Abstract
Background
Imported cases of infectious disease provide invaluable information about epidemiological conditions abroad, and should guide treatment decisions at home and abroad. Here, we examined cases of malaria imported from Africa to China for mutations eroding the efficacy of sulfadoxine-pyrimethamine (SP), sometimes used as an intermittent preventive treatment during for pregnant women and infants.
Methods
A total of 208 blood samples were collected from P. falciparum-infected workers who had returned from Western and Central Africa to Guangxi Province Frequency distribution. Samples were analyzed for the mutations in dhfr and dhps genes by PCR -sequencing. The prevalence of dhfr and dhps polymorphisms was analyzed. Among the isolates, polymorphisms were detected in mutants N51I, C59R, S108N and I164L of Pfdhfr and I431V, S436 A/F, A437G, K540 E/N, A581G and A613T of pfdhps.
Results
Mutations promoting drug resistance were widespread in this cohort. For pfdhfr and pfdhps, wild types were equally rare among patients returned from Western Africa and Central Africa. A triple-mutant dhfr haplotype was most prevalent (>70%). We report for the first time mutation I164L-dhfr and I431V-dhps in Ghana, and for the first time we found A581G to exceed a clinically-relevant threshold that may counter-indicate current clinical practices. For Pfdhps, the double-mutant IAGKAA was high prevalent haplotype in Ghana, Western Africa. The single-mutant ISGKAA was a majority haplotype in Cameroon. Alarmingly, a “super resistance” quintuple mutant was detected, for the first time, in parasites of West African origin (defined by IAGKAA/IRNI in combination with pfdhps 581G and dhfr I164L). This may limit the efficacy of this drug combination for even intermittent clinical applications.
Conclusions
These data are cause for great concern and call for continued surveillance of the efficacy of SP in source and recipient populations, and should be considered when developing treatment policy for imported malaria cases in China and elsewhere.
Keywords: Africa, Anti-malarial drug resistance, Sulfadoxine–pyrimethamine, Plasmodium falciparum
Graphical abstract
1. Introduction
Malaria is the most important vector-borne disease and remains a preeminent global public health problem. In 2016, 91 countries reported approximately 216 million cases of malaria and a global death toll of about 445,000 (World Health Organization, 2017a) African countries, especially in sub-Saharan Africa, account for 90% of malaria cases and deaths (World Health Organization, 2017a) The majority of human malaria is caused by five species, namely, Plasmodium falciparum, Plasmodium vivax, Plasmodium malaria, Plasmodium ovale and Plasmodium knowelsi (Miller et al., 2002). In Africa, P. falciparum is the predominant species of malaria (World Health Organization, 2017a).
Malaria used to be one of the most important infectious disease in China (LI Jun and Zhang, 1360). Since the Chinese Government initiated the National Malaria Elimination Program in 2010, malaria transmission has obviously been reduced. There has been no indigenous cases reported in China since 2017 (Zhang et al., 2018). However, migrant workers and travelers between China and other countries have increased in recent years. Hence, imported malaria cases have markedly increased in the Guangxi Zhuang Autonomous Region. And the most of imported cases were P. falciparum from Africa (Zhang et al., 2018). An outbreak of imported malaria took place in 2013 in Shanglin, Guangxi Zhuang Autonomous Region (Wei Shujiao et al., 2013).
Sulfadoxine-pyrimethamine (SP) is no longer used for treatment of clinical malaria in Africa because of widespread resistance (Curtis et al., 1998; Sibley et al., 2001). However, it continues to be used as an intermittent preventive treatment during pregnancy (IPTp) in malaria-endemic areas in Africa. In sub-Saharan Africa, IPTp-SP has been shown to reduce maternal anemia, low birth weight and perinatal mortality (World Health Organization, 2018). Seasonal malaria chemoprevention (SMC) with amodiaquine (AQ) plus SP (AQ + SP) for children aged 3–59 months reduces the incidence of clinical attacks and severe malaria by about 75% (World Health Organization, 2018). SP is also provided as intermittent preventive treatment in infants (IPTi) in areas of moderate-to-high malaria transmission of Africa, however, no countries have reported implementation of an IPTi policy as of 2015, Sierra Leone began implementing IPTi on a pilot basis in one district in 2016 (World Health Organization, 2017a, 2017b, 2018). WHO is calling on all malaria-affected countries in sub-Saharan Africa and their development partners to adopt and implement IPTi-SP (World Health Organization, 2017b). Yet the decline in the efficacy of SP had already emerged in Africa, and levels of SP resistance vary within Africa (Naidoo and Roper, 2013), boding ill for the future.
