Abstract
Background
In response to various environmental stresses, many plant species synthesize L-proline in the cytosol and accumulates in the chloroplasts. L-Proline accumulation in plants is a well-recognized physiological reaction to osmotic stress prompted by salinity, drought and other abiotic stresses. L-Proline plays several protective functions such as osmoprotectant, stabilizing cellular structures, enzymes, and scavenging reactive oxygen species (ROS), and keeps up redox balance in adverse situations. In addition, ample-studied osmoprotective capacity, L-proline has been also ensnared in the regulation of plant improvement, including flowering, pollen, embryo, and leaf enlargement.
Scope and conclusions
Albeit, ample is now well-known about L-proline metabolism, but certain characteristics of its biological roles are still indistinct. In the present review, we discuss the L-proline accumulation, metabolism, signaling, transport and regulation in the plants. We also discuss the effects of exogenous L-proline during different environmental conditions. L-Proline biosynthesis and catabolism are controlled by several cellular mechanisms, of which we identify only very fewer mechanisms. So, in the future, there is a requirement to identify such types of cellular mechanisms.
Keywords: L-proline, Osmoprotectant, Environmental stresses, Cellular mechanisms, Signal transduction, Biochemistry, Molecular biology, Cell biology, Plant biology
L-Proline; Osmoprotectant; Environmental stresses; Cellular mechanisms; Signal transduction, Biochemistry, Molecular biology, Cell Biology, Plant Biology.
1. Introduction
Salinity, high temperature, dry season, heavy metals, nutrient inadequacy, UV-radiation and pathogenic diseases are various types of abiotic and biotic stresses which affect the plants throughout their life cycle (Siripornadulsil et al., 2002). These various abiotic stresses lead to changes in cellular level, secondary stresses comprising protein denaturation, memberane injury, destruction of reactive species and osmotic stress, which are interrelated to each other. During stress conditions, plants accumulate different osmolytes, especially L-proline, L-proline betaine, glycine betaine, glycerol, mannitol, sorbitol etc (Sharma et al., 2019). Plants use these osmolytes to cope up with the stress conditions by employing different mechanisms such as change in cell osmotic pressure, contribution in reactive oxygen species (ROS) detoxification, assurance of membrane respectability and adjustment of compounds/proteins (Ashraf and Foolad, 2007; Hayat et al., 2012). Among them, the aggregation of L-proline upon osmotic pressure are all around seen in an enormous number of various creatures, comprising protozoa (Poulin et al., 1987; Inbar et al., 2013), eubacteria (Csonka, 1989; Verbruggen et al., 1993; Otvos et al., 2018), marine invertebrates (Burton, 1991; Sperstad et al., 2011), and algae (Brown and Hellebust, 1978; Liang et al., 2013; Kumari et al., 2018a, b), as well as in various plant species (Nakashima et al., 1998; Mattioli et al., 2008; Székely et al., 2008; Chun and Chandrasekaran, 2018). Besides acting as an osmolyte, L-proline additionally assumes significant character throughout stress as a metal chelator and an antioxidative defense molecule. L-Proline is a proteinogenic amino acid with the α-amino group present as an auxiliary amine which is essential for primary metabolism (Verslues and Sharma, 2010; Barupal et al., 2019). L-Proline aggregation ordinarily happens in cytoplasm where it works as molecular chaperons settling the structure of proteins and its accumulation buffers cytosolic pH and maintains up cell redox status. L-Proline works as an osmolyte, radical scrounger, electron sink, stabilizer of different macromolecules, and component of cell wall (Matysik et al., 2002). Diverse reports indicated that exogenous consumption of L-proline stimulates abiotic stresses tolerance in plants. In various environmental stress situations, the free radical generation rise above the entire cellular antioxidative capability prominent to oxidative stress, which participates to antagonistic effects on plant development. Under salinity, up to 1 mol l−1 L-proline accumulates in entire tissue. Expanded degrees of L-proline is associated with upgraded salinity resilience (Munns, 2005), for instance, in tobacco cell culture communicating the regulatory Hal3 gene from Arabidopsis thaliana (Yonamine et al., 2004). The present review focuses on the synthesis, accumulation and metabolism of L-proline. The main emphasis of this review is based on the role of L-proline in stress resistance in plants during several environmental conditions.
2. L-Proline accumulation and stress tolerance in plants
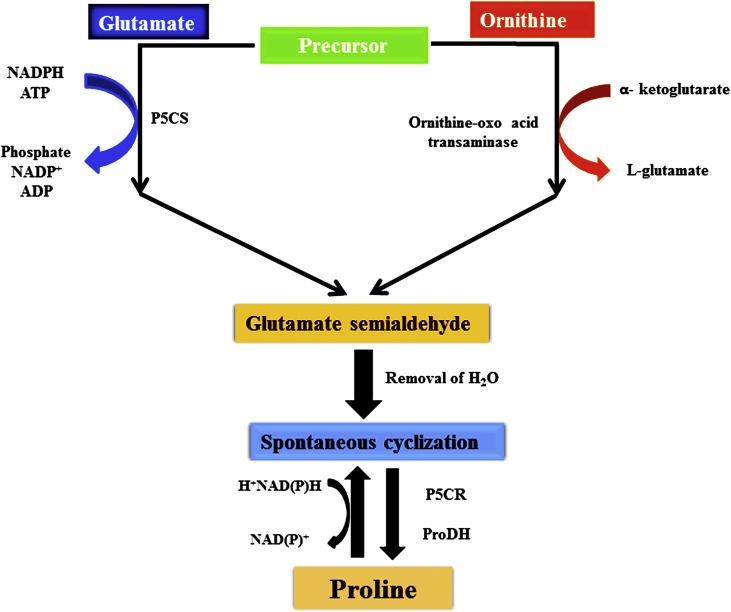
L-Proline comprises less than 5% of the total pool of the free amino acids in plants under regular conditions (Shahbaz et al., 2013). In numerous plants under different type of stress, the concentration increments up to 80% of the amino acid pool. Intracellular L-proline levels in plants are administered by biosynthesis, catabolism and transport among cells and various compartments of cell. L-proline is dominatingly incorporated from glutamate. Three enzymatic activities, in particular (i) the Δ1-γ-glutamyl kinase (EC 2.7.2.11) action of Δ1-pyrroline-5-carboxylate synthetase (At2g39800), (ii) the glutamic-γ-semialdehyde dehydrogenase (EC 1.2.1.41) movement of Δ1-pyrroline-5-carboxylate synthetase (P5CS), and (iii) two isogenes of Δ1-pyrroline-5-carboxylate reductase (P5CR; EC 1.5.1.2) convert glutamate to L-proline in three exergonic responses devouring 1 ATP and 2 NADPH per L-proline. The utilization of two moles of NADPH indicates that L-proline involves in electron sink mechanism. L-proline is synthesized from ornithine by ornithine-δ-aminotransferase (OAT), where Δ1-pyrroline-5-carboxylate (P5C) is delivered. In higher plants, L-proline biosynthesis happens either through the glutamate or the ornithine pathway (Figure 1). Contingent on ecological conditions, L-proline can be coordinated in various subcellular compartments. Housekeeping biosynthesis of L-proline happens in the cytosol, and in Arabidopsis it is constrained by the P5CS2 gene (Székely et al., 2008), which is dynamic in partitioning meristematic tissues, for example, shoot and root tips, and inflorescences (Madan et al., 1995; Deuschle et al., 2001; Tripathi and Gaur, 2004; Meena et al., 2018a, b). Both P5CS genes (involve in biosynthesis of L-proline) are dynamic in floral shoot apical meristems and contribute in bloom improvement (Csonka and Hanson, 1991). L-Proline integration in chloroplasts is constrained by the stress initiated gene pyrroline-5-carboxylate synthetase (P5CS1) in Arabidopsis (Savouré et al., 1995; Strizhov et al., 1997; Székely et al., 2008).
Figure 1.
Figure showing the metabolic pathway of L-proline through glutamate and ornithine. It also indicates the basic difference between the glutamate pathway and ornithine pathway for L-proline synthesis.
Any adjustment in the encompassing condition may upset homeostasis. Natural adjustment of homeostasis might be characterized as biological stress. One of the stress reactions in plants is the accelerated generation of ROS e.g., OH•, O2•, H2O2 and so on. These ROS cause extensive harm through membrane lipid peroxidation and furthermore through direct communication with different macromolecules. Cells have adjusted various components to hold the ROS level in check. In any case, less concentration of ROS partakes in a sign transduction component. L-Proline gives protection to plants from stress by adding to cell osmotic change, ROS detoxification, insurance of membrane uprightness and catalysts/protein adjustment (Figure 2). Saradhi et al. (1995) revealed the gathering of L-proline in rice, mustard and mung bean plants against UV radiation. Molecular mechanism of extinguishing of ROS by L-proline in plants has been reported by Matysik et al. (2002). L-Proline aggregation likewise happens in plants in light of drought stress. For instance, water shortfall rice plants collected high measures of L-proline in leaves (Hsu et al., 2003) which were credited to improved substance of the antecedents for L-proline biosynthesis, including glutamic acid, ornithine and arginine. On account of wheat, rate of L-proline aggregation and use was likewise impressively higher in the drought tolerant cultivar than drought sensitive cultivar (Nayyar and Walia, 2003). Besides, activities of L-proline biosynthetic enzymes P5CR and OAT expanded principally in tolerant lines in Brassica juncea plants developed under stressed condition; in any case, the action of catalyst which debases L-proline "L-proline oxidase" diminished in all lines (Madan et al., 1995; Meena et al., 2013). L-Proline collection has additionally been proposed to actuate elective detoxification pathway by ensuring and settling ROS scavenging enzymes. In salt stressed tobacco cells, L-proline has been shown to increase the activity of methyl-glyoxal detoxification enzymes; improve peroxidase, glutathione-S-transferase, superoxide dismutase and catalase activities; and increment the glutathione redox state (Hoque et al., 2008; Islam et al., 2009; Meena and Samal, 2019). Aggregation of P5CS1 and P5CR in chloroplasts during the states of salt stress shows that under such unfriendly conditions, glutamate-inferred L-proline biosynthesis is raised in plastids, where photosynthesis happens (Székely et al., 2008). The relationship between L-proline accumulation and abiotic stresses resistance in plants is not clear till now. Aggregation of L-proline in the leaves was accepted to be an indication of salt injury as opposed to an indication of salt resistance in rice plants developed under salinity (Lutts et al., 1999). Moreover, evaluation of L-proline accumulation and dissemination in two sorghum genotypes varying in salt resilience demonstrated that L-proline collection happened because of salt stress and not in light of salt resistance (de-Lacerda et al., 2003).
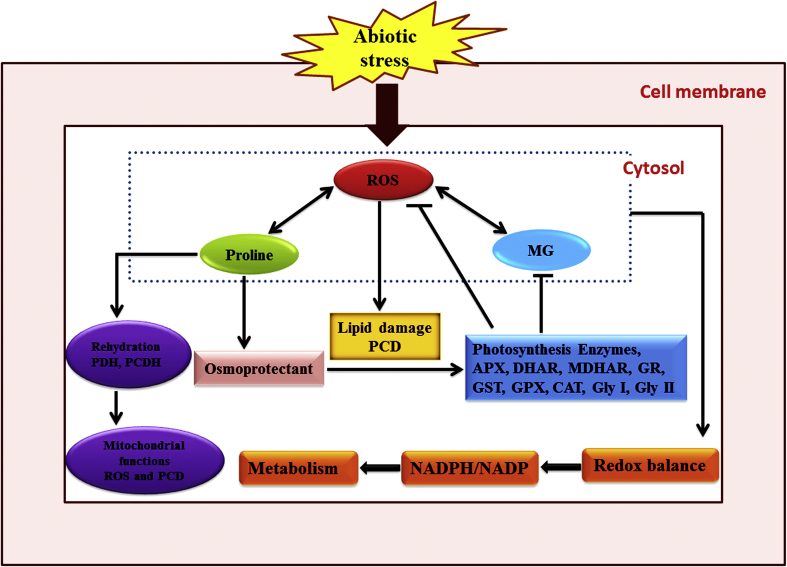
Figure 2.
Role of L-proline in plant growth and stress tolerance. L-proline plays important role as an osmolyte (protective purpose) and responsible to maintain the redox equilibrium through control the ROS and MG, increases photosynthetic production, can adjust development and metabolic signaling networks monitoring mitochondrial roles, stress utility and development (reformed from Szabados and Savouré, 2010).
3. L-proline metabolism
Elijah Adams and Harold Strecker first of all described the metabolic pathway of L-proline in the mid of year 1950's (Adams, 1970; Phang, 1985; Phang et al., 2001). L-proline secretion helps plant to combat under various abiotic stress conditions.
3.1. Effect of phytohormones on L-proline metabolism
Crops are exposed to various types of abiotic and biotic stresses. Abiotic stresses like salinity affects the growth of plants (positively or negatively) due to ionic imbalances/toxicity, osmotic stress, and nutritional deficiency, which ultimately disturb the metabolism and leads to oxidative stress (Van Zandt et al., 2003; Zhu, 2002; Nazar et al., 2011; Khan et al., 2012a, Khan et al., 2012b) that triggers DNA damage, lipid peroxidation, photosynthetic inhibition and disturbance in nutrient status (Nazar et al., 2011; Turan and Tripathy, 2012). There are various strategies has been adopted to overcome salt stress in plants such as accumulation of osmolytes works as osmoprotectants to maintain ionic and nutrient homeostasis (Nazar et al., 2011; Khan et al., 2012a, Khan et al., 2012b). Kishore et al. (1995) reported that under stress condition L-proline plays a very important role as osmolyte and their metabolism regulated by phytohormones (Verslues and Bray, 2005; Khan et al., 2012a, Khan et al., 2012b; Iqbal et al., 2014; Meena and Swapnil, 2019). These phytohormones are endogenous growth regulators which regulate germination, growth, metabolism, or other physiological activities and mechanisms of tolerance in plants to salinity stress (Khan et al., 2013). Phytohormones may regulate salinity tolerance through its influence on L-proline metabolism via regulating nitrogen (N2) or calcium (Ca2+) accumulation. Auxins (Jung and Park, 2011; Park et al., 2011), gibberellins (GA3) (Hisamatsu et al., 2000; Baek and Skinner, 2003; Siddiqui et al., 2008; Achard et al., 2006; Hamayun et al., 2010; Qin et al., 2011), abscisic acid (Narusaka et al., 2003; Szepesi et al., 2009; Gurmani et al., 2013), cytokinins (Walker and Dumbroff, 1981; Wu et al., 2013; Meena et al., 2017a,b) and ethylene (Zhao and Schaller, 2004; Cao et al., 2008; Wi et al., 2010; Khan et al., 2012a, Khan et al., 2012b; Iqbal et al., 2013; Meena and Zehra, 2019) are the phytoharmones known to regulate salinity stress effect in plants. Gibberellins (GA3) maintains membrane permeability and macro (P, N, Mg+2, Ca+2 and K+2) and micro (Fe, Mn and Zn) nutrient levels by increasing L-proline content under salt stress (Tuna et al., 2008). In Linum usitatissimum GA3 and Ca2+ promotes the L-proline level (Khan et al., 2010). Treatment of wheat plant with exogenous IAA (0.3 mM conc.) results in reduction in L-proline accumulation under drought (6% polyethylene glycol) and salinity (150 mM NaCl) (Sadiqov et al., 2002). In Triticum aestivum, under heat stress condition ethylene interact with other signalling molecules and hence enhances the L-proline metabolism results in increase in heat stress tolerance ability of plant (Khan et al., 2013). Accumulation of L-proline mediated by both ABA-independent and ABA-dependent and signaling pathway, and under abiotic stress condition, it enhances the L-proline accumulation in plants to down regulates the stress effects (Hare et al., 1999; Makela et al., 2003; Verslues and Bray, 2005). In Arabidopsis, P5CS1 L-proline accumulation was increased under both salt and osmotic stress (Strizhov et al., 1997; Abrahám et al., 2003; Verslues et al., 2007). ABA stimulates glutamic acid to synthesize L-proline (Stewart, 1980). Nitrogen is an integral component of L-proline so nitrogen content and its level in plants are correlated with the L-proline metabolism and L-proline level as well (Tarighaleslami et al., 2012). Free L-proline content is dependent on nitrogen availibility (Neuberg et al., 2010). Accumulation of L-proline is also dependent on the availability of potassium chloride (Chou et al., 1991) and calicium (Knight et al., 1997). More studies need to be focused to understand the mechanism of phytohormones interaction and L-proline metabolism for salt tolerance.
3.2. Core L-proline metabolic pathway
L-Proline synthesis involves the two pathways i.e., through glutamate cycle and ornithine cycle. Glutamate and ornithine are the precursors for L-proline biosynthesis. L-Proline metabolism consists of L-proline synthesis and L-proline catabolism. L-Proline synthesis usually occurs in cytoplasm or chloroplast, catalyzed by two enzymes P5CS1 and P5CS2 (Δ1-pyrroline-5-carboxlyate synthetase 1 and Δ1-pyrroline-5-carboxlyate synthetase 2), respectively and catabolism of L-proline occurs in mitochondria where L-proline catabolized back to glutamate and this overall process is also termed as “L-proline cycle” (Verslues and Sharma, 2010; Meena et al., 2017c, d). L-Proline synthesis is a reduction reaction process started from glutamate by glutamate dehydrogenase enzyme. It was reported that P5CS mRNA level increases and δ-OAT mRNA level decreases under salt stress and nitrogen deficient condition while it was reported that δ-OAT mRNA level is increases under excess nitrogen (Kishor et al., 2005).
3.3. L-Proline biosynthesis through glutamate pathway
L-Proline synthesis via glutamate is first of all reported in bacteria (Leisinger, 1987). The glutamate pathway normally occurs in the cytosol and chloroplast (Armengaud et al., 2004). Pathway of L-proline metabolism consist of following pathways; first of all glutamate synthesized L-proline in chloroplast or cytoplasm and this process is catalysed by two enzymes P5CS1 and P5CS2. Firstly, glutamate is phosphorylated and converted into γ-glutamyl phosphate and then P5CS converts the γ-glutamyl phosphate into the intermediate compound glutamic semialdehyde (GSA) which converts into Δ1-pyrroline-5-carboxlyate (P5C) which finally synthesizes L-proline. P5C is then reduced to L-proline by P5C reductase (P5CR) in both prokaryotes and eukaryotes. In plants and other eukaryotes glutamate directly converts into GSA catalysed by P5CS. P5CS is an important enzyme in L-proline biosynthesis as it acts as a rate limiting step and monitered by the feed-back inhibition and transcriptional regulation (Savouré et al., 1995; Yoshiba et al., 1995; Zhang et al., 2014; Meena et al., 2017e, f).
