preparation
NADES was prepared as described by Yuniarti et al. (2019) with some modifications. NADES was prepared by mixing choline chloride (ChCl), which acts as a hydrogen bond acceptor (HBA), with glycerol, which serves as an HBD, in a beaker glass using a magnetic stirrer (IKA® C-MAG HS 7, USA) at 80 °C with the speed scale set at No. 3. The Beaker glass was covered with Parafilm and then allowed to stand for approximately 1 h or until the mixture turned into a clear liquid solution, after which it was used immediately. ChCl was bought from Xi'an Rongsheng Biotechnology Co., Ltd. (Xi'an, China). Glycerol was purchased from PT. Brataco (Indonesia). Double-distilled water (ddH2O) was purchased from PT. Ikapharmindo Putramas (Indonesia).
2.3. Preparation of NADES extract
The NADES extraction process was performed using an ultrasonicator (Krisbow, China) with power 35 W that provide frequency 42.000 Hz. The powder samples were added to vials and then mixed with ddH2O as described in Table 1. NADES was then added to the vial. Ultrasonication-assisted extraction was performed for 30 and 50 min for cinnamon and sappan wood, respectively (Li et al., 2015; Xia et al., 2017). The mixtures were transferred to centrifuge tubes and then centrifuged (Hettich Universal 320 Centrifuge, Germany) for 10 min at 3.283 g to separate the NADES liquid extract from the waste. The liquid layer solution was then filtered using 0.45 μm Whatman micropore filter paper. The filtrate was collected and stored in a refrigerator at −20 °C until further analysis.
Table 1.
The experimental design for optimizing the extraction process using choline chloride-glycerol-based natural deep eutectic solvent (NADES) to extract bioactive substances from cinnamon and sappan wood.
| Extraction parameters | Units | Symbol | Range and level |
|||||
|---|---|---|---|---|---|---|---|---|
| Low |
Medium |
High |
||||||
| A | B | A | B | A | B | |||
| Ratio of glycerol vs ChCl in NADES (HBD-HBA molarity ratio) | %HBD | x1 | 66.00 | 66.00 | 50.00 | 33.00 | 33.00 | 20.00 |
| Ratio of NADES vs sample powders (liquid-solid ratio) | %Liquid | x2 | 66.00 | 83.30 | 75.00 | 90.90 | 80.00 | 93.75 |
| Presence of water in NADES | %Water content | x3 | 10.00 | 20.00 | 30.00 | 50.00 | 50.00 | 80.00 |
Bioactive contents are expressed as the mean ± SEM of two replicates. The extraction conditions that produced the (a) highest and (b) lowest bioactive compound yields are labeled.
2.4. Preparation of conventional extract
Maceration and reflux extraction methods were performed to obtain comparative data for NADES-based extraction. The conventional extraction procedure was performed as described by Wardatun et al. (2017) and Batubara et al. (2009) for cinnamon and sappan wood, respectively, with modifications. Maceration was performed by placing 25 g of powder samples into a distillation flask followed by the addition of 96% ethanol (1:10 w/v). The mixture was shaken for 3 h at room temperature and then allowed to stand for 9 h. Subsequently, the mixture was filtered, and the filtrate was collected. This procedure was repeated twice.
Using the same solvent, the reflux extraction method was performed at 80 °C and repeated for three cycles. The obtained filtrates were combined and then evaporated to dryness using a rotary vacuum evaporator (Rotavapor® R-215, Buchi, Switzerland) at 65 °C. The concentrated extracts were collected and stored in a refrigerator at −20 °C for use in further analysis.
2.5. Determination of bioactive substances via high-performance liquid chromatography (HPLC)
The bioactive substances present in NADES and conventional extracts were determined using a Shimadzu 20 LC-AT HPLC system (Shimadzu, Japan). The system was equipped with an LC-20AT pump and SPD 20A UV detector. Separation was performed using an Inertsil ODS C18 reverse-phase column (150 × 4.6 mm i.d., 5 μm particle size, GL Sciences, Japan). HPLC was performed as described by Ahmad et al. (2013) and Setyaningsih et al. (2019) for cinnamon and sappan wood, respectively, with the optimal wavelength set at 280 nm. All samples were dissolved in methanol. A solution of 0.04% acetic acid in water-acetonitrile (40:0 v/v) was used as the mobile phase for cinnamon sample analysis, whereas acetic acid 0.3% in water-acetonitrile (85.5:14.5 v/v) was used as the mobile phase for sappan wood analysis. The optimal flow rate was 1.0 ml/min, and the injection volume was 20 μl. The bioactive substance content was calculated using data from the standard curve (6 levels concentration for each standard) for trans-cinnamaldehyde (5.5–220 μg/ml; limit of quantitation [LOQ] = 23.26 μg/ml), coumarin (5.0–205 μg/ml; [LOQ] = 27.59 μg/ml), and brazilin (5.0–200 μg/ml; [LOQ] = 3.18 μg/ml). Trans-cinnamaldehyde (99%) and brazilin standards (≥98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and standard coumarin (≥98%) was purchased from Universitas Andalas (Padang, Indonesia). All reagents, such as HPLC-grade acetonitrile, HPLC-grade methanol, and concentrated acetic acid, were obtained from Sigma-Aldrich.