To inform current policy on continuing use of SP, surveillance of SP resistance levels must be achieved by monitoring of molecular markers and by describing its geographical distribution. SP resistance is linked with dihydrofolate reductase (dhfr) in the folate biosynthetic pathway, and substitutions of amino acids in the enzymes dihydropteroate synthetase (dhps) (Aubouy et al., 2003). Resistance to SP is caused by point mutations in the dhfr and dhps genes of P. falciparum (Roper et al., 2003). In the dhfr gene, the mutations N51I, C59R, S108N and I164L are associated with pyrimethamine resistance (Cowman et al., 1988; Peterson et al., 1988). In the dhps gene, mutations have been identified in codons 436, 437, 540, 581 and 613 that confer resistance to dihydropteroate (Brooks et al., 1994; Triglia et al., 1997). The combination of a triple dhfr (N51I/C59R/S108N) mutant with a double dhps (A437G/K540E) mutant is a useful predictor of clinical SP treatment failure and results in limited efficacy of SP-IPTi (Kublin et al., 2002). The triple mutant pfdhfr with the pfdhps single mutant 437G also has been found to be associated with treatment failure in Western and Central Africa (Kun et al., 1999; Dunyo et al., 2006; Picot et al., 2009). In addition, the WHO recommended that SP-IPTi should not be implemented when the prevalence of dhps K540E exceeds 50% (Intermittent Preventive). A recent meta-analysis demonstrated that IPTp efficacy was reduced when the prevalence of A581G exceeds 10% in Africa (Chico et al., 2015). This situation may suggest discontinuation of IPTp. In some parts of East Africa, other findings reported that SP-IPTi and IPTp have failed in the quintuple mutant (N51I/C59R/S108N + A437G/K540E) acquired pfdhps A581G (Picot et al., 2009; Gesase et al., 2009). According to reports, an unusual I431V mutation in dhps gene has been seen to emerge in combination with A436S, A437G, A581G and A613S in Africa (Sutherland et al., 2009; Oguike et al., 2016). Although there was no available data show that higher SP resistance is associated with an increasing number of these mutations, these mutations do favor resistance risk.
In Western Africa and Central Africa, the combination of dhfr N51I, N59R, and S108N with dhps A437G has been demonstrated to be prevalent (Naidoo and Roper, 2013). However, we know little about drug resistance imported from Western Africa to China in recent years (Yang et al., 2019; Xu et al., 2018). Therefore, we evaluated polymorphisms and the geographic distribution of haplotypes of pfdhfr and pfdhps imported to China from Western Africa, which would help provide information on imported P. falciparum malaria cases in China and future treatment policy with IPTp for the two clinical populations.
2. Methods
2.1. Samples collection and DNA extraction
Blood samples were obtained from malaria cases who returned from Africa to Guangxi Province, China between 2016 and 2018. Malaria infections were diagnosed by microscopic examination of Giemsa-stained thick and thin blood films. The questionnaires of all cases were recorded, including gender, ethnicity, occupation, microscopic examination results and travel history. The human subject protocol for this study was by the Shanglin Hospital Institutional Review Board. All participants supplied written informed consent. Prospective cases with complex travel histories were excluded from the study.
Parasite DNA was extracted from whole blood using the High Pure PCR Template Preparation Kit (Roche) according to the manufacturer's instructions. Then P. falciparum infection was confirmed using a nested polymerase chain reaction (PCR) targeting the small subunit ribosomal RNA gene as described previously (Snounou, 2002; Snounou and Singh, 2002). The DNA template was kept at −20 °C until use.
2.2. Amplification and sequencing of pfdhfr and pfhhps gene
Only infections containing strains P. falciparum by genotyping were used for sequencing analysis. Those associated with resistance to SP were investigated by examining four codons of pfdhfr (N51I, C59R, S108N and I164L) and six codons of pfdhps (I431V, S436 A/F, A437G, K540 E/N, A581G and A613T) (Cowman et al., 1988; Peterson et al., 1988; Brooks et al., 1994; Triglia et al., 1997). The pfdhfr and pfdhps gene were amplified using primers and conditions as described earlier (Garg et al., 2009). Briefly, primary PCR was performed in a total volume of 20 μL containing 2 μL DNA template, 10 μL Premix Taq Version 2.0 (TakaRa, USA), 1 μL of each primer, and 6 μl ddH2O. The secondary PCR was performed in a total volume of 40 μL containing 2 μL primary PCR product, 20 μL Premix Taq Version 2.0 (TakaRa, USA), 1 μL of each primer, and 19 μL ddH2O. Positive PCR products were sequenced by Generalbiol (Chuzhou, China). The forward and reverse sequences were aligned using DNASTAR (version 7.1) software and were manually refined to obtain a better consensus nucleotide sequence. The consensus sequences of pfdhfr and pfdhps gene were multiple-aligned with a reference sequence of the gene retrieved from the GeneBank database using BioEdit (version 7.2.5). The GeneBank accession numbers of the pfdhfr and pfdhps reference sequences were PF3D7_0417200.1 and PF3D7_0810800.1, respectively. The nucleotide sequences of the multiple alignments for each gene were translated into their corresponding amino acid sequences and manually analyzed for mutations at the specified codons.
2.3. Statistical analysis
Statistical analysis was carried out with the SPSS software (version 22). The chi-square test was used to compare the categorical variables among the groups. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Characteristics of study participants
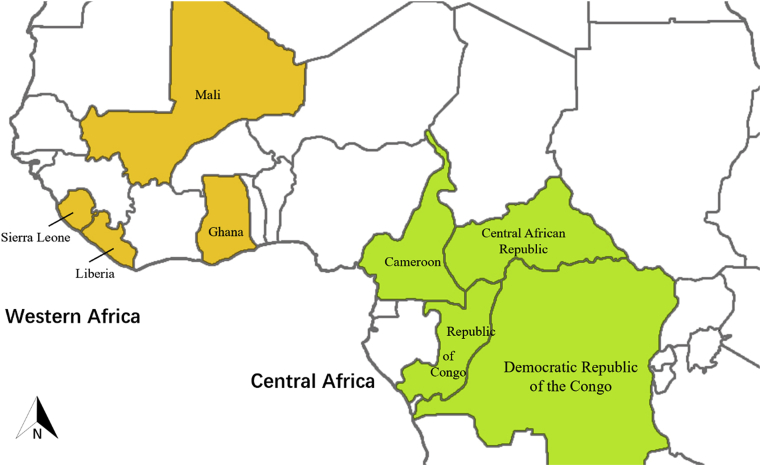
A total of 243 P. falciparum cases returning to Guangxi Province from 8 countries of Africa between March 2016 and September 2018 were enrolled in this study. These patients returned from four countries in Western Africa (78.6%, 191/243): Liberia, Sierra Leone, Mali, Ghana, and four countries located in Central Africa (21.4%, 52/243): Congo, Cameroon, the Democratic Republic of the Congo (DRC), Central African Republic (Fig. 1). Most of those from Ghana came from Kumasi or Asankragua. There were 235 male patients and 8 female patients. The distribution of patients by age ranged from 15 to 64, the mean age was 38.9 ± 9.9 years. All patients received targeted antimalarial treatment according to the guidelines of antimalarial drugs in China.