3.3.1. Δ1-pyrroline-5-carboxylate synthetase (P5CS)
P5CS act as a bifunctional enzyme which firstly converts glutamate (precursor of L-proline synthesis) into an intermediate compound glutamic semialdehyde which again converts into P5C through cyclization event. In Vigna aconitifolia the enzymatic domains of P5CS have leucine zipper sequence which helps in maintenance of tertiary structure and helps in protein-protein interaction of enzyme (Hu et al., 1992). Activity of P5CS is enhanced by light intensity (Hayashi et al., 2000) and nitric oxide (Zhao et al., 2009). Treatment of brassinosteroid is known to decrease the activity of P5CS1 (Ábrahám et al., 2003). Kishor et al. (1995) have studied that Δ1-pyrroline-5-carboxylate synthetase encoded by P5CS1 have indigenous expression as rate limiting enzyme for L-proline synthesis via glutamate. In Arabidopsis Δ1-pyrroline-5-carboxylate synthetase is encoded by P5CS2 was found to encode CONSTANS gene which is a transcription regulator (Samach et al., 2000).
3.3.2. Δ1-pyrroline-5-carboxylate reductase (P5CR)
P5CR mediate the conversion of intermediate compound P5C into L-proline. Zhang et al. (2014) have isolated two ClP5CS1 and ClP5CS2 genes which are homologous to P5CS, one C1PDH gene homologous to L-proline dehydrogenase and four ClProT1-4 genes which are homologous to L-proline transporter (GenBank accession numbers: KF743136–KF743142) in Chrysanthemum lavandulifolium, and found that these genes are more potent against abiotic stress and enhance resistance and stress tolerance ability in Chrysanthemum morifolium.
3.4. Chromosomal location of gene encoding the Δ1-pyrroline-5-carboxylate synthetase (P5CS)
At–P5C is the first gene which encodes P5CS and was isolated from A. thaliana (Savouré et al., 1995). Homology of P5CS is found at the N terminal of yeast γ-glutamyl kinase and C- terminus to bacterial γ-glutamyl phosphate reductase. The chromosomal location of At–P5C gene was found at the bottom of chromosome through southern analysis technique. In Arabidopsis, P5CS is encoded by two form of At–P5C gene that is gene AtP5CS1 and AtP5CS2 (Strizhov et al., 1997; Zhang et al., 1997). Gene AtP5CS1 is active in most of the plant parts but do not express in dividing cells, whereas gene AtP5CS2 is active in cell division and trancscribed in other plant tissue (Strizhov et al., 1997).
3.5. L-proline biosynthesis through ornithine pathway
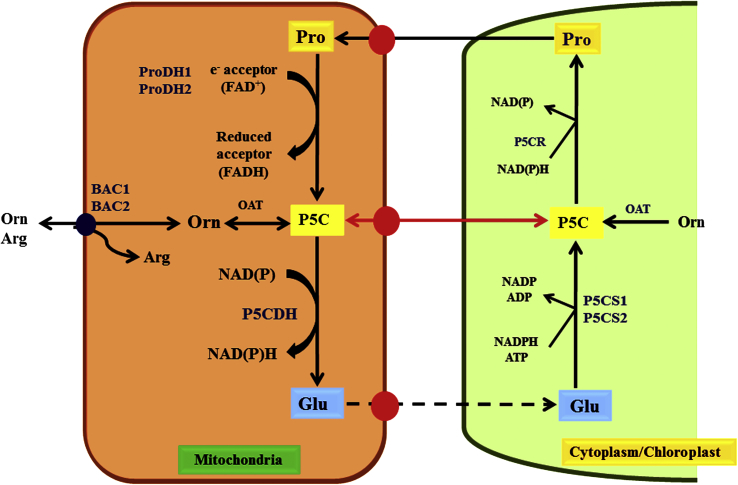
L-proline can be synthesized through an alternative pathway via ornithine (precursor of L-proline) and the role of ornithine is not well explained (Verslues and Sharma, 2010). Ornithine pathway involves two basic steps by transamination of α- or δ-NH2 moiety of ornithine (Mestichelli et al., 1979; Adams and Frank, 1980; Delauney and Verma, 1993). Ornithine transamination into glutamic-5-semialdehyde (GSA) is catalyzed by ornithine δ-aminotransferase and converted to L-proline through P5C enzyme (Szabados and Savouré, 2010). L-Proline metabolism is affected with respect to the expression of PDH in different organs and duplicated P5CS genes in Arabidopsis, it was reported that L-proline metabolism is a light dependent process stimulated by abscisic acid and in Arabidopsis under salt stress condition inhibited by brassinosteroid (Ábrahám et al., 2003). In Medicago truncatula, L-proline biosynthesis is a glutamate biosynthesis mediated pathway and it is a gene-specific pathway dependent on various plant growth conditions (Armengaud et al., 2004). It was found that Ornithine-d-aminotransferase (OAT) play catabolic role in A. thaliana mutants and their deficiency showed no change in the L-proline level (Funck et al., 2008, Figure 3).
Figure 3.
Schematic presentation of the ornithine pathway during L-proline synthesis which occurs in mitochondria and cytoplasm/chloroplast. Synthesis of L-proline occurs in the cytosol as well as in the chloroplast, while L-proline degradation is implemented in the mitochondrion.
4. L-Proline catabolism
L-Proline dehydrogenase (ProDH) is the first enzyme for L-proline catabolism. ERD5 (Early Response to Dehydration 5) encodes a protein which encodes ProDH enzyme. Reduction of ProDH enhances electron transport and ATP generation in mitochondria (Verslues and Sharma, 2010). L-Proline degradation pathway occurs in mitochondria (When there is no need of L-proline in plants at all or when there is excess amount of L-proline in plants, then L-proline catabolic activity start). L-Proline is oxidized to P5C by L-proline dehydrogenase enzyme, now P5C is converted back to the glutamate catalyzed by enzyme P5C dehydrogenase. The PDH enzyme is present in the inner mitochondrial membrane. Hence, PDH are the chief enzymes for L-proline catabolic pathway. There are two homologous genes which codes for the enzymes P5CS and PDH, in A. thaliana (Strizhov et al., 1997; Funck et al., 2010), M. truncatula (Armengaud et al., 2004) and Nicotiana tabacum (Ribarits et al., 2007). During stress conditions the L-proline content is enhanced by increasing the expression of P5CS gene or decreasing the expression of PDH gene (Verbruggen and Hermans, 2008; Mizoi and Yamaguchi-Shinozaki, 2013). Figure 4 indicates the L-proline metabolism during the course of phosphate starvation (Pi) and osmotic stressed conditions. In C. lavandulifolium, PorTs homologus gene were isolated and found to be associated with L-proline accumulation and also play a vital role during stress environment (Zhang et al., 2014). In Gram negative bacteria, there is a bifunctional enzyme L-proline utilization A (PutA) which is combinedly formed by fusion of both L-proline dehydrogenase (PRODH) and Δ1-pyrroline-5-carboxylate (P5C) dehydrogenase (P5CDH) which perform both the catabolism and synthesis (Sanyal et al., 2015). Reduction in ProDH enhances electron transport and ATP generation in mitochondria (Sharma and Verslues, 2010).
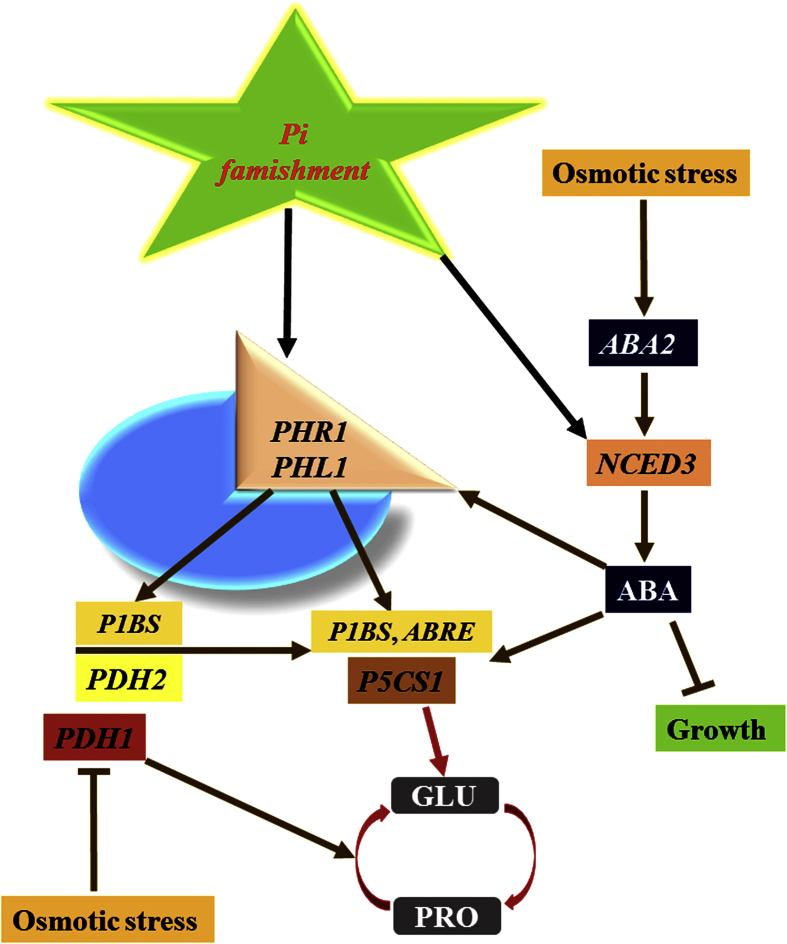
Figure 4.
Figure showing the regulation of L-proline metabolism during the course of phosphate starvation (Pi) and osmotic stressed conditions. During the course of osmotic stress, L-proline accumulation occurs and structured by the stimulation of P5CS1 and suppression of PDH1 genes, correspondingly. Activation of P5CS1 gene is regulated by ABA signals, probably completed by the ABRE cis acting motif in the promoter. Phosphate starvation stimulated PHR1 and PHL1, which induces by P5CS1 via binding to its P1BS motif. Similarly, PDH2 is also stimulates by PHR1 and PHL1 and Pi scarcity. During phosphate starvation, NCED3 also induced which can increase ABA levels. ABA signals control plant growth and activates several stress-associated genes, comprising PHL1 and P5CS1.
L-Proline metabolism is depends upon nitrogen uptake and works as a nitrogen-storage compound (Wyn Jones et al., 1977; Sánchez et al., 2001). It is found that under drought stress L-proline metabolism has a positive correlation with nitrogen supply in sugar beet plants; under drought condition in beet root increase in nitrogen uptake consequently enhance the L-proline level (Monreal et al., 2007). It is found that in nitrogen-fixing bacteria under salinity stress condition there is an increase in the activity of PDH enzyme and hence there is increase in L-proline metabolism (Kohl et al., 1994). The various doses of nitrogen positively correlated with the L-proline metabolism and it is found that in green bean (Phaseolus vulgaris) plants protein metabolism significantly affected by different nitrogen levels (Sánchez et al., 2001). The function of enzymes δ-OAT and P5CS involved in L-proline biosynthesis also depends on nitrogen level and hence affect the L-proline biosynthesis in plants (Sánchez et al., 2001). It was reported that L-proline biosynthesis play important role in breakdown of kiwi fruit bud without hydrogen cyanamide (HC) treatment (Walton et al., 1998).
5. Signal transduction
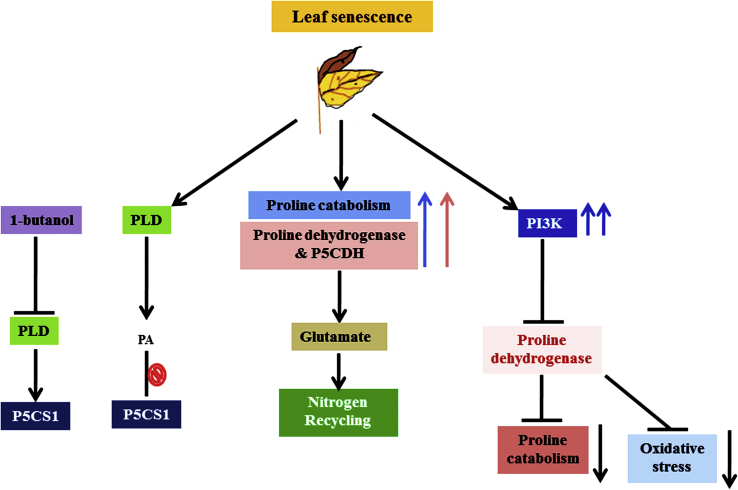
MAPK cascades respond to antioxidant signaling pathways (Pitzschke et al., 2009; Sinha et al., 2011). For L-proline biosynthesis under abiotic stress condition mitogen activated protein kinase (MAPK) is the prime signalling pathway. The receptor in this cascade is modulated by various stimuli. MAP kinase signalling pathway is a combination of MAP kinase kinase kinases (MAP3Ks/MAPKKKs/MEKKs), MAP kinase kinases (MAP2Ks/MAPKKs/MEKs/MKKs) and MAP kinases (MAPKs/MPKs) (Mishra et al., 2006; Rodriguez et al., 2010). Under stimulation through serine/threonine kinases phosphorylation, MAPK receptor undergoes the phosphorylation and become activate. MAPKs are phosphorylated by MAP2Ks on threonine and tyrosine residues at a conserved T-X-Y motif (Chang and Karin, 2001). MAP kinase phosphorylates various substrate, kinases and transcriptional factors under stress condition and also activates and synthesize the L-proline. Figure 5 represents connection between L-proline metabolism and leaf senescence signaling pathway where leaf undergo to senescence because of abiotic stresses and results in reduction of L-proline level, as a result there is increase in the activity of L-proline catabolic enzyme PRODH2 and P5CDH. Glutamate play role in nitrogen recycling process. Phospholipase D (PLD) is known to act as inhibitor of L-proline biosynthesis in Arabidopsis, 1-butanol act as a negative inhibitor of PLD and 1-butanol enhance the activity of P5CS1 and enhance L-proline accumulation (Thiery et al., 2004). PI3K enzyme inhibits the PRODH2 activity and hence reduces the L-proline catabolic activity as well as blocks the ROS and oxidative stress (Zhang and Becker, 2015).
Figure 5.
Flow diagram showing the signaling pathway for L-proline activation under leaf senescence.
6. Transport
To combat from various abiotic stresses, L-proline transportation plays a vital role. Transport of various essential amino acids is somehow depends on environmental signals. Various abiotic stresses affect the transport of L-proline (Kishor et al., 2005). Principal L-proline transporters (ProTs) were initially isolated from Arabidopsis as extremely selective transporters for L-proline (Rentsch et al., 1996). Transporters are required for L-proline transport and the L-proline transporter is divided into two super-families; (1) the amino acid, polyamine, and choline transport superfamily; and (2) the amino acid transporter family (ATF) superfamily. Further, the super family is sub divided into sub-classes; amino acid permeases, lysine, histidine transporters, L-proline transporters (ProTs), auxin transporters and a new member of the family (Fischer et al., 1998; Verbruggen et al., 1993; Ortiz-Lopez et al., 2000; Waditee et al., 2002). Several types of L-proline transporters are present in different plants which are discussed in Table 1. Eight L-proline transporters were identified in Arabidopsis, A ProT1 transporter was found in various organs of plants and its main role is found in L-proline transport to the pollen grain (Schwacke et al., 1999). During stress conditions, A. thaliana secretes ProT1 and ProT2 transporters, ProT1 mainly works under salt stress and ProT2 is mainly responsible for N transport during water stress condition (Rentsch et al., 1996). L-Proline transport through plasma membrane is achieved through two plant transporters family i.e., amino acid/auxinpermease (AAAP) family and APC (amino acid–polyamine choline) family. ProT is located at plasma membrane and is responsible for long distance intercellular transport (Rentsch et al., 2007). Glutamine synthetase influence and control the L-proline transport and amount in different organs of plant like in xylem and phloem cells (Brugière et al., 1999). The three L-proline transporters ProT1, ProT2 and AAP6 are reported in A. thaliana through C-DNA technology (Rentsch et al., 1996). HvProT2, is a novel gene, encode L-proline transporter in mestome sheath and lateral root cap cells of barley (Fujiwara et al., 2010). LeProTs L-proline transporter is also found in tomato for L-proline transport (Schwacke et al., 1999; Grallath et al., 2005). LeProT1 transporter present in both mature and germinating pollen of tomato, as demonstrated by RNA in situ hybridization (Schwacke et al., 1999). After biosynthesis of L-proline, silicone oil centrifugation technique has been used to understand the transportation of L-proline through intercellular space between chloroplast, cytosol and mitochondria. In mitochondria, L-proline uptake is an active transport because L-proline transport is achieved through many transporters (Yu et al., 1983). Yamada et al. (2011) studied on sugar beet and explained that a novel choline transport mechanism through substrate specificity and expression of L-proline transporter, L-proline and betaine transport is a pH dependent process and L-proline/betaine transport is inhibited by proton uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP) (Table 2).
Table 1.
Different L-proline transporters in various plants.
| Transporters | Plants | References |
|---|---|---|
| AtProT1-3 | Arabidopsis thaliana |
Rentsch et al. (1996) ; Grallath et al. (2005); Naidu et al. (1991) |
| LeProT1-3 | Tomato | Schwacke et al. (1999) |
| OsProT1 | Rice | Igarashi et al. (2000) |
| HvProT1-2 | Barley | Ueda et al. (2001) |
| ProT1 | Sugar beet | Yamada et al. (2011) |
| ClProT | Chrysanthemum lavandulifolium | Rentsch et al. (1996); Grallath et al. (2005) |
| Pro/Gluantiporter | Durum of wheat | Hare and Cress (1997) |
| EgProT1 | Oil palm Elaeisguineensis | Yamada et al. (2009) |
| ProT1–2 | Avicennia marina | Waditee et al. (2002) |
Table 2.
Effect of exogenous L-proline application and its role in protecting plants under various abiotic stresses.
| Name of the crop | Exogenous L-proline |
Result/Effects | References |
|---|---|---|---|
| Chickpea (Cicer arietinum) | 10 μM | Oxidative injury reduced by increasing enzymatic and non- enzymatic antioxidants | Kaushal et al. (2011) |
| Lentil (Lens culinaris) | 15 μM | Upregulation of glyoxalase and glutathione transferase | Molla et al. (2014) |
| Melon (Cucumis melo) | 200 μM | Increased PN, FV/FM, Chl content fresh and dry masses and also antioxidative enzyme activity but reduced levels of O2- and H2O2 content | Yan et al. (2011) |
| Mung bean (Phaseolus vulgaris) | 50 μM | Increase in activities of antioxidative enzymes, components of ascorbate-glutathione cycle, decrease in H2O2 content and lipid peroxidation | Aggarwal et al. (2011) |
| Olive (Olea europaea) | 50 μM | Modulation of anti-oxidative defense system, enhanced photosynthetic activity and plant growth and also maintenance of stable plant water status | Ahmed et al. (2011) |
| Sea daffodil (Pancratium maritimum) | 5 μM | Protection of protein turnover machinery against damage due to stress and also up-regulation of stressprotective proteins | Khedr et al. (2003) |
| Sugarcane (Saccharum officinarum) | 20 μM | Enhancing salt induced oxidative stress through alleviating guaicol peroxidase activity | Patade et al. (2014) |
| Tobacco (Nicotiana tabacum) | 20 μM | Alleviation antioxidant enzyme activity and fresh mass | Hoque et al., 2007a |
| Wheat (Triticum aestivum) | 20 μM | Improvement in root and shoot fresh and dry masses, shoot length and grain yield | Kamran et al. (2009) |
(PN - net photosynthesis rate; Fv/FM – maximum quantum yield of photosystem II photochemistry; Chl – chlorophyll).
