Abstract
Coenzyme Q10 (CoQ10) is an important component of the respiratory chain in humans and some bacteria. As a high-value-added nutraceutical antioxidant, CoQ10 has excellent capacity to prevent cardiovascular disease. The content of CoQ10 in the industrial Rhodobacter sphaeroides HY01 is hundreds of folds higher than normal physiological levels. In this study, we found that overexpression or optimization of the synthetic pathway failed CoQ10 overproduction in the HY01 strain. Moreover, under phosphate- limited conditions (decreased phosphate or in the absence of inorganic phosphate addition), CoQ10 production increased significantly by 12% to220 mg/L, biomass decreased by 12%, and the CoQ10 productivity of unit cells increased by 27%. In subsequent fed-batch fermentation, CoQ10 production reached 272 mg/L in the shake-flask fermentation and 1.95 g/L in a 100-L bioreactor under phosphate limitation. Furthermore, to understand the mechanism associated with CoQ10 overproduction under phosphate- limited conditions, the comparatve transcriptome analysis was performed. These results indicated that phosphate limitation combined with glucose fed-batch fermentation represented an effective strategy for CoQ10 production in the HY01. Phosphate limitation induced a pleiotropic effect on cell metabolism, and that improved CoQ10 biosynthesis efficiency was possibly related to the disturbance of energy metabolism and redox potential.
Keywords: R sphaeroides, CoQ10, Phosphate limitation, Overproduction, Scale-up, Transcriptome
1. Introduction
Coenzyme Q (CoQ) is a crucial component of the respiratory chain, which is responsible for oxidative phosphorylation and adenosine triphosphate (ATP) generation in all aerobic organisms. CoQ comprises a polyisoprenoid tail and a benzoquinone nucleus, and according to the number of isoprenoid moieties in different species, CoQ can be classified into different subtypes (e.g., CoQ6, CoQ8, CoQ9, and CoQ10). In humans and some microbes, CoQ10 is the major form of CoQ, and as a high value-added nutraceutical antioxidant, exhibits excellent capacity to prevent cardiovascular disease [1,2]. In Western countries, CoQ10 is among the most popular nutraceuticals and has been widely used for decades. In 2011, the CoQ10 market reached $500 million in United States [1].
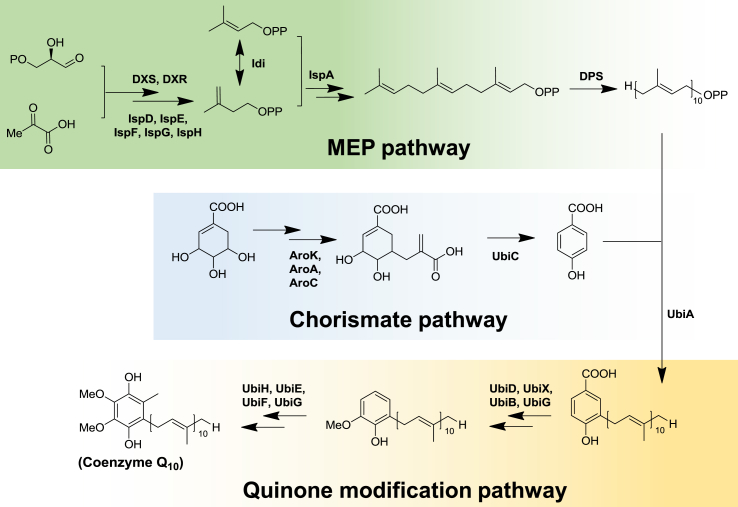
Microbes are a primary source of certain chemicals, nutraceuticals, and drugs/drug precursors [3,4]. Agrobacterium tumefaciens, Paracoccus denitrificans, Schizosaccharomyces pombe, Sporidiobolus johnsonii, and Rhodobacter sphaeroides can naturally produce CoQ10, with the associated biosynthetic pathway for CoQ10 has elucidated in these species [[5], [6], [7], [8], [9], [10], [11], [12]]. Fig. 1 shows that the benzoquinone nucleus of CoQ10, the para-hydroxybenzoic acid moiety derived from the shikimate pathway, and the 10-isoprenoid tail are synthesized via the 2-C-methyl-D-erythritol 4-phosphate pathway (MEP) [13,14]. This is followed by prenyltransferase (UbiA)-mediated transfer of the hydrophobic 10-isoprenoid chain onto the benzoquinone nucleus [15], and hydroxylation and methylation of the benzoquinone nucleus in the quinone-modification pathway to produce CoQ10 [10]. Additionally, cofactors, such as NADH, NADPH, and S-adenosyl methionine (SAM), are involved in the CoQ10 biosynthesis. The CoQ10 biosynthetic pathway has subsequently been engineered for heterologous production in other microbes, including Escherichia coli and Saccharomyces cerevisiae [[16], [17], [18], [19], [20], [21]].
Fig. 1.
Biosynthetic pathway of CoQ10 in bacteria. Schematic showing the pathway of metabolic precursors leading to the formation of para-hydroxybenzoic acid moiety, the 10-isoprenoid tail, and the final CoQ10 product.
R. sphaeroides has been used for industrial production of CoQ10 due to its high biosynthetic efficiency [22]. Metabolic engineering strategies have been applied to enhance CoQ10 production in R. sphaeroides. Lu et al. reported that overexpression of UbiG, which catalyzes O-methylation of the benzoquinone ring, significantly improved CoQ10 production to 65.8 mg/L in a wild type strain [23]. Another study showed that UbiG overexpression combined with MEP pathway optimization (finely tuned the expression of DXS, DXR, IDI, and IspD) further increased CoQ10 production to 93.3 mg/L [24]. Moreover, combining the optimized quinone- modification pathway with the MEP pathway resulted in a strain capable of yielding 138.7 mg/L CoQ10 [2]. Recently, Zhu et al. reported that synergistic regulation of redox potential (NADH/NAD+) and oxygen uptake yielded 163.5 mg/L of CoQ10 in shake-flask fermentation by R. sphaeroides [22,25]. However, the titer of CoQ10 in industrial strain (190 mg/L in shake-flask fermentation, Table 1) is higher than that in these genetically engineered strains. And up to now, none of the engineering endeavors achieved overproduction of CoQ10 in an industrial strain, indicating that the rate-limiting steps in the CoQ10 overproduction strains remain to be elucidated.