Fig. 1.
Map of isolates returned from Central Africa and Western Africa.
3.2. P. falciparum pfdhfr gene analysis
A total of 208 cases were successfully sequenced for codons 51, 59, 108, 164 of pfdhfr gene, 165 in Western African and 43 in Central Africa. A high prevalence of Pfdhfr mutant isolates was observed in this study. The pfdhfr mutations at codons 51, 59 and 108 were prevalent in each geographic region (>85%) (Table 1). In addition, C59R and S108N were near saturation in our study. The I164L mutant was present, but rare, with no evident regional difference between Western and Central Africa (P > 0.05).
Table 1.
Prevalence distribution of pfdhfr and pfdhps gene mutations among two groups.
| Gene | Codon | % (n) of isolates |
P | |
|---|---|---|---|---|
| Western Africa | Central Africa | |||
| pfdhpr | N = 165 | N = 43 | ||
| N51I | 86.1 (142) | 90.7 (39) | 0.611 | |
| C59R | 90.3 (149) | 93.0 (40) | 0.799 | |
| S108N | 93.3 (154) | 93.0 (40) | 1 | |
| I164L | 11.5 (19) | 4.7 (2) | 0.295 | |
| pfdhps | N = 147 | N = 40 | ||
| I431V | 0.7 (1) | 0.0 (0) | 1.000 | |
| S436 A/F | 57.1 (84) | 45.0 (18) | 0.211 | |
| A437G | 87.8 (129) | 70.0 (28) | 0.015* | |
| K540 E/N | 12.9 (19) | 10.0 (4) | 0.820 | |
| A581G | 11.6 (17) | 0.0 (0) | 0.052 | |
| A613 S/T | 10.9 (16) | 5.0 (2) | 0.414 | |
*:P < 0.05.
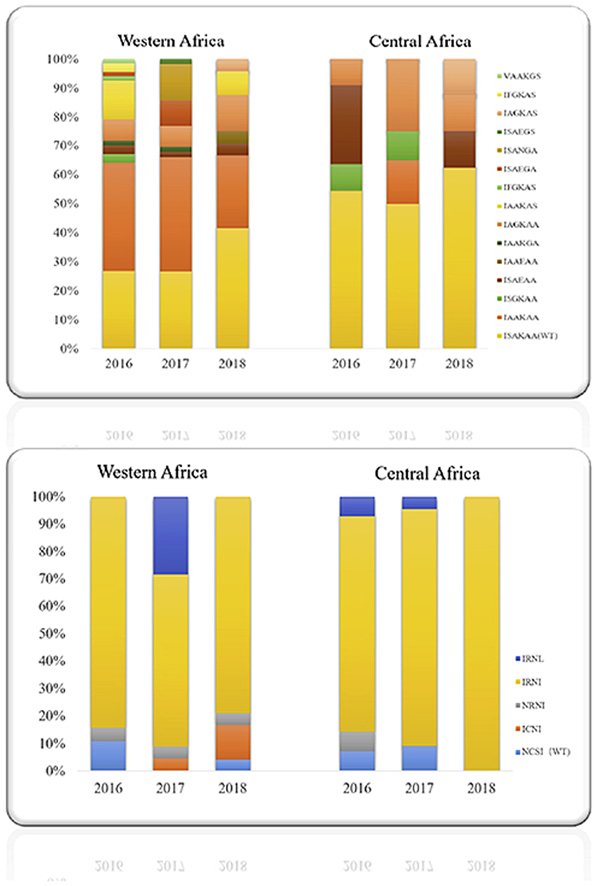
The genotypes of pfdhfr are presented in Table 2. About 165 isolates returned from 4 countries in Western Africa followed 5 haplotypes. Only 11 of 165 isolates (6.67%) were wild type N51C59S108I164, meaning nearly 95% harbored at least one mutation. The most prevalent of these (71.5%) was the triple-mutant haplotype triple-mutant haplotype IRNI, followed by a quadruple-mutant IRNL (11.5%), and two double mutants ICNI (3.0%) and NRNI (7.3%). As in Western Africa, wild-type haplotypes were only infrequently encountered in the 43 isolates derived from Central Africa 7%), Here too, the triple-mutant IRNI was also by far the most prevalent (86.1%). Other haplotypes included the quadruple-mutant IRNL (4.7%) and the double-mutant NRNI (2.3%). In addition, the double-mutant NRNI was only detected in five patients who returned from Ghana, Western Africa. Significant differences among groups were not observed with these mutations (P > 0.05). No mixed haplotypes were identified.
Table 2.
