Abstract
The tumor microenvironment (TME) is a hypoxic, acidic, and immune/inflammatory cell–enriched milieu that plays crucial roles in tumor development, growth, progression, and therapy resistance. Targeting TME is an attractive strategy for the treatment of solid tumors. Conventional cancer chemotherapies are mostly designed to directly kill cancer cells, and the effectiveness is always compromised by their penetration and accessibility to cancer cells. Small-molecule inhibitors, which exhibit good penetration and accessibility, are widely studied, and many of them have been successfully applied in clinics for cancer treatment. As TME is more penetrable and accessible than tumor cells, a lot of efforts have recently been made to generate small-molecule inhibitors that specifically target TME or the components of TME or develop special drug-delivery systems that release the cytotoxic drugs specifically in TME. In this review, we briefly summarize the recent advances of small-molecule inhibitors that target TME for the tumor treatment.
Highlights
-
•
Tumor microenvironment (TME) is an indispensable part of tumor and is an important therapeutic target.
-
•
TME is more penetrable and accessible than tumor cell area.
-
•
Small-molecule inhibitors that target TME are very promising.
-
•
The target efficiency can be improved by specific deliver and release systems.
Introduction
The tumor microenvironment (TME) is a hypoxic and acidic milieu constituted of cellular and noncellular components. The cellular component is composed of various stromal cells, including endothelial cells (ECs), cancer-associated fibroblasts (CAFs), myeloid-derived suppressor cells (MDSCs), tumor-infiltrating lymphocytes (TILs), and tumor-associated macrophages (TAMs). The noncellular component includes nonsoluble or semisoluble substances, such as the extracellular matrix (ECM), and soluble substances, such as interstitial fluids, various cytokines and chemokines, growth factors, and metabolites [[1], [2], [3], [4], [5]]. TME is not only intrinsically immunosuppressive to protect tumor cells from immune surveillance but also dynamically adaptive to accommodate rapid tumor growth and progression and to counter any stress and insult conditions, such as chemotherapy [6,7]. TME is an essential part of the tumor mass, which is important for tumor growth, progression, metastasis, and therapy resistance [4,6,8]. Therefore, targeting TME would be an efficient way for the treatment of cancer. Indeed, many strategies have been developed to target the TME. As small molecules can easily access TME than can penetrate into tumor cells, development of small-molecule inhibitors that specifically target TME is one of the rapidly growing areas in this field.
Small-molecule inhibitors are compounds with a small size (≤500 Da). Compared with macromolecule agents, small-molecule inhibitors are more penetrative to the targets and usually can be engineered to be suitable for oral administration [[9], [10], [11], [12]]. Many small-molecule inhibitors have been successfully applied to treat a wide range of cancers, and much more are currently in either clinical trials or ongoing development. For example, sunitinib (Sutent), a multiple-tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR), oncogene c-KIT (KIT), receptor tyrosine kinase and platelet-derived growth factor receptor (PDGFR), has been approved as a potent antiangiogenesis drug and is applied to treat various tumors [9,13].
Recently, many small-molecule inhibitors have been developed to specifically or mainly target TME. These small molecules are designed to interrupt the specific features of TME, including the hypoxic, acidic, inflammatory milieu, as well as the abnormal ECM network in TME. Here, we briefly review the recent advances in the development of therapeutic small-molecule inhibitors that target TME.
Targeting Hypoxia in the TME
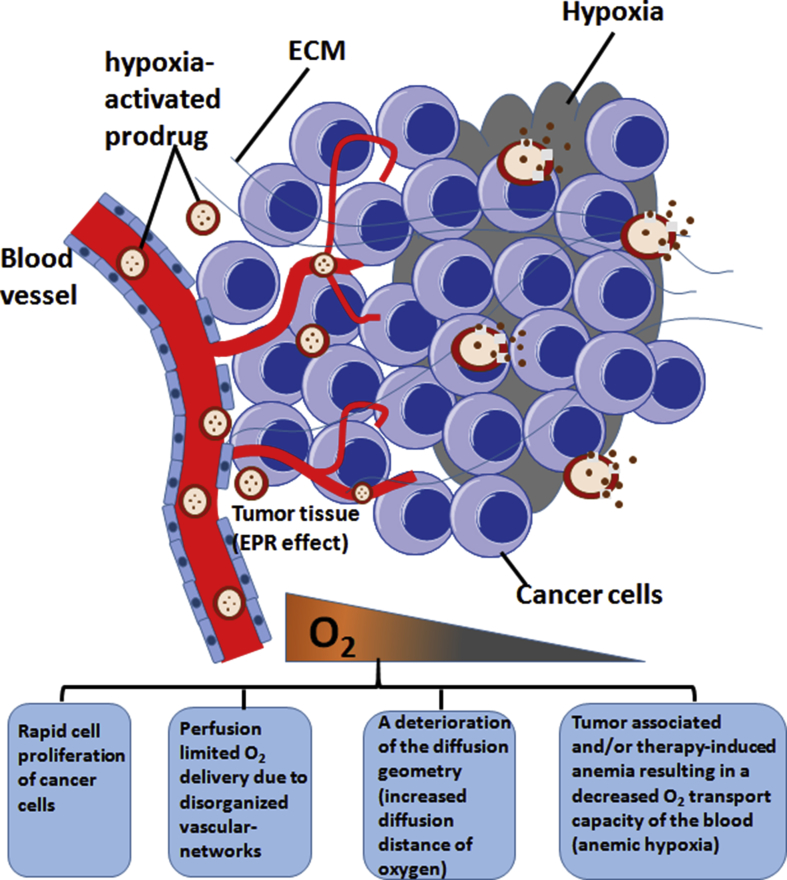
Hypoxia is one of the prominent features of TME. The rapid proliferation of cancer cells speeds up the consumption of oxygen, resulting in reduced oxygen level in solid tumor areas [14]. The disorganized vascular networks in tumor site that induce diffusion distance of oxygen also contribute to low oxygen level in TME [6,14,15]. In addition, tumor-associated and/or therapy-induced anemia causes a decreased O2 transport capacity of the blood, leading to hypoxia in tumor sites [16]. Hypoxia is associated with tumor metastasis, radiotherapy/chemotherapy resistance, and poor prognosis [15,17]. In hypoxic environment, tumor cells can use many mechanisms to survive, including shifting from aerobic to anaerobic metabolism, erythropoietin (EPO) production, deregulating DNA repair systems, recruiting the stromal components, as well as upregulating protooncogenes and hypoxia-inducible factor (HIF) 1α and HIF 2α [18,19]. For a detailed review of targeting hypoxia in cancer therapy, please refer to a recent publication by Wilson and Hay [20]. To exploit the unique feature of hypoxia in TME, the therapeutic agents are often designed as low-toxicity prodrugs in normoxia environment while selectively activated in hypoxic tumor areas (Figure 1). Papadopoulos et al. [21] designed the hypoxia-activated prodrug AQ4N (banoxantrone) that is converted into AQ4, a potent inhibitor of topoisomerase II, in hypoxic areas. This prodrug is applied to treat advanced solid tumors such as bronchoalveolar lung cancer and ovarian cancer. Weiss et al. [22] designed a hypoxia-activated prodrug TH-302 that is consisted of 2-nitroimidazole, a hypoxia trigger, and a brominated version of isophosphoramide mustard (Br-IPM). This prodrug remains intact in normal oxygen conditions and can be activated in severe hypoxic conditions (<0.5% O2) to release Br-IPM, a DNA cross-linking agent. TH-302 shows antitumor activities in metastatic melanoma and small cell lung cancer (SCLC). Another hypoxic cell toxin is tirapazamine (TPZ), which preferentially shows cytotoxic activity to hypoxic cancer cells. The underlying mechanism is that TPZ forms a radical by adding an electron under the catalytic action of various intracellular reductases. This TPZ radical is highly reactive and can lead to DNA single- or double-strand breaks in hypoxic environment. However, under aerobic conditions, the TPZ radical is back-oxidized into its nontoxic parent, and its cytotoxicity is rapidly reduced [15,23]. Another strategy is to design a delivery system that releases the carried-on drug preferentially in hypoxic microenvironment. For instance, Huo et al. [24] reported a size-tunable nanocluster bomb with an initial size of approximately 33 nm featuring a long half-life during blood circulation and destructed to release small hypoxia microenvironment-targeting nanoparticles (NPs) to achieve deep tumor penetration. The small-molecule inhibitors that target hypoxic TME are summarized in Table 1 [[21], [22], [23],25].
Figure 1.
Hypoxia-targeted therapy. The hypoxia in TME is resulted from several factors. Some hypoxia-activated prodrugs or hypoxia-targeting nanoparticle drug-delivery system are developed to inhibit the growth of cancer cells. TME, tumor microenvironment; ECM, extracellular matrix; EPR, enhanced permeability and retention effect.
Table 1.