2.6. Optimization of NADES extraction using response surface methodology (RSM)
Optimization of the NADES extraction parameters that affected the response variables (the content of extracted bioactive substances) was performed using RSM with Design-Expert® v12 trial version (Statease Inc. Minneapolis, MN, USA). A Box-Behnken design (three factors-three levels) was applied, resulting in 15 experimental conditions (Tables 2 and 3). The median values were obtained from the literature (Bajkacz and Adamek, 2017a, 2017b; Khezeli et al., 2016). This procedure optimized the ratio of ChCl to glycerol in NADES (HBD-HBA molarity ratio), the ratio of NADES to sample powders (liquid-solid ratio), and the amount of water in NADES. The experimental design schemes are presented in Table 1.
Table 2.
The results of the determination bioactive substances contained in Cinnamomum burmanii NADES extracts from experimental design suggested by RSM.
| Run | Independent Variables |
Responses |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (x1) Ratio of Glycerol vs ChCl in NADES (%) |
(x2) Ratio of NADES vs Sample powders (%) |
(x3) Percentage of water added (%) | R1: Trans-cinnamaldehyde levels (μg/ml) |
R2: Coumarin levels (μg/ml) |
||||||
| Glycerol | ChCl | NADES | Powder | Predicted | Actual | Predicted | Actual | |||
| 1 | 33.33 | 66.67 | 93.75 | 6.25 | 50.00 | 830.11 | 858.72 ± 11.33 | 678.37 | 643.35 ± 13.02 | |
| 2 | 56.66 | 43.34 | 93.75 | 6.25 | 80.00 | 416.83 | 453.59 ± 1.17b | 617.62 | 616.76 ± 7.42b | |
| 3 | 80.00 | 20.00 | 83.33 | 16.67 | 50.00 | 1710.55 | 1681.94 ± 12.18 | 1694.05 | 1729.07 ± 18.09 | |
| 4 | 80.00 | 20.00 | 88.54 | 11.46 | 80.00 | 906.97 | 956.60 ± 9.37 | 850.63 | 930.09 ± 11.18 | |
| 5 | 33.33 | 66.67 | 88.54 | 11.46 | 20.00 | 1175.83 | 1126.20 ± 24.56 | 952.15 | 872.69 ± 23.75 | |
| 6 | 33.33 | 66.67 | 88.54 | 11.46 | 80.00 | 1240.13 | 1174.75 ± 21.24 | 1107.12 | 1143.00 ± 27.19 | |
| 7 | 56.66 | 43.34 | 83.33 | 16.67 | 50.00 | 1041.86 | 1035.24 ± 29.71 | 1152.79 | 1173.40 ± 39.08 | |
| 8 | 33.33 | 66.67 | 83.33 | 16.67 | 50.00 | 1820.93 | 1907.32 ± 96.59a | 1702.26 | 1780.87 ± 112.51a | |
| 9 | 56.66 | 43.34 | 83.33 | 16.67 | 20.00 | 1566.13 | 1529.36 ± 6.06 | 1734.82 | 1735.68 ± 5.40 | |
| 10 | 80.00 | 20.00 | 93.75 | 6.25 | 50.00 | 686.054 | 599.66 ± 0.78 | 720.25 | 641.64 ± 2.20 | |
| 11 | 56.66 | 43.34 | 88.54 | 11.46 | 50.00 | 1041.86 | 972.21 ± 2.48 | 1152.79 | 1106.11 ± 3.76 | |
| 12 | 56.66 | 43.34 | 88.54 | 11.46 | 50.00 | 1041.86 | 1118.14 ± 20.52 | 1152.79 | 1178.87 ± 20.45 | |
| 13 | 56.66 | 43.34 | 83.33 | 16.67 | 80.00 | 1418.43 | 1397.41 ± 5.74 | 1408.93 | 1294.45 ± 5.74 | |
| 14 | 56.66 | 43.34 | 93.75 | 6.25 | 20.00 | 552.41 | 573.43 ± 5.23 | 528.45 | 642.93 ± 6.34 | |
| 15 | 80.00 | 20.00 | 88.54 | 16.67 | 20.00 | 1254.55 | 1319.93 ± 5.35 | 1242.32 | 1206.44 ± 4.30 | |
Bioactive contents are expressed as mean ± SEM (Standard Error Measurement) of two replicates. The results are labeled shows extraction conditions that produce the (a) highest and (b) lowest bioactive compounds.
Table 3.
Determination of brazilin content in Caesalpinia sappan natural deep eutectic solvent (NADES) extracts using different experimental designs.