Prevalence of Pfdhfr and Pfdhps haplotypes in isolates.
| Gene | Haplotype | Total |
Western Africa |
Total |
Central Africa |
Pa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liberia % (n) |
Sierra Leone % (n) |
Mali % (n) |
Ghana % (n) |
Congo % (n) |
Cameroon % (n) |
Congo, DRC % (n) |
Central African Republic % (n) |
|||||
| pfdhfr | N = 165 | N = 7 | N = 3 | N = 1 | N = 154 | N = 43 | N = 10 | N = 24 | N = 5 | N = 4 | ||
| NCSI (WT) | 6.7 (11) | 0 | 0 | 100.00 (1) | 6.5 (10) | 7.0 (3) | 10.00 (1) | 8.3 (2) | 0 | 0 | 0.792 | |
| ICNI | 3.0 (5) | 0 | 0 | 0 | 3.3 (5) | 0 | 0 | 0 | 0 | 0 | 0.551 | |
| NRNI | 7.3 (12) | 14.3 (1) | 100 (3) | 0 | 5.2 (8) | 2.3 (1) | 10.00 (1) | 0 | 0 | 0 | 0.401 | |
| IRNI | 71.5 (118) | 85.7 (6) | 0 | 0 | 72.7 (112) | 86.1 (37) | 70.00 (7) | 91.7 (22) | 80.0 (4) | 100.0 (4) | 0.051 | |
| IRNL | 11.5 (19) | 0 | 0 | 0 | 12.3 (19) | 4.7 (2) | 10.00 (1) | 0 | 20.0 (1) | 0 | 0.295 | |
| pfdhps | N = 147 | N = 8 | N = 3 | N = 1 | N = 135 | N = 40 | N = 4 | N = 25 | N = 6 | N = 5 | ||
| ISAKAA (WT) | 1.4 (2) | 1.3 (1) | 0 | 0 | 0.74 (1) | 5.0 (2) | 0 | 8.0 (2) | 0 | 0 | 0.201 | |
| IA/FAKAA | 8.8 (13) | 0 | 33.3 (1) | 0 | 8.9 (12) | 22.5 (9) | 0 | 32.0 (8) | 0 | 20.0 (1) | 0.036* | |
| ISGKAA | 29.3 (43) | 50.0 (4) | 33.3 (1) | 100.0 (1) | 27.4 (37) | 40.0 (16) | 0 | 40.0 (10) | 83.3 (5) | 20.0 (1) | 0.195 | |
| IAGKAA | 36.1 (53) | 37.5 (3) | 0 | 0 | 37.0 (50) | 17.5 (7) | 0 | 16.0 (4) | 4.0 (1) | 40.0 (2) | 0.026* | |
| IA/FAKAS | 2.0 (3) | 0 | 0 | 0 | 2.2 (3) | 2.5 (1) | 0 | 0 | 0 | 20.0 (1) | 1 | |
| ISGEAA | 2.7 (4) | 0 | 33.3 (1) | 0 | 2.2 (3) | 10.0 (4) | 100.00 (4) | 0 | 0 | 0 | 0.115 | |
| IAGKGA | 1.4 (2) | 0 | 0 | 0 | 1.4 (2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| IAGKES | 7.5 (11) | 0 | 0 | 0 | 8.2 (11) | 2.5 (1) | 0 | 4.0 (1) | 0 | 0 | 0.438 | |
| ISGN/EGA | 8.8 (13) | 0 | 0 | 0 | 9.6 (13) | 0 | 0 | 0 | 0 | 0 | 0.11 | |
| IAGEAA | 0.7 (1) | 0 | 0 | 0 | 0.7 (1) | 0 | 0 | 0 | 0 | 0 | 1 | |
| ISGEGS | 0.7 (1) | 0 | 0 | 0 | 0.7 (1) | 0 | 0 | 0 | 0 | 0 | 1 | |
| VAGKGS | 0.7 (1) | 0 | 0 | 0 | 0.7 (1) | 0 | 0 | 0 | 0 | 0 | 1 | |
*: P < 0.05.
Comparison between Western Africa and Central Africa.
3.3. Analysis of pfdhps gene
The pfdhps gene was successfully sequenced in 147 Western African and 40 Central African isolates. The prevalence distribution of the pfdhps mutation is summarized in Table 1. None of which were entirely wild-type.
The I431V and A581G mutant allele were observed only from Ghana in Western Africa. The most frequent pfdhps mutation (A437G) was prevalent everywhere, but significantly more so in Western Africa (87.8%) than in Central Africa (70.0%) (χ2 = 5.966, P < 0.05).
Fifteen different genotypes were found in patients returned from Western Africa (Table 2). Prevalence of the wild-type haplotype did not significantly vary regionally. In Western Africa, the double-mutant IAGKAA was most common in patients who returned from Ghana. It was significantly higher in Western than Central Africa (χ2 = 4.968, P < 0.05). There were 7 different genotypes of pfdhps gene found in patients returned from Central Africa. The common genotype was ISGKAA. In addition, single-mutant haplotypes IA/FAKAA was significantly higher in Central Africa than Western Africa (χ2 = 4.410, P < 0.05).
3.4. Pfdhfr and pfdhps haplotypes analysis
As summarized in Table 3, multiple mutations that contribute to SP resistance in both pfdhfr and pfdhps genes were detected in this study, comprising more than twenty pfdhfr/pfdhps haplotypes, none of which were entirely wild-type. The quadruple mutant IAAKAA/IRNI was significantly higher in Central Africa (χ2 = 8.623, P < 0.05). The quadruple mutant (IAAKAA/IRNI) was presented in high proportion (42.1%) in Cameroon, Central Africa. However, the quintuple mutant (IAGKAA/IRNI) haplotypes were significantly higher in Western Africa than Central Africa (χ2 = 7.801, P < 0.05). Results showed that a prevalence of 46.2% (42/91) pfdhfr/pfdhps quintuple (IAGKAA/IRNI) was especially prevalent in patients returned from Ghana, Western Africa. However, the quadruple mutant (IAAKAA/IRNI) was presented in high proportion (42.1%) in Cameroon, Central Africa.