Small-Molecule Inhibitors Target Hypoxia and Acidic Microenvironment
| Target | Small-molecular Inhibitor | Target Strategy | Mechanism of Action | Cancer Target | References |
|---|---|---|---|---|---|
| Hypoxia | Hypoxia-activated prodrug AQ4N (banoxantrone) | Inhibit tumor growth and progression | Be converted into AQ4, a potent inhibitor of topoisomerase II, in hypoxic areas | Bronchoalveolar lung cancer and ovarian cancer | [21] |
| Hypoxia-activated prodrug TH-302 | Inhibit tumor growth | Release brominated version of isophosphoramide mustard (Br-IPM) in hypoxic areas | Small cell lung cancer (SCLC) and melanoma | [22] | |
| Tirapazamine (TPZ) | Show preferentially cytotoxic activity to hypoxic cells | Form a reactive radical under the catalytic action of various intracellular reductases | Squamous cell carcinoma | [23] | |
| PR-104 [2-((2-bromoethyl)-2-[(2-hydroxyethyl)amino]carbonyl-4,6-dinitroanilino)ethyl methanesulfonatephosphateester] | Be converted into cytotoxic drug, hydroxylaminePR-104H, selectively under hypoxia, resulting in suppression of growth of hypoxic and aerobic cells | DNA cross-linking | Pancreatic and prostate tumors | [25] | |
| Acidic microenvironment | Esomeprazole (ESOM) | pH neutralization | Alter tumor pH by inhibiting proton extrusion | Melanoma | [40] |
| Omeprazole | pH neutralization | Inhibit V–H+-ATPase activity and alter extracellular pH | Colon, breast, ovarian cancer, melanoma | [41] | |
| Bicarbonate | pH neutralization | Increase tumor extracellular pH and reduce the formation of spontaneous metastases | Breast and prostate cancer | [42] | |
| 4,4′-Diisothiocyanatostilbene-2,20-disulfonic acid (DIDS) | Induce cell growth arrest and cell apoptosis | Inhibit anion exchangers (AEs) | Hepatocellular carcinoma | [55] | |
| α-Cyano-4-hydroxycinnamate (CHC) (combined with radiotherapy) | Retard tumor growth and render the remaining cancer cells sensitive to irradiation | Inhibit monocarboxylate transporter 1 (MCT1) | Lung carcinoma and colorectal adenocarcinoma | [56] | |
| Sulfonamide-based CAIX inhibitors (CAI17 and U-104) | Inhibit tumor growth, metastasis formation and deplete cancer stem cells | Inhibit CAIX activity | Breast cancer | [[43], [44], [45]] | |
| Glycosylcoumarins(GC-204 and GC-205) | Inhibit tumor growth and metastasis formation | Inhibit CAIX activity | Breast cancer | [43] | |
| Small organic ligands (such as AAZ) | Retard tumor growth, reduce metastasis and tumor stem cell expansion | Inhibit CAIX activity | Renal cell carcinoma | [50] | |
| Acetazolamide (combined with rapamycin) | Inhibit tumor growth and potentiate the anticancer activity of rapamycin | Inhibit CAIX activity | Colorectal adenocarcinoma | [51] | |
| SLC-0111 (combined with dacarbazine, temozolomide, doxorubicin, and 5-fluorouracil) | Potentiate the cytotoxic effects of conventional chemotherapeutic drugs | Inhibit CAIX activity | Melanoma, breast and colon cancer | [52] | |
| 2-Aminophenoxazine-3-one (Phx-3) | Disturb intracellular pH homeostasis, leading to apoptotic and cytotoxic events | Inhibit NHE1 activity | Gastric cancer | [46] | |
| Cariporide | Regulate intracellular pH reduce proliferation and induce apoptosis | Inhibit NHE1 activity | Cholangiocarcinoma, breast cancer | [[47], [48], [49]] | |
| S3705 | Regulate intracellular pH, reduce proliferation, and induce apoptosis | Inhibit the Na+-dependent Cl−/HCO3- exchanger activity | Cholangiocarcinoma | [48] |
Targeting the Acidic TME
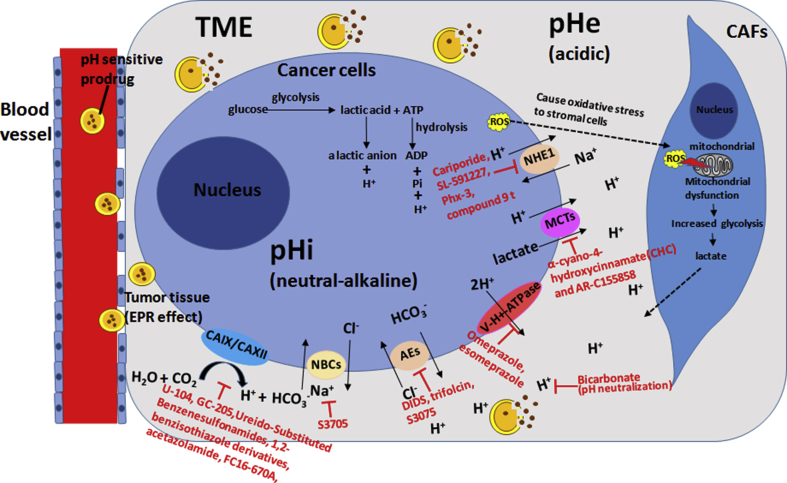
The extracellular pH in normal tissues is ∼7.4, while the pH value in TME is much lower (∼6.7–7.1) [26]. There are many mechanisms for the formation of acidic pH in tumors. Tumor cells use aerobic glycolysis as a major energy metabolism pathway in hypoxic environment, leading to increased production of lactic acid and H+ which are subsequently released in TME through passive diffusion and active membrane-based ion transport [27]. The H+-ATPases, Na+-H+ exchanger NHE1, as well as monocarboxylate-H+ efflux cotransporter MCT1 and MCT4 are highly increased or/and activated in tumor cells, which drive H+ efflux [26,[28], [29], [30]]. In addition, the carbonic anhydrase 9 (CA9), which is overexpressed in many types of cancer, also participates in the maintenance of low pH in TME [31,32]. In addition, tumor cells can induce oxidative stress to their neighboring stromal cells such as CAFs and TAMs by producing reactive oxygen species (ROS), which lead to mitochondrial dysfunction in CAFs and TAMs, resulting in accumulation of lactate in TME [33,34] (Figure 2). In addition, several mechanisms including the adaptation to hypoxia, oncogene activation, uncontrolled cell growth, and deficiencies in tumor perfusion due to the disorganized vascular networks also contribute to the tumor acidic microenvironment [35].
Figure 2.
Schematic diagram for the acidic-targeted therapy. When acid is produced due to the anaerobic glycolysis in tumor cells, several membrane transporters or exchangers, including NHE1, MCTs, V–H+-ATPase, AEs, and NBCs, transport the acid to extracellular microenvironment. In addition, the oxidative stress from tumor cells impairs the mitochondrial function of CAFs, resulting in the production and secretion of lactate. Some small-molecule inhibitors are designed to target these proton membrane transporters or exchangers to suppress tumor progression. And some pH-responsive prodrugs or pH-sensitive drug delivery systems are developed to specifically release drug in acidic TME. Abbreviation: pHi: intracellular pH; pHe: extracellular pH; NHE1: Na+/H+ exchanger 1; MCTs: monocarboxylate-H+ efflux cotransporters; V–H+-ATPase: vacuolar-type H-ATPase; AEs: anion exchangers; NBCs: Na+-HCO3- co-transporters; CAIX: carbonic anhydrase IX; CAXII: carbonic anhydrase XII; ROS: reactive oxygen species; TME, tumor microenvironment; CAFs, cancer-associated fibroblasts; EPR, enhanced permeability and retention effect.
The dysregulated pH in TME contributes to tumor progression, invasion, metastasis, and chemoresistance, and therefore, targeting acidic TME is a desirable tumor therapeutic strategy [26,[35], [36], [37], [38], [39]]. Some small-molecule inhibitors targeting acidic TME are developed (Table 1) (Figure 2) [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]]. In addition, efforts have recently been made to develop pH-responsive drug-release systems that deliver cytotoxic chemotherapy drugs specifically to acidic microenvironment (Figure 2). Zhang et al. [53] established a drug delivery system for targeting tumor acidic microenvironment via modifying pH (low) insertion peptide (pHLIP) on mesoporous organosilica nanoparticles (MONs), in which the doxorubicin (DOX) is loaded and can be released in response to glutathione and low pH in TME. Chen et al. developed another pH-responsive delivery system using polyethylene glycol (PEG)–DOX-encapsulated aza-BODIPY nanotheranostic agent. They linked DOX with PEG-benzaldehyde (PEG–CHO) via –HC=N– bond to form a Schiff's base, and then a near-infrared photosensitizer aza-BODIPY (AB) was encapsulated to form hydrophilic nanoparticles (DAB NPs). The –HC=N– bond can be broken in acidic TME, resulting in the release of DOX specifically in the tumor site [54].
Targeting Immune and Inflammatory Signaling in TME
The immune system is implicated in both tumor initiation and progression, and immune cells are enriched in TME in some solid tumor such as prostate cancer [57,58]. Some immune cells in TME, for instance TAMs and myeloid-derived suppressor cells (MDSCs), are tumor-promotive, while the immune activity of other cells, for instance CD8+ cells of TILs, is suppressed in TME [5,59,60]. Many therapeutic strategies have been tested for the treatment of cancer through inhibiting the tumor-promotive cells and their signaling or reversing/reconstituting the function of TILs. The small-molecule inhibitors that target immune cells or/and the inflammatory signaling are developed and summarized in Table 2 [[61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]].
Table 2.