Table 1.
List of R. sphaeroides strains and their CoQ10 production in shake-flask fermentationa.
| Strain | Descriptions | CoQ10 production (mg/L) | Plasmid source/Ref. |
|---|---|---|---|
| HY01 | CoQ10 industrial strain R. sphaeroides | 192.2 ± 3.7 | |
| HY01-pBBR | HY01 containing pBBR1MCS2 (plasmid control) | 192.9 ± 5.9 | [26] |
| MEP pathway overexpression | |||
| HY01-idi | idi overexpression in HY01 | 111.2 ± 4.4 | This study |
| HY01-dxs | dxs overexpression in HY01 | 159.5 ± 5.1 | This study |
| Quinone modification pathway overexpression | |||
| HY01-ubiCA | ubiC, ubiA overexpression in HY01 | 105.5 ± 4.9 | This study |
| HY01-ubiF | ubiF overexpression in HY01 | 86.5 ± 0.7 | This study |
| HY01-ubiH | ubiH overexpression in HY01 | 168.0 ± 8.5 | This study |
| HY01-ubiE | ubiE overexpression in HY01 | 210 ± 3.5 | This study |
| HY01-ubiG | ubiG overexpression in HY01 | 83.5 ± 3.5 | This study |
| MEP and quinone modification pathway optimization | |||
| HY01-MQc | HY01 containing pMCS-MQc (dxs, dxr, idi, ispD, ubiE, ubiG, lacIqRBSc) | 160.8 ± 1.1 | [2] |
| HY01-MQe | HY01 containing pMCS-MQc (dxs, dxr, idi, ispD, ubiE, ubiG, lacIqRBSe) | 90.5 ± 1.2 | [2] |
Detail information about construction and fermentation of these genetic engineered strains is provided in supplementary materials. Data are expressed as mean ± standard deviation (SD).
Inorganic phosphate is an important essential nutrient that determines cell physiology, nucleotide biosynthesis, and phospholipid and energy metabolism [[27], [28], [29], [30]]. Under natural conditions, phosphorus is often a limited nutrient in microorganisms. Moreover, bacteria have evolved mechanisms to sense, adapt and respond under phosphate-limited or starvation conditions. In previous studies, phosphate limitation was applied as a fermentation strategy to enhance the production of target products, such as propanediol [31,32], poly-3-hydroxybutyrate [33], and secondary metabolites [34]. Benning et al. found that R. sphaeroides could alter its membrane composition to adapt to phosphate-limited conditions [[35], [36], [37]]. As our desired product CoQ10 is a component of the respiratory chain closely associated with the membrane, the effect of phosphate on the production of CoQ10 in an industrial overproduction strain need to be well understood, and this endeavor might bring new insight into the metabolic engineering of the industrial strain.
In this study, we used HY01 as a CoQ10- overproduced derivative of the wild-type strain, and evaluated the previously described strategies to further enhance CoQ10 production. In addition, this study also found that the concentration of the inorganic phosphate in the medium significantly regulated the CoQ10-biosynthesis efficiency of HY01, and the strategy for CoQ10 overproduction in an industrial strain might be developed through regulation of the phosphate supply.
2. Materials and methods
2.1. Microorganisms and cultivation
E. coli DH10b was used for plasmid construction and propagation, and E. coli S17-1 was used for di-parental conjugation. All E. coli strains were cultivated in Luria–Bertani medium at 37 °C. HY01 and its derivatives were cultivated on agar plates (0.8% yeast extract, 0.3% glucose, 0.2% NaCl, 0.13% KH2PO4, 0.0125% MgSO4, and 1.5% agar, supplemented with 15 mg/L biotin, 1 mg/L nicotinic acid, and 1 mg/L thiamine hydrochloride). For shake-flask and bioreactor fermentation, HY01 and its derivatives were cultivated in fermentation medium [4% glucose, 0.4% corn steep liquor, 0.3% sodium glutamate, 0.3% (NH4)2SO4, 0.28% NaCl, 0.3% KH2PO4, 0.63% MgSO4, and 0.2% CaCO3 supplemented with 1 mg/L thiamine hydrochloride, 1 mg/L nicotinic acid, and 15 μg/L biotin). For phosphate- limited conditions, 50% or 100% KH2PO4 was removed from the fermentation medium, and potassium was replaced to the same level as that in the control group via the addition of potassium chloride.
2.2. Di-parental conjugation and gene overexpression
A pBBR1MCS2 derivative harboring a terminator from pTrc99a and the tac promoter from pGEX-4T1 was used for gene overexpression [22,26]. Targeted genes in the MEP pathway or quinone-modification pathway were amplified from R. sphaeroides 2.4.1 genomic DNA, and conjugation was performed, as described previously [24,38]. E. coli S17-1 was used as a donor for plasmid transformation into R. sphaeroides.
2.3. Cell growth and sugar analysis
Growth of R. sphaeroides cells was detected by measuring the optical density at 700 nm (OD700). Initially, 0.5 mL of culture broth was mixed with 0.2 mL of 0.1 N HCl to completely dissolve CaCO3, followed by dilution with deionized water and measurement of the OD700 using a spectrophotometer. Residual glucose in the culture broth was measured using an SBA-40D biological sensing analyzer (Biology Institute of the Shangdong Academy of Science, Jinan, China) according to manufacturer instructions.
2.4. Phosphate analysis
Residual phosphate in the fermentation broth was analyzed using ammonium molybdate, as previously reported [39]. A KH2PO4 standard (Sangon Biotech, Shanghai, China) was used for preparation of a standard curve. The absorption of samples was measured at 400 nm using a FLUOstra microplate reader (BMG Labtech, Cary, NC, USA).