| Run | Independent Variables |
Response R1: Brazilin levels (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|
| (x1) Ratio of glycerol vs ChCl in NADES (%) |
(x2) Ratio of NADES vs sample powders (%) |
(x3) Percentage of water added (%) | |||||
| Glycerol | ChCl | NADES | Powder | Predicted | Actual | ||
| 1 | 49.50 | 50.50 | 80.00 | 20.00 | 10.00 | 80.62 | 74.21 ± 0.19b |
| 2 | 49.50 | 50.50 | 80.00 | 20.00 | 50.00 | 139.97 | 141.39 ± 1.27 |
| 3 | 49.50 | 50.50 | 66.00 | 34.00 | 10.00 | 169.49 | 179.02 ± 0.71 |
| 4 | 33.00 | 67.00 | 80.00 | 20.00 | 30.00 | 121.89 | 132.66 ± 1.98 |
| 5 | 49.50 | 50.50 | 73.33 | 26.67 | 30.00 | 185.35 | 174.04 ± 0.87 |
| 6 | 49.50 | 50.50 | 73.33 | 26.67 | 30.00 | 185.35 | 177.74 ± 0.47 |
| 7 | 33.00 | 67.00 | 73.33 | 26.67 | 50.00 | 274.02 | 268.94 ± 0.78 |
| 8 | 66.00 | 34.00 | 66.00 | 34.00 | 30.00 | 233.17 | 233.25 ± 1.92 |
| 9 | 66.00 | 34.00 | 73.33 | 26.67 | 10.00 | 114.61 | 112.11 ± 0.27 |
| 10 | 66.00 | 34.00 | 73.33 | 26.67 | 50.00 | 217.25 | 206.91 ± 15.48 |
| 11 | 33.00 | 67.00 | 66.00 | 34.00 | 30.00 | 287.63 | 282.45 ± 2.10 |
| 12 | 49.50 | 50.50 | 66.00 | 34.00 | 50.00 | 351.31 | 368.67 ± 1.66a |
| 13 | 66.00 | 34.00 | 80.00 | 20.00 | 30.00 | 98.69 | 114.72 ± 2.54 |
| 14 | 49.50 | 50.50 | 73.33 | 26.67 | 30.00 | 185.35 | 175.84 ± 1.06 |
| 15 | 33.00 | 67.00 | 73.33 | 26.67 | 10.00 | 135.50 | 138.26 ± 1.26 |
Bioactive contents are expressed as the mean ± SEM of two replicates. The extraction conditions that produced the (a) highest and (b) lowest bioactive compound yields are labeled.
2.7. Statistical analysis
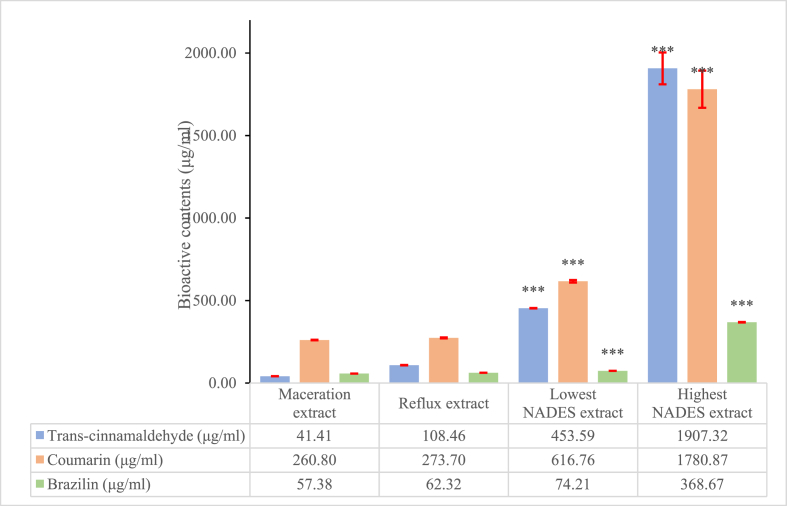
The data for the determination of bioactive contents are presented as the mean ± SEM of two replicates. The differences in means (Figure 1) were analyzed via one-way analysis of variance (ANOVA) followed by Tukey's test, and Fisher's least significant difference post hoc test using SPSS v.22. p ≤ 0.001 denoted statistical significance. The result of the optimization and the lack of fit of the model were also analyzed by ANOVA using Design-Expert® v.12. Statistical significance was interpreted as p ≤ 0.05.
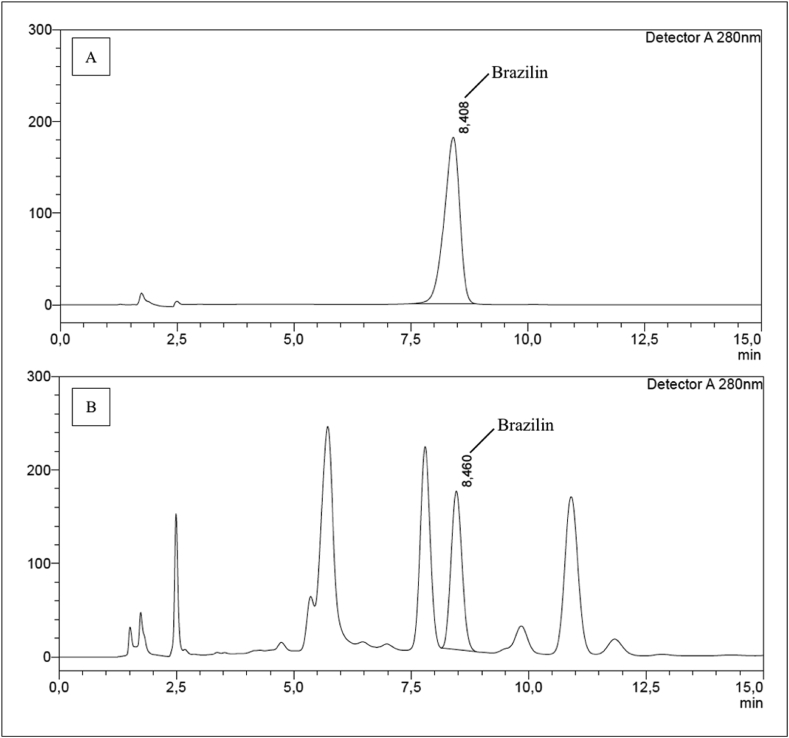
Figure 3.