Table 3.
Prevalence of Pfdhfr/Pfdhps genotype.
| Gene |
No. of mutations | Total | Western Africa |
Total | Central Africa |
Pa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| pfdhfr/pfdhps genetype | Liberia % (n) |
Sierra Leone % (n) | Ghana % (n) |
Congo % (n) |
Congo, DRC % (n) |
Cameroon % (n) |
||||
| N = 98 | N = 5 | N = 2 | N = 91 | N = 21 | N = 1 | N = 1 | N = 19 | |||
| IAGKAA/NCSI | 1 | 0 | 0 | 0 | 0 | 4.8 (1) | 0 | 0 | 5.3 (1) | 0.394 |
| IAGKAA/NCSI | 2 | 0 | 0 | 0 | 0 | 4.8 (1) | 0 | 0 | 5.3 (1) | 0.394 |
| ISGKAA/NRNI | 3 | 4.1 (4) | 20.0 (1) | 0 | 3.3 (3) | 4.8 (1) | 0 | 0 | 5.3 (1) | 1 |
| ISAKAA/IRNI | 3 | 1.0 (1) | 20.0 (1) | 0 | 0 | 9.5 (2) | 0 | 0 | 10.5 (2) | 0.137 |
| IAAKAA/NRNI | 3 | 2.0 (2) | 0 | 0 | 2.2 (2) | 0 | 0 | 0 | 0 | 1 |
| IAGKAS/NRNI | 4 | 2.0 (2) | 0 | 0 | 2.2 (2) | 0 | 0 | 0 | 0 | 1 |
| IAGKAA/NRNI | 4 | 1.0 (1) | 0 | 0 | 1.1 (1) | 0 | 0 | 0 | 0 | 1 |
| IAGKAA/ICNI | 4 | 4.1 (4) | 0 | 0 | 4.4 (4) | 0 | 0 | 0 | 0 | 0.794 |
| IAAKAA/IRNI | 4 | 10.2 (10) | 0 | 50.00 (1) | 9.9 (9) | 38.1 (8) | 0 | 0 | 42.1 (8) | 0.003* |
| IA/FAKAS/IRNI | 5 | 2.0 (2) | 0 | 0 | 2.2 (2) | 4.8 (1) | 0 | 0 | 5.3 (1) | 0.445 |
| IAGKAA/IRNI | 5 | 45.9 (45) | 60.0 (3) | 0 | 46.2 (42) | 14.3 (3) | 0 | 50.0 (1) | 10.5 (2) | 0.001* |
| ISGEAA/IRNI | 5 | 3.1 (3) | 0 | 50.00 (1) | 2.2 (2) | 14.3 (3) | 0 | 0 | 15.7 (3) | 0.113 |
| ISGEAA/IRNL | 5 | 0 | 0 | 0 | 0 | 4.8 (1) | 50.0 (1) | 0 | 0 | – |
| IAGKAS/IRNI | 5 | 9.2 (9) | 0 | 0 | 9.9 (9) | 0 | 0 | 0 | 0 | 0.322 |
| IAGKGA/IRNI | 6 | 2.0 (2) | 0 | 0 | 2.2 (2) | 0 | 0 | 0 | 0 | 1 |
| ISGNGA/IRNI | 6 | 1.0 (1) | 0 | 0 | 1.10 (1) | 0 | 0 | 0 | 0 | 1 |
| IAGEAA/IRNI | 6 | 1.0 (1) | 0 | 0 | 1.10 (1) | 0 | 0 | 0 | 0 | 1 |
| ISGEGA/IRNL | 7 | 9.2 (9) | 0 | 0 | 9.9 (9) | 0 | 0 | 0 | 0 | 0.322 |
| VAGKGS/IRNI | 7 | 1.0 (1) | 0 | 0 | 1.10 (1) | 0 | 0 | 0 | 0 | 1 |
| ISGEGS/IRNI | 7 | 1.0 (1) | 0 | 0 | 1.10 (1) | 0 | 0 | 0 | 0 | 1 |
*: P < 0.05.
Comparison between Western Africa and Central Africa.
4. Discussion
The evolution and widespread dissemination of malarial resistance to sulfadoxine-pyrimethamine has restricted its clinical use in Africa to intermittent preventive treatment during pregnancy (IPTp), in infants (IPTi) and SMC (Curtis et al., 1998; Sibley et al., 2001). In 2016, 15 million children in 12 countries in Africa's Sahel subregion were protected through SMC (World Health Organization, 2018). Among 39 African countries on IPTp in 2017, about 22% of pregnant women received three or more doses of IPTp, compared with 17% in 2015 and 0% in 2010 (World Health Organization, 2018). Current guidelines in China call for the use of ACT treatment (Ministry of Health, 2009). For uncomplicated P. falciparum patients, ACTs routinely include oral compound tablets of dihydroarte-misinin plus piperaquine (DHA + PQ), artesunate plus amodiaquine (AS + AQ), and ART + PQ (Ministry of Health, 2016). All patients in this study were treated with intravenous (IV) injections of artesunate for 3 days (on day 0 with 120 mg, and 60 mg on subsequent 2 days) in the hospital. Upon resolution of their symptoms, patients were discharged and two days of oral treatment with dihydroartemisinin-piperaquine (DHP) was prescribed.