2.5. High-performance liquid chromatography (HPLC) analysis
CoQ10 production was measured by HPLC. First, 1 mL of culture broth was mixed with 10 μL of 6 N HCl and 0.2 mL 30% hydrogen peroxide, followed by the addition of 2 mL acetone and vortexing for 1 min. The volume was subsequently adjusted to 10 mL with ethanol, followed by incubation in an ultrasonic bath for 45 min at room temperature. Supernatant was collected following centrifugation (12,000 rpm for 10 min at 4 °C) and filtered using a 0.45-μm filter (Merck Millipore). The resulting samples were then used for CoQ10 detection by HPLC. A YMC-Pack ODS-A C18 column (150 mm × 4.6 mm; YMC Co., Ltd., Tokyo, Japan) for HPLC analysis on an Agilent 1260 system (Agilent Technologies, Santa Clara, CA, USA). The mobile phase (methanol: ethanol; 65: 35) was applied at a flow rate of 1.5 mL/min at room temperature, and the eluate was monitored at 275 nm using a photodiode array detector (Agilent Technologies, Santa Clara, CA, USA).
2.6. Fed-batch fermentation
Fed-batch fermentation was performed in a 100-L stirred bioreactor (Shanghai Guoqiang Bioengineering Equipment CO., LTD, Shanghai, China) with an initial working volume of 40 L. Foam formation was prevented by the addition of antifoam 204 (Sigma–Aldrich, St. Louis, MO, USA). The temperature was maintained at a constant 32 °C, aeration at 1.0 VVM, agitation at 650 rpm, and pH at 6.5 by automatic injection of acetic acid or ammonia. The fed-batch process was initiated after 16 h of cultivation from a 600 g/L concentrated glucose stock solution.
2.7. RNA sequencing (RNA-seq) and transcriptome analysis
RNA-seq was performed as described previously [40]. Total RNA was isolated using a Redzol reagent kit from SBS Genetech Co. Ltd (Beijing, China). The quality of the RNA samples was analyzed using an Agilent Bioanalyzer 2100 system (Agilent Technologies), and mRNA was enriched by rRNA depletion and followed by mRNA fragmentation, cDNA strand synthesis and library construction. The RNA-seq and transcriptomic analyses were performed by Novogene Co., Ltd (Beijing, China).
2.8. Statistical analysis
Statistical analyses were performed using Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA). Unless otherwise indicated, data are expressed as mean ± standard error of mean (SEM) and were analyzed by an unpaired two-tailed Student's t-test. P < 0.05 indicated statistical significance.
3. Results
3.1. Overexpression of enzymes associated with the MEP and quinone-modification pathways failed CoQ10 overproduction in HY01
HY01 was screened and obtained by N-methyl-Nʹ-nitro-N-nitrosoguanidine mutagenesis of wild-type R. sphaeroides 2.4.1. The content of CoQ10 in HY01 is hundreds of folds higher than normal physiological levels [41]. In the shake-flask fermentation, the initial production of CoQ10 was about 190 mg/L (Table 1). We evaluated previously reported enzymes involved in CoQ10 biosynthesis, including UbiG, UbiE, and UbiH etc., by overexpressing them in pBBR1MCS2. As shown in Table 1, overexpression of these enzymes did not affect CoQ10 production in HY01, suggesting that in this CoQ10 industrial strain, the biosynthetic pathways were not the rate-limiting steps in CoQ10 overproduction.
3.2. Phosphate-limitation increases CoQ10-biosynthesis efficiency in HY01
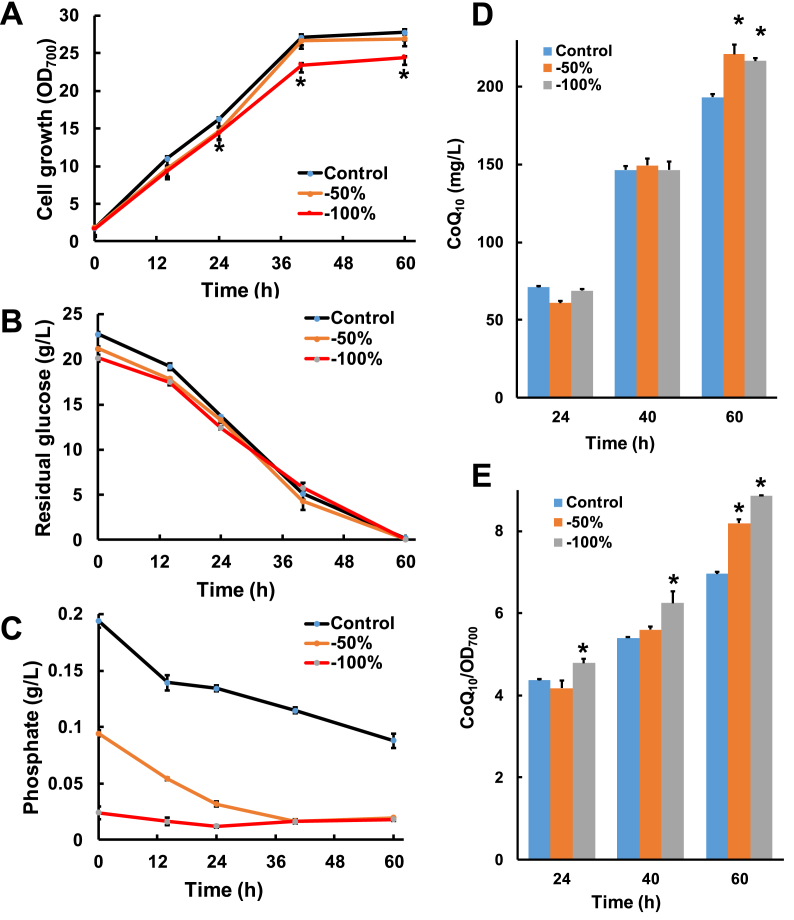
In this study, we found that the reduction of inorganic phosphate in media significantly decreased HY01 growth during fermentation (Fig. 2A), and that the consumption of glucose was slightly elevated at the early stage (before 24 h) of fermentation (Fig. 2B). Additionally, the consumption of inorganic phosphate decreased along with the addition of phosphate, with residual phosphate in the fermentation broth in the absence of inorganic phosphate addition remaining stable at low levels (Fig. 2C). However, compared with the control group (with phosphate addition), phosphate limitation resulted in a significant increase in CoQ10 production at the end of fermentation (60 h) by 12% (P < 0.05). Moreover, CoQ10 production per unit cell significant increased from 10% (24 h; P < 0.05) to 27% (60 h; P < 0.05) during fermentation (Fig. 2D & E). These results indicated that under inorganic phosphate-limited conditions, CoQ10 production by HY01 could be increased by improving the productivity ratio of the unit cell.