7. Effect of exogenous L-proline during various environmental conditions
Plants when exposed to stressful environment accumulates range of metabolites especially amino acids. Generally, amino acids are considered as precursors and components of proteins and also play a major and vital role in plants development and metabolism (Hayat et al., 2012). A large data suggests there is direct association amid L-proline accumulation and plant stress because L-proline as an amino acid plays crucial role in plants exposed to various stresses (Iqbal et al., 2019). L-Proline not only serves as an excellent osmolyte, but also plays three major roles during stress i.e., as a metal chelator, an oxidative defense molecule and most important a signaling molecule (Liang et al., 2013). Literature review indicated that stressful condition imparts overproduction of L-proline which in turn results an overproduction of L-proline by sustaining osmotic balance or cell turgor pressure, provides membrane stabilization thus preventing electrolyte leakage, and transporting the ROS concentration within regular ranges, therefore inhibiting oxidative burst in plants (Hayat et al., 2012). Various reports showed that when L-proline supplied exogenously in adequate amount or low concentration enhanced the stress tolerance in plants wherein when supplied with higher concentration showed toxicity (Fougère et al., 1991; Petrusa and Winicov, 1997; Hossain et al., 2012; Aslam et al., 2017; Table 2).
7.1. Effect exogenous L-proline on growth of plants under varying environment
Plants when exposed to various abiotic stresses, they generally experiences growth inhibition/growth retardation. Application of exogenous L-proline provides the osmoprotection and also enhanced the plant growth under salinity stress (Csonka and Hanson, 1991; Abdelhamid et al., 2013; Yancey, 1994; Peng et al., 1996; Rhodes et al., 2002; Sharma and Dietz, 2006). Roy et al. (1993) reported that when L-proline applied exogenously at a lower concentration, enhanced the adverse effects of salinity in rice. In Arachis hypogea, when L-proline provided in lower concentration in the culture medium, it efficiently enhanced salinity induced deterioration in fresh weight, also diminished peroxidative damage of lipid membrane. However, there is no beneficial effect was obtained at higher concentration of L-proline while under salinity stress in the similar experiment there was an increase in the dry weight and also free L-proline content in the callus cells of alfa alfa (Medicago sativa) was increased (Jain et al., 2001; Ehsanpour and Fatahian, 2003). In maize (Zea mays) when applied exogenously in immature embryos it stimulated somatic embryogenesis (Armstrong et al., 1985; Duncan and Widholm, 1987; Claparols et al., 1993; Ali et al., 2015). According to Ali et al. (2008) when L-proline applied exogenously as spray treatment at seedling and/or at vegetative stage on Zea mays showed enhanced growth of maize under water deficient condition. On T. aestivum when exogenous L-proline was applied on as pre-sowing seed soaking treatment it enhanced the growth and yield of wheat (Kamran et al., 2009). Exogenous L-proline when supplied exogenously on maize it alleviated the growth and maintained the nutrient status by promoting the uptake of K+, Ca+, P and N in drought stress (Ali et al., 2008). Application of exogenous L-proline on tobacco Bright Yellow-2 (BY-2) mitigated cadmium induced inhibitory effect (Islam et al., 2009).
7.2. Effect of exogenous L-proline on oxidative stress and the antioxidant system
As a result of various metabolic processes plants continuously synthesize ROS (Foyer and Harbinson, 1994). Oxidative stress occurs when the ROS formation exceeds its scavenging potential. Therefore, plants have developed many protective methods so as to reduce or eliminate excess harmful ROS by various antioxidant enzymes for example superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR) also by non-enzymatic antioxidants like ascorbate, tocopherol, glutathione etc (Beak and Skinner, 2003; Woo et al., 2007; Ben Rejeb et al., 2014). Through the process of physical quenching progression L-proline deactivates 1O2 (Mohanty and Matysik, 2001; Matysik et al., 2002). But recently it has been reported that L-proline cannot quench 1O2 in an aqueous buffer which leads to a consideration that L-proline scavenges 1O2 in plants under stress (Signorelli et al., 2013). According to mechanistic approach collected by DFT/PCM (Density functional theory coupled with polarizable continuum model) to prove the role of L-proline during stress as a protective OH˙ scavenger in plants. In proline cycle, L-proline captures the first OH˙ via H abstraction followed by another or second H abstraction where it captures another OH releasing P5C that is again recycled back to L-proline through the action of NADPH/P5CR enzymes (Signorelli et al., 2013). ROS significantly provides protection against pathogens to plants (Alvarez and Lamb, 1997; Bolwell et al., 2002). Besides protection, ROS plays a major role in various developmental processes, formation of treachery elements and lignification (Tevini et al., 1991; Jacobson, 1996; Fath et al., 2002). High or excessive level of ROS causes the oxidative damage like DNA/RNA damage, oxidation of proteins and lipids, and also chlorolphyll pigment degradation (Fridovich, 1986; Imlay and Linn, 1988; Davies, 1987; Schützendübel and Polle, 2002). Under optimum conditions different antioxidants defence molecules scavenge ROS (Alscher et al., 1997). However, plants exposed to various biotic and abiotic stresses caused generation of ROS, excessive release of ROS (Dat et al., 2000; Mano, 2002; Mittler, 2002; Meena et al., 2017g) not only damages the cell physiology at genomic and proteomic level but also causes the release or efflux of K+ from cells (Shabala, 2006). Exogenous L-proline when applied to Arabidopsis roots reduced the level of ROS indicated the scavenging potential of L-proline and also reduces ROS – induced K+ efflux (Cuin and Shabala, 2007; Bisen et al., 2015, 2016). Application of exogenous L-proline were significantly increased the anti-oxidative enzyme activities such as catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD) in tobacco suspension cultures when exposed to salinity stress (Hoque et al., 2007a, Hoque et al., 2007b). One of another very important defence response of plants against harmful ROS is ascorbate-glutathione (ASC-GSH) cycle (Noctor and Foyer, 1998). Application of exogenous L-proline up-regulates the enzymatic activities of ASC-GSH cycle. APX (ascorbate peroxidase), MDHAR (monohydroascorbate reductase) and DHAR (dihydroascorbare reductase) in tobacco cultures that were exposed to salinity stresses (Hoque et al., 2007a, Hoque et al., 2007b; Chitara et al., 2017). Exogenous application of L-proline and betaine conferred the cadmium stress tolerance in cultured tobacco cells through increasing activates of SOD and CAT, and also there was decrease in the lipid peroxidation rate (Islam et al., 2009). Many studies showed that exogenous L-proline application enhanced the activity of antioxidative enzymes in several plants under stress condition, for examples, chickpea (Kaushal et al., 2011), sea daffodil (Khedr et al., 2003), sugarcane (Patade et al., 2014), lentil (Molla et al., 2014), olive (Ben Ahmed et al., 2010), melon (Yan et al., 2011) etc (Table 1). Exogenous application of L-proline on the salt treated mung bean showed increased level of ascorbate and glutathione/disulfide ratio and also high levels of GR, CAT and APX but reduced levels of H2O2 and malondialdehyde (Table 1). Endogenous L-proline application in the transgenic citrus plants carrying heterologous gene P5CS112A leads to increase in the transcription of cytosolic APX and chloroplast Gr and Cu/Zn SOD isoforms (De Carvalho et al., 2013).
Effect of exogenous L-proline on P. Vulgaris, alleviated salt-induced oxidative stress through the increase in various growth parameters, endogenous L-proline, photosynthetic pigments, nitrate, nitrite, ascorbic acid and mineral nutrient concentrations such as P, K, Na, and lastly enhance the activity of antioxidantive enzymes such as SOD, CAT, and POD as well as increase in concentrations of carotenoids, endogenous L-proline and ascorbic acid (Abdelhamid et al., 2013). The exogenous application of L-proline enhance the biochemical features, morphological characteristics, growth and yield of the two inbred rice cultivars viz. BRRI dhan 29 (salt sensitive) and Binadhan-8 (moderately salt-tolerant). There was increase in chlorophyll, ascorbate contents, intracellular L-proline K+/N+ ratio and also antioxidative enzymes (Keswani et al., 2014, 2016; Kumar et al., 2017). In maize seedlings, exogenous L-proline application enhanced the antioxidant and detoxification systems along with this showed a better protective role in improving the physiological and biochemical adaptations (Rohman et al., 2015). The effects of exogenous L-proline in the transgenic sorghum (Sorghum bicolor (L.) Moench) results in decreased chlorophyll content up to 30–38% in transgenic than in untransformed but the carotenoid level was not altered. There was complete reduction in the photosynthetic rate (PSII activity) in untransformed completely while it declined to 62–88% in various transgenic lines. Salinity stress induced 100% stomatal closure in non-transformed plants but after few days stomatal conductance reduced to 64–81% in transgenic. Level of intercellular CO2 lowered by 30% in transgenic. Leaves exposed to 100 mM NaCl leads to an increase in the level of CAT, SOD and glutathione reductase but lowere the value of MDA in transgenics (Reddy et al., 2015).
7.3. Effect of exogenous L-proline on plant-water relation and photosynthesis
Generally, stress hampers the plant water relations (Barceló and Poschenrieder, 1990) and due to this may affect uptake of water, ascent of sap stomatal functioning (Poschenrieder, and Barcelo, 2004) and chlorophyll biosynthesis retardation (Singh and Tewari, 2003) ultimately leading to decreased photosynthesis. Decrease in water potential in leaf is associated with stress. When the plants are exposed to heavy metals there was disturbance in plant-water relation but this exposure was triggered by L-proline accumulation as observed in Lectuca sativa in response to Cd (Costa and Morel, 1994). In Vicia faba exogenous L-proline application enhanced the leaf water potential during salinity stress (Gadallah, 1999). Application of exogenous L-proline in a concentration dependent manner mitigated the decrease in photosynthetic activity in and also leaf water potential in Olea europaea L. cv Chemlali under salt stress condition (Ben Ahmed et al., 2010). Various research showed that L-proline is well known to protect under stress by various methods such as mitochondrial ETS complex II (Hamilton and Heckathorn, 2001), enzymes like RUBISCO (Allen et al., 1997) and membrane and proteins (Paleg et al., 1984; Mansour, 1998; McNeil et al., 1999; Holmström et al., 2000). In comparison with other osmolytes such as glycine, betaine, L-proline application was very effective in alleviating NaCl-generated stress in cells of tobacco (Ashraf and Foolad, 2007). In V. faba when exogenously L-proline applied to upper and lower stomata responded differently in different concentrations either to intact or detached leaves (Rajagopal, 1981; S. Kumar et al., 2017) as a result the stomata on abaxial surface exhibited higher resistance than adaxial surface. Furthermore, it was also seen that when lower dose of L-proline was supplied it proved effective in increasing the stomatal resistance than spraying ABA (Rajagopal, 1981). In leaves of wheat and barley undergoing stress an exogenous L-proline application maintained turgidity (Rajagopal and Sinha, 1980). Structurally L-proline possesses a rigid structure that carries no charge at neutral pH and also maintains high solubility in water. L-proline alleviates the activity of ribulose-1,5-bisphosphate even at a concentration of 50 mM NaCl and therefore plays its role in protecting photosynthetic activity under various stresses as elucidated (Sivakumar et al., 2000). According to Wu and Bolen (2006) L-proline stabilizes protein structures by buried the peptide backbone and expedites protein folding. Under salt stress L-proline prevents aggregation of P39A cellular retinoic acid binding protein (Ignatova and Gierasch, 2006). L-proline accumulation results in enhancing the cellular osmolarity that increases water influx and reduces efflux thus, provides pressure potential that is needed for cell expansion (Joseph et al., 2015).
7.4. Effect of exogenous L-proline on plants exposed to salinity
One of the major and serious problems associated with plants worldwide is high salinity which results in serious metabolic disturbance and reduction in plant's growth, yield and productivity. It also hampers protein content in Pancratium maritium. But effect was reverse significantly when exogenous L-proline was applied to the plants (Khedr et al., 2003). Due to salinity stress there was reduction in the ubiquitin conjugate content ad also inhibition of antioxidative enzymes catalase and peroxidase in P. maritimum but after exogenous L-proline application the effect was significantly overcome (Khedr et al., 2003). In Vicia faba, exogenous L-proline application alleviated the salinity-induced stress (Gadallah, 1999) and also membrane disruptions. In the similar experiment, he showed that there was increase in leaf chlorophyll content, relative water content of leaf and thus overall plant growth. Exogenous application of L-proline also increased percentage and root length in salinity stressed pea plant (Bar-Nun and Poljakoff-Mayber, 1977; Singh et al., 2019). In a study on Medicago sativa callus cells, exogenous L-proline application resulted in an increase in dry weight and also increased free proilne content in salinity induced stress callus cells (Ehsanpour and Fatahian, 2003). In Mesembryanthemum crystallinum L. addition of exogenous L-proline into nutrient medium drastically decreased oxidative damage due to stress caused via salinity thus resulting in decreased lipid peroxidation rate but chlorophyll content was increased in leaves of salt stressed plants (Shevyakova et al., 2009). In Cucumis sativus L. exogenous L-proline application alleviated growth inhibition that was induced via NaCl, and was also accompanied by higher POD activity and relative water content of leaf (Huang et al., 2009). L-proline acts as a compatible solute and is upregulated in plants under various abiotic stresses. During salt stress exogenous L-proline application reduced the Na+/K+ ratio and this alleviated the endogenous L-proline and also transcription levels of P5CS and P5CR but in the meantime upregulated the some antioxidant enzymes and also genes encoding these were upregulated. Effect of exogenous L-proline improved the adverse effect of L-proline by improving the RWC, chlorophyll, carotenoid, photosynthetic activity and starch content in two year old olive tree (Ahmed et al., 2011). Application of exogenous L-proline contributes to plant's protection against the salt stress through the induction of antioxidant defense systems in the two cultivars of rice, one salt sensitive species (BRRI dhan29) and other moderately salt-tolerant (BRRI dhan47) species by significantly increase in plant growth, increased grain yield in the salt sensitive cultivar. Salt stress decreased chlorophyll and ascorbate contents, straw Na+/K+ ratio and significantly lowered the activity of antioxidant enzyme guaiacol peroxidase (POX) in the two cultivars. But when L-proline was applied exogenously it increased the chlorophyll, ascorbate contents, intracellular L-proline and K+/Na+ ratio and activities of antioxidant enzymes in salt sensitive rice cultivar (Bhusan et al., 2016). In Eurya emarginata application of exogenous L-proline in the salt stress condition allievated the fresh weight, endogenous L-proline and K+ concentration but in same time decreased the Na+ and MDA concentration. Thus, high K+ and low MDA was associated with increased H+-ATPase activity and also antioxidant enzymes except GPX. There was sharply decrease in the P5CS activity but PDH activity was remaining unmodulated. Due to exogenous L-proline there was decrease in the endogenous synthetic L-proline showing that more energy was stored in the form of nitrogen and can be used for growth of plants (Zheng et al., 2015). One of the study showed that sugarcane (Saccharum officinarum L.) productivity is highly influenced by salt stress as salinity stress induces oxidative stress that in turn damages cellular structures and biomolecules but on the application of exogenous L-proline Na+ accumulation in the plant was reduced in the mean while salt stressed plants showed high Na + accumulation other than this enzymatic activity was evaluated that showed activity of CAT, APX and POD was increased (Medeiros et al., 2015). In Oryza sativa when L-proline was exogenously applied it enhanced the plant's height, number of roots, root nitrate content, root NR, and root GS activities under the salt stress in rice cultivars (Teh et al., 2016). Exogenous application of L-proline enhanced the proline content due to elevated level of P5CS also disfunction on enzyme L-proline dehydrogenase (PDH) or L-proline oxidase (Iqbal et al., 2015; Singh et al., 2016). Effect of exogenous L-proline on Solanum melongena L. counteracted the adverse effects of salt stress on the shoot fresh weight of the eggplant cultivars and also on the A/E ratio in cv. Round only (Shahbaz et al., 2013). Exogenous L-proline application on the seedlings of two Lupinus termis L. varieties under salt stress showed remarkable enhancement in the growth attributes, physiological characteristics and yield, and also on the various anatomical attributes of the plant under study. It was examined that 6 Mm concentration of L-proline caused highest level of plant growth, total soluble sugar, leaf photosynthetic pigments, endogenous L-proline, yield and the best anatomical study was made at this concentration (Rady et al., 2016). In chilli exogenous L-proline application enhanced the shoot root length, fresh weight and dry mass of plant, photosynthetic rate, antioxidant enzyme (SOD and CAT) activity and transpiration rate under salinity stress (Butt et al., 2016). Effect of exogenous L-proline on salt tolerant species Tetragenococcus halophilus showed accumulation of intracellular L-proline which leads to increase in biomass. According to metabolomic approach that was applied revealed protective mechanism and cellular metabolic response of L-proline under salt stress. Results showed that both metabolite profiling and cellular membrane fatty acid composition increased unsaturated and cyclopropane fatty acid proportion and accumulation of certain specific intracellular metabolites i.e., environment stress protector. Cells supplemented with L-proline showed increased level in the intermediates of glycolysis, TCA cycle and pentose phosphate pathway. Also, application of L-proline resulted in enhanced concentration of many organic osmolytes such as alanine, glutamate, N-acetyl-trytophan, citrulline and mannitol necessary for osmotic homeostasis (He et al., 2017). Effect of exogenous L-proline on the Capsicum annum L. Showed increased levels of Na+ and Na+/K+ ratio with increasing levels of salinity also there was increased growth, yield and quality of plants on the application of osmolyte under salinity stress (Meena et al., 2016a, b; Jamil et al., 2018).
L-proline performs a protective function such as it serves as an osmolyte, helps in the maintenance of redox balance through the regulation of ROS and magnesium metabolism (MG) metabolism, also boosts the photosynthetic performance in the plants, also can regulate development and is also a component of various metabolic signaling networks therefore controlling the several mitochondrial functions, stress relief and development.