Our purpose was to better understand the spectrum of malarial parasites being imported to China from Africa, where such resistance is widespread, in order to shed light on clinical options and limitations for such patients, and to assess risks associated with the spread of resistant malarial parasites. Such molecular epidemiology is needed in order to elucidate dynamic epidemiological features and to provide policy makers with information needed to develop better safeguards for such travelers and for the populations to which they return. Unfortunately, we cannot determine from these data, whether in the same locality, certain resistance haplotypes are co-circulating, which would further accelerate the development of resistance phenotypes comprised of combinations of mutations not previously known to co-occur in the same parasite genome.
Historically, malaria was highly prevalent in the Guangxi Zhuang Autonomous Region of China. Subsequent national anti-malarial campaigns proved highly successful in this region, stemming indigenous transmission. Recently, however, the region has experienced a dramatic rise in imported cases, owing to an increase in migrant workers who have lived in regions that remain endemic for malaria. In China, before travelling to Africa, all patients received health education conducted by local CDCs, including training about malaria transmission and personal protection. Tablets of chloroquine or piperaquine were recommended as prophylaxis.
Here, we investigated mutations of pfdhfr and pfdhps in the parasites of migrant workers returning to Shanglin, Guangxi Zhuang Autonomous Region from Africa (World Health Organization, 2017b) between 2016 and 2018. As The WHO recommends that SP-IPTi has been reported from recent surveys in Africa, we confirmed that virtually no cases imported into China in this temporal interval lacked drug-resistance mutations. For context, mutations N51I, C59R and S108N were reported as universal (or nearly so) in Gabon and southeast Nigeria (Bouyou-Akotet et al., 2015; Esu et al., 2018).
Notably, we report for the first time mutation I164L in patients who come from Ghana, Western Africa (12.34%) even though previous data suggested this mutation was rare in Western Africa, but more prevalent in East Africa, and only 0.6% continent-wide between 2004 and 2008 (Naidoo and Roper, 2013). This patient had not worked outside of China in any other country excepting Ghana. No I164L had been detected in samples collected in Ghana between 2003 and 2010 (Duah et al., 2012). We observed this mutation in only one isolate returned from DRC; this mutation was initially reported there in 2014 at a national prevalence of only 0.1% (1/1715) (Nkoli Mandoko et al., 2018). Hence, our survey of returning Chinese migrant laborers may indicate that this mutation is on the increase in Western Africa. For genotypes of Pfdhfr, five kinds of genotypes were detected in our study. Our results showed that the triple mutant IRNI was the predominant haplotype (>70%) in workers returned from both Central and Western Africa. It was more prevalent among these patients (72.73%) than had been reported in Ghana in 2010, 50%–60% (Duah et al., 2012).
In contrast to the mutations of Pfdhfr gene, mutations of the Pfdhps were observed less frequently. Mutation I431V was observed in only one isolate who returned from Ghana, the first report of this mutation from this country to date. Before working in Ghana, this patient had never traveled to any other country outside of China. The I431V mutation has previously been reported in Nigeria (46%), DRC (0.8%), and Cameroon (9.8%) (Oguike et al., 2016; Nkoli Mandoko et al., 2018; Chauvin et al., 2015). It was widespread in Nigeria, with highest prevalence in 2014–2015 (Oguike et al., 2016). Thus, this mutation may be increasing in Western Africa. Although the effect of I431V mutation on antifolate resistance has not yet been established, it may interfere with the efficient binding of sulfadoxine, conferring increased sulfadoxine/pyrimethamine resistance (Oguike et al., 2016). The mutant A437G is associated with resistance to sulfadoxine in endemic areas and A437G selection by sulfadoxine/pyrimethamine has been previously reported during IPTi (Marks et al., 2005; Mayor et al., 2008). The pfdhps A437G mutant is very common across Africa and in much of Western and Central Africa (Pearce et al., 2009). Similarly, its prevalence is over 70% in our study. According to reported survey, the prevalence of K540 E/N was high in East Africa and exceeded 50% in Uganda and Tanzania (Naidoo and Roper, 2013; Lynch et al., 2008; Alifrangis et al., 2009). The WHO recommends that SP-IPTi should be implemented only when the prevalence of the K540E mutation is not exceed 50% (Intermittent Preventive). In this study, mutant K540 E/N did not exceed 50% among migrants returned from Central and Western Africa. This may favor continued efficacy of SP-IPTi in this region, but its prevalence in these workers (12.9%) is much higher than 0.00%–3.00% prevalence reported in Ghana 2005–2010, when sulphadoxine/pyrimethamine SP first replaced chloroquine as the drug for IPTp in 2005 (Duah et al., 2012; Koram et al., 2005). A recent analysis concluded that IPTp efficacy was reduced when the prevalence of A581G exceeds 10% (Chico et al., 2015). Ours is the first to suggest prevalence above that threshold in Ghana (11.6%), calling for increased surveillance to monitor the efficacy of sulfadoxine/pyrimethamine there.
Among the migrant workers studied here, the single-mutant type ISGKAA was the predominant haplotype in Cameroon, consistent with published data in this region during 2014–2015 (Nkoli Mandoko et al., 2018). This single-mutant was the most prevalent haplotype in Ghana in 2005 (Pearce et al., 2009), however, in our study, double-mutant type IAGKAA became the predominant haplotype (37.0%) in patients returned from Ghana, indicating growing drug resistance there, driven by an increase in A436G.