Small-Molecule Inhibitors Target Immune Cells and Inflammatory Signaling in TME
| Target | Small-molecular Inhibitor | Target Strategy | Mechanism of Action | Cancer Target | References |
|---|---|---|---|---|---|
| TAMs | Bindarit | Decrease infiltration of TAMs and impair inflammatory cell responses | Inhibit the synthesis of MCP-1/CCL2 | The prostate and breast cancer | [61] |
| AS1517499 | Inhibit the differentiation of mouse macrophages into the M2-type | Target Stat6 pathway and reduce expression of Arg-1 and Mrc-1 expression and arginase activity | Breast cancer | [62] | |
| Tasquinimod | Inhibit MDSCs and TAMs of the M2-polarized phenotype | Target the inflammatory protein S100A9 | Prostate cancer and melanoma | [63] | |
| Trabectedin | Selective cytotoxicity to TAMs and circulating monocytes, resulting in TAMs depletion | Activates extrinsic TRAIL apoptotic pathway and inhibits production of cytokines such as CCL2 and IL-6 | Fibrosarcoma, ovarian and lung carcinoma | [64,65] | |
| BLZ-945 | TAMs depletion | Inhibit CSF-1R (colony-stimulating factor 1 receptor) | Breast and colon cancer | [66] | |
| Sorafenib | Reverse the immunosuppressive cytokine profile of TAMs | Restore secretion of IL-12, suppress IL-10 production | Breast tumor | [67] | |
| MDSCs | GW2580 | Reduce MDSC infiltration to tumors | Inhibit CSF1/CSF1R signaling | Prostate cancer | [68] |
| Sunitinib | Reduce MDSCs and tumor T regulatory cells and inhibit angiogenesis | Inhibit Stat3 in MDSCs and downregulate angiogenic gene expression | Metastatic renal cell carcinoma and pancreatic neuroendocrine tumor | [69,103] | |
| Axitinib | Decrease in the number of MDSCs in the spleens and tumor site | Downregulate STAT3 expression and reverse MDSC-mediated tumor-induced immunosuppression | Metastatic renal cell carcinoma (RCC) | [70] | |
| Sildenafil | Reduce MDSC function and enhance intratumoral T-cell infiltration and activation | Inhibit phosphodiesterase-5 and downregulate arginase 1 and nitric oxide synthase-2 expression | Multiple myeloma and head and neck cancer | [71] | |
| DCs | Paclitaxel (noncytotoxic dose) | Attenuate the propagation of regDC | Target Rho GTPase signaling | Lung cancer | [72] |
| TILs | SB415286 | Enhance the function of CD8+ T cells | Downregulate PD-1 expression through targeting GSK-3α/β | Melanoma and lymphoma | [73] |
| Trametinib | Inhibit CD4+ T-cell proliferation and cytokine production | Inhibit MEK | Colon cancer | [74] | |
| Cyclophosphamide (CTX) (combined with an agonist antibody OX40) | Induce Treg cell depletion | Induce Treg cell–specific apoptosis and enhance effector CD8+ T cells | Melanoma | [75] | |
| IC87114 | Inhibit activation and proliferation of Tregs and enhance CD8+ responses | Inhibit the PI3K-Akt pathway | Colon cancer and melanoma | [76] |
TAMs, tumor-associated macrophages; TILs, tumor-infiltrating lymphocytes; DCs, dendritic cells; CSF, cerebrospinal fluid; MDSCs, myeloid-derived suppressor cells; IL, interleukin.
TAMs are one of the most abundant and crucial cell components in TME. A high presence of TAMs in TME is significantly associated with poor prognosis of patients [77,78]. The phenotype of TAMs is diverse and plastic. There are 17 TAM phenotypes being identified based on single-cell analysis [79], and these subsets are potential targets for therapy. The strategies that suppress M2-type TAM recruitment, survival, and the relevant signaling cascades or repolarize M2-type TAMs to an M1 phenotype have been proposed for the treatment of cancer [3,[80], [81], [82]]. Some small-molecule inhibitors that target TAMs or TAM-associated signals have been developed (Table 2) [[61], [62], [63], [64], [65], [66], [67]].
MDSCs are a heterogeneous group of myeloid cells with an immature phenotype that expand in response to various tumor-derived cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-6 [[83], [84], [85], [86]]. MDSCs are associated with tumor progression, metastasis, and poor clinical prognosis [[87], [88], [89]]. MDSCs play important roles in the maintenance of the immunosuppressive TME through affecting the interactions between cancer cells and immune effectors [90]. MDSCs can release high levels of Arginase-1 in TME, leading to L-arginine depletion (a crucial nutrient for lymphocytes) that inhibits T-cell function. MDSCs also suppress dendritic cells (DCs)–mediated activation of T cells [[91], [92], [93]]. DCs act as a key cellular sensor to capture danger events such as invading microbes in the environment and provide necessary signals for T-cell activation, thereby shaping immune responses [94,95]. Targeting MDSC expansion or inhibiting their protumorigenic functions is a promising strategy to inhibit tumor progression. Some small-molecule inhibitors that target MDSCs or/and their associated signals have been developed and are summarized in Table 2 [[68], [69], [70], [71], [72]].
TILs are a complex group of T lymphocytes infiltrated in TME [96]. TILs have different subpopulations with different even opposite roles in immune responses. For instance, the subset of CD4+CD25+FOXP3+ regulatory T (Treg) cells suppress tumor-specific T-cell immunity and are associated with poor prognosis in ovarian carcinoma [97], and tumor-infiltrating CD4+CD25+FOXP3+ Treg cells showed prometastatic function in receptor activator of nuclear factor-κB (RANK)-expressing breast/mammary cancer cells [98]. However, the CD8+ TILs are positively correlated with patients’ overall survival in many cancer types, including cutaneous angiosarcoma, esophageal carcinomas, and non-small cell lung cancers (NSCLCs) [[99], [100], [101]]. Some small-molecule inhibitors that either enhance the function of CD8+ T cells or inhibit Treg cell proliferation and cytokine production have been developed (Table 2) [[73], [74], [75], [76]].
Targeting immune checkpoint, for instance, antibodies binding to programmed death 1 (PD-1) or programmed cell death 1 ligand 1 (PD-L1), has shown remarkable efficacy for cancer therapy. However, most immune checkpoint inhibitors currently used in clinic or in clinical trials are antibody drugs, which have some disadvantages such as immunogenicity. Immune checkpoint small-molecule inhibitors could offer inherent advantages in terms of pharmacokinetics and druggability. Many efforts have been made to develop immune checkpoint small-molecule inhibitors. The small-molecule inhibitors targeting PD-1 or PD-L1 have been well summarized by Li and Tian [102] in a recent review.
Targeting CAFs and ECs
CAFs are heterogeneous with various origins, including resident fibroblasts, mesenchymal cells, epithelia, and endothelia cells via epithelial/endothelial–mesenchymal transition [104,105]. CAFs are an essential component of TME and play an indispensable role in tumor development [105,106]. CAFs secret various cytokines, chemokines, growth factors, and other factors such as WNT16B, which promote tumorigenesis, metastasis, chemoresistance, angiogenesis, and cancer stem cell self-renewal [105,107]. CAFs express several specific markers, such as fibroblast activation protein (FAP), alpha smooth muscle actin (α-SMA), vimentin, S100A4 protein, fibroblast-specific protein–1 (FSP-1), insulin-like growth factor–binding protein 7 (IGFBP7), and Thy-1 [[108], [109], [110], [111], [112]]. These CAF markers not only make CAFs identifiable from normal counterparts but also can be used as specific therapeutic targets for tumor treatment. Some small-molecule inhibitors that target CAFs have been developed (Table 3) [[113], [114], [115], [116], [117], [118], [119], [120]]. In addition, the CAFs markers can be exploited as a drug delivery tool. For example, an FAP-specific peptide is coupled to a potent cytotoxic natural plant product thapsigargin (TG), which can be cleaved by the membrane-bound post-prolyl endopeptidase FAP in TME, resulting in TG release specifically in TME [121].
Table 3.