Fig. 2.
Effects of phosphate-limitation on HY01 fermentation. Time course of cell growth (A), residual phosphate (B) and residual glucose (C) levels, CoQ10 production (D) and the productivity of unit cells (E) in shake-flask fermentation. *P < 0.05. Control: normal culture conditions (fermentation medium containing 0.3% KH2PO4); −50%: removal of 50% KH2PO4 (fermentation medium containing 0.15% KH2PO4); −100%: without KH2PO4 addition.
3.3. CoQ10 production during glucose fed-batch fermentation under phosphate-limited conditions
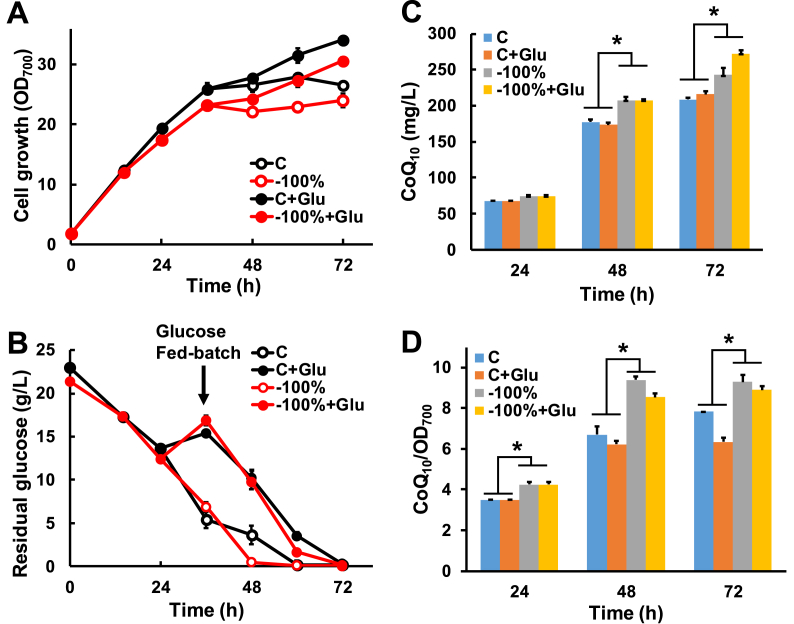
To evaluate CoQ10 fermentation potential under inorganic phosphate-limiting conditions, we performed glucose fed-batch culture in flasks. We found that in either the presence or absence of phosphate, glucose feeding at 10 g/L for 36 h significantly increased end-stage cell growth (after 48 h) (Fig. 3A). Additionally, under phosphate-limited conditions, the glucose-consumption rate increased after glucose feeding (Fig. 3B). After 48 h in the absence of phosphate addition, CoQ10 production increased significantly, resulting in the largest increase in production at the end of fermentation (26%; −100% + Glu vs. C + Glu; up to 272 mg/L CoQ10) (Fig. 3C). Moreover, under these conditions, the CoQ10-productivity ratio of the unit cells increased significantly, regardless of glucose feeding. Notably, glucose feeding slight decreased the CoQ10-productivity ratio of the unit cells under phosphate-limited conditions (Fig. 3D). These results suggested that during scale-up fermentation, conditions related to glucose feeding and phosphate-limitation should be carefully optimized to maximized CoQ10-production efficiency.
Fig. 3.
Effect of glucose feeding on HY01 fermentation in the present or absence of inorganic phosphate addition. Time course of cell growth (A), residual glucose level (B), CoQ10 production (C), and the productivity of the unit cells (D) during shake-flask fermentation. *P < 0.05. C: control, normal culture conditions; −100%: without KH2PO4 addition; +Glu: addition of glucose (fed-batch culture with a final glucose concentration of 10 g/L) for 36 h.
3.4. Scale-up fermentation under phosphate-limited conditions in a 100-L bioreactor
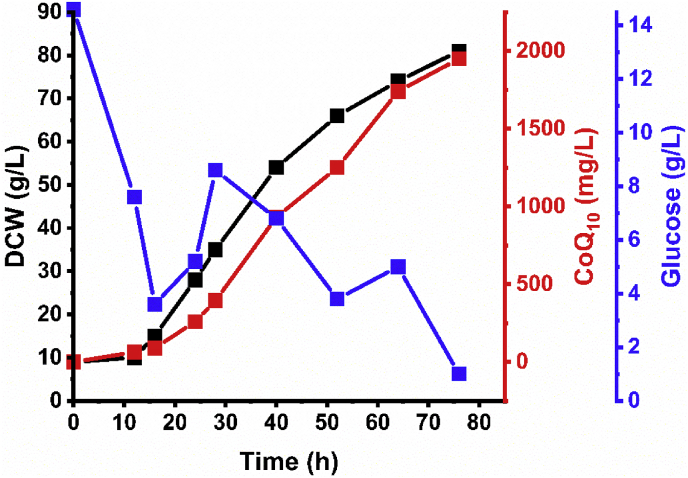
To demonstrate the application of a phosphate-limiting strategy, we used a 100-L stirred bioreactor for CoQ10 scale-up fermentation. Time course of CoQ10 fermentation (Fig. 4) showed that under phosphate-limited conditions (<0.15 g/L), CoQ10 production reached 1.95 g/L by the end of fermentation, which represents the highest reported total to date. This result demonstrated phosphate-limitation as an efficient strategy for CoQ10 production in HY01.
Fig. 4.
Time course of CoQ10 fermentation under phosphate-limited conditions in a 100-L stirred bioreactor. The fed-batch process was initiated after 16 h of cultivation from a 600 g/L concentrated glucose stock solution. The concentration of residual phosphate remained <0.15 g/L.
