Abstract
Sustainability of cork oak (Quercus suber) forests is threatened by biotic and abiotic factors and characterization of potentially differing genetic resources has therefore gained importance. This work addresses the chemical variation of the three tissues of cork oak stems – cork, phloem and wood – in relation to tree and provenance, looking for genetic chemical diversity and for physiological derived differences. The three tissues differ with cork clearly differentiating regarding summative composition, component ratios and monomeric composition. Cork is the only tissue where suberin is present (42.3% o.d. mass) as the main cell wall component, and it has a high content of extractives (11.7%) with significant proportion of lipophilic compounds. Phloem is more lignified than wood (38.0% vs. 23.4%) and has less polysaccharides (49.1% vs. 64.6%) with glucose-to-other sugars relation of 1:1.3 in phloem and 1:0.7 in wood. Analytical pyrolysis showed that lignification is a heterogeneous process and the lignin monomeric composition depends on tissue and cell type: cork lignin has a H:G:S ratio of 1:2.5:0.3 and S/G ratio of 0.12, while phloem and wood lignins have mainly G and S units with a S/G ratio of respectively 1.1 and 2.3. No significant differences were found between the three provenances, but some chemical variation occurred between the trees within a provenance. NIR spectroscopy and principal component analysis differentiated cork, phloem and wood, while the dispersion within each group highlighted the significant tree variability, while provenances were a non-significant factor of chemical variation.
Keywords: Chemical engineering, Analytical chemistry, Plant biology, Lignin, Suberin, S/G ratio, Cork oak, NIR, Py-GC/MS
Chemical engineering; Analytical chemistry; Plant biology; Lignin suberin S/G ratio cork oak NIR Py-GC/MS
1. Introduction
Cork oak (Quercus suber) forests are spread across the western Mediterranean areas of Southern Europe and North Africa, where they play a substantial ecological role. They are part of montado, a multifunctional agro-forestry-pastoral system that is classified as a High Nature Value Farming System by the European Environmental Agency (Pinto-Correira et al., 2011) and listed in the Habitats Directive as conservation value habitats (Catry et al., 2012). However, this ecosystem is threatened by biotic and abiotic factors such as insect pests and wildfires (Catry et al., 2017). The importance of differing genetic resources for improving sustainability of cork oak forests is stressed and a multi-locality provenance trial started in 1998, as part of the European EUFORGEN Network (Almeida et al., 2005).
The cork oak is characterized by a substantial formation of cork in its periderm. The observation of a cross-section of a cork oak tree stem (Figure 1) shows distinctively the wood at the inside, and the bark located to the outside including the phloem and the cork tissues. Both are accumulated during tree growth by the functioning of two meristems: the cambium which forms the wood cells to the inside and phloem cells to the outside; and the phellogen which forms the periderm with phelloderm cells to the inside and cork cells to the outside (Pereira, 2007). The cork oak phellogen has characteristics that are at the base of the species exploitation for cork.
Figure 1.
Stem cross-sections of the three 6-year-old Quercus suber trees of provenance P15.
Cork production is the major economic activity in this non-wood forest system. The cork chain from forest to consumer relies on the regular and sustainable production of cork with the quality required by the increasingly demanding consumers of cork products. Cork is a cellular material with a very interesting and unique set of physical, biological and chemical properties (Pereira, 2007; Fortes et al., 2004), known worldwide for the “corking” of wine bottles. Applications as a thermal insulator and for vibrational and sound absorption have developed from classic and historical uses to high-tech developments (e.g. space ablative insulators, equipment sealants).
In this framework, many studies were done on cork e.g. formation (Graça and Pereira, 2004), structure (Pereira et al., 1987; Oliveira et al., 2016), chemical composition (Graça and Pereira, 1997; Pereira, 1988, 2013), and properties (Pereira et al., 1992; Oliveira et al., 2014) that have contributed to the technological innovation of the cork chain. Cork performance depends on structure and chemistry (Pereira, 2015), although the impacts of their variation are far from being well established, e.g. it is believed that the cell wall chemical variation related to contents in suberin (23.1%–54.2%) and lignin (17.1%–36.4%) and the ratio suberin-to-lignin plays a determining role in properties namely in compression (Pereira, 1988, 2013; Oliveira et al., 2014).
Cork oak wood was less studied despite its quality for shipbuilding, tools manufacturing and construction, and only a few works summarized several wood properties including chemical composition (Knapic et al., 2012; Leal et al., 2008a). On the other hand, phloem was studied for the first time by Lourenço et al. (2016) who compared its chemical composition with that of wood and cork in one individual tree.
The present study addresses the chemical variation of the three tissues of cork oak stems – cork, phloem and wood – in relation to tree and provenance, looking for genetic chemical diversity and for physiological derived differences. Summative chemical composition methods were used as well as analytical pyrolysis for compositional analysis. NIR spectral measurements were performed, having in mind the potential applicability of NIR to determine chemical parameters and screen Q. suber samples.