Small-Molecule Inhibitors Target Cancer-associated Fibroblasts and Endothelial Cells in TME
| Target | Small-molecular Inhibitor | Target Strategy | Mechanism of Action | Cancer Target | References |
|---|---|---|---|---|---|
| CAFs | PT-100 (combined with oxaliplatin) | Inhibit CAFs and reduce chemoresistance | Target fibroblast activation protein | Colon cancer | [113] |
| CAFs | RKN5755 | Inhibit CAF migration | Bind to β-arrestin 1 and interfere withβ-arrestin 1–mediated cofilin signaling pathways. | Breast cancer | [114] |
| CAFs | mPGES-1 inhibitor compound III (CIII) | Reduce tumor growth, impair angiogenesis, inhibit CAFs migration and infiltration, and favor shift in the M1/M2 macrophage ratio | Block CAF-derived prostaglandin E2 (PGE2) production | Neuroblastoma tumor | [115] |
| CAFs | Scriptaid | Repress TGFβ-mediated CAF differentiation and inhibit ECM secretion | Alter the cellular epigenetic regulatory machinery via HDAC inhibition | Melanoma | [116] |
| CAFs | LE135 and bicalutamide (combined with cisplatin) | Suppress CAF-facilitated oncogenesis and reduce chemoresistance | Retinoic acid receptor β and androgen receptor antagonists | Squamous cell carcinoma | [117] |
| CAFs | AC1MMYR2 (combined with taxol) | Reprogram CAFs, suppress tumor migration and invasion ability | Reprogram CAFs via the NF-kB/miR-21/VHL axis | Breast cancer | [118] |
| CAFs | SOM230 (combined with gemcitabine) | Reprogram CAFs and reduce chemoresistance | Activate the sst1 receptor and inhibit the mTOR/4E-BP1 pathway and the resultant synthesis of secreted CAF proteins | Pancreatic cancer | [139] |
| CAFs | Navitoclax | Trigger CAF apoptosis and suppress tumor outgrowth | Upregulate the proapoptotic protein Bax and diminish expression of the desmoplastic extracellular matrix protein tenascin C | Cholangiocarcinoma | [119] |
| CAFs | WRG-28 | Inhibit tumor invasion and migration | Inhibit receptor–ligand interactions via allosteric modulation of the collagen receptor discoidin domain receptor 2 (DDR2) | Breast cancer | [140] |
| CAFs and ECs | PD173074 | Reduce proliferation of CAFs and ECs suppress fibroblast-enhanced tumor cell growth and inhibit tumor growth | Inhibit fibroblast growth factor receptor (FGFR) | Head and neck squamous cell carcinoma (HNSCC) | [120] |
| ECs | DIMP53-1 | Induce cancer cell apoptosis, inhibit the migration and tube formation of ECs and inhibit angiogenesis | Bind to p53 inhibiting its interaction with MDM2 and MDMX | Colon cancer | [130] |
| ECs | BEZ235 (combined with verteporfin) | Enhance vascular-targeted photodynamic therapy inhibit endothelial cell proliferation and suppress tumor regrowth | Inhibit PI3K pathway activation | Prostate cancer | [141] |
| ECs | Biochanin A | Inhibit ECs functions such as cell viability, migration, invasion, and tumor progression | Inhibit activation of proangiogenic proteins (ERK/AKT/mTOR), inhibit chemical hypoxia-inducible factor-1α and vascular endothelial growth factor | Angiogenic gliomas | [131] |
| ECs | LLL12 | Reduce proliferation/migration of ECs and inhibit VEGF-induced tube formation, suppress tumor growth | Inhibit VEGF-stimulated STAT3phosphorylation in ECs | Osteosarcoma | [132] |
| ECs | TW-37 (combined with radiotherapy) | Abrogate new endothelial cell sprouting, inhibit tumor growth | Inhibit Bcl-2 | Head and neck cancer | [133] |
| ECs | CX-4945 | Inhibit EC migration, tube formation, cause cell-cycle arrest and selectively induce apoptosis in cancer cells | Attenuate PI3K/Akt signaling and block CK2-dependent HIF-1α transcription | Breast and pancreatic cancer | [134] |
| ECs | CC-5079 | Inhibit ECs, fibroblast, cancer cell proliferation and migration, inhibit microvessel formation | Stimulate MKP1 expression in ECs and fibroblast | Colon cancer | [135] |
| ECs | Dasatinib | Inhibit motility and other functions of ECs and myeloid cells, suppress tumor growth associated with increased tumor cell apoptosis, decreased microvessel density | Inhibit phosphorylation of SFKs and downstream signaling, reduce matrix metalloproteinase (MMP)-9 levels in TME | Prostate cancer and colon cancer | [136] |
| ECs | Pazopanib (GW786034B) | Block cancer cell growth, survival, and migration, and inhibit VEGF-induced up-regulation of adhesion molecules on ECs and tumor cells and decrease angiogenesis | Inhibit VEGF-triggered signaling pathways | Multiple myeloma and metastatic renal-cell cancer | [137,142] |
| ECs | TNP-470 | Inhibit vascular hyperpermeability of tumor blood vessels | Inhibit VPF/VEGF-induced phosphorylation of vascular endothelial growth factor receptor-2, calcium influx, and RhoA activation in ECs | Melanoma, glioblastoma and breast cancer | [138] |
| ECs | Sunitinib(SU11248) | Cause regression, growth arrest, or substantially reduced growth of cancer cells | Target the vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), KIT, and FLT3 receptor tyrosine kinases | Epidermoid carcinoma, colon carcinoma and metastatic renal-cell cancer | [142,143] |
CAFs, cancer-associated fibroblasts; ECs, endothelial cells; TME, tumor microenvironment.
Endothelial cells (ECs) are mostly quiescent and slowly proliferated in normal tissues of adults [122,123], while ECs in tumors are activated and possess high proangiogenic properties [124]. As a result, the morphologies and gene expression of tumor-associated ECs are very different compared with those in normal ECs. The tumor-associated ECs upregulate several angiogenesis-related genes and markers, such as aminopeptidase N (APN) and tumor endothelial marker 8 (TEM8) [125]. The tumor vessels are disorganized, irregular, fragile, and leaky, resulting in abnormal blood flow in tumor [126]. The disorganized tumor vessels hinder the delivery of drugs to some tumor sites and impair the efficacy of chemotherapy. Therefore, some therapeutic strategies are designed to normalize tumor vasculature, which can alleviate hypoxia in tumor and increase the efficacy of therapies [127]. Because the tumor endothelium dysfunction helps to sculpt the microenvironment and establish an immunosuppressed TME necessary for tumor progression and metastasis [128], targeting tumor-associated ECs is a very promising strategy for the tumor treatment. It has been reported that some naturally occurring endogenous angiogenesis inhibitors act as tumor suppressor proteins or peptides that block the angiogenic switch in tumors [129]. Small-molecule inhibitors designed to specifically target tumor-associated ECs are summarized in Table 3 [[130], [131], [132], [133], [134], [135], [136], [137], [138]].
Targeting the Abnormal ECM Network of TME
The ECM contains more than one hundred proteins, which are organized into a structural framework and act as a scaffold [144,145]. The major components of the ECM are fibrous proteins and proteoglycans including fibronectin, collagen, and hyaluronan (HA). The ECM also contains various growth factors, cytokines, and chemokines secreted by tumor cells and stromal cells. For example, vascular endothelial growth factor (VEGF) in TME, secreted by tumor cells, CAFs, and inflammatory cells, plays important roles in remodeling the ECM [6,146]. Some small-molecule inhibitors have been developed to target ECM components (Table 4) [[147], [148], [149], [150], [151], [152], [153], [154]].
Table 4.
Small-Molecule Inhibitors Target Extracellular Matrix Components
| Target | Small-molecular Inhibitor | Target Strategy | Mechanism of Action | Cancer Target | References |
|---|---|---|---|---|---|
| Hyaluronan (HA) | 4-methylumbelliferone (MU) | Lower HA levels | Inhibit HAS to synthesize HA | Prostate, breast, pancreatic cancer | [[147], [148], [149]] |
| Matrix metalloproteinases (MMPs) | cis-ACCP | Inhibit MMPs and show antimetastatic activity | Sustain and prolong absorption occurred via paracellular | Prostate and melanoa cancer | [150] |
| N-[4-(difluoromethoxy)phenyl]-2-[(4-oxo-6-propyl-1Hpyrimidin-2-yl)sulfanyl]-acetamide | Abrogate MMP-9 homodimerization and block MMP-9–mediated cell migration related signaling pathway | Target hemopexin (PEX) domain of matrix metalloproteinase-9 | Breast cancer | [151] | |
| Lysophosphatidic acid (LPA) | ONO-8430506 | Decrease lysophosphatidate signaling | Inhibit activity of secreted enzyme, autotaxin (ATX) | Breast cancer | [152] |
| PF-8380 | Abrogate radiation-induced tumor neovascularization and delay tumor growth | Inhibit activity of secreted enzyme, autotaxin (ATX) | Glioblastoma multiforme | [153] | |
| Collagen | 3-[4″-methoxy-3,2′-dimethyl-(1,1'; 4′,1″)terphenyl-2″-yl]propionic acid (T12) | Impair the formation of mesh collagen IV and impede tumor EMT | Target mesenchymal goodpasture antigen-binding protein (GPBP) and disturb its multimerization | Non-small cell lung carcinoma (NSCLC) | [154] |
Hyaluronan
HA, a major ECM component, is synthesized by HA synthases (HAS) and degraded by hyaluronidase (HYAL). HA is elevated in many solid tumors, which leads to increased interstitial fluid pressure and reduced elasticity of tumor tissue [155,156]. Based on its function in ion exchange, HA level may act as a molecular sieve, which blocks perfusion of anticancer drugs and contributes to chemoresistance. High HA levels are correlated with poor prognosis in breast, gastric, colorectal, and bladder cancer [157,158]. Targeting HA has been used to manipulate tumor stroma remodeling and to facilitate drug delivery. For instance, initial reports demonstrated that the addition of corticosteroids to chemotherapeutic regimens, which rapidly decreases HA via inhibiting its synthesis, could significantly improve therapeutic efficacy [156]. Pegvorhyaluronidase alfa (PEGPH20), an enzymatic agent that can ablate stromal HA level and remodel TME, facilitates drug delivery by overcoming physical barriers in the ECM. The combination of chemotherapeutic regimen gemcitabine with PEGPH20 for the treatment of pancreatic ductal adenocarcinoma has shown objective tumor responses and nearly doubled overall survival [159,160]. HYAL, an enzyme that catalyzes the degradation of HA, promotes DOX penetration and its cell killing effect [161]. In addition, HA is an anionic cell surface–associated polysaccharide, which facilitates HA binding to the CD44 receptor overexpressed in most of cancer cells. Thus, HA has been exploited as a part of delivery tool to transport drug to tumor cells [[162], [163], [164]].
Matrix Metalloproteinase Protein
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases. MMPs are synthesized as inactive zymogens, which are subsequently activated by serine proteinases or other MMPs in the microenvironment [165]. Because MMPs can cleave almost all components of the ECM, they play important roles in ECM remodeling. Accumulated evidence has shown that increased expression or/and activation of MMPs is involved in the processes of carcinogenesis, invasion, and metastasis [166]. Several small-molecule inhibitors selectively targeting MMPs have been developed (Table 4) [150,151]. In addition to being a direct target for cancer therapy, MMPs have also been used as part of drug delivery tools that release the cytotoxic drugs specifically in TME. Sun et al. integrated the chemotherapy drug paclitaxel into nanoparticles that is modified with an MMP-cleavable linker and a cell-penetrating peptide. This functionalized nanoparticle showed a high affinity to both tumor cells and TAMs, and the integrated paclitaxel was effectively delivered and released into the tumor site owing to high levels of MMPs in TME [167].
Lysophosphatidic Acid
Lysophosphatidic acid (LPA) is a crucial component of TME. LPA is mainly produced from lysophosphatidylcholine (LPC) by a secreted enzyme, autotaxin (ATX). LPA activates six G protein–coupled receptors to regulate cell division, survival, and migration; LPA signaling is essential for vasculogenesis in embryonic development [168]. LPA acts as a profibrotic and prooncogenic factor and plays important roles in tumor progression, metastasis, angiogenesis, and therapy resistance [2,[169], [170], [171], [172], [173]]. Targeting LPA signaling has been reported to be a promising method to inhibit tumor growth and to improve the efficacies of chemotherapy and radiotherapy (Table 4) [152,153].