7.5. Regulation of L-proline metabolism during the salt stress condition
The amount of L-proline content has been increased during the salinity stress condition (Jamil et al., 2018). The higher concentration of L-proline is responsible for the tolerance of the plant under the salinity stress condition (Sairam et al., 1998). In various stress conditions due to the genetic manipulation of L-proline metabolism, L-proline accumulation takes place (Tari, 2002). During the salt stress condition, gene zinc finger TF (ZFP3), changed L-proline accumulation in Arabidopsis in comparison to normal condition (Zhang et al., 2016). Genes expression of P5CS and ProDH upregulated by 5-aminolevulinic acid (ALA), and changed the L-proline concentration because these genes encoding the L-proline metabolic enzymes, during the salt stress condition (Xiong et al., 2018). ALA treatment increased the salt tolerance, by enhancing the L-proline accumulation in Brassica napus (Xiong et al., 2018). The L-proline accumulation in the rice plant has been increased 5- fold during the high concentration of salt in comparison to non-stress condition (Ito et al., 2006). The accumulation of L-proline has been increased by the overexpression of gene OsNAC5 and improved tolerance under the high salt concentration (Todaka et al., 2012). The osmolyte such as L-proline play a role in cellular homeostasis maintenance via osmotic regulation under salt stress condition and induces physiological process properly (Iqbal et al., 2014). In Brassica napus, salt stress activated the L-proline biosynthesis and inhibited the L-proline degradation (Xue et al., 2009). L-proline is an important osmolyte in osmotic adjustment under the salinity stress condition (Ashraf and Foolad, 2007). Sodium nitro prusside (SNP) increased the tolerance activity in cucumber seedling against the salt stress condition by adjusting the biosynthesis of L-proline by enhance the P5CS activity and reduced the L-proline dehydrogenase (PDH) activity (Fan et al., 2012). Under the salt stress condition, 24-Epibrassinolide enhanced the activity of antioxidant system by increasing the level of L-proline accumulation in Cucumis sativus (Fariduddin et al., 2013). The synthesis of L-proline by the slightly increase in the expression of KvOAT with increasing the salinity stress, But for the biosynthesis of L-proline in Kosteletzkya virginica, up regulate the expression of KvP5CS1 played the more important role compare to KvOAT for the accumulation of L-proline under the high salt concentration (Wang et al., 2015). Plant tolerates under osmotic condition due to overexpression of bHLH protein and results in the increase in the L-proline level (Liu et al., 2014, 2015a; Meena et al., 2015, 2016c) in cold stress (Jin et al., 2016). When Arabidopsis faced the high salt condition, then LY294002 inhibits the PI3K, hence results decreased to P5CS1 and enhanced the PRODH1 expression and lower the level of L-proline content (Leprince et al., 2015). Phytohormones up-regulates the L-proline biosynthesis and enhanced salt tolerance (Iqbal et al., 2014). Table 3 showed the regulatory factors and their expression in concerning to L-proline and related gene activities.
Table 3.
Regulatory factors and their gene expression in regarding L-proline accumulation, biosynthesis under different environmental stress conditions.
| Regulatory factors | Expression | References |
|---|---|---|
| ALA | Up- regulated the P5CS, proDH expression | Xiong et al. (2018) |
| Gene OsNAC5 | Increased the L-proline accumulation | Iqbal et al. (2014) |
| SNP | Enhanced the P5CS activity | Fan et al. (2012) |
| SNP | Reduced the PDH activity | Fan et al. (2012) |
| 24-Epibrassinolide | Increased the L-proline accumulation | Fariduddin et al. (2013) |
| High expression of KvP5CS1 and KvOAT and low expression of KvPDH | Increased the L-proline accumulation under salt stress | Wang et al. (2015) |
| Overexpression of bHLH protein | Enhanced the L-proline accumulation under osmotic condition | Liu et al. (2014), 2015 |
| LY294002 overexpressed | Down regulated the P5CS1 and Up-regulated the PRODH1 expression under salt stress | Leprince et al. (2015) |
| Phytohormones | Up-regulates the L-proline biosynthesis under salt stress | Iqbal et al. (2014) |
| P5CS1 and P5CR are overexpressed | Increased the biosynthesis of L-proline under drought stress | Szabados and Savouré (2010); Lehmann et al. (2010) |
| ABI1 and CaM4 calmodulin-MYB2 pathway | Up-regulates the P5CS1 transcription | Knight et al. (1997); Strizhov et al. (1997); Yoo et al. (2005); Parre et al. (2007) |
| CAU1 | Up-regulates ANAC055–P5CS1 | Fu et al. (2017) |
The higher accumulation of L-proline during the drought tolerance condition is due to its utility during drought recovery period. Unfortunately, some drought susceptible genotypes like Scarlett failed to utilized the reserved L-proline efficiently due to early leaf (wilting) and leaf death which results in L-proline reduction during the drought stress condition in barley (Sayed et al., 2012). In the case of drought tolerance, barley crop have higher L-proline level in the drought susceptible genotypes (Singh et al., 1972; Hanson et al., 1979). During the water stress condition L-proline act as a compatible osmolyte and it may also source of carbon, nitrogen and energy during the recovery of the plant (Trotel et al., 1996; Szabados and Savouré, 2010). During water stress condition L-proline act ass signal molecules that control the various genes expression and enhance the plant growth and development such as flowering and seed set (Mattioli et al., 2008; Székely et al., 2008). L-proline synthesized from glutamate by the enzymes D1-pyrroline-5-carboxylate (P5C) synthetase (P5CS; EC 2.7.2.11) and P5C reductase (P5CR; EC 1.5.1.2) and reversed to again glutamate by enzymes L-proline dehydrogenase in A. thaliana (Székely et al., 2008).
Under drought stress condition L-proline act as an osmoprotectant and accumulation of the L-proline by the expression of some genes and it regulated at the transcriptional level (Delauney and Verma, 1993; Yoshiba et al., 1997). High L-proline accumulation was observed in pigeon pea under polyethylene glycol (PEG) induced water stress condition (Fazeli et al., 2007). Insertion of heterogenous gene P5CS in to the tobacco plants results to increase the L-proline accumulation under the water limiting condition than the control plant (Zhu et al., 1998; Konstantinova et al., 2002; Pospisilova et al., 2011). The accumulation of L-proline in rice can increase the expression of the drought stress genes (Iyer and Caplan, 1998). In the case of Arabidopsis, three enzymes coding genes P5CS1, P5CS2 and P5CR regulates the L-proline metabolism under the drought stress. Out of these three genes two genes (P5CS1 and P5CR) increased with the biosynthesis of L-proline in chloroplast and one gene (P5CS2) act as a housekeeping gene for the L-proline biosynthesis under the drought stress condition (Szabados and Savouré, 2010; Lehmann et al., 2010). Under drought stress condition L-proline act as reliable marker for the measurement of drought stress, because QPC.S42.3H, QPC.S42.4H and QPC.S42.6H increased the total L-proline content in comparison to control (Sayed et al., 2012). Melatonin treatment is responsible for the increased accumulation of L-proline in comparison to non-treated plants under drought stress condition (Antoniou et al., 2017). Melatonin treatments was supported by the result of P5CS activity assay, as well as by transcriptional level of P5CS and P5CR, these two enzymes responsible for the biosynthesis of L-proline under drought stress (Hayat et al., 2012). In the several plant, ABI1 (ABA-INSENSITIVE 1) and the CaM4 calmodulin-MYB2 pathway regulates the transcription of P5CS1 (encoding the L-proline biosynthetic enzyme Δ1-pyrroline-5-carboxylate synthetase 1) regulatory pathway are involved in the control of P5CS1 transcription (Knight et al., 1997; Strizhov et al., 1997; Yoo et al., 2005; Parre et al., 2007). The CAU1 regulates the ANAC055–P5CS1 (downstream pathway) which leads to L-proline accumulation under drought stress condition and this result indicates that ANAC055 and CAS are genetically independent genes of CAU1 and CAU1–ANAC055 plays the major role under the drought stress conditions (Fu et al., 2017). P5CR activity regulated by L-proline and chloride ions which depend on whether NADH or NADPH was used as the cofactor (Giberti et al., 2014). The L-proline metabolism affects the NADH/NADPH ratio under the stress condition (Sharma et al., 2011). Under the water stress condition decrease the L-proline accumulation because of alternative splicing at the P5CS1 locus (Kesari et al., 2012). When the plant under the drought stress condition have higher level of PDH1 at the same time when the accumulation of L-proline level has been increased (Kaplan et al., 2007; Schertl et al., 2014). Three mutants such as PP2Cs, highly ABA-Induced 1 (HAI1), HAI2 (also known as AKT-Interacting Phosphatase1, AIP1) and HAI3 increased the accumulation of L-proline under the low water potential (Bhaskara et al., 2012). Under the water stress condition ABA-induced protein phosphatase 2Cs (PP2Cs) affect the P5CS1 and PDH1 at protein level and similarly increased the L-proline accumulation (Bhaskara et al., 2015). ProDH1 mainly involved in L-proline degradation, under water stress condition, accumulation of L-proline has been increased, because of proDH1 down regulated and up regulated by L-proline (Kiyosue et al., 1996). In the cold tolerant plant Adonis amurensis increased the two fold of L-proline accumulation due to the overexpression of AaDREB1 protien in rice under both uncool and cold stress conditions (Zong et al., 2016). L-proline level increased by degradation of L-proline biosynthesis inhibiting enzymes, so enhanced the tolerance of Nicotiana tabacum against drought stress (Hong et al., 2000; Goudarzi and Pakniyat, 2009). Under chilling condition the L-proline metabolism and its accumulation is depends on its degradation by L-proline dehydrogenase (PDH) (Baek and Skinner, 2003). Under cold stress condition enhanced the L-proline metabolism in peaches, which was happened due to increased glutamate decarboxylase (GAD), P5CS, and ornithine δ-aminotransferase (OAT) and decreased the PDH expression (Shang et al., 2011).
7.6. Effect of exogenous L-proline on plants exposed to temperature stress
Plants growth and development are severely affected by high/low temperature. Deviation from optimum temperature results membrane disruption, variations in protein content, enzymatic activity and amino acid and electrolyte leakage from cells (Hayat et al., 2012). The tropical and subtropical plants for example soybean and mung bean treated with low temperature resulted in severe physiological and biochemical alterations, in which maximum alterations are arbitrated by ROS (Mittler, 2002).
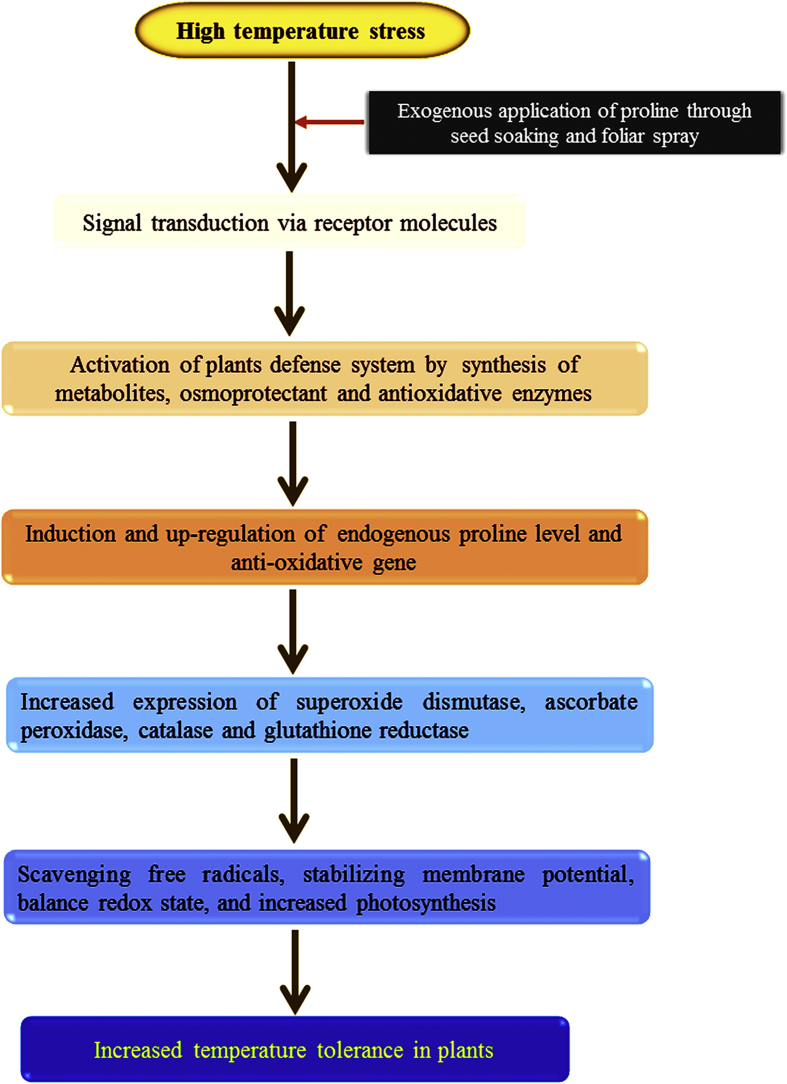
Seed treated with low temperature result in decrease in percent germination (Bramlage et al., 1978; Leopold, 1980; Larcher, 1981) and inhibit growth of plants thus decreasing yield (Larcher, 1981). Hare et al. (2003) observed that in A. thaliana exogenously applied L-proline enhances seed germination. The exogenously applied L-proline enhances plant growth (Fedina et al., 1993) and crop productivity (Itai and Paleg, 1982) under chilling stress conditions. The exogenous application of L-proline was provide tolerance to chilling stress at 5 °C for 2–6 days (Hayat et al., 2012) as well as had a dose-dependent stimulatory effect on seeds germination of Vigna radiata (Posmyk and Janas, 2007). The role of exogenous L-proline in stabilizing membrane potential which was changed from permeable and leaky to stable and non-leaky (Webster and Leopold, 1977, Figure 6). In V. radiata, chilling (Parkin and Kuo, 1989) induces lipid peroxidation due to production of ROS was overcome by exogenously applied L-proline. Van Swaaij et al. (1985) observed that exogenously applied L-proline enhanced frost tolerance in leaves of Solanum. Exogenous L-proline is a reactive oxygen scavenger, reduced lipid peroxidation and it can also act as a source of carbon and nitrogen that improve seedling growth in V. radiata exposed to chilling stress (Posmyk and Janas, 2007). In Lablab purpureus L. (Hyacinth bean) exogenously applied L-proline mitigates high-temperature induced generation of active oxygen species such as H2O2, O2•−, 1O2, and OH• responsible for chlorophyll degradation, membrane disruption, alter the structure of proteins and enzymes (Demirevska-Kepova et al., 2005) and reduces the redox state, growth and productivity (Rai et al., 2018) (Figure 6). The chickpea plant treated with L-proline alleviates the production of hydrogen peroxide and malondialdehyde (MDA) at 40/35 °C and 45/40 °C temperatures (Kaushal et al., 2011). Kaur et al. (2011) reported that exogenously applied L-proline alleviates chilling stress and enhanced plant growth and yield in chickpea. Exogenous application of L-proline enhanced resistance of barley leaves to high temperature at 45 °C by stabilizing the complex II of electron transport system (Oukarroum et al., 2012).
Figure 6.
Hypothetical model showing L-proline mediated high temperature tolerance in plants (Adopted from Rai et al., 2018).
7.7. Effect of exogenous L-proline on plants exposed to heavy metal stress
The heavy metal is increasing day by day in soil environment due to mining, soil amended with sewage-sludge, manufacturing, waste disposal practices, surface mineralization and forest fires etc (Liu et al., 2015b; Maleki et al., 2017). Heavy metals deteriorate the soil, water and air environment and it harmful to plants and humans. Heavy metals are those metals which have density higher than 5 g/cm3 causes serious problem in plants (Tutic et al., 2015). It affects the various metabolic pathways in plant including blockage of photosynthetic pathways, chlorosis of leaf, disruption of conducting tissue, reduced plant growth, decreases stomatal density, disruption of cellular components, and etc. The plants exposed to a number of heavy metals such as Cu, Cd, Cu, Zn, Pb, Co, Ni, Mn, As, and others present in the environment that affect plant growth and their metabolism (Aslam et al., 2017). Uptake of Excess concentration of heavy metals in plant results the production of superoxides (O2-) anions, hydroxyl (OH−) radicals and H2O2 that causes lipid peroxidation, disturb cellular ionic homeostasis and damage to protein, DNA, lipid and carbohydrates (Alyemeni et al., 2016). Heavy metals are categorized into essential micronutrients such as Co, Fe, Mn, Mo, Ni, Zn, and Cu needed for plants growth and development and nonessential micronutrients such as Pd, Cd, As, Cr, and Hg are highly toxic for plants (Sebastiani et al., 2004; Rai et al., 2004; Foyer and Noctor, 2005). Under heavy metals stress plants have evolved enzymatic and non-enzymatic antioxidants to detoxify the ROS and methylglyoxal (Hossain et al., 2012). These antioxidants include the enzymes SOD, CAT, GPX, glutathione S-transferases (GST), APX, DHAR, and GR, water-soluble compounds such as ascorbate (AsA), glutathione (GSH) and flavonoids, and L-proline and glycinebetaine (Hossain and Fujita, 2010). In plants accumulate osmoprotectant such as L-proline subjected to heavy metals stress condition (Sakamoto and Murata, 2000). L-proline is a multifunctional amino acid acting as an excellent osmolyte, protein stabilizer, a metal chelator, inhibitor of lipid peroxidation, redox signaling molecule and free radicals scavenger (Trovato et al., 2008). Exogenous application of L-proline play an important role in tolerance of plants exposed to heavy metal stress. In microalgae, L-proline reduces the Cd-induced ROS and maintaining the reducing environment (Siripornadulsil et al., 2002). In Solanum nigrum (hyperaccumulator of Cd) higher concentration of L-proline level was reported as compared to Solanum melongena (nonaccumulator) indicating its role in detoxification of heavy metals (Sun et al., 2007). Exogenous L-proline and betaine enhances tolerance and mitigate growth inhibition in cultured tobacco Bright Yellow (BY-2) against Cd stress (Islam et al., 2009). The exogenously applied L-proline reduces lipid peroxidation and K+ efflux in Chlorella vulgaris in response to heavy metal exposure (Mehta and Gaur, 1999). The Foliar application of L-proline increases growth and photosynthetic parameters in Chickpea plant exposed to cadmium stress (Hayat et al., 2013). Heavy metal tolerant Deschampsia and Silene accumulates a higher concentration of L-proline as compared to heavy metal sensitive plants (Schat et al., 1997). It has been reported that L-proline accumulation increases in Cajanus cajan, Vigna mungo, Helianthus annuus and Triticum aestivum exposed to heavy metals stress environment (Saradhi, 1991; Kastori et al., 1992; Bassi and Sharma, 1993a, 1993b; Doke, 1997; Meena et al., 2019). In Anacystis nidulans (Wu et al., 1995), Chlorella sp. (Wu et al., 1998) and C. vulgaris (Mehta and Gaur, 1999) accumulation of L-proline increases under Cu stress condition.
The exogenous application of L-proline in plants protects the antioxidative enzyme (CAT, POX and SOD), membrane damage, Rubisco enzyme degradation and reducing the chlorophyllase and free radicals generation. In S. nigrum the exogenously applied L-proline enhances tolerance against cadmium stress by reducing the ROS formation and cellular damages (Xu et al., 2009). Exogenous application of L-proline in mung bean is up-regulates the antioxidative defense enzymes synthesis, reducing lipid peroxidation and free radicals accumulation (Hossain and Fujita, 2010), and in chickpea plant, it enhances the efficiency of nitrogen fixation and nitrogen metabolizing enzymes subjected to cadmium stress (Alyemeni et al., 2016).