Chromatograms of (A) standard brazilin and (B) sappan wood natural deep eutectic solvent extract containing brazilin and several unknown substances.
3. Results and discussion
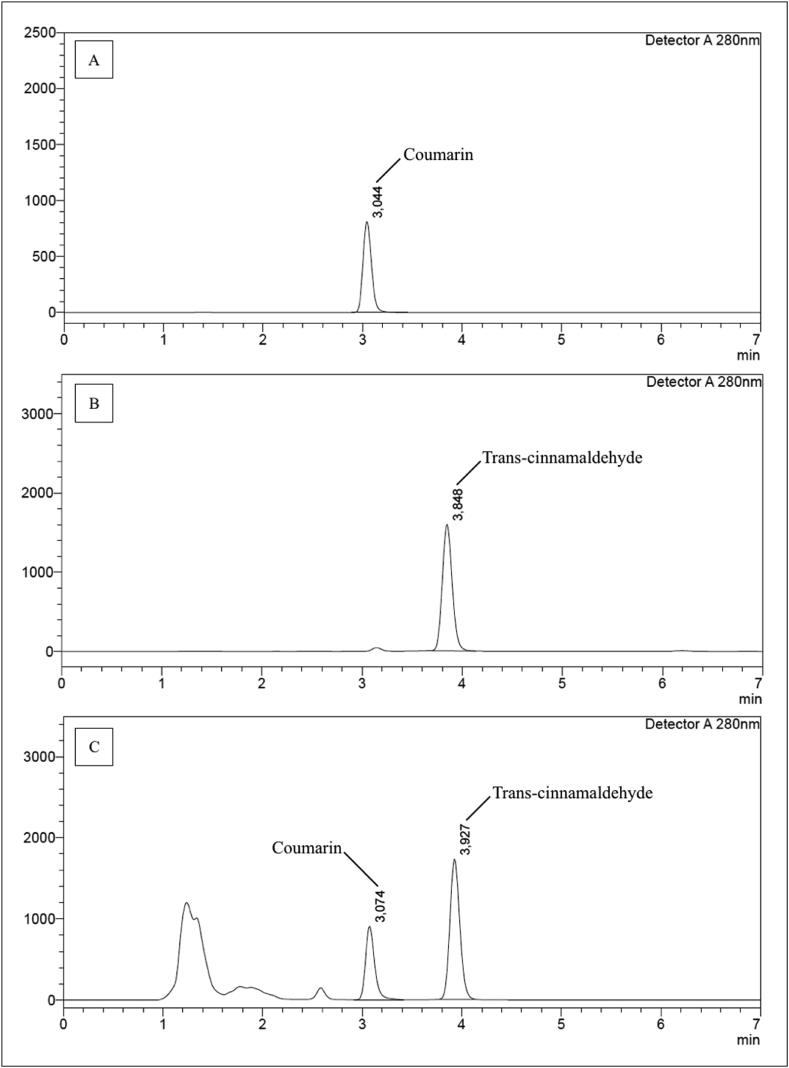
In this study, NADES was successfully applied to extract the active compounds contained in cinnamon and sappan wood. As observed in Figure 2C, coumarin (Rt = 3.074 min) and trans-cinnamaldehyde (Rt = 3.927 min) were present in the NADES extract. Both compounds were well separated with a resolution value of 4.6. This illustrates that the applied chromatography method for analyzing cinnamon extract successfully separated the two compounds contained in the extract mixture. A resolution value ≥ 2.0 indicates that the peak compounds are separated by a baseline gap (Ahuja, 2002), whereas 1.2 is the minimum value needed for two peak compounds to completely separate (Hanai, 1999). Brazilin was also successfully extracted using NADES (Rt = 8.460) (Figure 3C). However, the resolution value was only 1.5, illustrating that although brazilin was separated, further optimization is required to avoid overlap with other peaks. The presence of other peaks from unknown compounds in the chromatogram (Figure 3C) was suspected to represent the major phytochemical compounds contained in sappan wood, namely, hematoxylin, episappanol, sappanin, sappanchalcone, sappanone A, and protosappanins A–E, which also exhibit absorbance at approximately 280 nm (Mueller et al., 2016; Tulin et al., 2017; Xia et al., 2017). Further research is needed to determine the unknown compounds.
Figure 2.
Chromatograms of (A) standard coumarin, (B) standard trans-cinnamaldehyde, and (C) cinnamon natural deep eutectic solvent extract containing both coumarin and trans-cinnamaldehyde.
The content of bioactive substances in NADES extracts is presented in Tables 2 and 3. The results revealed that the extraction conditions for run 8 produced the highest yields of bioactive compounds from cinnamon, including yields of 1907.32 ± 96.59 and 1780.87 ± 112.51 μg/ml for trans-cinnamaldehyde and coumarin, respectively. Contrarily, run 2 produced the lowest yields of bioactive substances, namely, trans-cinnamaldehyde and coumarin levels of 453.59 ± 1.17 and 616.76 ± 7.42 μg/ml, respectively. The highest brazilin content was obtained via NADES-based extraction using the extraction conditions for run 12 (Table 3). A ChCl-glycerol ratio of 1:1 (v/v), a NADES-sample powder ratio of 2:1 (v/w), and the addition 50% water were applied to obtain 368.67 ± 1.66 μg/ml brazilin. The lowest brazilin content was obtained using the extraction conditions of run 1, which yielded only 74.21 ± 0.19 μg/ml brazilin. These illustrated that the actual responses were close to the predicted responses, indicating that the experimental design model has good linearity (R2 = 0.9998, Table 4).