Collagen
Collagen is an indispensable component of the ECM. Collagen is composed of 3 polypeptide α chains that form a triple helical structure. In vertebrates, 46 distinct collagen chains assemble to form 28 collagen types that are categorized into fibril-forming collagens, network-forming collagens, fibril-associated collagens with interruptions in their triple helices (FACITs), and others [145,174,175]. Multiple reports have shown that collagen is associated with tumor initiation and progression. Collagen level and cross-linking are strongly correlated with tumor stiffness. Increased stiffness is associated with poor prognosis of breast tumor [[176], [177], [178], [179]]. Targeting collagen is a promising strategy to inhibit tumor growth (Table 4) [154].
Conclusions and Perspectives
Conventional cancer chemotherapies are mostly designed to directly kill cancer cells, and the effectiveness is always compromised by their penetration and accessibility to cancer cells. Small-molecule inhibitors, which exhibit good penetration and accessibility as compared with other large molecules, are widely studied, and most of them are designed to attack cancer cells directly. TME is a complicated and dynamic system, which is an indispensable part of tumor as a whole. As TME is more penetrable and accessible than tumor cells, many efforts have been made to develop therapeutic strategies that target TME. A large number of small-molecule inhibitors that target TME have been developed, many of which are still at the early stages in preclinical and clinical trials. These small-molecule inhibitors are designed to specifically target TME or the components of TME or to be delivered and released specifically in TME.
As there are rapid advances in understanding the underlying mechanisms of the interaction between tumor cells and TME, more and more specific targets in TME will be emerged as druggable targets for cancer therapy. New small-molecule inhibitors that specifically interrupt these new targets will be highly appreciable. To improve the tumor-killing potency and reduce side toxicity effect of the TME-oriented small-molecule inhibitors, we should also consider combining the "three specifics" for the same inhibitor (namely, targeted specifically, delivered specifically, and released specifically in TME). For instance, modifying a small-molecule inhibitor with tumor-selective ligands (such as folate, transferrin, peptides) and PEGylation will improve target specificity, while integrating this inhibitor into nanoparticle delivery systems will increase the delivery efficiency. In addition, exploiting environmental stimuli–responsive delivery systems will enhance inhibitors’ target selectivity.
Inhibition of immune regulatory checkpoints, such as CTLA-4 and the PD-1–PD-L1 axis, is currently at the forefront of immunotherapy for cancers of various histological types. However, the CTLA-4 and PD-1/PD-L1 antibodies currently available for tumor immunotherapy are only effective for 20–30% of patients with solid tumor [[180], [181], [182]]. The complexity and heterogeneity of the TME suggest that there would be other unknown important mechanisms leading to inhibition of T-cell killing and immune suppression. For the immune system to mount an adequate response to cancer, it must overcome a slew of obstacles. First, T cells that recognize tumor antigens must be sufficiently activated by antigen-presenting cells, and they need to (leave lymphoid system and reside beyond the lymphoid system) migrate and amass within specific solid tumors to destroy tumor cells. However, numerous factors from tumors and TME can dampen and suppress antitumor T-cell responses by preventing T cells from amassing into tumors [183]. Second, signals present within the harsh TME undermine the ability of the tumor-infiltrating lymphocytes (TILs), the CD8+ T cells, to fight cancer. Therefore, any strategies, particularly small-molecule inhibitors (or activators) that target TME to promote CD8+ T-cell infiltration in tumors and to prevent the development of a dysfunctional or "exhausted" T-cell state or reverse the function of "exhausted" T cells, would be highly desirable.
Conflicts of interests
The authors declare no conflicts of interests.
Acknowledgements
This work is supported by a postdoctoral scholarship from China Science Foundation (2018M633001) and the grant from the Frenchman's Creek Women for Cancer Research. Our study was also supported by the Natural Science Foundation of China (81573012).
Contributor Information
Zhikang Chen, Email: 403445@csu.edu.cn.
Zihua Chen, Email: zihuachenxy@126.com.
Jun-Li Luo, Email: jlluo@scripps.edu.
References
- 1.Fan F., Schimming A., Jaeger D., Podar K. Targeting the tumor microenvironment: focus on angiogenesis. J Oncol. 2012;2012:281261. doi: 10.1155/2012/281261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benesch M.G.K., Yang Z., Tang X., Meng G., Brindley D.N. Lysophosphatidate signaling: the tumor microenvironment's new nemesis. Trends Cancer. 2017;3:748–752. doi: 10.1016/j.trecan.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M., Zhao J., Zhang L., Wei F., Lian Y., Wu Y., Gong Z., Zhang S., Zhou J., Cao K. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rokavec M., Wu W., Luo J.L. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilkes D.M., Semenza G.L., Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J.L., Tan W., Ricono J.M., Korchynskyi O., Zhang M., Gonias S.L., Cheresh D.A., Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 8.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai K., Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie M.J., Hirte H.W., Glenwood G., Jean M., Goel R., Major P.P., Miller W.H., Jr., Panasci L., Lorimer I.A., Batist G. A phase II trial of ZD1839 (Iressa) 750 mg per day, an oral epidermal growth factor receptor-tyrosine kinase inhibitor, in patients with metastatic colorectal cancer. Investig New Drugs. 2005;23:165–170. doi: 10.1007/s10637-005-5862-9. [DOI] [PubMed] [Google Scholar]
- 11.Dancey J., Sausville E.A. Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov. 2003;2:296–313. doi: 10.1038/nrd1066. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Huang J., Jeong J.H., Park S.J., Wei R., Peng J., Luo Z., Chen Y.T., Feng Y., Luo J.L. Selective TBK1/IKKi dual inhibitors with anticancer potency. Int J Cancer. 2014;134:1972–1980. doi: 10.1002/ijc.28507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx J. Cancer. Encouraging results for second-generation antiangiogenesis drugs. Science. 2005;308:1248–1249. doi: 10.1126/science.308.5726.1248. [DOI] [PubMed] [Google Scholar]
- 14.Vaupel P., Mayer A., Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 15.Brown J.M. Hypoxic cytotoxic agents: a new approach to cancer chemotherapy. Drug Resist Updates. 2000;3:7–13. doi: 10.1054/drup.2000.0120. [DOI] [PubMed] [Google Scholar]
- 16.Hockel M., Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 17.Vaupel P. Prognostic potential of the pre-therapeutic tumor oxygenation status. Adv Exp Med Biol. 2009;645:241–246. doi: 10.1007/978-0-387-85998-9_36. [DOI] [PubMed] [Google Scholar]
- 18.Casazza A., Di Conza G., Wenes M., Finisguerra V., Deschoemaeker S., Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33:1743–1754. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 19.Bristow R.G., Hill R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 20.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos K.P., Goel S., Beeram M., Wong A., Desai K., Haigentz M., Milian M.L., Mani S., Tolcher A., Lalani A.S. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res. 2008;14:7110–7115. doi: 10.1158/1078-0432.CCR-08-0483. [DOI] [PubMed] [Google Scholar]
- 22.Weiss G.J., Infante J.R., Chiorean E.G., Borad M.J., Bendell J.C., Molina J.R., Tibes R., Ramanathan R.K., Lewandowski K., Jones S.F. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 23.Brown J.M. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 24.Huo D., Liu S., Zhang C., He J., Zhou Z., Zhang H., Hu Y. Hypoxia-targeting, tumor microenvironment responsive nanocluster bomb for radical-enhanced radiotherapy. ACS Nano. 2017;11:10159–10174. doi: 10.1021/acsnano.7b04737. [DOI] [PubMed] [Google Scholar]
- 25.Patterson A.V., Ferry D.M., Edmunds S.J., Gu Y., Singleton R.S., Patel K., Pullen S.M., Hicks K.O., Syddall S.P., Atwell G.J. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 26.Webb B.A., Chimenti M., Jacobson M.P., Barber D.L. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 27.Tannock I.F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 28.Martinez-Zaguilan R., Lynch R.M., Martinez G.M., Gillies R.J. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265:C1015–C1029. doi: 10.1152/ajpcell.1993.265.4.C1015. [DOI] [PubMed] [Google Scholar]
- 29.McLean L.A., Roscoe J., Jorgensen N.K., Gorin F.A., Cala P.M. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol. 2000;278:C676–C688. doi: 10.1152/ajpcell.2000.278.4.C676. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro C., Longatto-Filho A., Ferreira L., Pereira S.M., Etlinger D., Moreira M.A., Jube L.F., Queiroz G.S., Schmitt F., Baltazar F. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int J Gynecol Pathol. 2008;27:568–574. doi: 10.1097/PGP.0b013e31817b5b40. [DOI] [PubMed] [Google Scholar]
- 31.Swietach P., Patiar S., Supuran C.T., Harris A.L., Vaughan-Jones R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem. 2009;284:20299–20310. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiche J., Ilc K., Laferriere J., Trottier E., Dayan F., Mazure N.M., Brahimi-Horn M.C., Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 33.Bohme I., Bosserhoff A.K. Acidic tumor microenvironment in human melanoma. Pigment Cell Melanoma Res. 2016;29:508–523. doi: 10.1111/pcmr.12495. [DOI] [PubMed] [Google Scholar]