In our study, the mutation IAAKAA/IRNI is significantly higher in Central Africa, and the mutation IAGKAA/IRNI is significantly higher in Western Africa. The quadruple ISGKAA/IRNI was not obviously different from previous reports in Ghana (Duah et al., 2012). For the first time, a high prevalence (46.2%) of quintuple pfdhfr/pfdhps resistance (IAGKAA/IRNI) is evident in Ghana. Available data do not definitively associate the above mutations with strong drug resistance, so the clinical implications of such new haplotypes require further study. The difference haplotype of pfdhfr and pfdhps that we have observed among imported samples may be related to differences in treatment schemes among the countries in Africa. According to reports, the ISGEAA/IRNI mutation is a significant predictor of clinical treatment failure and results in limited protective value of SP-IPTi (Kublin et al., 2002; Gosling et al., 2009). In our study, this quintuple mutant was observed in Ghana (2.2%) and Cameroon (15.7%) with rare prevalence. It may suggest that SP for IPTi and IPTp is still efficient among these countries. ISGEAA/IRNI, adds pfdhps 581G and/dhfr I164L were linked very high levels of SP failure, called ‘super resistance’ (Gesase et al., 2009). For the first time, we found 1.1%, 9.9% respectively for IRNI/ISGEGA and IRNL/ISGEGA.
5. Conclusions
Taken together, our study reinforces that resistance of P. falciparum to SP continues to increase in Central and Western Africa, and that study of imported cases can provide valuable information on the genetic basis for such clinically-relevant phenotypic evolution in source and recipient countries. The high prevalence of resistance may now, or in the near future, abrogate the efficacy of even intermittent treatment of pregnant women or infants with sulfadoxine-pyrimethamine. This is a matter of great clinical consequence and bears on public health policy concerning treatment policy for imported malaria in China. Fortunately, ACTs treatment remains effective in China.
Acknowledgments
The authors thank all the patients for volunteering to participate in the study. This study was supported by the National Science Foundation of China (31860604 and U1802286), Major science and technology projects of Yunnan Province (2018ZF0081). CL, XC and YZ were supported by Yunnan Applied Basic Research Projects-Union Foundation (2017FE468-185, 2018FE001-190, 2015FB034 respectively. YQ was supported by Guangxi Zhuang Autonomous Region Health Commission of Scientific Research Project (Z20190892). YL was funded by grants of the Science and Technology Bureau Programs for Science and Technology Development of Nanning, Guangxi Zhuang Autonomous Region, China (ZC20153012).
References
- Alifrangis M., Lusingu J.P., Mmbando B. Five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe District, Tanzania: accumulation of the 581G mutation in the P. falciparum dihydropteroate synthase gene. Am. J. Trop. Med. Hyg. 2009;80(4):523–527. [PubMed] [Google Scholar]
- Aubouy A., Jafari S., Huart V. DHFR and DHPS genotypes of Plasmodium falciparum isolates from Gabon correlate with in vitro activity of pyrimethamine and cycloguanil, but not with sulfadoxine-pyrimethamine treatment efficacy. J. Antimicrob. Chemother. 2003;52(1):43–49. doi: 10.1093/jac/dkg294. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Bouyou-Akotet M.K., Tshibola M.L., Mawili-Mboumba D.P. Frequencies of dhfr/dhps multiple mutations and Plasmodium falciparum submicroscopic gametocyte carriage in Gabonese pregnant women following IPTp-SP implementation. Acta Parasitol. 2015;60(2):218–225. doi: 10.1515/ap-2015-0031. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Wang P., Read M. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur. J. Biochem. 1994;224(2):397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- Chauvin P., Menard S., Iriart X. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaounde, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J. Antimicrob. Chemother. 2015;70(9):2566–2571. doi: 10.1093/jac/dkv160. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Chico R.M., Cano J., Ariti C. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop. Med. Int. Health : TM & IH. 2015;20(12):1621–1633. doi: 10.1111/tmi.12595. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Cowman A.F., Morry M.J., Biggs B.A. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 1988;85(23):9109–9113. doi: 10.1073/pnas.85.23.9109. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Duraisingh M.T., Warhurst D.C. In vivo selection for a specific genotype of dihydropteroate synthetase of Plasmodium falciparum by pyrimethamine-sulfadoxine but not chlorproguanil-dapsone treatment. J. Infect. Dis. 1998;177(5):1429–1433. doi: 10.1086/517831. [DOI] [PubMed] [Google Scholar]
- Duah N.O., Quashie N.B., Abuaku B.K. Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine 5 years after the change of malaria treatment policy in Ghana. Am. J. Trop. Med. Hyg. 2012;87(6):996–1003. doi: 10.4269/ajtmh.2012.12-0202. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunyo S., Ord R., Hallett R. Randomised trial of chloroquine/sulphadoxine-pyrimethamine in Gambian children with malaria: impact against multidrug-resistant P. falciparum. PLoS Clin. Trials. 2006;1(3):e14. doi: 10.1371/journal.pctr.0010014. [published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esu E., Tacoli C., Gai P. Prevalence of the Pfdhfr and Pfdhps mutations among asymptomatic pregnant women in Southeast Nigeria. Parasitol. Res. 2018;117(3):801–807. doi: 10.1007/s00436-018-5754-5. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Garg S., Saxena V., Kanchan S. Novel point mutations in sulfadoxine resistance genes of Plasmodium falciparum from India. Acta Trop. 2009;110(1):75–79. doi: 10.1016/j.actatropica.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gesase S., Gosling R.D., Hashim R. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One. 2009;4(2) doi: 10.1371/journal.pone.0004569. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling R.D., Gesase S., Mosha J.F. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2009;374(9700):1521–1532. doi: 10.1016/S0140-6736(09)60997-1. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Intermittent Preventive Treatment for Infants Using Sulfadoxine-Pyrimethamine (SP-IpIi) for Malaria Control in Africa: Implementation Field Guide.