Table 4.
Regression model formula for predicting independent variables and responses from the experimental model.
| Responses | Regression model formula (y) | Linearity (R2) |
|---|---|---|
| Trans-cinnamaldehyde contenta | y = 1041.86-63.61-503.83-70.82-8.42-102.97 + 3.03+187.98 + 32.06–85.48 | 0.9812 |
| Coumarin contenta | y = 1152.79 + 8.42-499.42-59.18 + 12.52–136.66 + 103.77+5.77 + 40.17–120.51 | 0.9737 |
| Brazilin contentb | y = 185.35-19.42-75.05 + 60.29+7.82-8.97-30.62 | 0.9848 |
The yields of bioactive substances (responses) were represented as y, whereas x1, x2, and x3 represent factors A (percentage of glycerol [hydrogen bond donor] in NADES), B (percentage of natural deep eutectic solvent [NADES] without sample powder), and C (percentage of water in NADES), respectively. The analysis was performed via analysis of variance for the aquadratic and btwo-factor interactions models.
The ability of NADES to extract bioactive substances from the natural product matrix correlates with its physical and chemical properties such as hydrogen bonding, polarity, acidity, and viscosity (Marcus, 2019). As reported by Kim et al. (2016), the combination of ChCl-glycerol (1:2 w/w) has solvatochromic indices, including the index (Dimorth model), and Kamlet–Taft parameters, consisting of indexes that represent the polarity and polarizability of NADES (π*), hydrogen bond donation (α), and acceptance (β) as methanol and water, indicating that the ChCl-glycerol–based NADES has high polarity and good ability to donate and accept hydrogen through the natural product matrix (Kim et al., 2016; Marcus, 2019) (Figure 1). Figure 3 shows that NADES extract contains higher bioactive substances level compared with conventional extracts (p ≤ 0.001). Even if we compare with the lowest bioactive substances level NADES extract, it was still superior to conventional extracts. The concentration of conventional extract, that performed as comparative data was 1000 ppm and 5000 ppm respectively, for cinnamon and sappan wood. The concept of π-π interaction with target analytes that performed to extract bioactive substances from Cinnamomum burmannii bark and Caesalpinia sappan wood also applied for a number of purposes, such as to extract chromium species contamination in water samples (Yilmaz and Soylak, 2016). As a new type of solvent that is more ecofriendly, DES is also suitable to extract Malachite Green contaminant deposits in aquarium (Aydin et al., 2017). This interesting fact makes DES become a prospective solvent to detemine contaminants and bioactive substances from natural products as we have done.
Figure 1.
Comparison of bioactive contents extracted using conventional and natural deep eutectic solvent (NADES) extraction methods. Significant differences were found between NADES and conventional extracts (p ≤ 0.001). Asterisks (*) denote significantly different data.
The RSM optimization process using the Box-Behnken design provided a significant model with a non-significant lack of fit (p ≤ 0.05). This model suggests that the optimal conditions for extracting trans-cinnamaldehyde from cinnamon include a ChCl-glycerol ratio of 2:1 (w/w), a powder-NADES ratio of 1:4 (w/w), and 20% water content in NADES. These extraction parameters are predicted to produce trans-cinnamaldehyde and coumarin levels of as high as 1150.14 and 920.43 μg/ml, respectively. Meanwhile, to maximize the brazilin yield, the extraction conditions should be a ChCl-glycerol ratio of 1:2 (w/w), a powder-NADES ratio of 2:1 (w/w), and 47.57% water content in NADES. These conditions are predicted to produce 373.28 μg/ml brazilin. To predict the extraction parameters that produce the expected responses, a manual calculation can be performed using the equation presented in Table 4.