- 34.Pavlides S., Whitaker-Menezes D., Castello-Cros R., Flomenberg N., Witkiewicz A.K., Frank P.G., Casimiro M.C., Wang C., Fortina P., Addya S. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 35.De Milito A., Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 36.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiche J., Brahimi-Horn M.C., Pouyssegur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14:771–794. doi: 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo A., Yamamoto S., Nakaki R., Shimamura T., Hamakubo T., Sakai J., Kodama T., Yoshida T., Aburatani H., Osawa T. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 2017;18:2228–2242. doi: 10.1016/j.celrep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Taylor S., Spugnini E.P., Assaraf Y.G., Azzarito T., Rauch C., Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updates. 2015;23:69–78. doi: 10.1016/j.drup.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 40.De Milito A., Canese R., Marino M.L., Borghi M., Iero M., Villa A., Venturi G., Lozupone F., Iessi E., Logozzi M. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010;127:207–219. doi: 10.1002/ijc.25009. [DOI] [PubMed] [Google Scholar]
- 41.Luciani F., Spada M., De Milito A., Molinari A., Rivoltini L., Montinaro A., Marra M., Lugini L., Logozzi M., Lozupone F. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 42.Robey I.F., Baggett B.K., Kirkpatrick N.D., Roe D.J., Dosescu J., Sloane B.F., Hashim A.I., Morse D.L., Raghunand N., Gatenby R.A. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou Y., McDonald P.C., Oloumi A., Chia S., Ostlund C., Ahmadi A., Kyle A., Auf dem Keller U., Leung S., Huntsman D. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71:3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 44.Pacchiano F., Carta F., McDonald P.C., Lou Y., Vullo D., Scozzafava A., Dedhar S., Supuran C.T. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011;54:1896–1902. doi: 10.1021/jm101541x. [DOI] [PubMed] [Google Scholar]
- 45.Lock F.E., McDonald P.C., Lou Y., Serrano I., Chafe S.C., Ostlund C., Aparicio S., Winum J.Y., Supuran C.T., Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- 46.Nagata H., Che X.F., Miyazawa K., Tomoda A., Konishi M., Ubukata H., Tabuchi T. Rapid decrease of intracellular pH associated with inhibition of Na+/H+ exchanger precedes apoptotic events in the MNK45 and MNK74 gastric cancer cell lines treated with 2-aminophenoxazine-3-one. Oncol Rep. 2011;25:341–346. doi: 10.3892/or.2010.1082. [DOI] [PubMed] [Google Scholar]
- 47.Amith S.R., Fliegel L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res. 2013;73:1259–1264. doi: 10.1158/0008-5472.CAN-12-4031. [DOI] [PubMed] [Google Scholar]
- 48.Di Sario A., Bendia E., Omenetti A., De Minicis S., Marzioni M., Kleemann H.W., Candelaresi C., Saccomanno S., Alpini G., Benedetti A. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Dig Liver Dis. 2007;39:60–69. doi: 10.1016/j.dld.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Harguindey S., Arranz J.L., Polo Orozco J.D., Rauch C., Fais S., Cardone R.A., Reshkin S.J. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs--an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J Transl Med. 2013;11:282. doi: 10.1186/1479-5876-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wichert M., Krall N. Targeting carbonic anhydrase IX with small organic ligands. Curr Opin Chem Biol. 2015;26:48–54. doi: 10.1016/j.cbpa.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Faes S., Planche A., Uldry E., Santoro T., Pythoud C., Stehle J.C., Horlbeck J., Letovanec I., Riggi N., Datta D. Targeting carbonic anhydrase IX improves the anti-cancer efficacy of mTOR inhibitors. Oncotarget. 2016;7:36666–36680. doi: 10.18632/oncotarget.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreucci E., Ruzzolini J., Peppicelli S., Bianchini F., Laurenzana A., Carta F., Supuran C.T., Calorini L. The carbonic anhydrase IX inhibitor SLC-0111 sensitises cancer cells to conventional chemotherapy. J Enzym Inhib Med Chem. 2019;34:117–123. doi: 10.1080/14756366.2018.1532419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., Dang M., Tian Y., Zhu Y., Liu W., Tian W., Su Y., Ni Q., Xu C., Lu N. Tumor acidic microenvironment targeted drug delivery based on pHLIP-modified mesoporous organosilica nanoparticles. ACS Appl Mater Interfaces. 2017;9:30543–30552. doi: 10.1021/acsami.7b10840. [DOI] [PubMed] [Google Scholar]
- 54.Chen D., Tang Q., Zou J., Yang X., Huang W., Zhang Q., Shao J., Dong X. pH-responsive PEG-doxorubicin-encapsulated aza-BODIPY nanotheranostic agent for imaging-guided synergistic cancer therapy. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201701272. [DOI] [PubMed] [Google Scholar]
- 55.Liu C.J., Hwang J.M., Wu T.T., Hsieh Y.H., Wu C.C., Hsieh Y.S., Tsai C.H., Wu H.C., Huang C.Y., Liu J.Y. Anion exchanger inhibitor DIDS induces human poorly-differentiated malignant hepatocellular carcinoma HA22T cell apoptosis. Mol Cell Biochem. 2008;308:117–125. doi: 10.1007/s11010-007-9619-y. [DOI] [PubMed] [Google Scholar]
- 56.Sonveaux P., Vegran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N., De Saedeleer C.J., Kennedy K.M., Diepart C., Jordan B.F. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Investig. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ammirante M., Luo J.L., Grivennikov S., Nedospasov S., Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vikas P., Borcherding N., Zhang W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag Res. 2018;10:6823–6833. doi: 10.2147/CMAR.S185176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo J.L., Maeda S., Hsu L.C., Yagita H., Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Majety M., Runza V., Lehmann C., Hoves S., Ries C.H. A drug development perspective on targeting tumor-associated myeloid cells. FEBS J. 2018;285:763–776. doi: 10.1111/febs.14277. [DOI] [PubMed] [Google Scholar]
- 61.Zollo M., Di Dato V., Spano D., De Martino D., Liguori L., Marino N., Vastolo V., Navas L., Garrone B., Mangano G. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis. 2012;29:585–601. doi: 10.1007/s10585-012-9473-5. [DOI] [PubMed] [Google Scholar]
- 62.Binnemars-Postma K., Bansal R., Storm G., Prakash J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018;32:969–978. doi: 10.1096/fj.201700629R. [DOI] [PubMed] [Google Scholar]
- 63.Shen L., Sundstedt A., Ciesielski M., Miles K.M., Celander M., Adelaiye R., Orillion A., Ciamporcero E., Ramakrishnan S., Ellis L. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res. 2015;3:136–148. doi: 10.1158/2326-6066.CIR-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Germano G., Frapolli R., Belgiovine C., Anselmo A., Pesce S., Liguori M., Erba E., Uboldi S., Zucchetti M., Pasqualini F. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Liguori M., Buracchi C., Pasqualini F., Bergomas F., Pesce S., Sironi M., Grizzi F., Mantovani A., Belgiovine C., Allavena P. Functional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironment. Oncotarget. 2016;7:41662–41676. doi: 10.18632/oncotarget.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen S., Li H.J., Chen K.G., Wang Y.C., Yang X.Z., Lian Z.X., Du J.Z., Wang J. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17:3822–3829. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 67.Edwards J.P., Emens L.A. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE(2) in murine macrophages. Int Immunopharmacol. 2010;10:1220–1228. doi: 10.1016/j.intimp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J., Escamilla J., Mok S., David J., Priceman S., West B., Bollag G., McBride W., Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xin H., Zhang C., Herrmann A., Du Y., Figlin R., Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan H., Cai P., Li Q., Wang W., Sun Y., Xu Q., Gu Y. Axitinib augments antitumor activity in renal cell carcinoma via STAT3-dependent reversal of myeloid-derived suppressor cell accumulation. Biomed Pharmacother. 2014;68:751–756. doi: 10.1016/j.biopha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Serafini P., Meckel K., Kelso M., Noonan K., Califano J., Koch W., Dolcetti L., Bronte V., Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong H., Gutkin D.W., Han B., Ma Y., Keskinov A.A., Shurin M.R., Shurin G.V. Origin and pharmacological modulation of tumor-associated regulatory dendritic cells. Int J Cancer. 2014;134:2633–2645. doi: 10.1002/ijc.28590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor A., Rothstein D., Rudd C.E. Small-molecule inhibition of PD-1 transcription is an effective alternative to antibody blockade in cancer therapy. Cancer Res. 2018;78:706–717. doi: 10.1158/0008-5472.CAN-17-0491. [DOI] [PubMed] [Google Scholar]
- 74.Liu L., Mayes P.A., Eastman S., Shi H., Yadavilli S., Zhang T., Yang J., Seestaller-Wehr L., Zhang S.Y., Hopson C. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639–1651. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 75.Hirschhorn-Cymerman D., Rizzuto G.A., Merghoub T., Cohen A.D., Avogadri F., Lesokhin A.M., Weinberg A.D., Wolchok J.D., Houghton A.N. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abu-Eid R., Samara R.N., Ozbun L., Abdalla M.Y., Berzofsky J.A., Friedman K.M., Mkrtichyan M., Khleif S.N. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steidl C., Lee T., Shah S.P., Farinha P., Han G., Nayar T., Delaney A., Jones S.J., Iqbal J., Weisenburger D.D. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campbell M.J., Tonlaar N.Y., Garwood E.R., Huo D., Moore D.H., Khramtsov A.I., Au A., Baehner F., Chen Y., Malaka D.O. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Canc Res Treat. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chevrier S., Levine J.H., Zanotelli V.R.T., Silina K., Schulz D., Bacac M., Ries C.H., Ailles L., Jewett M.A.S., Moch H. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169:736–749 e718. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis C., Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poh A.R., Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pyzer A.R., Cole L., Rosenblatt J., Avigan D.E. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer. 2016;139:1915–1926. doi: 10.1002/ijc.30232. [DOI] [PubMed] [Google Scholar]