- Koram K.A., Abuaku B., Duah N. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop. 2005;95(3):194–203. doi: 10.1016/j.actatropica.2005.06.018. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Kublin J.G., Dzinjalamala F.K., Kamwendo D.D. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 2002;185(3):380–388. doi: 10.1086/338566. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Kun J.F., Lehman L.G., Lell B. Low-dose treatment with sulfadoxine-pyrimethamine combinations selects for drug-resistant Plasmodium falciparum strains. Antimicrob. Agents Chemother. 1999;43(9):2205–2208. doi: 10.1128/aac.43.9.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Jun YY-c, Zhang Wei-wei. Mid-term assessment report of malaria elimination action plan in Guangxi.China Trop. Med. doi: 10.13604/j.cnki.46-1064/r.2016.04.03[published Online First: Epub Date]|.
- Lynch C., Pearce R., Pota H. Emergence of a dhfr mutation conferring high-level drug resistance in Plasmodium falciparum populations from southwest Uganda. J. Infect. Dis. 2008;197(11):1598–1604. doi: 10.1086/587845. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Marks F., von Kalckreuth V., Kobbe R. Parasitological rebound effect and emergence of pyrimethamine resistance in Plasmodium falciparum after single-dose sulfadoxine-pyrimethamine. J. Infect. Dis. 2005;192(11):1962–1965. doi: 10.1086/497698. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Mayor A., Serra-Casas E., Sanz S. Molecular markers of resistance to sulfadoxine-pyrimethamine during intermittent preventive treatment for malaria in Mozambican infants. J. Infect. Dis. 2008;197(12):1737–1742. doi: 10.1086/588144. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Miller L.H., Baruch D.I., Marsh K. The pathogenic basis of malaria. Nature. 2002;415(6872):673–679. doi: 10.1038/415673a. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Ministry of Health . 2009. Beijing, China.Antimalarial Drug Policy in China. [Google Scholar]
- Ministry of Health . 2016. Beijing, China.Antimalarial Drug Policy in China. [Google Scholar]
- Naidoo I., Roper C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013;29(10):505–515. doi: 10.1016/j.pt.2013.08.002. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Nkoli Mandoko P., Rouvier F., Matendo Kakina L. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in the Democratic Republic of the Congo: emergence of highly resistant pfdhfr/pfdhps alleles. J. Antimicrob. Chemother. 2018;73(10):2704–2715. doi: 10.1093/jac/dky258. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Oguike M.C., Falade C.O., Shu E. Molecular determinants of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum in Nigeria and the regional emergence of dhps 431V. Int. J. Parasitol. Drugs Drug Resist. 2016;6(3):220–229. doi: 10.1016/j.ijpddr.2016.08.004. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce R.J., Pota H., Evehe M.S. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med. 2009;6(4) doi: 10.1371/journal.pmed.1000055. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D.S., Walliker D., Wellems T.E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. U.S.A. 1988;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot S., Olliaro P., de Monbrison F. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar. J. 2009;8:89. doi: 10.1186/1475-2875-8-89. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper C., Pearce R., Bredenkamp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet (London, England) 2003;361(9364):1174–1181. doi: 10.1016/S0140-6736(03)12951-0. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Sibley C.H., Hyde J.E., Sims P.F. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17(12):582–588. doi: 10.1016/s1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- Snounou G. Genotyping of Plasmodium spp. nested PCR. Methods Mol. Med. 2002;72:103–116. doi: 10.1385/1-59259-271-6:103. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Snounou G., Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol. Med. 2002;72:189–203. doi: 10.1385/1-59259-271-6:189. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Sutherland C.J., Fifer H., Pearce R.J. Novel pfdhps haplotypes among imported cases of Plasmodium falciparum malaria in the United Kingdom. Antimicrob. Agents Chemother. 2009;53(8):3405–3410. doi: 10.1128/AAC.00024-09. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Menting J.G., Wilson C. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 1997;94(25):13944–13949. doi: 10.1073/pnas.94.25.13944. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Shujiao LJ, Lin Kangming, Li Jinhui, Guo Chuankun, Huang Ya. Analysis of malaria epidemic situation in Guangxi in 2013. Int. J. Med. Paraist. Dis. doi: 10.3760/cma.j.issn.1673-4122.2015.04.009[published Online First: Epub Date]|.
- World Health Organization . 2017. Malaria Report. [Google Scholar]
- World Health Organization . 2017. Malaria Prevention Works Let's Close the Map. [Google Scholar]
- World Health Organization . 2018. World Malaria Report. [Google Scholar]
- Xu C., Wei Q., Yin K. Surveillance of antimalarial resistance Pfcrt, Pfmdr1, and Pfkelch13 polymorphisms in African Plasmodium falciparum imported to Shandong Province, China. Sci. Rep. 2018;8(1):12951. doi: 10.1038/s41598-018-31207-w. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Zhou R., Liu Y. Molecular investigation of the Pfmdr1 gene of Plasmodium falciparum isolates in Henan Province imported from Africa. Parasitology. 2019;146(3):372–379. doi: 10.1017/S0031182018001609. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- Zhang Li FJ., Zhang Shao-Sen, Xia Zhi-Gui, Zhou Shui-Sen. The progress of national malaria elimination and epodemiological characteristics of malaria in China in 2017. Chin. J. Parasitol. Parasit. Dis. 2018 1000-7423(2018)-03-0201-09. [Google Scholar]