3.5. Transcriptome analysis of HY01 under phosphate-limited conditions
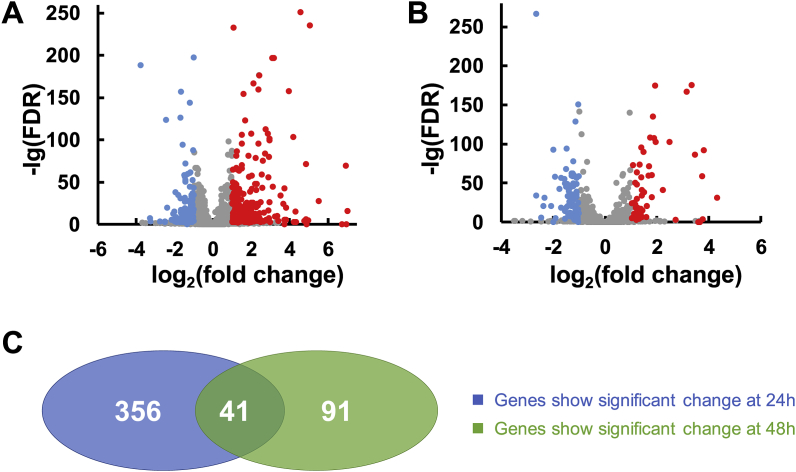
To investigate the effects of phosphate limitation on cell metabolism, we performed RNA-seq analysis to compare the transcriptomes in the presence and absence of inorganic phosphate addition at two time-points during fermentation. As shown in Fig. 5A, we found that 397 genes exhibited a 2-fold change in transcription under phosphate-limited conditions, with 133 genes upregulated and 264 downregulated over 24 h. During the later stage of fermentation (48 h), 132 genes exhibited significantly altered expression, with 78 genes upregulated and 54 genes downregulated (Fig. 5B). Fig. 5C shows the overlap of 41 genes exhibiting changes in transcription between 24 h and 48 h in the presence or absence of phosphate addition. As expected, significantly upregulated genes were involved in energy/carbohydrate/lipid/peptidoglycan metabolism, transporter, signal transduction, and the pilus system under phosphate-limited conditions, whereas only a few genes involved in oxidative degradation and stress response were significantly downregulated (Table 2). These findings indicated that phosphate limitation caused a pleiotropic physiological effect in HY01.
Fig. 5.
Comparative transcriptomic analysis in HY01 in the presence or absence of phosphate addition. Volcano plot showing gene transcription with phosphate addition over 24 h (A) and 48 h (B) of fermentation. Green: upregulated genes in the control group (+phosphate); red: downregulated genes in the control group (+phosphate). (C) Venn diagram showing the overlapping genes exhibiting significant alterations of transcription between 24 h and 48 h in the presence or absence of phosphate addition.
Table 2.
Selected genes that are probably affected by phosphate limitation in HY01.
| Gene | Description | Function annotation | log2FDa |
|---|---|---|---|
| RSP_2020 | DHC diheme cytochrome C | Energy metabolism | −7.01 |
| nuoI1 | NADH-quinone oxidoreductase | Energy metabolism | 1.95 |
| RSP_1848 | Pyruvate kinase | Glycolysis | −6.97 |
| glgC | ADP-glucose pyrophosphorylase | Glycogen metabolism | 1.47 |
| btaB | SAM-diacylgycerolhomoserine-N-methyltransferase | Lipid metabolism | −4.86 |
| btaA | SAM-diacylglycerol 3-amino-3-carboxypropyl transferase | Lipid metabolism | −4.18 |
| dgkA | Diacylglycerol kinase | Lipid metabolism | −3.54 |
| murC | UDP-N-acetylmuramate--alanine ligase | Peptidoglycan metabolism | −1.22 |
| murG | UDP-N-acetylglucosamine--N-acetylmuramyl-pyrophosphoryl-undecaprenol N-acetylglucosamine transferase | Peptidoglycan metabolism | −1.13 |
| RSP_2543 | Peptidoglycan-binding LysM | Peptidoglycan metabolism | −1.23 |
| RSP_1794 | Putative lytic transglycosylase | Peptidoglycan metabolism | −1.22 |
| expE1 | Hemolysin-type calcium-binding region | Galactoglucan metabolism | −1.99 |
| RSP_2320 | TRAP-T family transporter | Transporter | −2.38 |
| RSP_1883 | ABC polyamine/opine transporter | Transporter | −2.12 |
| RSP_1613 | TRAP-T family transporter | Transporter | −1.74 |
| xylF | d-xylose transport system substrate-binding protein | Transporter | −1.50 |
| RSP_3701 | Monosaccharide ABC transporter substrate-binding protein | Transporter | −1.27 |
| dctP | TRAP-T family transporter | Transporter | −1.04 |
| RSP_0454 | Two-component system | Signal transduction | −3.18 |
| ctrA | Two-component system | Signal transduction | −2.40 |
| RSP_3975 | Two-component system | Signal transduction | −2.29 |
| RSP_2177 | DNA protecting protein DprA | Replication and repair | −2.96 |
| RSP_3094 | Putative transmembrane anti-sigma factor | Transcription machinery | −2.43 |
| RSP_3095 | RNA polymerase sigma-70 factor | Transcription machinery | −1.24 |
| rpsK | 30S ribosomal protein S11 | Ribosome | 1.14 |
| RSP_3802 | Universal stress protein UspA-like protein | Stress | 1.14 |
| RSP_3180 | Transglutaminase-like enzyme | Stress | 1.35 |
| RSP_1909 | Pilus assembly protein CpaC | Pilus system | −1.67 |
| RSP_1908 | Outer membrane protein | Pilus system | −1.59 |
| RSP_0443 | Rrf2 family transcriptional regulator | Transcription factors | −1.34 |
| RSP_7510 | Hypothetical protein | Unknown | −6.94 |
| RSP_1521 | Hypothetical protein | Unknown | −3.82 |
| RSP_3092 | Hypothetical protein | Unknown | −2.49 |
| RSP_3363 | Hypothetical protein | Unknown | −1.17 |
| RSP_7526 | Hypothetical protein | Unknown | 1.44 |
| RSP_6120 | Protein of unknown function (DUF3309) | Unknown | 1.18 |
| RSP_2019 | Protein of unknown function (DUF3478) | Unknown | −3.68 |
FD = FPKM(-phosphate)/FPKM(+phosphate); FPKM: fragments per kilobase of transcript per million fragments mapped.