- 85.Vuk-Pavlovic S., Bulur P.A., Lin Y., Qin R., Szumlanski C.L., Zhao X., Dietz A.B. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aliper A.M., Frieden-Korovkina V.P., Buzdin A., Roumiantsev S.A., Zhavoronkov A. Interactome analysis of myeloid-derived suppressor cells in murine models of colon and breast cancer. Oncotarget. 2014;5:11345–11353. doi: 10.18632/oncotarget.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang G., Huang H., Zhu Y., Yu G., Gao X., Xu Y., Liu C., Hou J., Zhang X. A novel subset of B7-H3(+)CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells are associated with progression of human NSCLC. OncoImmunology. 2015;4 doi: 10.4161/2162402X.2014.977164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z.L., Ye S.B., OuYang L.Y., Zhang H., Chen Y.S., He J., Chen Q.Y., Qian C.N., Zhang X.S., Cui J. COX-2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid-derived suppressor cells. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2015.1044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang H., Zhang G., Li G., Ma H., Zhang X. Circulating CD14(+)HLA-DR(-/low) myeloid-derived suppressor cell is an indicator of poor prognosis in patients with ESCC. Tumour Biol. 2015;36:7987–7996. doi: 10.1007/s13277-015-3426-y. [DOI] [PubMed] [Google Scholar]
- 90.Schupp J., Krebs F.K., Zimmer N., Trzeciak E., Schuppan D., Tuettenberg A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol. 2017 doi: 10.1016/j.cellimm.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Wu G., Morris S.M., Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Popovic P.J., Zeh H.J., 3rd, Ochoa J.B. Arginine and immunity. J Nutr. 2007;137:1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 93.Hu C.E., Gan J., Zhang R.D., Cheng Y.R., Huang G.J. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46:156–164. doi: 10.3109/00365521.2010.516450. [DOI] [PubMed] [Google Scholar]
- 94.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tran Janco J.M., Lamichhane P., Karyampudi L., Knutson K.L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J., Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol. 2014;31:82. doi: 10.1007/s12032-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 97.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 98.Tan W., Zhang W., Strasner A., Grivennikov S., Cheng J.Q., Hoffman R.M., Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujii H., Arakawa A., Utsumi D., Sumiyoshi S., Yamamoto Y., Kitoh A., Ono M., Matsumura Y., Kato M., Konishi K. CD8(+) tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134:2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 100.Schumacher K., Haensch W., Roefzaad C., Schlag P.M. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 101.Zhuang X., Xia X., Wang C., Gao F., Shan N., Zhang L., Zhang L. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18:24–28. doi: 10.1097/PAI.0b013e3181b6a741. [DOI] [PubMed] [Google Scholar]
- 102.Li K., Tian H.Q. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J Drug Target. 2019;27:244–256. doi: 10.1080/1061186X.2018.1440400. [DOI] [PubMed] [Google Scholar]
- 103.Faivre S., Niccoli P., Castellano D., Valle J.W., Hammel P., Raoul J.L., Vinik A., Van Cutsem E., Bang Y.J., Lee S.H. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol. 2017;28:339–343. doi: 10.1093/annonc/mdw561. [DOI] [PubMed] [Google Scholar]
- 104.Anderberg C., Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;8:1461–1462. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- 105.Xing F., Saidou J., Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed) 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J., Chen S., Wang W., Ning B.F., Chen F., Shen W., Ding J., Chen W., Xie W.F., Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 107.Tao L., Huang G., Song H., Chen Y., Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugimoto H., Mundel T.M., Kieran M.W., Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 109.Park J.E., Lenter M.C., Zimmermann R.N., Garin-Chesa P., Old L.J., Rettig W.J. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 110.Kim H.M., Jung W.H., Koo J.S. Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: an immunohistochemical analysis. J Transl Med. 2015;13:222. doi: 10.1186/s12967-015-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rupp C., Scherzer M., Rudisch A., Unger C., Haslinger C., Schweifer N., Artaker M., Nivarthi H., Moriggl R., Hengstschlager M. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene. 2015;34:815–825. doi: 10.1038/onc.2014.18. [DOI] [PubMed] [Google Scholar]
- 112.Schliekelman M.J., Creighton C.J., Baird B.N., Chen Y., Banerjee P., Bota-Rabassedas N., Ahn Y.H., Roybal J.D., Chen F., Zhang Y. Thy-1(+) cancer-associated fibroblasts adversely impact lung cancer prognosis. Sci Rep. 2017;7:6478. doi: 10.1038/s41598-017-06922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li M., Li M., Yin T., Shi H., Wen Y., Zhang B., Chen M., Xu G., Ren K., Wei Y. Targeting of cancerassociated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment. Mol Med Rep. 2016;13:2476–2484. doi: 10.3892/mmr.2016.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suvarna K., Honda K., Kondoh Y., Osada H., Watanabe N. Identification of a small-molecule ligand of beta-arrestin1 as an inhibitor of stromal fibroblast cell migration accelerated by cancer cells. Cancer Med. 2018;7:883–893. doi: 10.1002/cam4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kock A., Larsson K., Bergqvist F., Eissler N., Elfman L.H.M., Raouf J., Korotkova M., Johnsen J.I., Jakobsson P.J., Kogner P. Inhibition of microsomal prostaglandin E synthase-1 in cancer-associated fibroblasts suppresses neuroblastoma tumor growth. EBioMed. 2018;32:84–92. doi: 10.1016/j.ebiom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim D.J., Dunleavey J.M., Xiao L., Ollila D.W., Troester M.A., Otey C.A., Li W., Barker T.H., Dudley A.C. Suppression of TGFbeta-mediated conversion of endothelial cells and fibroblasts into cancer associated (myo)fibroblasts via HDAC inhibition. Br J Canc. 2018;118:1359–1368. doi: 10.1038/s41416-018-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan J.S.K., Sng M.K., Teo Z.Q., Chong H.C., Twang J.S., Tan N.S. Targeting nuclear receptors in cancer-associated fibroblasts as concurrent therapy to inhibit development of chemoresistant tumors. Oncogene. 2018;37:160–173. doi: 10.1038/onc.2017.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren Y., Zhou X., Liu X., Jia H.H., Zhao X.H., Wang Q.X., Han L., Song X., Zhu Z.Y., Sun T. Reprogramming carcinoma associated fibroblasts by AC1MMYR2 impedes tumor metastasis and improves chemotherapy efficacy. Cancer Lett. 2016;374:96–106. doi: 10.1016/j.canlet.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Mertens J.C., Fingas C.D., Christensen J.D., Smoot R.L., Bronk S.F., Werneburg N.W., Gustafson M.P., Dietz A.B., Roberts L.R., Sirica A.E. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sweeny L., Liu Z., Lancaster W., Hart J., Hartman Y.E., Rosenthal E.L. Inhibition of fibroblasts reduced head and neck cancer growth by targeting fibroblast growth factor receptor. Laryngoscope. 2012;122:1539–1544. doi: 10.1002/lary.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brennen W.N., Rosen D.M., Wang H., Isaacs J.T., Denmeade S.R. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;104:1320–1334. doi: 10.1093/jnci/djs336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aird W.C. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98:159–162. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 123.Hida K., Maishi N., Annan D.A., Hida Y. Contribution of tumor endothelial cells in cancer progression. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 125.Matsuda K., Ohga N., Hida Y., Muraki C., Tsuchiya K., Kurosu T., Akino T., Shih S.C., Totsuka Y., Klagsbrun M. Isolated tumor endothelial cells maintain specific character during long-term culture. Biochem Biophys Res Commun. 2010;394:947–954. doi: 10.1016/j.bbrc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 126.Dudley A.C. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 128.De Sanctis F., Ugel S., Facciponte J., Facciabene A. The dark side of tumor-associated endothelial cells. Semin Immunol. 2018;35:35–47. doi: 10.1016/j.smim.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 129.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 130.Soares J., Espadinha M., Raimundo L., Ramos H., Gomes A.S., Gomes S., Loureiro J.B., Inga A., Reis F., Gomes C. DIMP53-1: a novel small-molecule dual inhibitor of p53-MDM2/X interactions with multifunctional p53-dependent anticancer properties. Mol Oncol. 2017;11:612–627. doi: 10.1002/1878-0261.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jain A., Lai J.C., Bhushan A. Biochanin A inhibits endothelial cell functions and proangiogenic pathways: implications in glioma therapy. Anti Cancer Drugs. 2015;26:323–330. doi: 10.1097/CAD.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 132.Bid H.K., Oswald D., Li C., London C.A., Lin J., Houghton P.J. Anti-angiogenic activity of a small molecule STAT3 inhibitor LLL12. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeitlin B.D., Spalding A.C., Campos M.S., Ashimori N., Dong Z., Wang S., Lawrence T.S., Nor J.E. Metronomic small molecule inhibitor of Bcl-2 (TW-37) is antiangiogenic and potentiates the antitumor effect of ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;78:879–887. doi: 10.1016/j.ijrobp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siddiqui-Jain A., Drygin D., Streiner N., Chua P., Pierre F., O'Brien S.E., Bliesath J., Omori M., Huser N., Ho C. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70:10288–10298. doi: 10.1158/0008-5472.CAN-10-1893. [DOI] [PubMed] [Google Scholar]