7.8. Effect of exogenous L-proline on plants exposed to radiation stress
The UV-B (280–315nm) radiation is harmful and produces physiological, biochemical, morphological, and anatomical changes in the plants (Searles et al., 2001; Rajendiran and Ramanujam, 2003). Ultraviolet radiation 290 nm reaches at the earth surface and its target to damage the DNA, protein, and membrane lipid. Under UV-B exposure plant accumulates an osmolyte such as L-proline, a free radicals scavenger (Arora and Saradhi, 2002; Zlatev et al., 2012). UV-B exposure also increases the concentrations of flavonoids and decreases the chlorophyll and Rubisco content in pea plant (Tevini et al., 1991; He et al., 1993; Jordan et al., 1994; Pardha Saradhi et al., 1995; Khan and Khan, 2013; Sharma et al., 2019). Under UV-B stress peroxidative process might be reduced by the accumulation of L-proline to protect the plant cells (Pardha Saradhi et al.,1995). Singh et al., 2009 reported the increment of L-proline content under UV-B stress in maize, pea and wheat (Zlatev et al., 2012). In Medicago saliva (alfalfa) high concentration of prolin content were determined under water stress (Irigoyen et al., 1992). Kentucky bluegrass, Creeping bentgrass, tall fescue, and perennial ryegrass plugs were kept under artificial UV-B exposure for one week with 10-h photoperiod and showed high accumulation of L-proline (Sarkar et al., 2011). Spathiphyllum treated with ultraviolet radiation (UV) at different time interval 0, 15, 30, 45 min and recorded the maximum value of L-proline (Metwally et al., 2019). It has been observed that in barley seedlings treated with NaCl provide more resistant to UV-B radiation (Arora and Saradhi, 2002). In barley, exogenous application of L-proline reduced chlorophyll/carotenoid ratio due to synthesis of pigments that provided protection to UV-B radiation exposure (Fedina et al., 2003). In Salvia officinalis exogenously applied L-proline reduces the free radicals accumulation in response to exposure of UV-B radiation (Radyukina et al., 2011).
7.9. Effect of L-proline on enzymes and metabolites
Exogenously applied L-proline enhances the activity of various antioxidative enzymes and metabolites which are involved in mitigates abiotic stresses have been listed in Table 4.
Table 4.
Effect of exogenous L-proline on various antioxidative enzyme and metabolites.
| Plants | Response/Effect | Reference |
|---|---|---|
| Cicer arietinum | Enhanced SOD, CAT and POD enzymes activities under Cd stress | Hayat et al. (2013) |
| Eurya emarginata | Alleviated salt stress by increasing the CAT, POD, and GPX enzymes activities | Zheng et al. (2015) |
| Glycine max | Enhanced nitrogenase activity under drought stress in bacteroid nodule | Pedersen et al. (1996) |
| Mung bean | Increased phenolics content under chilling stress | Posmyk and Janas (2007) |
| Alleviated salt stress by enhancement of glutathione rductase (GR), glutathione peroxidase (GPX), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), and glutathione S-transferase (GST) activities | Hossain and Fujita (2010) | |
| Nicotiana tabacum | Alleviated salt stress by reducing membrane damage | Okuma et al. (2004) |
| Increased superoxide dismutase, L-proline oxidase and catalase activities in salt stress condition | Hoque et al. (2007a) | |
| Nicotiana tabacum cv. Bright Yellow-2 (BY-2) cells | Enhanced antioxidative enzymes activities (CAT and SOD) under Cd stress | Islam et al. (2009) |
| Olea europaea cv. Chemlali | Increased SOD, CAT and APX enzymes activities under salt stress | Ben Ahmed et al. (2010) |
| Mitigated Cd stress by improvement of antioxidative enzymes activities such as CAT, SOD, GPX and APX | Zouari et al. (2016) | |
| Pancratium maritimum | Mitigated salt stress by protecting enzymes | Khedr et al. (2003) |
| Phoenix dactylifera | Enhanced catalase, glutathione peroxidase and superoxide dismutase activities in both leaves and roots under Cd stress | Zouari et al. (2016) |
| Vicia faba | Increased soluble sugar, hydrolysable sugar and soluble protein contents under salt stress | Gadallah (1999) |
| Zea mays | Protect 3D structure of enzymes against cold stress | Schobert (1977); Paleg et al. (1984) |
| L-proline-primed seed increases POD and APX activity while soil watered with 5 μM and 50 μM concentration of Cd | Karalija and Selovic (2018) |
7.10. Effect of excess L-proline
L-proline is an anti-stress compound which plays a multi-functional role in plant against stresses. The Basal and excess level of L-proline in plant is reported approximate 93.2% and 98.6 % respectively (Metwally et al., 2019). Exogenous L-proline may be having side effect to plant instead of protective function under stress condition (Bonner et al., 1996; Hellmann et al., 2000; Deuschle et al., 2001). The external supply of L-proline to Arabidopsis is suppressed the At-PDH, called AtProDH (Mani et al., 2002). Root and shoot growth were inhibited due to PDH showed the hypersensitivity to the application of exogenous L-proline (Nanjo et al., 2003). If accumulated concentration of L-proline is very high then it showed the negative effect on tomato, where an imbalanced the inorganic ions (Heuer, 2003). The excessive concentration of exogenous L-proline inhibits the growth whereas at a low concentration of L-proline expanded in vitro shoot organogenesis in Arabidopsis hypocotyls explants (Hare et al., 2001).
If the accumulation of exogenous L-proline in higher concentration, then it inhibits the P5CS, thus inhibited the organogenesis as in Arabidopsis (Zheng et al., 1995; García-Ríos et al., 1997; Ali et al., 2007; Harsh et al., 2016). Applied of exogenous L-proline may be highly toxic for plant metabolism (Bonner et al., 1996). The degradation of Pro into P5C and GSA toxic intermediates, which can causes the cell death (Hellmann et al., 2000). P5C cycle was increased due to application of high level of exogenous L-proline, which increased the transport of electron into ROS (Miller et al., 2009). High concentrations of L-proline stimulate the toxic effects of Cd in rice seedlings.
8. Conclusion
L-proline plays a significant role in ROS scavenging besides this it may become a convenient tool to pawn the adverse consequence of stressful surroundings thus reducing yearly losses to agriculture. Albeit, the various physio-morphological effects of L-proline on plants, ample work is still required for further complete understating of its effects on plant response to external stress. From an applied science viewpoint, L-proline is still of curiosity as an approach for enhancing plant stress tolerance, however intensifying elementary information should lead to new distinguished appearances of engineering L-proline metabolism. The administrative instruments controlling L-proline metabolism, intercellular and intracellular transport and associations of L-proline to other new metabolic pathways are extremely imperative to the in vivo roles of L-proline metabolism. Associations of L-proline metabolism to the oxidative pentose phosphate pathway and glutamate-glutamine metabolism are specific attention. The N-acetyl glutamate pathway can similarly generate ornithine and feasibly L-proline, but yet its function and activity are indistinct. Utilization of model frameworks, for example, A. thaliana to well understand both these prolonged studied and recently developing roles of L-proline can support in the structure of next-generation investigation although L-proline metabolism is an auspicious metabolic engineering goal for refining stress resistance of productively significant plants.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by UGC-Startup Research Grant and sustained by Mohanlal Sukhadia University, Udaipur, Rajasthan, India. Authors are highly grateful to the authorities of respective departments for support in doing this work.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdelhamid M.T., Rady M.M., Osman A.S., Abdalla M.A. Exogenous application of proline alleviates salt-induced oxidative stress in Phaseolus vulgaris L. plants. J. Hortic. Sci. Biotechnol. 2013;88(4):439–446. [Google Scholar]
- Ábrahám E., Rigó G., Székely G., Nagy R., Koncz C., Szabados L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003;51(3):363–372. doi: 10.1023/a:1022043000516. [DOI] [PubMed] [Google Scholar]
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Adams E. International Review of Connective Tissue Research. Vol. 5. Elsevier; 1970. Metabolism of proline and of hydroxyl proline; pp. 1–91. [DOI] [PubMed] [Google Scholar]
- Adams E., Frank L. Metabolism of proline and the hydroxyprolines. Annu. Rev. Biochem. 1980;49(1):1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Aggarwal M., Sharma S., Kaur N., Pathania D., Bhandhari K., Kaushal N., Kaur R., Singh K., Srivastava A., Nayyar H. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res. 2011;140(3):354–367. doi: 10.1007/s12011-010-8699-9. [DOI] [PubMed] [Google Scholar]
- Ahmed C.B., Magdich S., Rouina B.B., Sensoy S., Boukhris M., Abdullah F.B. Exogenous proline effects on water relations and ions contents in leaves and roots of young olive. Amino Acids. 2011;40(2):565–573. doi: 10.1007/s00726-010-0677-1. [DOI] [PubMed] [Google Scholar]
- Ali M.A., Kumar D.P., Murata Y., Hoque M.A. Effect of soil salinity and exogenous proline application on rice growth, yield, biochemical and antioxidant enzyme activities. EC Agriculture. 2015;2:229–240. [Google Scholar]
- Ali Q., Ashraf Muhammad, Shahbaz M., Humera Hafiza. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 2008;40(1):211–219. [Google Scholar]
- Ali Q., Ashraf M., Athar H.U.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007;39:1133–1144. [Google Scholar]
- Allen D.J., McKee I.F., Farage P.K., Baker N.R. Analysis of the limitation to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ. 1997;20:633–640. [Google Scholar]
- Alscher R.G., Donahue J.L., Cramer C.L. Reactive oxygen species and antioxidants: relationships in green cells. Physiol. Plant. 1997;100(2):224–233. [Google Scholar]
- Alvarez M.E., Lamb C. Oxidative burst-mediated defense responses in plant disease resistance. In: Scandalios J.G., editor. Vol. 34. Cold Spring Harbor: Cold spring Harbor Press; 1997. pp. 815–840. (Oxidative Stress and the Molecular Biology of Antioxidant Defenses). [Google Scholar]
- Alyemeni M.N., Hayat Q., Hayat S., Faizan M., Faraz A. Exogenous proline application enhances the efficiency of nitrogen fixation and assimilation in chickpea plants exposed to cadmium. Legume Res.: Int. J. 2016;39(2):221–227. [Google Scholar]
- Antoniou C., Chatzimichail G., Xenofontos R., Pavlou J.J., Panagiotou E., Christou A., Fotopoulos V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017;62(4) doi: 10.1111/jpi.12401. [DOI] [PubMed] [Google Scholar]
- Armengaud P., Thiery L., Buhot N., Grenier-de March G., Savouré A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol. Plant. 2004;120(3):442–450. doi: 10.1111/j.0031-9317.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C.L., Green C.E. Establishment and maintenance of friable, embryogenic maize callus and the involvement of proline. Planta. 1985;164(2):207–214. doi: 10.1007/BF00396083. [DOI] [PubMed] [Google Scholar]
- Arora S., Saradhi P.P. Light induced enhancement in proline levels under stress is regulated by non-photosynthetic events. Biol. Plant. 2002;45(4):629–632. [Google Scholar]
- Ashraf M.F.M.R., Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59(2):206–216. [Google Scholar]
- Aslam M., Saeed M.S., Sattar S., Sajad S., Sajjad M., Adnan M., Iqbal M., Sharif M.T. Specific role of proline against heavy metals toxicity in plants. Ind. J. Pure App. Biosci. 2017;5:27–34. [Google Scholar]
- Baek K.H., Skinner D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003;165(6):1221–1227. [Google Scholar]
- Barupal T., Meena M., Sharma K. Inhibitory effects of leaf extract of Lawsonia inermis on Curvularia lunata and characterization of novel inhibitory compounds by GC–MS analysis. Biotechnol. Rep. 2019;23 doi: 10.1016/j.btre.2019.e00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló Juan, Poschenrieder C. Plant water relations as affected by heavy metal stress: a review. J. Plant Nutr. 1990;13(1):1–37. [Google Scholar]
- Bar-Nun N., Poljakoff-Mayber Alexandra. Salinity stress and the content of proline in roots of Pisum sativum and Tamarix tetragyna. Ann. Bot. 1977;41(1):173–179. [Google Scholar]
- Bassi R., Sharma S.S. Proline accumulation in wheat seedlings exposed to zinc and copper. Phytochemistry. 1993;33(6):1339–1342. [Google Scholar]
- Bassi R., Sharma S.S. Changes in proline content accompanying the uptake of zinc and copper by Lemna minor. Ann. Bot. 1993;72(2):151–154. [Google Scholar]
- Beak K.H., Skinner D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003;165:1221–1227. [Google Scholar]
- Ben Ahmed C., Ben Rouina B., Sensoy S., Boukhriss M., Ben Abdullah F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010;58(7):4216–4222. doi: 10.1021/jf9041479. [DOI] [PubMed] [Google Scholar]
- Ben Rejeb K., Abdelly C., Savouré A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014;80:278–284. doi: 10.1016/j.plaphy.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Bhaskara G.B., Nguyen T.T., Verslues P.E. Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol. 2012;160(1):379–395. doi: 10.1104/pp.112.202408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara G.B., Yang T.H., Verslues P.E. Dynamic proline metabolism: importance and regulation in water limited environments. Front. Plant Sci. 2015;6:484. doi: 10.3389/fpls.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhusan D., Das D.K., Hossain M., Murata Y., Hoque M.A. Improvement of salt tolerance in rice (Oryza sativa L.) by increasing antioxidant defense systems using exogenous application of proline. Aust. J. Crop. Sci. 2016;10(1):50–56. [Google Scholar]
- Bisen K., Keswani C., Mishra S., Saxena A., Rakshit A., Singh H.B. Unrealized potential of seed biopriming for versatile agriculture. In: Rakshit A., Singh H.B., Sen A., editors. Nutrient Use Efficiency: from Basics to Advances. Springer India; 2015. pp. 193–206. [Google Scholar]
- Bisen K., Keswani C., Patel J.S., Sarma B.K., Singh H.B. Trichoderma spp.: efficient inducers of systemic resistance in plants. In: Choudhary D.K., Verma A., editors. Microbial-mediated Induced Systemic Resistance in Plants. Springer; Singapore: 2016. pp. 185–195. [Google Scholar]
- Bolwell G.P., Bindschedler L.V., Blee K.A., Butt V.S., Davies D.R., Gardner S.L., Gerrish C., Minibayeva F. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 2002;53(372):1367–1376. [PubMed] [Google Scholar]
- Bonner C.A., Williams D.S., Aldrich H.C., Jensen R.A. Antagonism by L-glutamine of toxicity and growth inhibition caused by other amino acids in suspension cultures of Nicotiana silvestris. Plant Sci. 1996;113(1):43–58. [Google Scholar]
- Bramlage W.J., Leopold A.C., Parrish D.J. Chilling stress to soybeans during imbibition. Plant Physiol. 1978;61(4):525–529. doi: 10.1104/pp.61.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.M., Hellebust J.A. Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can. J. Bot. 1978;56(6):676–679. [Google Scholar]
- Brugière N., Dubois F., Limami A.M., Lelandais M., Roux Y., Sangwan R.S., Hirel B. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell. 1999;11(10):1995–2011. doi: 10.1105/tpc.11.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R.S. Regulation of proline synthesis in osmotic response: effects of protein synthesis inhibitors. J. Exp. Zool. 1991;259(2):272–277. [Google Scholar]
- Butt M., Ayyub C.M., Amjad M., Ahmad R. Proline application enhances growth of chilli by improving physiological and biochemical attributes under salt stress. Pak. J. Agric. Sci. 2016;53(1):43–49. [Google Scholar]
- Cao Y.R., Chen S.Y., Zhang J.S. Ethylene signaling regulates salt stress response. Plant Signal. Behav. 2008;3:761–763. doi: 10.4161/psb.3.10.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chitara M.K., Keswani C., Bisen K., Singh V., Singh S.P., Sarma B.K., Singh H.B. Improving crop performance under heat stress using thermo tolerant agriculturally important microorganisms. In: Singh H.B., Sarma B.K., Keswani C., editors. Advances in PGPR Research. CABI; UK: 2017. pp. 296–305. [Google Scholar]
- Chou I.T., Chen C.T., Kao C.H. Characteristics of the induction of the accumulation of proline by abscisic acid and isobutyric acid in detached rice leaves. Plant Cell Physiol. 1991;32:269–272. [Google Scholar]
- Chun S.C., Chandrasekaran M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018;9:2525. doi: 10.3389/fmicb.2018.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claparols I., Santos M.A., Torné J.M. Influence of some exogenous amino acids on the production of maize embryogenic callus and on endogenous amino acid content. Plant Cell Tissue Organ Cult. 1993;34:1–11. [Google Scholar]
- Costa G., Morel J.L. Water relations, gas exchange and amino acid content in Cd-treated lettuce. Plant Physiol. Biochem. 1994;32:561–570. [Google Scholar]
- Csonka L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L.N., Hanson A.D. Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Cuin T.A., Shabala S. Compatible solutes reduce ROS induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 2007;30:875–885. doi: 10.1111/j.1365-3040.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- Dat J., Vandenabeele S., Vranová E., Van Montagu M., Inzé D., Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K.J. Protein damage and degradation by oxygen radicals. I. general aspects. J. Biol. Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- De Carvalho K., De Campos M.K.F., Domingues D.S., Pereira L.F.P., Vieira L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013;40:3269–3279. doi: 10.1007/s11033-012-2402-5. [DOI] [PubMed] [Google Scholar]
- de-Lacerda C.F., Cambraia J., Oliva M.A., Ruiz H.A., Prisco J.T. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ. Exp. Bot. 2003;49:107–120. [Google Scholar]
- Delauney A.J., Verma D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;4(2):215–223. [Google Scholar]
- Demirevska-Kepova K., Holzer R., Simova-Stoilova L., Feller U. Heat stress effects on ribulose-1, 5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol. Plant. 2005;49(4):521–525. [Google Scholar]
- Deuschle K., Funck D., Hellmann H., Däschner K., Binder S., Frommer W.B. A nuclear gene encoding mitochondrial Delta-1-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 2001;27:345–356. doi: 10.1046/j.1365-313x.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- Doke N. The oxidative burst: role in signal transduction and plant stress. In: Scandalios J.G., editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor. Cold Spring Harbor Press; 1997. pp. 785–813. [Google Scholar]
- Duncan D.R., Widholm J.M. Proline accumulation andits implication in cold tolerance of regenerable maize callus. Plant Physiol. 1987;83:703–708. doi: 10.1104/pp.83.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanpour A.A., Fatahian N. Effects of salt and proline on Medicago sativa callus. Plant Cell Tissue Organ Cult. 2003;73(1):53–56. [Google Scholar]