4. Discussion
Improvement of high yield industrial strains often requires system-wide engineering and optimization of cellular metabolism [42]. Following several rounds of mutagenesis and selection, the biosynthetic efficiency of CoQ10 in HY01 has been dramatically increased relative to that observed in an engineered strain derived from R. sphaeroides 2.4.1 [22]. In the present study, we initially focused on evaluation of the CoQ10 biosynthetic pathway, finding that previous methods [2,[22], [23], [24]] were unable to enhance production (Table 1) in HY01. We speculated that the biosynthetic pathway was likely not a bottleneck for CoQ10 overproduction in the industrial strain HY01. And based on previous observations, the accumulation of CoQ10 in HY01 was much more like the phenomenon associated with physiological responses to oxygen supply or energy (ATP) generation [43,44].
Phosphate is important for cell-membrane structure, nucleotide biosynthesis and ATP metabolism in cells. To further enhance CoQ10 production in HY01, the effects of phosphate concentration on HY01 fermentation has been investigated. We subsequently found that under phosphate-limited conditions, CoQ10 production at the end of fermentation (60 h) significantly increased at the expense of reduced cell growth (Fig. 2). Phosphate starvation leading to reduced growth has been widely reported [45]. Surprisingly, CoQ10 productivity of the unit cell increased significantly during fermentation (Fig. 2E), suggesting that by balancing cell growth and unit-cell productivity, this industrial strain should show improved fermentation performance. As expected, glucose fed-batch fermentation in a shake flask resulted in increased cell growth and a 26% increase in CoQ10 production relative to the control group, reaching 272 mg/L (Fig. 3). These findings showed that combined glucose feeding and phosphate limitation was an efficient strategy for CoQ10 production. We then applied this strategy in a pilot scale-up fermentation using a 100-L bioreactor (Fig. 4), resulting in the highest recorded of production of CoQ10 in R. sphaeroides [22]. Consequently, the role of phosphate limitation in accumulation of CoQ10 is worth to be further investigated and a future strategy for metabolic engineering of HY01 for CoQ10 overproduction would be developed based on the understanding of this mechanism.
Subsequent transcriptome analysis to determine the transcriptional mechanisms associated with altered CoQ10 production verified the induction of a pleiotropic effect on gene expression by phosphate limitation resulting from changes in the expression of hundreds of genes (Fig. 5). Table 1 shows the genes exhibiting transcriptional alteration between 24 h and 48 h in the presence or absence of phosphate addition. Previous studies report that under stress associated with phosphate-limited conditions, membrane phospholipids are partially replaced by lipids containing no phosphorus [e.g. betaine lipid diacylglyceryl-O-4’-(N,N,N,-trimethyl)homoserine and diacylglyceryl-O-2’-(hydroxymethyl) (N,N,N-trimethyl)-β-alanine] [35,36]. Moreover, btaA/btaB were identified as genes essential for biosynthesis of these betaine lipids [37] and demonstrated as significantly upregulated under phosphate-limited conditions in HY01. Moreover, previous studies report that levels of the ABC transporter and TRAP-T family transporters were upregulated and increased phosphate (or other substrate) acquisition from medium [46,47]. These findings indicated that HY01 was capable of regulating the mechanisms of phosphate assimilation and uptake in order to adapt to phosphate-limited conditions. Additionally, we found that oxidative phosphorylation and diheme cytochrome C biosynthesis were significantly affected by phosphate limitation. Previous studies reported the involvement of these two proteins in energy metabolism [48,49], and recent studies showed that CoQ10 yield in R. sphaeroides could be improved by modifying the redox respiration chain or redox potential [22,50]. Therefore, a possible explanation for why phosphate limitation increased CoQ10 biosynthesis might involve disturbance of energy metabolism or redox potential in the industrial strain. Additionally, as shown in Table 2, the significant changes in carbohydrate metabolism (e.g., glycolysis and/or glycogen metabolism), the two-component system, cell stress, and the pilus system indicated ubiquitous and profound effects from phosphate limitation on cell metabolism. These mechanisms remain to be further elucidated in future work.
5. Conclusion
Based on these results, we concluded that the biosynthetic pathway was likely not a bottleneck for CoQ10 overproduction in HY01. And these results also demonstrated phosphate limitation combined with glucose fed-batch fermentation as a useful strategy for CoQ10 production in the industrial strain HY01, and that this strategy might be applicable for industrial scale-up manufacture of CoQ10. Additionally, we found that phosphate limitation induced a pleiotropic effect on cell metabolism, and that improved CoQ10 biosynthesis efficiency was possibly related to the disturbance of energy metabolism and redox potentials. Further study is necessary to elucidate the mechanisms associated with upregulated CoQ10 production in HY01.
Declaration of competing interest
The authors declare that they have no competing financial interests.