2. Material and methods
Six-year-old Quercus suber trees were sampled from a provenance trial located in Santiago do Cacém, Southern Portugal. This provenance trial was established in March 1998 as part of an European project (FAIR 1 CT 95–0202) with cork oak seeds collected from 35 provenances from Portugal, Spain, Italy, France, Morocco, Tunisia and Algeria (Sampaio et al., 2017). The present study used three provenances from Portugal, from the same cork producing region where the trial was established: Alcácer do Sal (P14), Azeitão (P15) and Santiago do Cacém (P19). The location, and climatic and soil characterization of the seed provenance sites as well as of the trial site are presented in Table 1.
Table 1.
Information on the cork oak provenances (P14, P15, P19) regarding location and main ecological features. Tm – long-term annual average air temperature; PPT – long-term annual average precipitation; sPPT – long-term annual summer precipitation.
| Provenances | P14 | P15 | P19 | Stablished trial site |
|---|---|---|---|---|
| Site | Herdade da Palma | Quinta da Serra | Monte Branco | Monte da Fava |
| Nearest locality | Alcácer do Sal | Azeitão | Santiago do Cacém | Santiago do Cacém |
| Latitude | 38°29′N | 38°30′N | 38°01′N | 37°56′N |
| Longitude | 8°35′W | 9°02′W | 8°42′W | 8°27′W |
| Altitude (m) | 30 | 120 | 140 | 79 |
| Tm (°C) | 16.3 | 14.3 | 15.6 | 15.8 |
| PPT (mm) | 577 | 681 | 736 | 557 |
| Soil type | Sedimentary of silica | Sandy | ||
Three trees were sampled from each provenance. From each tree, a disc was taken between 1.0 and 1.3 m of stem height (Figure 1). The wood, phloem and cork areas were determined using image analysis of the discs, acquired by a Kaiser RS1 board connected to a computer using AnalySIS® image processing software (Germany, version 3.1). The wood diameter, phloem thickness and cork proportion in the total cross-sectional area was calculated and the results are included in Table 2.
Table 2.
Cork oak cross-sectional dimensions of the trees from each of the three provenances (P14, P15, P19) (mean values and standard deviation).
| Tree cross-section dimensions | P14 | P15 | P19 |
|---|---|---|---|
| Wood area (cm2) | 21.4 (11.3) | 25.1 (1.5) | 13.1 (7.8) |
| Phloem area (cm2) | 5.5 (3.2) | 4.4 (8.0) | 3.3 (7.8) |
| Cork area (cm2) | 24.2 (13.2) | 21.2 (6.2) | 17.6 (8.8) |
| Total stem area (cm2) | 51.1 (27.7) | 50.7 (8.4) | 34.0 (18.5) |
| Wood diameter (mm) | 51.1 (5.1) | 56.5 (5.6) | 39.2 (3.9) |
| Phloem thickness (mm) | 3.1 (0.9) | 2.4 (0.4) | 2.4 (0.7) |
| Cork proportion (%) | 47.3 (1.1) | 41.4 (4.9) | 53.2 (4.1) |
The wood, phloem and cork were manually separated with a chisel from each disc. The samples were milled in a cutting mill from Retsch (SM2000), first passing through a 6 × 6 mm sieve and then by a 1 × 1 mm sieve. The samples were dried in an oven at 60 °C and kept for analysis.
2.1. Chemical analysis
The summative chemical analysis was made in two aliquots of the wood, phloem and cork samples (after manual separation with a chisel) using procedures adapted from TAPPI standards: ash content by incineration (TAPPI T15 os-58), extractives by solvent extraction with a sequence of dichloromethane, ethanol and water during 16 h for each solvent (TAPPI T204 cm-07), Klason lignin (TAPPI T222 om-11) and soluble lignin (TAPPI UM 205 om-93). The composition of monosaccharides, acetic acid and uronic acids were determined in the hydrolysate obtained from lignin analysis using a Dionex ICS-3000 system in HPIC-PAD and an Aminotrap plus Carbopac PA10 column (250 × 4 mm) for monosaccharides and uronic acids, and by HIPCE-UV using a Waters 600 system with a Biorad Aminex 87H column (300 × 7.8 mm) for acetic acid.
For the cork samples, the suberin content was determined in the extractive-free material by methanolysis, as presented by Pereira (2013). The subsequent determinations of total lignin, monosaccharides, uronic acids and acetic acid were made in the suberin-free material, as above described. All the chemical results were calculated and reported as percentage of the initial material.
2.2. Analytical pyrolysis
The extractive-free samples were dried and powdered in a Retsch MM200 mixer ball during 10 min. Approximately 100 μg were weighted and pyrolysed at 650 °C during 10 s in a CDS platinum coil pyroprobe linked to a 5150 CDS apparatus, and connected to a GC (Agilent 7890B) equipped with a mass detector (Agilent 5977B, EI at 70 eV). The injector and the GC/MS interface were kept at 270 °C and 280 °C, respectively. The oven program and the pyrolysis products identification are described in more detail by Lourenço et al., (2017). The percentage of each compound was calculated using the corresponding peak area in relation to the total area of the chromatogram. The percentage of hydroxyphenyl (H), guaiacyl (G) and syringyl (S) lignin-derived products were separately summed, thus allowing calculation of the S/G ratio and the H:G:S relation.