- Fan H.F., Du C.X., Guo S.R. Effect of nitric oxide on proline metabolism in cucumber seedlings under salinity stress. J. Am. Soc. Hortic. Sci. 2012;137(3):127–133. [Google Scholar]
- Fariduddin Q., Khalil R.R., Mir B.A., Yusuf M., Ahmad A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013;185(9):7845–7856. doi: 10.1007/s10661-013-3139-x. [DOI] [PubMed] [Google Scholar]
- Fath A., Bethke P., Beligni V., Jones R. Active oxygen and cell death in cereal aleurone cells. J. Exp. Bot. 2002;53:1273–1282. [PubMed] [Google Scholar]
- Fazeli F., Ghorbanli M., Niknam V. Effect of drought on water relations, growth and solute accumualation in two sesame cultivars. Pak. J. Biol. Sci. 2007;9:1829–1835. [Google Scholar]
- Fedina I.S., Georgieva K., Grigorova I. Response of barley seedlings to UV-B radiation as affected by proline and NaCl. Biol. Plant. 2003;47(4):549–554. doi: 10.1078/0176-1617-00760. [DOI] [PubMed] [Google Scholar]
- Fedina I.S., Tsonev T.S., Guleva E.I. The effect of pre-treatment with proline on the responses of Pisum sativum to salt stress. Photosynthetica. 1993;29:521–527. [Google Scholar]
- Fischer W.N., Andre B., Rentsch D., Krolkiewicz S., Tegedar M., Breitkreuz K., Frommer W.B. Amino acid transport in plants. Trends Plant Sci. 1998;3:188–195. [Google Scholar]
- Fougère F., Le Rudulier D., Streeter J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.) Plant Physiol. 1991;96:1228–1236. doi: 10.1104/pp.96.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Harbinson J.C. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer C.H., Mullineaux P.M., editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. CRC Press; Boca Raton, FL, USA: 1994. pp. 1–42. [Google Scholar]
- Foyer C.H., Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17(7):1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv. Enzymol. Relat. Area Mol. Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Fu Y., Ma H., Chen S., Gu T., Gong J. Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. J. Exp. Bot. 2017;69(3):579–588. doi: 10.1093/jxb/erx419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Mitsuya S., Miyake H., Hattori T., Takabe T. Characterization of a novel glycinebetaine/proline transporter gene expressed in the mestome sheath and lateral root cap cells in barley. Planta. 2010;232(1):133–143. doi: 10.1007/s00425-010-1155-4. [DOI] [PubMed] [Google Scholar]
- Funck D., Eckard S., Müller G. Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis. BMC Plant Biol. 2010;10:70. doi: 10.1186/1471-2229-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck D., Stadelhofer B., Koch W. Ornithine-δ-aminotransferase is essential for arginine catabolism but not for proline biosynthesis. BMC Plant Biol. 2008;8(1):40. doi: 10.1186/1471-2229-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadallah M.,A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999;42(2):249–257. [Google Scholar]
- García-Ríos M., Fujita T., LaRosa P.C., Locy R.D., Clithero J.M., Bressan R.A., Csonka L.N. Cloning of a polycistronic cDNA from tomato encoding γ-glutamyl kinase and γ-glutamyl phosphate reductase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8249–8254. doi: 10.1073/pnas.94.15.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giberti S., Funck D., Forlani G. Δ1-pyrroline-5-carboxylatereductase from Arabidopsis thaliana: stimulation or inhibition by chloride ion sand feedback regulation by proline depend on whether NADPH or NADH acts as co substrate. New Phytol. 2014;202:911–919. doi: 10.1111/nph.12701. [DOI] [PubMed] [Google Scholar]
- Goudarzi M., Pakniyat H. Salinity causes increase in proline and protein contents and peroxidase activity in wheat cultivars. J. Appl. Sci. 2009;9:348–353. [Google Scholar]
- Grallath S., Weimar T., Meyer A., Gumy C., Suter-Grotemeyer M., Neuhaus J.M., Rentsch D. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005;137(1):117–126. doi: 10.1104/pp.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurmani A.R., Bano A., Ullah N., Khan H., Jahangir M., Flowers T.J. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (Oryza sativa indica) Aust. J. Crop. Sci. 2013;7:1219–1226. [Google Scholar]
- Hamayun M., Khan S.A., Khan A.L., Shin J.H., Ahmad B., Shin D.H., Lee I.J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010;58:7226–7232. doi: 10.1021/jf101221t. [DOI] [PubMed] [Google Scholar]
- Hamilton E.W., Heckathorn S.A. Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol. 2001;126(3):1266–1274. doi: 10.1104/pp.126.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A.D., Nelsen C.E., Pedersen A.R., Everson E.H. Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance. Crop Sci. 1979;19:489–493. [Google Scholar]
- Hare P.D., Cress W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997;21(2):79–102. [Google Scholar]
- Hare P.D., Cress W.A., van Staden J. The effects of exogenous proline and proline analogues on in vitro shoot organogenesis in Arabidopsis. Plant Growth Regul. 2001;34:203–207. [Google Scholar]
- Hare P.D., Cress W.A., Van Staden J. A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul. 2003;39(1):41–50. [Google Scholar]
- Hare P., Cress W., van Staden J. Proline synthesis and degradation, a model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999;50:413–434. [Google Scholar]
- Harsh A., Sharma Y.K., Joshi U., Rampuria S., Singh G., Kumar S., Sharma R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia) Ann. Agric. Sci. 2016;61(1):57–64. [Google Scholar]
- Hayashi F., Ichino T., Osanai R., Wada K. Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol. 2000;41:1096–1101. doi: 10.1093/pcp/pcd036. [DOI] [PubMed] [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Ahmad A. Proline enhances antioxidative enzyme activity, photosynthesis and yield of Cicer arietinum L. exposed to cadmium stress. Acta Bot. Croat. 2013;72(2):323–335. [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G., Wu C., Huang J., Zhou R. Effect of exogenous proline on metabolic response of Tetragenococcus halophilus under salt stress. J. Microbiol. Biotechnol. 2017;27(9):1681–1691. doi: 10.4014/jmb.1702.02060. [DOI] [PubMed] [Google Scholar]
- He J., Huang L.K., Chow W.S., Whitecross M.I., Anderson J.M. Effects of supplementary ultraviolet-B radiation on rice and pea plants. Funct. Plant Biol. 1993;20(2):129–142. [Google Scholar]
- Hellmann H., Funck D., Rentsch D., Frommer W.B. Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol. 2000;123:779–790. doi: 10.1104/pp.123.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003;165:693–699. [Google Scholar]
- Hisamatsu T., Koshioka M., Kubota S., Fujime Y., King R.W., Mander L.N. The role of gibberellin in the control of growth and flowering in Matthiola incana. Physiol. Plant. 2000;109:97–105. [Google Scholar]
- Holmström K.O., Somersalo S., Mandal A., Palva T.E., Welin B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000;51:177–185. doi: 10.1093/jexbot/51.343.177. [DOI] [PubMed] [Google Scholar]
- Hong Z., Lakkineni K., Zhang Z., Verma D.P.S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylase synthetase (P5CS) results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122(4):1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M.A., Banu M.N., Nakamura Y., Shimoishi Y., Murata Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008;165:813–824. doi: 10.1016/j.jplph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Hoque M.A., Banu M.N., Okuma E., Amako K., Nakamura Y., Shimoishi Y., Murata Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol. 2007;164:1457–1468. doi: 10.1016/j.jplph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Hoque M.A., Okuma E., Banu M.N.A., Nakamura Y., Shimoishi Y., Murata Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007;164:553–561. doi: 10.1016/j.jplph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hossain M.A., Fujita M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol. Mol. Biol. Plants. 2010;16(1):19–29. doi: 10.1007/s12298-010-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.A., Piyatida P., da Silva J.A.T., Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012;2012:1–37. 872875. [Google Scholar]
- Hsu S.Y., Hsu Y.T., Kao C.H. The effect of polyethylene glycol on proline accumulation in rice leaves. Biol. Plant. 2003;46(1):73–78. [Google Scholar]
- Hu C.A., Delauney A.J., Verma D.P.S. A bifunctional enzyme (Δ1-pyrroline-5-carboxylate synthase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Bie Z., Liu Z., Zhen A., Wang W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2009;55(5):698–704. [Google Scholar]
- Igarashi Y., Yoshiba Y., Takeshita T., Nomura S., Otomo J., Yamaguchi-Shinozaki K., Shinozaki K. Molecular cloning and characterization of a cDNA encoding proline transporter in rice. Plant Cell Physiol. 2000;41:750–756. doi: 10.1093/pcp/41.6.750. [DOI] [PubMed] [Google Scholar]
- Ignatova Z., Gierasch L.M. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J.A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Inbar E., Schlisselberg D., Grotemeyer M.S., Rentsch D., Zilberstein D. A versatile proline/alanine transporter in the unicellular pathogen Leishmania donovani regulates amino acid homoeostasis and osmotic stress responses. Biochem. J. 2013;449(2):555–566. doi: 10.1042/BJ20121262. [DOI] [PubMed] [Google Scholar]
- Iqbal N., Fatma M., Khan N.A., Umar S. Plant Signaling Molecules. Woodhead Publishing; 2019. Regulatory role of proline in heat stress tolerance: modulation by salicylic acid; pp. 437–448. [Google Scholar]
- Iqbal N., Masood A., Khan N.A. Phytohormones in salinity tolerance: ethylene and gibberellins cross talk. In: Khan N., Nazar R., Iqbal N., Anjum N.A., editors. Phytohormones and Abiotic Stress Tolerance in Plants. Springer-Verlag; Berlin, Heidelberg: 2013. [Google Scholar]
- Iqbal N., Umar S., Khan N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea) J. Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Iqbal N., Umar S., Khan N.A., Khan M.I.R. A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ. Exp. Bot. 2014;100:34–42. [Google Scholar]
- Irigoyen J.J., Einerich D.W., Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992;84(1):55–60. [Google Scholar]
- Islam M.M., Hoque M.A., Okuma E., Banu M.N.A., Shimoishi Y., Nakamura Y., Murata Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009;166:1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Itai C., Paleg L.G. Responses of water-stressed Hordeum distichum L. and Cucumis sativus to proline and betaine. Plant Sci. Lett. 1982;25(3):329–335. [Google Scholar]
- Ito Y., Katsura K., Maruyama K., Taji T., Kobayashi M., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold responsive gene expression in transgenic rice. Plant Cell Physiol. 2006;47:141–153. doi: 10.1093/pcp/pci230. [DOI] [PubMed] [Google Scholar]
- Iyer S., Caplan A. Products of proline catabolism can induce osmotically regulated genes. Plant Physiol. 1998;116:203–211. [Google Scholar]
- Jacobson M.D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 1996;21(3):83–86. [PubMed] [Google Scholar]
- Jain M.J., Mathur G.M., Koul S.K., Sarin N.S. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.) Plant Cell Rep. 2001;20(5):463–468. [Google Scholar]
- Jamil M., Kharal M.A., Ahmad M., Abbasi G.H., Nazli F., Hussain A., Akhtar M.F. Inducing salinity tolerance in red pepper (Capsicum annuum L.) through exogenous application of proline and L-tryptophan. Soil Environ. 2018;37(2):160–168. [Google Scholar]
- Jin C., Huang X.S., Li K.Q., Yin H., Li L.T., Yao Z.H., Zhang S.L. Overexpression of a bHLH1 transcription factor of Pyrus ussuriensis confers enhanced cold tolerance and increases expression of stress-responsive genes. Front. Plant Sci. 2016;7:441. doi: 10.3389/fpls.2016.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B.R., James P.E., Strid A., Anthony R.G. The effect of ultraviolet-B radiation on gene expression and pigment composition in etiolated and green pea leaf tissue: UV-B-induced changes are gene-specific and dependent upon the developmental stage. Plant Cell Environ. 1994;17(1):45–54. [Google Scholar]
- Joseph E.A., Radhakrishnan V.V., Mohanan K.V. A study on the accumulation of proline - an osmoprotectant amino acid under salt stress in some native rice cultivars of North Kerala, India. Univ. J. Agric. Res. 2015;3:15–22. [Google Scholar]
- Jung J., Park C. Auxin modulation of salt stress signaling in Arabidopsis seed germination. Plant Signal. Behav. 2011;6:1198–1200. doi: 10.4161/psb.6.8.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran M., Shahbaz M., Ashraf M., Akram N.A. Alleviation of drought- induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot. 2009;41:621–632. [Google Scholar]
- Keswani C., Bisen K., Singh S.P., Sarma B.K., Singh H.B. A proteomic approach to understand the tripartite interactions between plant-Trichoderma-pathogen: investigating the potential for efficient biological control. In: Hakeem K.R., Akhtar Mohd. Sayeed, editors. Vol. 2. Springer USA; 2016. pp. 79–93. (Plant, Soil and Microbes: Mechanisms and Molecular Interactions). [Google Scholar]
- Keswani C., Mishra S., Sarma B.K., Singh S.P., Singh H.B. Unravelling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol. Biotechnol. 2014;98:533–544. doi: 10.1007/s00253-013-5344-5. [DOI] [PubMed] [Google Scholar]
- Kumar G., Patel J.S., Maharshi A., Mukherjee A., Keswani C., Singh S.P., Singh H.B., Sarma B.K. PGPR-mediated defence responses in plants under biotic and abiotic stresses. In: Singh H.B., Sarma B.K., Keswani C., editors. Advances in PGPR Research. CABI; UK: 2017. pp. 427–438. [Google Scholar]
- Kumari P., Meena M., Upadhyay R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean) Biocatal. Agric. Biotechnol. 2018;16:155–162. [Google Scholar]
- Kumari P., Meena M., Gupta P., Dubey M.K., Nath G., Upadhyay R.S. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek) Biocatal. Agric. Biotechnol. 2018;16:163–171. [Google Scholar]
- Kaplan F., Kopka J., Sung D.Y., Zhao W., Popp M., Porat R., Guy C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007;50:967–981. doi: 10.1111/j.1365-313X.2007.03100.x. [DOI] [PubMed] [Google Scholar]
- Karalija E., Selovic A. The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ. Sci. Pollut. Res. Int. 2018;25(33):33370–33380. doi: 10.1007/s11356-018-3220-7. [DOI] [PubMed] [Google Scholar]
- Kastori R., Petrović M., Petrović N. Effect of excess lead, cadmium, copper, and zinc on water relations in sunflower. J. Plant Nutr. 1992;15(11):2427–2439. [Google Scholar]
- Kaur G., Kumar S., Thakur P., Malik J.A., Bhandhari K., Sharma K.D., Nayyar H. Involvement of proline in response of chickpea (Cicer arietinum L.) to chilling stress at reproductive stage. Sci. Hortic. 2011;128(3):174–181. [Google Scholar]
- Kaushal N., Gupta K., Bhandhari K., Kumar S., Thakur P., Nayyar H. Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol. Mol. Biol. Plants. 2011;17(3):203–213. doi: 10.1007/s12298-011-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesari R., Lasky J.R., Villamor J.G., Marais D.L.D., Chen Y.J.C., Liu T.W., Juenger T.E., Verslues P.E. Intron-mediated alternative splicing of Arabidopsis P5CS1 and its association with natural variation in proline and climate adaptation. Proc. Natl. Acad. Sci. U.S.A. 2012;109:9197–9202. doi: 10.1073/pnas.1203433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.I.R., Khan N.A. Salicylic acid and jasmonates: approaches in abiotic stress tolerance. J. Plant Biochem. Physiol. 2013;1:4. [Google Scholar]
- Khan M.I.R., Iqbal N., Masood A., Khan N.A. Variation in salt tolerance of wheat cultivars: role of glycinebetaine and ethylene. Pedosphere. 2012;22:746–754. [Google Scholar]
- Khan M.I.R., Iqbal N., Masood A., Per T.S., Khan N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013;8(11) doi: 10.4161/psb.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.A., Nazar R., Iqbal N., Anjum N.A., editors. Phytohormones and Abiotic Stress Tolerance in Plants. Springer Science & Business Media; 2012. [Google Scholar]
- Khan N., Syeed S., Masood A., Nazar R., Iqbal N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010;1(e1):1–8. [Google Scholar]
- Khedr A.H.A., Abbas M.A., Wahid A.A.A., Quick W.P., Abogadallah G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003;54(392):2553–2562. doi: 10.1093/jxb/erg277. [DOI] [PubMed] [Google Scholar]
- Kishor P.B.K., Hong Z., Miao G.H., Hu C.A.A., Verma D.P.S. Overexpression of Δ1-pyrroline-5-carboxylatesynthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor P.K., Sangam S., Amrutha R.N., Laxmi P.S., Naidu K.R., Rao K.R.S.S., Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005;88(3):424–438. [Google Scholar]
- Kiyosue T., Yoshiba Y., Yamaguchi-Shinozaki K., Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell. 1996;8(8):1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H., Trewavas A.J., Knight M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12(5):1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Kohl D.H., Straub P.F., Shearer G. Does proline play a special role in bacteroid metabolism? Plant Cell Environ. 1994;17(12):1257–1262. [Google Scholar]
- Konstantinova T., Parvanova D., Atanassov A., Djilianov D. Freezing tolerant tobacco, transformed to accumulate osmoprotectans. Plant Sci. 2002;163(1):157–164. [Google Scholar]
- Kumar S., Beena A.S., Awana M., Singh A. Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front. Plant Sci. 2017;8:1151. doi: 10.3389/fpls.2017.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher W. Effects of low temperature stress and frost injury on plant productivity. Physiological Processes Limiting Plant Productivity. 1981:253–269. [Google Scholar]
- Lehmann S., Funck D., Szabados L., Rentsch D. Proline metabolism and transport in plant development. Amino Acids. 2010;39:949–962. doi: 10.1007/s00726-010-0525-3. [DOI] [PubMed] [Google Scholar]
- Leisinger T. Biosynthesis of proline. In: Neidhardt F.C., Ingraham J.L., Low K.B., Magasanik B., Schaechter M., Umbarger H.E., editors. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology; Washington D.C.: 1987. pp. 345–351. [Google Scholar]
- Leopold A.C. Temperature effects on soybean imbibition and leakage. Plant Physiol. 1980;65(6):1096–1098. doi: 10.1104/pp.65.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince A.S., Magalhaes N., De Vos D., Bordenave M., Crilat E., Clément G., Meyer C., Munnik T., Savouré A. Involvement of phosphatidylinositol 3-kinase in the regulation of proline catabolism in Arabidopsis thaliana. Front. Plant Sci. 2015;5:772. doi: 10.3389/fpls.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zhang L., Natarajan S.K., Becker D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013;19(9):998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tai H., Li S., Gao W., Zhao M., Xie C., Li W.X. bHLH122 is important for drought and osmotic stress resistance in Arabidopsis and in the repression of ABA catabolism. New Phytol. 2014;201:1192–1204. doi: 10.1111/nph.12607. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ji X., Nie X., Qu M., Zheng L., Tan Z., Zhao H., Huo L., Liu S., Zhang B., Wang Y. Arabidopsis AtbHLH112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs. New Phytol. 2015;207:692–709. doi: 10.1111/nph.13387. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang X., Liang L., Chen C., Wei S., Zhou Q. Reactive Oxygen Species and Oxidative Damage in Plants under Stress. Springer; Cham: 2015. Phytochelatin and oxidative stress under heavy metal stress tolerance in plants; pp. 191–217. [Google Scholar]