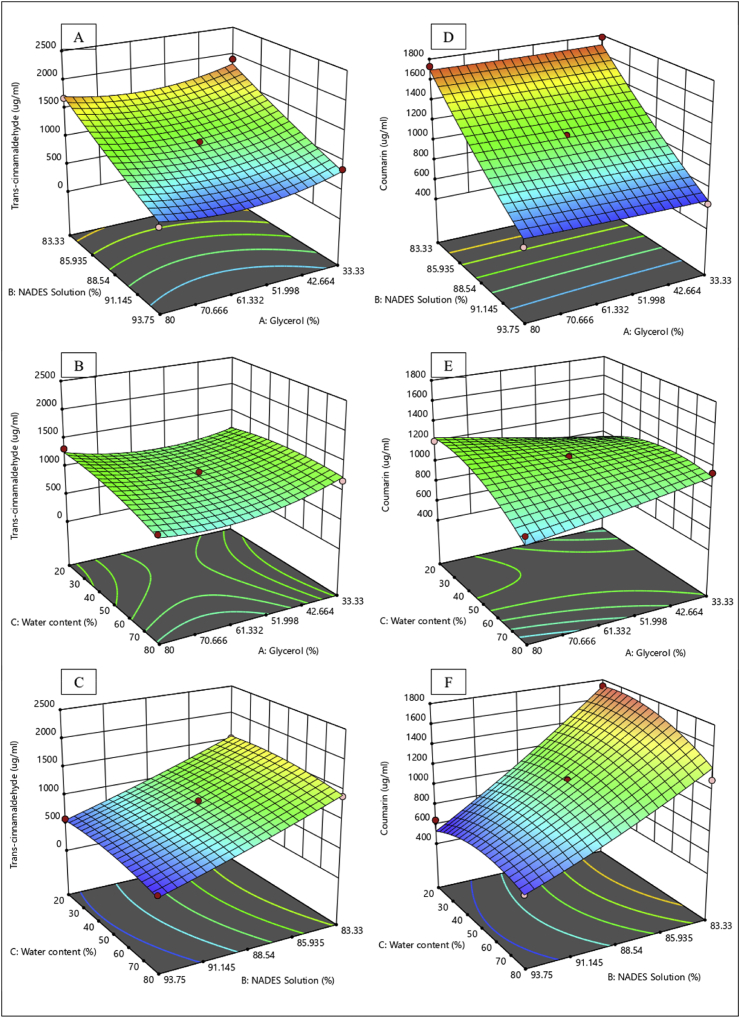
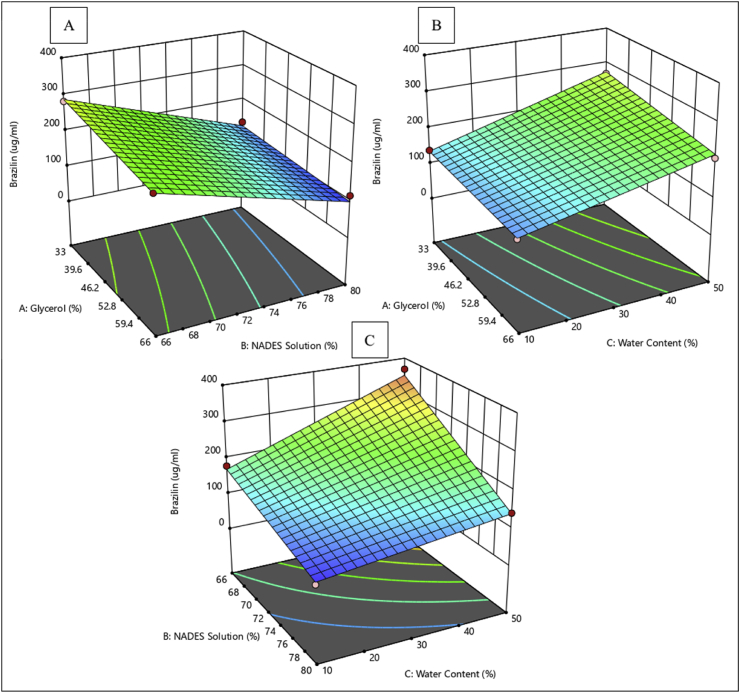
Coumarin, a phytochemical commonly found in cinnamon, is reported to have antiplatelet properties (Wu et al., 2009). However, other reports indicated that coumarin also has hepatotoxic and carcinogenic effects, which led to restrictions on its use, including a ban of its use as a food preservative by the FDA (Abraham et al., 2010). Interesting, 8 of the 15 NADES extracts generated in this study exhibited lower coumarin levels than trans-cinnamaldehyde levels. Conversely, coumarin levels were higher than trans-cinnamaldehyde levels in all conventional extracts. It can be observed in Figure 4 that the liquid-solid ratio (ratio of NADES to sample powders) strongly influenced the amount of coumarin extracted. The use of a smaller amount of NADES will lead to higher levels of extracted coumarin. From a solvatochromic perspective, 96% ethanol as a solvent in conventional extraction has the following Kamlet-Talf parameters: π* = 0.54, α = 0.83, and β = 0.77 (Trišović et al., 2011). Meanwhile, these values for a ChCl-glycerol ratio of 1:2 (w/w) were π* = 1.18, α = 0.78, and β = 0.55 (Marcus, 2019). This finding reveals the involvement and importance of various non-specific interactions besides the hydrogen interaction in the extraction of coumarin from natural products (Maity et al., 2014). ChCl-glycerol–based NADES has higher polarity than water (Marcus, 2019; Zakerhamidi et al., 2010), explaining why coumarin levels were lower than trans-cinnamaldehyde levels under the various extraction conditions. Figure 5 shows that all NADES extraction parameters are correlated with the brazilin level; however, it appears that the molarity ratio of NADES (ratio of ChCl to glycerol in NADES) slightly affected the amount of brazilin extracted.
Figure 4.
Three-dimensional surface response plots presenting the effects of the extraction parameters on the responses. (A), (B), and (C) present the effects on trans-cinnamaldehyde levels, and (D), (E), and (F) illustrate the effect son coumarin levels.
Figure 5.
Three-dimensional surface response plots presenting the effect of the extraction parameters on the responses for brazilin levels.
4. Conclusion
The use of ChCl-glycerol-based NADES to extract bioactive substances from Indonesian cassia and Sappan wood increased the extraction of bioactive substances compared with conventional extraction methods significantly (p ≤ 0.001). The optimization provided a significant model (p ≤ 0.05) with a regression model equation to predict the expected yields. To obtain maximum Trans-Cinnamaldehyde content with minimum Coumarin content, the extraction parameters performed suggested use ratio of Gycerol vs ChCl 1:2 (w/w); ratio of powder vs NADES 1:8 (w/w); and 20% water content in NADES. That are conditions predicted will produce Trans-Cinnamaldehyde and Coumarin as large as 1150.14 and 920.43 μg/ml respectively. Meanwhile, to maximize brazilin content, ratio Gycerol vs ChCl 1:2 (w/w); ratio of powder vs NADES 2:1 (w/w); and 47.57% water content in NADES are predicted will produce 373.28 μg/ml Brazilin.