Acknowledgements
The authors appreciate Dr. Jin Miao for the help to construct engineered strains in Table 1. The author also appreciates Prof. Hongwei Yu for providing plasmid materials. This work was supported by the National Natural Science Foundation of China [31870040, 31430002, 31720103901], the 111 Project of China [B18022], the Fundamental Research Funds for the Central Universities [22221818014], the Natural Science Foundation of Shandong Province [ZR2017ZB0206], and the Shandong Taishan Scholar Award to Lixin Zhang.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2019.11.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ayer A., Macdonald P., Stocker R. CoQ10 function and role in heart failure and ischemic heart disease. Annu Rev Nutr. 2015;35:175–213. doi: 10.1146/annurev-nutr-071714-034258. [DOI] [PubMed] [Google Scholar]
- 2.Lu W., Ye L., Lv X., Xie W., Gu J., Chen Z. Identification and elimination of metabolic bottlenecks in the quinone modification pathway for enhanced coenzyme Q10 production in Rhodobacter sphaeroides. Metab Eng. 2015;29:208–216. doi: 10.1016/j.ymben.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Tan G.Y., Liu T. Rational synthetic pathway refactoring of natural products biosynthesis in actinobacteria. Metab Eng. 2017;39:228–236. doi: 10.1016/j.ymben.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo Y., Zhang W., Chen D., Gao H., Tao J., Liu M. Reverse biological engineering of hrdB to enhance the production of avermectins in an industrial strain of Streptomyces avermitilis. Proc Natl Acad Sci USA. 2010;107:11250–11254. doi: 10.1073/pnas.1006085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuratsu Y., Inuzuka K. Factors affecting broth viscosity and coenzyme Q10 production by Agrobacterium species. Appl Microbiol Biotechnol. 1985;21:55–59. [Google Scholar]
- 6.Jung H.M., Kim S.Y., Moon H.J., Oh D.K., Lee J.K. Optimization of culture conditions and scale-up to pilot and plant scales for vancomycin production by Amycolatopsis orientalis. Appl Microbiol Biotechnol. 2007;77:789–795. doi: 10.1007/s00253-007-1221-4. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura M., Kobayashi T., Aiba S. Anaerobic production of ubiquinone-10 by Paracoccus denitrificans. Eur J Appl Microbiol Biotechnol. 1983;17:85–89. [Google Scholar]
- 8.Yen H.W., Shih T.Y. Coenzyme Q10 production by Rhodobacter sphaeroides in stirred tank and in airlift bioreactor. Bioproc Biosyst Eng. 2009;32:711–716. doi: 10.1007/s00449-008-0294-5. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H., Kotani Y., Ochiai K., Araki K. Production of ubiquinone-10 using bacteria. J Gen Appl Microbiol. 1998;44:19–26. doi: 10.2323/jgam.44.19. [DOI] [PubMed] [Google Scholar]
- 10.Cluis C.P., Burja A.M., Martin V.J.J. Current prospects for the production of coenzyme Q10 in microbes. Trends Biotechnol. 2007;25:514–521. doi: 10.1016/j.tibtech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D., Shrestha B., Niu W., Tian P., Tan T. Phenotypes and fed-batch fermentation of ubiquinone-overproducing fission yeast using ppt1 gene. J Biotechnol. 2007;128:120–131. doi: 10.1016/j.jbiotec.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Dixson D.D., Boddy C.N., Doyle R.P. Reinvestigation of coenzyme Q10 isolation from Sporidiobolus johnsonii. Chem Biodivers. 2011;8:1033–1051. doi: 10.1002/cbdv.201000278. [DOI] [PubMed] [Google Scholar]
- 13.Liu X., Wu H., Ye J., Yuan Q., Zhang H. Cloning and characterization of the ddsA gene encoding decaprenyl diphosphate synthase from Rhodobacter capsulatus B10. Can J Microbiol. 2006;52:1141–1147. doi: 10.1139/w06-080. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.K., Her G., Kim S.Y., Seo J.H. Cloning and functional expression of the dps gene encoding decaprenyl diphosphate synthase from Agrobacterium tumefaciens. Biotechnol Prog. 2004;20:51–56. doi: 10.1021/bp034213e. [DOI] [PubMed] [Google Scholar]
- 15.Huang H., Levin E.J., Liu S., Bai Y., Lockless S.W., Zhou M. Structure of a membrane-embedded prenyltransferase homologous to UBIAD1. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cluis C.P., Ekins A., Narcross L., Jiang H., Gold N.D., Burja A.M. Identification of bottlenecks in Escherichia coli engineered for the production of CoQ10. Metab Eng. 2011;13:733–744. doi: 10.1016/j.ymben.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.J., Kim M.D., Choi J.H., Kim S.Y., Ryu Y.W., Seo J.H. Amplification of 1-deoxy-d-xyluose 5-phosphate (DXP) synthase level increases coenzyme Q10 production in recombinant Escherichia coli. Appl Microbiol Biotechnol. 2006;72:982–985. doi: 10.1007/s00253-006-0359-9. [DOI] [PubMed] [Google Scholar]
- 18.Zahiri H.S., Yoon S.H., Keasling J.D., Lee S.H., Won Kim S., Yoon S.C. Coenzyme Q10 production in recombinant Escherichia coli strains engineered with a heterologous decaprenyl diphosphate synthase gene and foreign mevalonate pathway. Metab Eng. 2006;8:406–416. doi: 10.1016/j.ymben.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Okada K., Kainou T., Matsuda H., Kawamukai M. Biological significance of the side chain length of ubiquinone in Saccharomyces cerevisiae. FEBS Lett. 1998;431:241–244. doi: 10.1016/s0014-5793(98)00753-4. [DOI] [PubMed] [Google Scholar]
- 20.Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem. 2009;53:217–226. doi: 10.1042/BA20090035. [DOI] [PubMed] [Google Scholar]
- 21.Xu W., Yuan J., Yang S., Ching C.B., Liu J. Programming saposin-mediated compensatory metabolic sinks for enhanced ubiquinone production. ACS Synth Biol. 2016;5:1404–1411. doi: 10.1021/acssynbio.6b00148. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y., Ye L., Chen Z., Hu W., Shi Y., Chen J. Synergic regulation of redox potential and oxygen uptake to enhance production of coenzyme Q10 in Rhodobacter sphaeroides. Enzym Microb Technol. 2017;101:36–43. doi: 10.1016/j.enzmictec.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Lu W., Shi Y., He S., Fei Y., Yu K., Yu H. Enhanced production of CoQ10 by constitutive overexpression of 3-demethyl ubiquinone-9 3-methyltransferase under tac promoter in Rhodobacter sphaeroides. Biochem Eng J. 2013;72:42–47. [Google Scholar]