- 135.Vu H.N., Miller W.J., O'Connor S.A., He M., Schafer P.H., Payvandi F., Muller G.W., Stirling D.I., Libutti S.K. CC-5079: a small molecule with MKP1, antiangiogenic, and antitumor activity. J Surg Res. 2010;164:116–125. doi: 10.1016/j.jss.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 136.Liang W., Kujawski M., Wu J., Lu J., Herrmann A., Loera S., Yen Y., Lee F., Yu H., Wen W. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–935. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Podar K., Tonon G., Sattler M., Tai Y.T., Legouill S., Yasui H., Ishitsuka K., Kumar S., Kumar R., Pandite L.N. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc Natl Acad Sci USA. 2006;103:19478–19483. doi: 10.1073/pnas.0609329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Satchi-Fainaro R., Mamluk R., Wang L., Short S.M., Nagy J.A., Feng D., Dvorak A.M., Dvorak H.F., Puder M., Mukhopadhyay D. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7:251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 139.Duluc C., Moatassim-Billah S., Chalabi-Dchar M., Perraud A., Samain R., Breibach F., Gayral M., Cordelier P., Delisle M.B., Bousquet-Dubouch M.P. Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol Med. 2015;7:735–753. doi: 10.15252/emmm.201404346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grither W.R., Longmore G.D. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc Natl Acad Sci USA. 2018;115:E7786–E7794. doi: 10.1073/pnas.1805020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kraus D., Palasuberniam P., Chen B. Targeting phosphatidylinositol 3-kinase signaling pathway for therapeutic enhancement of vascular-targeted photodynamic therapy. Mol Cancer Ther. 2017;16:2422–2431. doi: 10.1158/1535-7163.MCT-17-0326. [DOI] [PubMed] [Google Scholar]
- 142.Motzer R.J., Hutson T.E., Cella D., Reeves J., Hawkins R., Guo J., Nathan P., Staehler M., de Souza P., Merchan J.R. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 143.Mendel D.B., Laird A.D., Xin X., Louie S.G., Christensen J.G., Li G., Schreck R.E., Abrams T.J., Ngai T.J., Lee L.B. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 144.Naba A., Clauser K.R., Hoersch S., Liu H., Carr S.A., Hynes R.O. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteom. 2012;11 doi: 10.1074/mcp.M111.014647. M111 014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naba A., Clauser K.R., Ding H., Whittaker C.A., Carr S.A., Hynes R.O. The extracellular matrix: tools and insights for the "omics" era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brown L.F., Guidi A.J., Schnitt S.J., Van De Water L., Iruela-Arispe M.L., Yeo T.K., Tognazzi K., Dvorak H.F. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 147.Lokeshwar V.B., Lopez L.E., Munoz D., Chi A., Shirodkar S.P., Lokeshwar S.D., Escudero D.O., Dhir N., Altman N. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010;70:2613–2623. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yoshihara S., Kon A., Kudo D., Nakazawa H., Kakizaki I., Sasaki M., Endo M., Takagaki K. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005;579:2722–2726. doi: 10.1016/j.febslet.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 149.Nakazawa H., Yoshihara S., Kudo D., Morohashi H., Kakizaki I., Kon A., Takagaki K., Sasaki M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;57:165–170. doi: 10.1007/s00280-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 150.Hoffman A., Qadri B., Frant J., Katz Y., Bhusare S.R., Breuer E., Hadar R., Reich R. Carbamoylphosphonate matrix metalloproteinase inhibitors 6: cis-2-aminocyclohexylcarbamoylphosphonic acid, a novel orally active antimetastatic matrix metalloproteinase-2 selective inhibitor--synthesis and pharmacodynamic and pharmacokinetic analysis. J Med Chem. 2008;51:1406–1414. doi: 10.1021/jm701087n. [DOI] [PubMed] [Google Scholar]
- 151.Dufour A., Sampson N.S., Li J., Kuscu C., Rizzo R.C., Deleon J.L., Zhi J., Jaber N., Liu E., Zucker S. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011;71:4977–4988. doi: 10.1158/0008-5472.CAN-10-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Benesch M.G., Tang X., Dewald J., Dong W.F., Mackey J.R., Hemmings D.G., McMullen T.P., Brindley D.N. Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J. 2015;29:3990–4000. doi: 10.1096/fj.15-274480. [DOI] [PubMed] [Google Scholar]
- 153.Bhave S.R., Dadey D.Y., Karvas R.M., Ferraro D.J., Kotipatruni R.P., Jaboin J.J., Hallahan A.N., Dewees T.A., Linkous A.G., Hallahan D.E. Autotaxin inhibition with PF-8380 enhances the radiosensitivity of human and murine glioblastoma cell lines. Front Oncol. 2013;3:236. doi: 10.3389/fonc.2013.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Revert F., Revert-Ros F., Blasco R., Artigot A., Lopez-Pascual E., Gozalbo-Rovira R., Ventura I., Gutierrez-Carbonell E., Roda N., Ruiz-Sanchis D. Selective targeting of collagen IV in the cancer cell microenvironment reduces tumor burden. Oncotarget. 2018;9:11020–11045. doi: 10.18632/oncotarget.24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Toole B.P. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 156.Whatcott C.J., Han H., Posner R.G., Hostetter G., Von Hoff D.D. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Minchinton A.I., Tannock I.F. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 158.Singha N.C., Nekoroski T., Zhao C., Symons R., Jiang P., Frost G.I., Huang Z., Shepard H.M. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol Cancer Ther. 2015;14:523–532. doi: 10.1158/1535-7163.MCT-14-0580. [DOI] [PubMed] [Google Scholar]
- 159.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jacobetz M.A., Chan D.S., Neesse A., Bapiro T.E., Cook N., Frese K.K., Feig C., Nakagawa T., Caldwell M.E., Zecchini H.I. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kohno N., Ohnuma T., Truog P. Effects of hyaluronidase on doxorubicin penetration into squamous carcinoma multicellular tumor spheroids and its cell lethality. J Cancer Res Clin Oncol. 1994;120:293–297. doi: 10.1007/BF01236386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song S., Chen F., Qi H., Li F., Xin T., Xu J., Ye T., Sheng N., Yang X., Pan W. Multifunctional tumor-targeting nanocarriers based on hyaluronic acid-mediated and pH-sensitive properties for efficient delivery of docetaxel. Pharm Res. 2014;31:1032–1045. doi: 10.1007/s11095-013-1225-y. [DOI] [PubMed] [Google Scholar]
- 163.Cohen Z.R., Ramishetti S., Peshes-Yaloz N., Goldsmith M., Wohl A., Zibly Z., Peer D. Localized RNAi therapeutics of chemoresistant grade IV glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano. 2015;9:1581–1591. doi: 10.1021/nn506248s. [DOI] [PubMed] [Google Scholar]
- 164.Yang L., Song X., Gong T., Jiang K., Hou Y., Chen T., Sun X., Zhang Z., Gong T. Development a hyaluronic acid ion-pairing liposomal nanoparticle for enhancing anti-glioma efficacy by modulating glioma microenvironment. Drug Deliv. 2018;25:388–397. doi: 10.1080/10717544.2018.1431979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 167.Sun Z., Li R., Sun J., Peng Y., Xiao L., Zhang X., Xu Y., Wang M. Matrix metalloproteinase cleavable nanoparticles for tumor microenvironment and tumor cell dual-targeting drug delivery. ACS Appl Mater Interfaces. 2017;9:40614–40627. doi: 10.1021/acsami.7b11614. [DOI] [PubMed] [Google Scholar]
- 168.Benesch M.G., Zhao Y.Y., Curtis J.M., McMullen T.P., Brindley D.N. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J Lipid Res. 2015;56:1134–1144. doi: 10.1194/jlr.M057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rancoule C., Espenel S., Trone J.C., Langrand-Escure J., Vallard A., Rehailia-Blanchard A., El Meddeb Hamrouni A., Xia Y., Guy J.B., Ben-Mrad M. Lysophosphatidic acid (LPA) as a pro-fibrotic and pro-oncogenic factor: a pivotal target to improve the radiotherapy therapeutic index. Oncotarget. 2017;8:43543–43554. doi: 10.18632/oncotarget.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benesch M.G.K., MacIntyre I.T.K., McMullen T.P.W., Brindley D.N. Coming of age for autotaxin and lysophosphatidate signaling: clinical applications for preventing, detecting and targeting tumor-promoting inflammation. Cancers (Basel) 2018;10 doi: 10.3390/cancers10030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu Y.L., Tee M.K., Goetzl E.J., Auersperg N., Mills G.B., Ferrara N., Jaffe R.B. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 172.Sengupta S., Kim K.S., Berk M.P., Oates R., Escobar P., Belinson J., Li W., Lindner D.J., Williams B., Xu Y. Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene. 2007;26:2894–2901. doi: 10.1038/sj.onc.1210093. [DOI] [PubMed] [Google Scholar]
- 173.Schneider G., Sellers Z.P., Abdel-Latif A., Morris A.J., Ratajczak M.Z. Bioactive lipids, LPC and LPA, are novel prometastatic factors and their tissue levels increase in response to radio/chemotherapy. Mol Cancer Res. 2014;12:1560–1573. doi: 10.1158/1541-7786.MCR-14-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23:S20–S23. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cox T.R., Erler J.T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Riegler J., Labyed Y., Rosenzweig S., Javinal V., Castiglioni A., Dominguez C.X., Long J.E., Li Q., Sandoval W., Junttila M.R. Tumor elastography and its association with collagen and the tumor microenvironment. Clin Cancer Res. 2018 Sep 15;24(18):4455–4467. doi: 10.1158/1078-0432.CCR-17-3262. [DOI] [PubMed] [Google Scholar]
- 178.Evans A., Whelehan P., Thomson K., Brauer K., Jordan L., Purdie C., McLean D., Baker L., Vinnicombe S., Thompson A. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Canc. 2012;107:224–229. doi: 10.1038/bjc.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Raja A.M., Xu S., Zhuo S., Tai D.C., Sun W., So P.T., Welsch R.E., Chen C.S., Yu H. Differential remodeling of extracellular matrices by breast cancer initiating cells. J Biophot. 2015;8:804–815. doi: 10.1002/jbio.201400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Drake C.G., Lipson E.J., Brahmer J.R. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sowell R.T., Kaech S.M. Probing the diversity of T cell dysfunction in cancer. Cell. 2016;166:1362–1364. doi: 10.1016/j.cell.2016.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]