Declarations
Author contribution statement
Aditya Sindu Sakti: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fadlina Chany Sapurti, Abdul Mun'im: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Universitas Indonesia (grant Hibah Skema Q1Q2 No. NBK-01980/UN2.R3.1/HKP.05.00/2019).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abraham K., Wöhrlin F., Lindtner O., Heinemeyer G., Lampen A. Toxicology and risk assessment of coumarin: focus on human data. Mol. Nutr. Food Res. 2010;54(2):228–239. doi: 10.1002/mnfr.200900281. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Lim C., Akowuah G., Ismail N., Hashim M., Hor S., Ang L., Yam M. Safety assessment of standardised methanol extract of Cinnamomum burmannii. Phytomedicine. 2013;20(12):1124–1130. doi: 10.1016/j.phymed.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Ahuja S. first ed. Academic Press; San Diego: 2002. Chromatography and Separation Science; p. 99. [Google Scholar]
- Al-Dhubiab B.E. Pharmaceutical applications and phytochemical profile of Cinnamomum burmannii. Pharmacogn. Rev. 2012;6(12):125. doi: 10.4103/0973-7847.99946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alishlah T., Mun’im A., Jufri M. Optimization of urea-glycerin based NADES-UAE for oxyresveratrol extraction from Morus alba roots for preparation of skin whitening lotion. J. Young Pharm. 2019;11(2):155–160. [Google Scholar]
- Anderson R., Zhan Z., Luo R., Guo X., Guo Q., Zhou J., Kong J., Davis P., Stoecker B. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. Journal of Traditional and Complementary Medicine. 2016;6(4):332–336. doi: 10.1016/j.jtcme.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashakirin S., Tripathy M., Patil U., Majeed A. Chemistry and bioactivity of cinnamaldehyde: a natural molecule of medicinal importance. Int. J. Pharm. Sci. Res. 2017;8(6):2333–2340. [Google Scholar]
- Aydin F., Yilmaz E., Soylak M. A simple and novel deep eutectic solvent based ultrasound-assisted emulsification liquid phase microextraction method for malachite green in farmed and ornamental aquarium fish water samples. Microchem. J. 2017;132:280–285. [Google Scholar]
- Bajkacz S., Adamek J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Analytical Methods. 2017;11(5):1330–1344. [Google Scholar]
- Bajkacz S., Adamek J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta. 2017;168:329–335. doi: 10.1016/j.talanta.2017.02.065. [DOI] [PubMed] [Google Scholar]
- Batubara I., Mitsunaga T., Ohashi H. Screening antiacne potency of Indonesian medicinal plants: antibacterial, lipase inhibition, and antioxidant activities. J. Wood Sci. 2009;55(3):230–235. [Google Scholar]
- Bechtold T. first ed. Wiley; Chichester: 2009. Handbook of Natural Colorants; p. 70. [Google Scholar]
- Cao H., Polansky M., Anderson R. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007;459(2):214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Clarke C., Tu W., Levers O., Bröhl A., Hallett J. Green and sustainable solvents in chemical processes. Chem. Rev. 2018;118(2):747–800. doi: 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]
- Dai Y., Rozema E., Verpoorte R., Choi Y. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A. 2016;1434:50–56. doi: 10.1016/j.chroma.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Emilda Efek senyawa bioaktif kayu manis Cinnamomum burmanii nees Ex.Bl.) terhadap diabetes melitus: kajian pustaka [article in bahasa Indonesia] Jurnal Fitofarmaka Indonesia. 2018;5(1):246–252. [Google Scholar]
- Häckl K., Kunz W. Some aspects of green solvents. Compt. Rendus Chem. 2018;21(6):572–580. [Google Scholar]
- Hanai T. first ed. Royal Society of Chemistry; Cambridge: 1999. HPLC A Practical Guide; p. 1. [Google Scholar]
- Khezeli T., Daneshfar A., Sahraei R. A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta. 2016;150:577–585. doi: 10.1016/j.talanta.2015.12.077. [DOI] [PubMed] [Google Scholar]
- Kim S., Hyun S., Choung S. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Kim S., Park S., Yu H., Kim J., Kim H., Yang Y., Kim Y., Kim K., Kan E., Lee S. Effect of deep eutectic solvent mixtures on lipase activity and stability. J. Mol. Catal. B Enzym. 2016;128:65–72. [Google Scholar]
- Li H., Chen C., Hong Z., Huang J., Chen C. Chemical constituents from the leaves of Cinnamomum burmannii. Chem. Nat. Comp. 2012;48(5):873–874. [Google Scholar]
- Li P., Tian L., Li T. Study on ultrasonic-assisted extraction of essential oil from cinnamon bark and preliminary investigation of its antibacterial activity. In: Zhang T.C., Nakajima M., editors. Vol. 332. Springer; Berlin, Heidelberg: 2015. p. 353. (Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering). [Google Scholar]