- Lutts S., Majerus V., Kinet J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999;105:450–458. [Google Scholar]
- Madan S., Nainawatee H.S., Jain R.K., Chowdhury J.B. Proline and proline metabolising enzymes in in-vitro selected NaCl-tolerant Brassica juncea L. under salt stress. Ann. Bot. (Lond.) 1995;76:51–57. [Google Scholar]
- Makela P., Munns R., Colmer T.D., Peltonen-Sainio P. Growth of tomato and an ABA-deficient mutant (sitiens) under saline conditions. Physiol. Plant. 2003;117:58–63. [Google Scholar]
- Maleki M., Ghorbanpour M., Kariman K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene. 2017;11:247–254. [Google Scholar]
- Mani S., van de Cotte B., van Montagu M., Verbruggen N. Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiol. 2002;128:73–83. [PMC free article] [PubMed] [Google Scholar]
- Mano J. Early events in environmental stresses in plants: induction mechanisms of oxidative stress. In: Inze D., Montagu M.V., editors. Oxidative Stress in Plants. Taylor & Francis; London: 2002. pp. 217–246. [Google Scholar]
- Mansour M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998;36:767–772. [Google Scholar]
- Mattioli R., Marchese D., D’Angeli S., Altamura M.M., Costantino P., Trovato M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008;66:277–288. doi: 10.1007/s11103-007-9269-1. [DOI] [PubMed] [Google Scholar]
- Matysik J., Alia Bhalu B., Mohanty P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002;10:525–532. [Google Scholar]
- McNeil S.D., Nuccio M.L., Hanson A.D. Betaines and related osmoprotectants: targets for metabolic engineering of stress resistance. Plant Physiol. 1999;120:945–950. doi: 10.1104/pp.120.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros L., Jaislanny M., Medeiros De A Silva M., Granja C., Maria M., De Souza E., Silva Júnior G., Willadino L. Effect of exogenous proline in two sugarcane genotypes grown in vitro under salt stress. Acta Biol. Colomb. 2015;20(2):57–63. [Google Scholar]
- Meena M., Aamir M., Vikas K., Swapnil P., Upadhyay R.S. Evaluation of morpho-physiological growth parameters of tomato in response to Cd induced toxicity and characterization of metal sensitive NRAMP3 transporter protein. Environ. Exp. Bot. 2018;148:144–167. [Google Scholar]
- Meena M., Swapnil P., Zehra A., Dubey M.K., Aamir M., Patel C.B., Upadhyay R.S. Virulence factors and their associated genes in microbes. In: Singh H.B., Gupta V.K., Jogaiah S., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2018. pp. 181–208. [Google Scholar]
- Meena M., Samal S. Alternaria host-specific (HSTs) toxins: an overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019;6:745–758. doi: 10.1016/j.toxrep.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M., Swapnil P. Regulation of WRKY genes in plant defense with beneficial fungus Trichoderma: current perspectives and future prospects. Arch. Phytopathol. Plant Prot. 2019;52(1-2):1–17. [Google Scholar]
- Meena M., Zehra A. Tomato: a model plant to study plant-pathogen interactions. Food Sci. Nutr. Technol. 2019;4(1) [Google Scholar]
- Meena M., Swapnil P., Barupal T., Sharma K. A Review on infectious pathogens and mode of transmission. J. Plant Pathol. Microbiol. 2019;10:472. [Google Scholar]
- Meena M., Zehra A., Dubey M.K., Aamir M., Gupta V.K., Upadhyay R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME) Front. Plant Sci. 2016;7:1408. doi: 10.3389/fpls.2016.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M., Zehra A., Dubey M.K., Upadhyay R.S. Mannitol and proline accumulation in Lycopersicum esculentum during infection of Alternaria alternata and its toxins. Int. J. Biomed. Sci. Bioinformatics. 2016;3:64–68. [Google Scholar]
- Meena M., Prasad V., Upadhyay R.S. Assessment of the bioweedicidal effects of Alternaria alternata metabolites against Parthenium species. Bull. Environ. Sci. Res. 2016;5(1):1–7. [Google Scholar]
- Meena M., Gupta S.K., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Alternaria toxins: potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017;8:1451. doi: 10.3389/fmicb.2017.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M., Swapnil P., Upadhyay R.S. Isolation, characterization and toxicological potential of tenuazonic acid, alternariol and alternariol monomethyl ether produced by Alternaria species phytopathogenic on plants. Sci. Rep. 2017;7:8777. doi: 10.1038/s41598-017-09138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M., Prasad V., Upadhyay R.S. Evaluation of Alternaria alternata isolates for metabolite production isolated from different sites of Varanasi, India. J. Agric. Res. 2017;2 [Google Scholar]
- Meena M., Prasad V., Upadhyay R.S. Evaluation of biochemical changes in leaves of tomato infected with Alternaria alternata and its metabolites. Vegetos. 2017;30:2. [Google Scholar]
- Meena M., Zehra A., Swapnil P., Dubey M.K., Patel C.B., Upadhyay R.S. Effect on lycopene, β-carotene, ascorbic acid and phenolic content in tomato fruits infected by Alternaria alternata and its toxins (TeA, AOH and AME) Arch. Phytopathol. Plant Prot. 2017;50:317–329. [Google Scholar]
- Meena M., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Prot. 2017;50(13-14):629–648. [Google Scholar]
- Meena M., Dubey M.K., Swapnil P., Zehra A., Singh S., Kumari P., Upadhyay R.S. The rhizosphere microbial community and methods of its analysis. In: Singh H.B., Sarma B.K., Keswani C., editors. Advances in PGPR Research. CAB International; 2017. pp. 275–295. [Google Scholar]
- Meena M., Prasad V., Zehra A., Gupta V.K., Upadhyay R.S. Mannitol metabolism during pathogenic fungal–host interactions under stressed conditions. Front. Microbiol. 2015;6:1019–1026. doi: 10.3389/fmicb.2015.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M., Tiwari A., Zehra A., Prasad V., Upadhyay R.S. Proceeding in International Conference on Global Scenario of Traditional System of Medicine, Ayurveda, Agriculture and Education, RGSC, Barkachha, BHU. Vol. 1. 2013. Morphological and molecular identification of Alternaria alternata from tomato; pp. 506–509. [Google Scholar]
- Mehta S.K., Gaur J.P. Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 1999;143(2):253–259. [Google Scholar]
- Mestichelli L.J.J., Gupta R.N., Spencer I.D. The biosynthetic route from ornithine to proline. J. Biol. Chem. 1979;254:640–647. [PubMed] [Google Scholar]
- Metwally S.A., Shoaib R.M., Hashish K.I., El-Tayeb T.A. In vitro ultraviolet radiation effects on growth, chemical constituents and molecular aspects of Spathiphyllum plant. Bull. Natl. Res. Cent. 2019;43(1):94. [Google Scholar]
- Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009;2(84) doi: 10.1126/scisignal.2000448. ra45-ra45. [DOI] [PubMed] [Google Scholar]
- Mishra N.S., Tuteja R., Tuteja N. Signaling through MAP kinase networks in plants. Arch. Biochem. Biophys. 2006;452:55–68. doi: 10.1016/j.abb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mizoi J., Yamaguchi-Shinozaki K. Rice Protocols 2013. Humana Press; Totowa, NJ: 2013. Molecular approaches to improve rice abiotic stress tolerance; pp. 269–283. [DOI] [PubMed] [Google Scholar]
- Mohanty P., Matysik J. Effect of proline on the production of singlet oxygen. Amino Acids. 2001;21(2):195–200. doi: 10.1007/s007260170026. [DOI] [PubMed] [Google Scholar]
- Molla M.R., Ali M.R., Hasanuzzaman M., Al-Mamun M.H., Ahmed A., Nazim-Ud-Dowla M.A.N., Rohman M.M. Exogenous proline and betaine-induced upregulation of glutathione transferase and glyoxalase I in lentil (Lens culinaris) under drought stress. Not. Bot. Horti Agrobot. Cluj-Napoca. 2014;42:73–80. [Google Scholar]
- Monreal J.A., Jimenez E.T., Remesal E., Morillo-Velarde R., García-Mauriño S., Echevarría C. Proline content of sugar beet storage roots: response to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot. 2007;60(2):257–267. [Google Scholar]
- Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167(3):645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Naidu B.P., Paleg L.G., Aspinall D., Jennings A.C., Jones G.P. Amino acid and glycine betaine accumulation in cold stressed wheat seedlings. Phytochemistry. 1991;30:407–409. [Google Scholar]
- Nakashima K., Satoh R., Kiyosue T., Yamaguchi-Shinozaki K., Shinozaki K. A gene encoding proline dehydrogenase is not only induced by proline and hypo osmolarity, but is also developmentally regulated in the reproductive organs of Arabidopsis. Plant Physiol. 1998;118:1233–1241. doi: 10.1104/pp.118.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjo T., Fujita M., Seki M., Kato T., Tabata S., Shinozaki K. Toxicity of free proline revealed in an Arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant Cell Physiol. 2003;44(5):541–548. doi: 10.1093/pcp/pcg066. [DOI] [PubMed] [Google Scholar]
- Narusaka Y., Nakashima K., Shinwari Z.K., Sakuma Y., Furihata T., Abe H., Narusaka M., Shinozaki K., Yamaguchi-Shinozaki K. Interaction between two cisacting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003;34:137–148. doi: 10.1046/j.1365-313x.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- Nayyar H., Walia D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plant. 2003;46:275–279. [Google Scholar]
- Nazar R., Iqbal N., Syeed S., Khan N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011;168:807–815. doi: 10.1016/j.jplph.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Neuberg M., Pavlikova D., Pavlik M., Balik J. The effect of different nitrogen nutrition on proline and asparagine content in plant. Plant Soil Environ. 2010;56:305–311. [Google Scholar]
- Noctor G., Foyer C.H. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Okuma E., Murakami Y., Shimoishi Y., Tada M., Murata Y. Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci. Plant Nutr. 2004;50(8):1301–1305. [Google Scholar]
- Ortiz-Lopez A., Chang H.C., Bush D.R. Amino acid transporters in plants. Biochim. Biophys. Acta Biomembr. 2000;1465(1-2):275–280. doi: 10.1016/s0005-2736(00)00144-9. [DOI] [PubMed] [Google Scholar]
- Otvos L., Jr., Ostorhazi E., Szabo D., Zumbrun S.D., Miller L.L., Halasohoris S.L., Desai P.D., Int Veldt S.M., Kraus C.N. Synergy between proline -rich antimicrobial peptides and small molecule antibiotics against selected Gram-negative pathogens in vitro and in vivo. Front. Chem. 2018;6:309. doi: 10.3389/fchem.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukarroum A., El Madidi S., Strasser R.J. Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): a chlorophyll a fluorescence study. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology. 2012;146(4):1037–1043. [Google Scholar]
- Paleg L.G., Stewart G.R., Bradbeer J.W. Proline and glycine betaine influence protein solvation. Plant Physiol. 1984;75:974–978. doi: 10.1104/pp.75.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Kim Y.S., Kim S.G., Jung J.H., Woo J.C., Park C.M. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol. 2011;2156:537–549. doi: 10.1104/pp.111.177071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin K.L., Kuo S.J. Chilling-induced lipid degradation in cucumber (Cucumis sativa L. cv Hybrid C) fruit. Plant Physiol. 1989;90(3):1049–1056. doi: 10.1104/pp.90.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parre E., Ghars M.A., Leprince A.S. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007;144:503–512. doi: 10.1104/pp.106.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partha Saradhi P.P., Alia P.P., Arora S., Prasad K.V. Proline accumulates in plants exposed to UV radiation and protects them against UV-induced peroxidation. Biochem. Biophys. Res. Commun. 1995;209(1):1–5. doi: 10.1006/bbrc.1995.1461. [DOI] [PubMed] [Google Scholar]
- Patade V.Y., Lokhande V.H., Suprasanna P. Exogenous application of proline alleviates salt induced oxidative stress more efficiently than glycine betaine in sugarcane cultured cells. Sugar Tech. 2014;16:22–29. [Google Scholar]
- Pedersen A.L., Feldner H.C., Rosendahl L. Effect of proline on nitrogenase activity in symbiosomes from root nodules of soybean (Glycine max L.) subjected to drought stress. J. Exp. Bot. 1996;47:1533–1539. [Google Scholar]
- Peng Z., Lu Q., Verma D.P.S. Reciprocal regulation of Delta (1)-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol. Genet. Genom. 1996;253:334–341. doi: 10.1007/pl00008600. [DOI] [PubMed] [Google Scholar]
- Petrusa L.M., Winicov I. Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol. Biochem. 1997;35:303–310. [Google Scholar]
- Phang J.M. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr. Top. Cell. Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Phang J.M., Hu C.A., Valle D. Disorders of proline and hydroxyproline metabolism. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. Metabolic and Molecular Basis of Inherited Disease. McGraw Hill Press; New York: 2001. pp. 1821–1838. [Google Scholar]
- Pitzschke A., Djamei A., Bitton F., Hirt H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant. 2009;2:120–137. doi: 10.1093/mp/ssn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschenrieder C.H., Barcelo J. Water relations in heavy metal stressed plants. In: Prasad M.N.V., editor. Heavy Metal Stress in Plants: from Biomolecules to Ecosystems. Narosa Publishing House; New Delhi: 2004. pp. 249–270. [Google Scholar]
- Posmyk M.M., Janas K.M. Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiol. Plant. 2007;29(6):509–517. [Google Scholar]
- Pospisilova J., Haisel D., Vankova D. Responses of transgenic tobacco plants with increased proline content to drought and/or heat stress. Am. J. Plant Sci. 2011;2(3):318–324. [Google Scholar]
- Poulin R., Larochelle J., Hellebust J.A. The regulation of amino acid metabolism during hyperosmotic stress in Acanthamoeba castellanii. J. Exp. Zool. 1987;243:365–378. [Google Scholar]
- Qin F., Kodaira K.S., Maruyama K., Mizoi J., Tran L.S., Fujita Y., Morimoto K., Shinozaki K., Yamaguchi-Shinozaki K. SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Plant Physiol. 2011;157:1900–1913. doi: 10.1104/pp.111.187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady M.M., Taha R.S., Mahdi A.H. Proline enhances growth, productivity and anatomy of two varieties of Lupinus termis L. grown under salt stress. South Afr. J. Bot. 2016;102:221–227. [Google Scholar]
- Radyukina N.L., Shashukova A.V., Makarova S.S., Kuznetsov V.V. Exogenous proline modifies differential expression of superoxide dismutase genes in UV-B-irradiated Salvia officinalis plants. Russ. J. Plant Physiol. 2011;58(1):51–59. [Google Scholar]
- Rai K.K., Rai N., Rai S.P. Response of Lablab purpureus L. to high temperature stress and role of exogenous protectants in mitigating high temperature induced oxidative damages. Mol. Biol. Rep. 2018;45(5):1375–1395. doi: 10.1007/s11033-018-4301-x. [DOI] [PubMed] [Google Scholar]
- Rai V., Vajpayee P., Singh S.N., Mehrotra S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004;167(5):1159–1169. [Google Scholar]
- Rajagopal V. The influence of exogenous proline on the stomatal resistance in Vicia faba. Physiol. Plant. 1981;52:292–296. [Google Scholar]
- Rajagopal V., Sinha S.K. Influence of exogenously supplied proline on relative water content in wheat and barley. Indian J. Exp. Biol. 1980;18:1523–1524. [Google Scholar]
- Rajendiran K., Ramanujam M.P. Alleviation of ultraviolet-B radiation-induced growth inhibition of green gram by triadimefon. Biol. Plant. 2003;46(4):621–624. [Google Scholar]
- Reddy P.S., Jogeswar G., Rasineni G.K., Maheswari M., Reddy A.R., Varshney R.K., Kishor P.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench] Plant Physiol. Biochem. 2015;94:104–113. doi: 10.1016/j.plaphy.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Rentsch D., Hirner B., Schmelzer E., Frommer W.B. Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell. 1996;8:1437–1446. doi: 10.1105/tpc.8.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch D., Schmidt S., Tegeder M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2007;581(12):2281–2289. doi: 10.1016/j.febslet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Nadolska-Orczyk A., Rich P.J. Salinity, osmolytes and compatible solutes. In: Lauchli A., Luttge U., editors. Salinity, Environment, Plant, Molecules. Al-Kluwer Academic Publishers; Netherlands: 2002. pp. 181–204. [Google Scholar]
- Ribarits A., Abdullaev A., Tashpulatov A., Richter A., Heberle-Bors E., Touraev A. Two tobacco proline dehydrogenases are differentially regulated and play a role in early plant development. Planta. 2007;225(5):1313–1324. doi: 10.1007/s00425-006-0429-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.C., Petersen M., Mundy J. Mitogen activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- Rohman M.M., Begum S., Akhi A.H., Ahsan A.F.M.S., Uddin M.S., Amiruzzaman M., Banik B.R. Protective role of antioxidants in maize seedlings under saline stress: exogenous proline provided better tolerance than betaine. Bothalia J. 2015;45(4):17–35. [Google Scholar]
- Roy D., Basu N., Bhunia A., Banerjee S. Counteraction of exogenous proline with NaCl in salt-sensitive cultivar of rice. Biol. Plant. 1993;35:69–72. [Google Scholar]
- Sadiqov S.T., Akbulut M., Ehmedov V. Role of Ca2+ in drought stress signaling in wheat seedlings. Biochemistry (Mosc.) 2002;67(4):491–497. doi: 10.1023/a:1015298309888. [DOI] [PubMed] [Google Scholar]
- Sairam R.K., Deshmukh P.S., Saxena D.C. Role of antioxidant systems in wheat genotype tolerance to water stresses. Biol. Plant. 1998;41(3):387–394. [Google Scholar]
- Sakamoto A., Murata N. Genetic engineering of glycine betaine synthesis in plants: current status and implications for enhancement of stress tolerance. J. Exp. Bot. 2000;51(342):81–88. [PubMed] [Google Scholar]
- Samach A., Onouchi H., Gold S.E., Ditta G.S., Schwarz-Sommer S., Yanofsky M.F., Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Sánchez E., López-Lefebre L.R., García P.C., Rivero R.M., Ruiz J.M., Romero L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike) J. Plant Physiol. 2001;158:593–598. [Google Scholar]
- Sanyal N., Arentson B.W., Luo M., Tanner J.J., Becker D.F. First evidence for substrate channeling between proline catabolic enzymes a validation of domain fusion analysis for predicting protein-protein interactions. J. Biol. Chem. 2015;290(4):2225–2234. doi: 10.1074/jbc.M114.625483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradhi P.P. Proline accumulation under heavy metal stress. J. Plant Physiol. 1991;138(5):554–558. [Google Scholar]