- 24.Lu W., Ye L., Xu H., Xie W., Gu J., Yu H. Enhanced production of coenzyme Q10 by self-regulating the engineered MEP pathway in Rhodobacter sphaeroides. Biotechnol Bioeng. 2014;11:761–769. doi: 10.1002/bit.25130. [DOI] [PubMed] [Google Scholar]
- 25.Koo B.S., Gong Y.J., Kim S.Y., Kim C.W., Lee H.C. Improvement of coenzyme Q10 production by increasing the NADH/NAD+ ratio in Agrobacterium tumefaciens. Biosci Biotechnol Biochem. 2010;74:895–898. doi: 10.1271/bbb.100034. [DOI] [PubMed] [Google Scholar]
- 26.Kovach M.E., Elzer P.H., Steven Hill D., Robertson G.T., Farris M.A., Roop R.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 27.Yadav K.K., Singh N., Rajasekharan R. Responses to phosphate deprivation in yeast cells. Curr Genet. 2016;62:301–307. doi: 10.1007/s00294-015-0544-4. [DOI] [PubMed] [Google Scholar]
- 28.Brown M.R.W., Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci USA. 2004;101:16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornberg A., Rao N.N., Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;8:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 30.Xie L., Jakob U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J Biol Chem. 2019;294:2180–2190. doi: 10.1074/jbc.REV118.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran-Din K., Gottschalk G. Formation of d(-)-1,2-propanediol and d(-)-lactate from glucose by Clostridium sphenoides under phosphate limitation. Arch Microbiol. 1985;142:87–92. [Google Scholar]
- 32.Cameron D.C., Altaras N.E., Hoffman M.L., Shaw A.J. Metabolic engineering of propanediol pathways. Biotechnol Prog. 1998;14:116–125. doi: 10.1021/bp9701325. [DOI] [PubMed] [Google Scholar]
- 33.Shang L., Jiang M., Chang H.N. Poly(3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol Lett. 2003;25:1415–1419. doi: 10.1023/a:1025047410699. [DOI] [PubMed] [Google Scholar]
- 34.Sola-Landa A., Moura R.S., Martín J.F. The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proc Natl Acad Sci USA. 2003;100:6133–6138. doi: 10.1073/pnas.0931429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benning C., Beatty J.T., Prince R.C., Somerville C.R. The sulfolipid sulfoquinovosyldiacylglycerol is not required for photosynthetic electron transport in Rhodobacter sphaeroides but enhances growth under phosphate limitation. Proc Natl Acad Sci USA. 1993;90:1561–1565. doi: 10.1073/pnas.90.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benning C., Huang Z.H., Gage D.A. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch Biochem Biophys. 1995;317:103–111. doi: 10.1006/abbi.1995.1141. [DOI] [PubMed] [Google Scholar]
- 37.Klug R.M., Benning C. Two enzymes of diacylglyceryl-O-4'-(N,N,N,-trimethyl)homoserine biosynthesis are encoded by btaA and btaB in the purple bacterium Rhodobacter sphaeroides. Proc Natl Acad Sci USA. 2001;98:5910–5915. doi: 10.1073/pnas.101037998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon R., Priefer U., Pühler A.A. Broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol. 1993;1:784–791. [Google Scholar]
- 39.Rouser G., Fleischer S., Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 40.Tan G.Y., Peng Y., Lu C., Bai L., Zhong J.J. Engineering validamycin production by tandem deletion of γ-butyrolactone receptor genes in Streptomyces hygroscopicus 5008. Metab Eng. 2015;28:74–81. doi: 10.1016/j.ymben.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary M., Fu Y.X., Mackenzie C., Kaplan S. DNA sequence duplication in Rhodobacter sphaeroides 2.4.1: evidence of an ancient partnership between chromosomes I and II. J Bacteriol. 2004;186:2019–2027. doi: 10.1128/JB.186.7.2019-2027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S.Y., Kim H.U. Systems strategies for developing industrial microbial strains. Nat Biotechnol. 2015;33:1061–1072. doi: 10.1038/nbt.3365. [DOI] [PubMed] [Google Scholar]
- 43.Yen H.W., Chiu C.H. The influences of aerobic-dark and anaerobic-light cultivation on CoQ10 production by Rhodobacter sphaeroides in the submerged fermenter. Enzym Microb Technol. 2007;41:600–604. [Google Scholar]
- 44.Sakato K., Tanaka H., Shibata S., Kuratsu Y. Agitation-aeration studies on coenzyme Q10 production using Rhodopseudomonas spheroides. Biotechnol Appl Biochem. 1992;16:19–28. [Google Scholar]
- 45.Geske T., Vom Dorp K., Dörmann P., Hölzl G. Accumulation of glycolipids and other non-phosphorous lipids in Agrobacterium tumefaciens grown under phosphate deprivation. Glycobiology. 2012;23:69–80. doi: 10.1093/glycob/cws124. [DOI] [PubMed] [Google Scholar]
- 46.Krol E., Becker A. Global transcriptional analysis of the phosphate starvation response in Sinorhizobium meliloti strains 1021 and 2011. Mol Genet Genom. 2004;272:1–17. doi: 10.1007/s00438-004-1030-8. [DOI] [PubMed] [Google Scholar]
- 47.Gebhard S., Tran S.L., Cook G.M. The Phn system of Mycobacterium smegmatis: a second high-affinity ABC-transporter for phosphate. Microbiology. 2006;152:3453–3465. doi: 10.1099/mic.0.29201-0. [DOI] [PubMed] [Google Scholar]
- 48.Peng G., Meyer B., Sokolova L., Liu W., Bornemann S., Juli J. Identification and characterization two isoforms of NADH:ubiquinone oxidoreductase from the hyperthermophilic eubacterium Aquifex aeolicus. Biochem Eng J. 2018;1859:366–373. doi: 10.1016/j.bbabio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Li B.R., Anderson J.L.R., Mowat C.G., Miles C.S., Reid G.A., Chapman S.K. Rhodobacter sphaeroides haem protein: a novel cytochrome with nitric oxide dioxygenase activity. Biochem Eng J. 2008;36:992–995. doi: 10.1042/BST0360992. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Gao D., Cai J., Liu H., Qi Z. Improving coenzyme Q10 yield of Rhodobacter sphaeroides via modifying redox respiration chain. Biochem Eng J. 2018;135:98–104. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.