- Liu Y., Friesen J., McAlpine J., Lankin D., Chen S., Pauli G. Natural deep eutectic solvents: properties, applications, and perspectives. J. Nat. Prod. 2018;81(3):679–690. doi: 10.1021/acs.jnatprod.7b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity B., Chatterjee A., Seth D. Photophysics of a coumarin in different solvents: use of different solvatochromic models. Photochem. Photobiol. 2014;90:734–746. doi: 10.1111/php.12258. [DOI] [PubMed] [Google Scholar]
- Marcus Y. first ed. Springer; Cham: 2019. Deep Eutectic Solvents; pp. 88–89. [Google Scholar]
- Mueller M., Weinmann D., Toegel S., Holzer W., Unger F., Viernstein H. Compounds from Caesalpinia sappan with anti-inflammatory properties in macrophages and chondrocytes. Food & Function. 2016;7(3):1671–1679. doi: 10.1039/c5fo01256b. [DOI] [PubMed] [Google Scholar]
- Panić M., Radić Stojković M., Kraljić K., Škevin D., Radojčić Redovniković I., Gaurina Srček V., Radošević K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019;283:628–636. doi: 10.1016/j.foodchem.2019.01.061. [DOI] [PubMed] [Google Scholar]
- Rao P., Gan S. Cinnamon: a multifaceted medicinal plant. Evid. Based Complement Altern. Med. 2014;2014:1–12. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyaningsih E., Saputri F., Mun’im A. The antidiabetic effectivity of Indonesian plants extracts via DPP-IV inhibitory mechanism. J. Young Pharm. 2019;11(2):161–164. [Google Scholar]
- Setyowati F., Utami R., Khasanah L., Manuhara G. Optimization of two-stage cinnamon bark (Cinnamomum burmanii) oleoresin maceration extraction process with ethanol solvent using response surface methodology (RSM) AIP Conference Proceedings. 2018 [Google Scholar]
- Srikrishna D., Godugu C., Dubey P. A review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 2018;18(2):1–29. doi: 10.2174/1389557516666160801094919. [DOI] [PubMed] [Google Scholar]
- Trišović N., Valentić N., Erović M., Đaković-Sekulić T., Ušćumlić G., Juranić I. Synthesis, structure, and solvatochromic properties of pharmacologically active 5-substituted 5-phenylhydantoins. Monatshefte für Chemie - Chemical Monthly. 2011;142(12):1227–1234. [Google Scholar]
- Tulin E., Loreto M., Tulin E. Alpha-glucosidase inhibitory activity and fractionation of bioactive compounds from bark extracts of sibucao (Caesalpinia sappan L.) in the Philippines. Pharmacogn. J. 2017;9(3):356–360. [Google Scholar]
- Venugopala K., Rashmi V., Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013;2013:1–14. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Bauer R., Melchart D., Staudinger A. first ed. Vol. 4. Springer; Switzerland: 2016. p. 126. (Chromatographic Fingerprint Analysis of Herbal Medicines, Thin-Layer and High Performance Liquid Chromatography of Chinese Drugs). [Google Scholar]
- Wardatun S., Rustiani E., Alfiani N., Rissani D. Study effect type of extraction method and type of solvent to cinnamaldehyde and trans-cinnamic acid dry extract cinnamon (Cinnamomum burmanii [nees & T, nees] Blume) J. Young Pharm. 2017;9(1s):s49–s51. [Google Scholar]
- Wu L., Wang X., Xu W., Farzaneh F., Xu R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009;16(32):4236–4260. doi: 10.2174/092986709789578187. [DOI] [PubMed] [Google Scholar]
- Xia Z., Li D., Li Q., Zhang Y., Kang W. Simultaneous determination of brazilin and protosappanin B in Caesalpinia sappan by ionic-liquid dispersive liquid-phase microextraction method combined with HPLC. Chem. Cent. J. 2017;11(114):1–11. doi: 10.1186/s13065-017-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E., Soylak M. Ultrasound assisted-deep eutectic solvent based on emulsification liquid phase microextraction combined with microsample injection flame atomic absorption spectrometry for valence speciation of chromium(III/VI) in environmental samples. Talanta. 2016;160:680–685. doi: 10.1016/j.talanta.2016.08.001. [DOI] [PubMed] [Google Scholar]
- You E., Khil L., Kwak W., Won H., Chae S., Lee B., Moon C. Effects of brazilin on the production of fructose-2,6-bisphosphate in rat hepatocytes. J. Ethnopharmacol. 2005;102(1):53–57. doi: 10.1016/j.jep.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Yuniarti E., Saputri F., Mun’im A. Application of the natural deep eutectic solvent choline chloride-sorbitol to extract chlorogenic acid and caffeine from green coffee beans (Coffea canephora) J. Appl. Pharm. Sci. 2019;9(3):82–90. [Google Scholar]
- Zakerhamidi M., Ghanadzadeh A., Tajalli H., Moghadam M., Jassas M., Hosseini nia R. Substituent and solvent effects on the photo-physical properties of some coumarin dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010;77(2):337–341. doi: 10.1016/j.saa.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lin L., Ye W. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018;13(20):1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Liu H., Liu C., Wang L., Ma R., Chen B., Li L., Niu J., Fu M., Zhang D., Gao S. Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017;122:78–89. doi: 10.1016/j.phrs.2017.05.019. [DOI] [PubMed] [Google Scholar]