- Sarkar D., Bhowmik P.C., Shetty K. The role of proline -associated pentose phosphate pathway in cool-season turfgrasses after UV-B exposure. Environ. Exp. Bot. 2011;70(2-3):251–258. [Google Scholar]
- Savouré A., Jaoua S., Hua X.E., Ardiles W., Van Montagu M., Verbruggen N. Isolation, characterization, and chromosomal location of a gene encoding the delta 1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1995;372(1):13–19. doi: 10.1016/0014-5793(95)00935-3. [DOI] [PubMed] [Google Scholar]
- Sayed M.A., Schumann H., Pillen K., Naz A.A., Léon J. AB-QTL analysis reveals new alleles associated to proline accumulation and leaf wilting under drought stress conditions in barley (Hordeum vulgare L.) BMC Genet. 2012;13(1):61. doi: 10.1186/1471-2156-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat H., Sharma S.S., Vooijs R. Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiol. Plant. 1997;101(3):477–482. [Google Scholar]
- Schertl P., Cabassa C., Saadallah K., Bordenave M., Savouré A., Braun H.P. Biochemical characterization of proline dehydrogenase in Arabidopsis mitochondria. FEBS J. 2014;281:2794–2804. doi: 10.1111/febs.12821. [DOI] [PubMed] [Google Scholar]
- Schobert B. Is there an osmotic regulatory mechanism in algae and higher plants? J. Theor. Biol. 1977;68(1):17–26. doi: 10.1016/0022-5193(77)90224-7. [DOI] [PubMed] [Google Scholar]
- Schützendübel A., Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002;53:1351–1365. [PubMed] [Google Scholar]
- Schwacke R., Grallath S., Breitkreuz K.E., Stransky E., Stransky H., Frommer W.B., Rentsch D. LeProT1, a transporter for proline, glycine betaine, and γ-amino butyric acid in tomato pollen. Plant Cell. 1999;11(3):377–391. doi: 10.1105/tpc.11.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles P.S., Flint S.D., Caldwell M.M. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia. 2001;127(1):1–10. doi: 10.1007/s004420000592. [DOI] [PubMed] [Google Scholar]
- Sebastiani L., Scebba F., Tognetti R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoids × maximowiczii) and I-214 (P. × euramericana) exposed to industrial waste. Environ. Exp. Bot. 2004;52(1):79–88. [Google Scholar]
- Shabala S. Non-invasive microelectrode ion flux measurements in plant stress physiology. In: Volkov A., editor. Plant Electrophysiology – Theory and Methods. Springer-Verlag; Berlin: 2006. pp. 35–71. [Google Scholar]
- Shahbaz M., Mushtaq Z., Andaz F., Masood A. Does proline application ameliorate adverse effects of salt stress on growth, ions and photosynthetic ability of eggplant (Solanum melongena L.) Sci. Hortic. 2013;164:507–511. [Google Scholar]
- Shang H., Cao S., Yang Z., Cai Y., Zheng Y. Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J. Agric. Food Chem. 2011;59(4):1264–1268. doi: 10.1021/jf104424z. [DOI] [PubMed] [Google Scholar]
- Sharma A., Shahzad B., Kumar V., Kohli S.K., Sidhu G.P.S., Bali A.S., Handa N., Kapoor D., Bhardwaj R., Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):E285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.S., Dietz K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- Sharma S., Verslues P.E. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ. 2010;33:1838–1851. doi: 10.1111/j.1365-3040.2010.02188.x. [DOI] [PubMed] [Google Scholar]
- Sharma S., Villamor J.G., Verslues P.E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011;157:292–304. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevyakova N., Bakulina E., Kuznetsov V. Proline antioxidant role in the common ice plant subjected to salinity and paraquat treatment inducing oxidative stress. Russ. J. Plant Physiol. 2009;56:663–669. [Google Scholar]
- Siddiqui M.H., Khan M.N., Mohammad F., Khan M.M.A. Role of nitrogen and gibberellic acid (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008;194:214–224. [Google Scholar]
- Signorelli S., Arellano J.B., Melø T.B., Borsani O., Monza J. Proline does not quench singlet oxygen: evidence to reconsider its protective role in plants. Plant Physiol. Biochem. 2013;64:80–83. doi: 10.1016/j.plaphy.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Singh H., Singh N.B., Singh A., Hussain I., Yadav V. Physiological and biochemical effects of salicylic acid on Pisum sativum exposed to isoproturon. Arch. Agron Soil Sci. 2016;62:1425–1436. [Google Scholar]
- Singh H.B., Keswani C., Reddy M.S., Royano E.S., García-Estrada C., editors. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications. Springer-Nature; Singapore: 2019. p. 392. [Google Scholar]
- Singh P.K., Tewari R.K. Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J. Environ. Biol. 2003;24:107–112. [PubMed] [Google Scholar]
- Singh T.N., Aspinall D., Paleg L.G. Proline accumulation and varietal adaptability to drought in barley: a potential metabolic measure of drought resistance. Nat. New Biol. 1972;236:188–190. doi: 10.1038/newbio236188a0. [DOI] [PubMed] [Google Scholar]
- Sinha A.K., Jaggi M., Raghuram B., Tuteja N. Mitogen-activated protein kinase signalling in plants under abiotic stress. Plant Signal. Behav. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripornadulsil S., Traina S., Verma D.P.S., Sayre R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 2002;14(11):2837–2847. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar P., Sharmila P., Saradhi P.P. Proline alleviates salt-stress induced enhancement in ribulose-1,5-bisphosphate oxygenase activity. Biochem. Biophys. Res. Commun. 2000;279:512–515. doi: 10.1006/bbrc.2000.4005. [DOI] [PubMed] [Google Scholar]
- Sperstad S.V., Haug T., Blencke H.M., Styrvold O.B., Li C., Stensvåg K. Antimicrobial peptides from marine invertebrates: challenges and perspectives in marine antimicrobial peptide discovery. Biotechnol. Adv. 2011;29(5):519–530. doi: 10.1016/j.biotechadv.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Stewart C.R. The mechanism of abscisic acid-induced proline accumulation in barley leaves. Plant Physiol. 1980;66:230–233. doi: 10.1104/pp.66.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizhov N., Abrahám E., Ökrész L., Blickling S., Zilberstein A., Schell J., Koncz C., Szabados L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997;12:557–569. doi: 10.1046/j.1365-313x.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- Sun R.L., Zhou Q.X., Sun F.H., Jin C.X. Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ. Exp. Bot. 2007;60(3):468–476. [Google Scholar]
- Szabados L., Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Székely G., Ábrahám E., Cséplő A., Rigó G., Zsigmond L., Csiszár J., Ayaydin F., Strizhov N., Jásik J., Schmelzer E., Koncz C., Szabados L. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- Szepesi A., Csiszar J., Gemes K., Horvarth E., Horvath F., Simon L.M., Tari I. Salicylic acid improves the acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ contents of the leaves without toxicity symptoms in Solanum lycopersicum L. J. Plant Physiol. 2009;166:914–925. doi: 10.1016/j.jplph.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Tari I. Acclimation of tomato plants to salinity stress after a salicylic acid pre-treatment. Acta Biol. Szeged. 2002;46:55–56. [Google Scholar]
- Tarighaleslami M., Zarghami R., Boojar M.M.A., Oveysi M. Effects of drought stress and different nitrogen levels on morphological traits of proline in leaf and protein of corn seed (Zea mays L.) Am. Eurasian J. Agric. Environ. Sci. 2012;12:49–56. [Google Scholar]
- Teh C.Y., Shaharuddin N.A., Ho C.L., Mahmood M. Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiol. Plant. 2016;38(6):151. [Google Scholar]
- Tevini M., Braun J., Fieser G. The protective functions of epidermal layer of rice seedlings against ultraviolet-B radiation. Photochem. Photobiol. 1991;53:329–333. [Google Scholar]
- Thiery L., Leprince A.S., Lefebvre D., Ghars M.A., Debarbieux E., Savouré A. Phospholipase D is a negative regulator of proline biosynthesis in Arabidopsis. J. Chem. Biochem. 2004;279:14812–14818. doi: 10.1074/jbc.M308456200. [DOI] [PubMed] [Google Scholar]
- Todaka D., Nakashima K., Shinozaki K., Yamaguchi-Shinozaki K. Toward understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice. 2012;5(1):6. doi: 10.1186/1939-8433-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi B.N., Gaur J.P. Relationship between copper and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta. 2004;219:397–404. doi: 10.1007/s00425-004-1237-2. [DOI] [PubMed] [Google Scholar]
- Trotel P., Bouchereau A., Niogret M.F., Larher F.R. The fate of osmo-accumulated proline in leaf discs of rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci. 1996;118:31–45. [Google Scholar]
- Trovato M., Mattioli R., Costantino P. Multiple roles of proline in plant stress tolerance and development. Rendiconti Lincei. 2008;19(4):325–346. [Google Scholar]
- Tuna A.L., Kaya C., Dikilitas M., Higgs D.E.B. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008;62:1–9. [Google Scholar]
- Turan S., Tripathy B.C. Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma. 2012;250:209–222. doi: 10.1007/s00709-012-0395-5. [DOI] [PubMed] [Google Scholar]
- Tutic A., Novakovic S., Lutovac M., Biocanin R., Ketin S., Omerovic N. The heavy metals in agrosystems and impact on health and quality of life. OA Maced. J. Med. Sci. (OAMJMS) 2015;3(2):345–355. doi: 10.3889/oamjms.2015.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A., Shi W., Sanmiya K., Shono M., Takabe T. Functional analysis of salt-inducible proline transporter of barley roots. Plant Cell Physiol. 2001;42:1282–1289. doi: 10.1093/pcp/pce166. [DOI] [PubMed] [Google Scholar]
- Van Swaaij A.C., Jacobsen E., Feenstra W.J. Effect of cold hardening, wilting and exogenously applied proline on leaf proline content and frost tolerance of several genotypes of Solanum. Physiol. Plant. 1985;64(2):230–236. [Google Scholar]
- Van Zandt P.A., Tobler M.A., Mouton E., Hasenstein K.H., Mopper S. Positive and negative consequences of salinity stress for the growth and reproduction of the clonal plant, Iris hexagona. J. Ecol. 2003;91(5):837–846. [Google Scholar]
- Verbruggen N., Hermans C. Proline accumulation in plants: a review. Amino Acids (Vienna) 2008;35(4):753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Verbruggen N., Villarroel R., Van Montagu M. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis. Plant Physiol. 1993;103:771–781. doi: 10.1104/pp.103.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P.E., Bray E.A. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J. Exp. Bot. 2005;57(1):201–212. doi: 10.1093/jxb/erj026. [DOI] [PubMed] [Google Scholar]
- Verslues P.E., Sharma S. Vol. 8. The Arabidopsis Book/American Society of Plant Biologists; 2010. Proline metabolism and its implications for plant-environment interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P.E., Kim Y.S., Zhu J.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate: glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007;64:205–217. doi: 10.1007/s11103-007-9145-z. [DOI] [PubMed] [Google Scholar]
- Waditee R., Hibino T., Tanaka Y., Nakamura T., Incharoensakdi A., Hayakawa S., Takabe T. Functional characterization of betaine/proline transporters in betaine-accumulating mangrove. J. Biol. Chem. 2002;277(21):18373–18382. doi: 10.1074/jbc.M112012200. [DOI] [PubMed] [Google Scholar]
- Walker M.A., Dumbroff E.B. Effects of salt stress on abscisic acid and cytokinin levels in tomatos. Zeitschrift fur Pflanzenphysiologie. 1981;101:461–470. [Google Scholar]
- Walton E.F., Podivinsky E., Wu R.M., Reynolds P.H., Young L.W. Regulation of proline biosynthesis in kiwifruit buds with and without hydrogen cyanamide treatment. Physiol. Plant. 1998;102(2):171–178. [Google Scholar]
- Wang H., Tang X., Wang H., Shao H.B. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front. Plant Sci. 2015;6:792. doi: 10.3389/fpls.2015.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B.D., Leopold A.C. The ultrastructure of dry and imbibed cotyledons of soybean. Am. J. Bot. 1977;64(10):1286–1293. [Google Scholar]
- Wi S.J., Jang S.J., Park K.Y. Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol. Cells. 2010;30:37–49. doi: 10.1007/s10059-010-0086-z. [DOI] [PubMed] [Google Scholar]
- Woo S.Y., Lee D.K., Lee Y.K. Net photosynthetic rate, ascorbate peroxidase and glutathione reductase activities of Erythrina orientalis in polluted and non-polluted areas. Photosynthetica. 2007;45:293–295. [Google Scholar]
- Wu J.T., Chang S.C., Chen K.S. Enhancement of intracellular proline level in cells of Anacystis nidulans (cyanobacteria) exposed to deleterious concentrations of copper 1. J. Phycol. 1995;31(3):376–379. [Google Scholar]
- Wu J.T., Hsieh M.T., Kow L.C. Role of proline accumulation in response to toxic copper in Chlorella sp. (Chlorophyceae) cells. J. Phycol. 1998;34(1):113–117. [Google Scholar]
- Wu P., Bolen D.W. Osmolyte-induced protein folding free energy changes. Proteins. 2006;63(2):290–296. doi: 10.1002/prot.20868. [DOI] [PubMed] [Google Scholar]
- Wu X., He J., Chen J., Yang S., Zha D. Alleviation of exogenous 6- benzyladenine on two genotypes of egg plant (Solanum melongena Mill.) growth under salt stress. Protoplasma. 2013;251(1):169–176. doi: 10.1007/s00709-013-0535-6. [DOI] [PubMed] [Google Scholar]
- Wyn Jones R.G., Storey R., Leigh R.A., Ahmad N., Pollard A. A hypothesis on cytoplasmic osmoregulation. In: Marré E., Ciferri O., editors. Regulation of Cell Membrane Activity in Plants. Elsevier/North Holland Biomedical Press; Amsterdam: 1977. pp. 121–136. [Google Scholar]
- Xiong J.L., Wang H.C., Tan X.Y., Zhang C.L., Naeem M.S. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018;124:88–99. doi: 10.1016/j.plaphy.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Xu J., Yin H., Li X. Protective effects of Proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009;28(2):325–333. doi: 10.1007/s00299-008-0643-5. [DOI] [PubMed] [Google Scholar]
- Xue X., Liu A., Hua X. Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 2009;42(1):28–34. doi: 10.5483/bmbrep.2009.42.1.028. [DOI] [PubMed] [Google Scholar]
- Yamada N., Promden W., Yamane K., Tamagake H., Hibino T., Tanaka Y., Takabe T. Preferential accumulation of betaine uncoupled to choline monooxygenase in young leaves of sugar beet—importance of long-distance translocation of betaine under normal and salt-stressed conditions. J. Plant Physiol. 2009;166:2058–2070. doi: 10.1016/j.jplph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Yamada N., Sakakibara S., Tsutsumi K., Waditee R., Tanaka Y., Takabe T. Expression and substrate specificity of betaine/proline transporters suggest a novel choline transport mechanism in sugar beet. J. Plant Physiol. 2011;168(14):1609–1616. doi: 10.1016/j.jplph.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Yan Z., Guo S., Shu S., Sun J., Tezuka T. Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr. J. Biotechnol. 2011;10:18381–18390. [Google Scholar]
- Yancey P.H. Compatible and counteracting solutes. In: Strange K., editor. Cellular and Molecular Physiology of Cell Volume Regulation. CRC Press; Boca Raton, FL: 1994. pp. 81–109. [Google Scholar]
- Yonamine I., Yoshida K., Kido K., Nakagawa A., Nakayama H., Shinmyo A. Overexpression of NtHAL3 genes confers increased levels of proline biosynthesis and the enhancement of salt tolerance in cultured tobacco cells. J. Exp. Bot. 2004;55(396):387–395. doi: 10.1093/jxb/erh043. [DOI] [PubMed] [Google Scholar]
- Yoo J.H., Park C.Y., Kim J.C. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J. Biol. Chem. 2005;280:3697–3706. doi: 10.1074/jbc.M408237200. [DOI] [PubMed] [Google Scholar]
- Yoshiba Y., Kiyosue T., Katagiri T., Ueda H., Mizoguchi T., Yamaguchi-Shinozaki K., Wada K., Harada Y., Shinozaki K. Correlation between the induction of a gene for D1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. 1995;7:751–760. doi: 10.1046/j.1365-313x.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- Yoshiba Y., Kiyosue V., Nakashima K., Yamaguchi-Shinozki K., Shinozaki K. Regulation of levels of proline as an osmolytes in plants under water stress. Plant Cell Physiol. 1997;38(10):1095–1102. doi: 10.1093/oxfordjournals.pcp.a029093. [DOI] [PubMed] [Google Scholar]
- Yu C., Claybrook D.L., Huang A.H. Transport of glycine, serine, and proline into spinach leaf mitochondria. Arch. Biochem. Biophys. 1983;227(1):180–187. doi: 10.1016/0003-9861(83)90361-2. [DOI] [PubMed] [Google Scholar]
- Zhang A., Liu D., Hua C., Yan A., Liu B., Wu M., Liu Y., Huang L., Ali I., Gan Y. The arabidopsis gene zinc finger protein 3(ZFP3) is involved in salt stress and osmotic stress response. PLoS One. 2016;11(12):1–16. doi: 10.1371/journal.pone.0168367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.S., Lu Q., Verma D.P. Characterisation of Δ1-pyrroline-5-carboxylate synthetase gene promoter in transgenic Arabidopsis thaliana subjected to water stress. Plant Sci. 1997;129(1):81–89. [Google Scholar]
- Zhang L., Becker D. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015;6:552. doi: 10.3389/fpls.2015.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Huang H., Dai S. Isolation and expression analysis of proline metabolism-related genes in Chrysanthemum lavandulifolium. Gene. 2014;537(2):203–213. doi: 10.1016/j.gene.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Zhao M.G., Chen L., Zhang L.L., Zhang W.H. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009;151:755–767. doi: 10.1104/pp.109.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.C., Schaller G.E. Effect of salt and osmotic stress upon expression of the ethylene receptor ETR1 in Arabidopsis thaliana. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2004;562:189–192. doi: 10.1016/S0014-5793(04)00238-8. [DOI] [PubMed] [Google Scholar]
- Zheng J.L., Zhao L.Y., Wu C.W., Shen B., Zhu A.Y. Exogenous proline reduces NaCl-induced damage by mediating ionic and osmotic adjustment and enhancing antioxidant defense in Eurya emarginata. Acta Physiol. Plant. 2015;37(9):181. [Google Scholar]
- Zhu B., Su J., Chang M., Verma D.P.S., Fan Y.L., Wu R. Overexpression of Δ1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water and salt-stressed in transgenic rice. Plant Sci. 1998;139(1):41–48. [Google Scholar]
- Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatev Z.S., Lidon F.J., Kaimakanova M. Plant physiological responses to UV-B radiation. Emir. J. Food Agric. 2012;24(6):481–501. [Google Scholar]
- Zong J.M., Li X.W., Zhou Y.H., Wang F.W., Wang N., Dong Y.Y., Yuan Y.X., Chen H., Liu X.M., Yao N., Li H.Y. The AaDREB1 transcription factor from the cold-tolerant plant Adonis amurensis enhances abiotic stress tolerance in transgenic plant. Int. J. Mol. Sci. 2016;17(4):611. doi: 10.3390/ijms17040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouari M., Ben Ahmed C., Elloumi N., Bellassoued K., Delmail D., Labrousse P., Ben Abdallah F., Ben Rouina B. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv. Chemlali exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2016;128:195–205. doi: 10.1016/j.ecoenv.2016.02.024. [DOI] [PubMed] [Google Scholar]