2.3. NIR spectroscopy
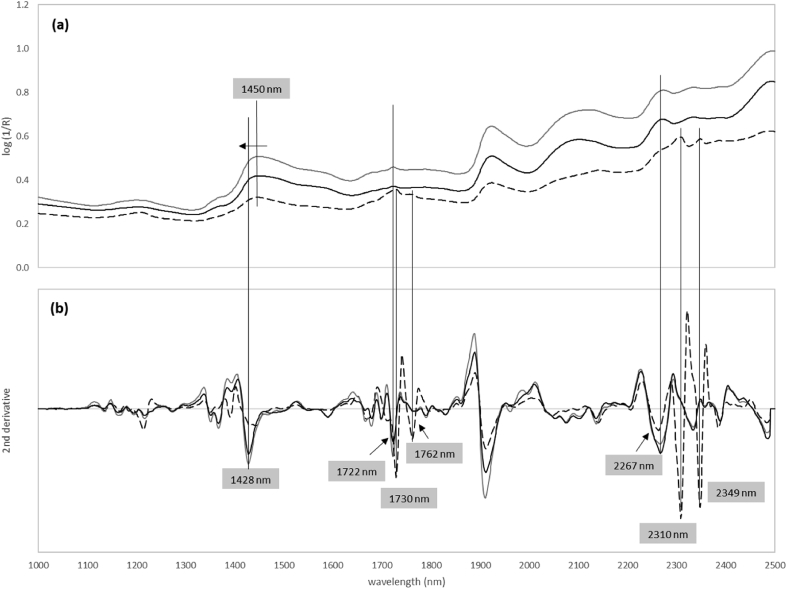
Near-infrared spectra were obtained for the wood, phloem and cork samples (Figure 3a). The samples were scanned using a Near Infrared Reflectance Accessory (NIRA) attached to a Perkin Elmer Frontier TM model FT-NIR spectrometer and the spectra collected over a wavenumber range of 1000–2500 nm, with 8 cm−1 spectral resolution and 32 scans per spectrum.
Figure 3.
(a) NIR raw spectra and (b) second derivative of the three tissues cork, phloem and wood from 6-year-old Quercus suber trees. Black and grey solid line indicate wood and phloem tissues, respectively, and dash line indicate cork tissue.
A blank spectrum was acquired after every 10 samples to remove any random effects associated with the instrument or environment (e.g., room temperature or humidity). Two spectra per sample were recorded at room temperature (20–22 °C).
The use of chemometric techniques has been the choice for extracting information from NIR spectra in which absorption bands correspond mainly to overtones and combinations of fundamental vibrations, and are relatively weak in intensity (Blanco and Villarroya, 2002). Since NIR spectra of solid samples are susceptible to scattering effects, such as offset, slope, and non-linear effects, spectral pre-treatments were performed to minimize those effects (Blanco and Villarroya, 2002; Rinnan et al., 2009). In this study the second-order derivative was selected, resulting in a spectral pattern display of absorption peaks in the negative direction, without false peaks (Schwanninger et al., 2011).
2.4. Statistical analysis
The chemical variability between tissues and provenances assessed by NIR spectroscopy was performed with principal component analysis (PCA) using The Unscrambler® (CAMO AS, Norway) software, version 10.5. The variation of chemical features within each tissue regarding the different cork oak provenances was statistically analysed using an analysis of variance with a nested design of trees within provenances. The effects were considered as statistically significant when the p-value was less than or equal to 0.05. All the statistical analysis was performed using SPSS® statistical software (version 25.0; SPSS Inc., Chicago IL).
3. Results and discussion
This study focused on young Quercus suber trees of different provenances, aiming at ascertaining chemical differences between the stem components i.e. cork, phloem and wood and the potential impact of provenance. A previous study (Lourenço et al., 2016) indicated that there were chemical differences between the three tissues but the limited sampling of only one tree did not allow to assess between tree variability. Here, a more robust sampling was made of three trees from each of three provenances with the elimination of the potential influence of tree age and growth conditions by taking 6-year-old plants from the same trial (Table 1).
The trees developed differently both within provenance as between provenances, with mean total stem cross-sectional areas of 51.1 cm2, 50.7 cm2 and 34.0 cm2 for P14, P15 and P19 respectively (Table 2). The dimensional measurements taken at the breast height cross-section showed between tree variation of radial growth on all tissues. The average annual wood growth was 4.1 mm (varying from 3.3 to 4.7 mm/yr for P19 and P15 respectively). This compares with the 2.7 mm/yr reported for trees with 35 years, while cork oak wood annual growth is lower (1.6 mm/yr) for 60-year-old trees (Leal et al., 2008b). Phloem growth was on average 0.43 mm per year, meaning that the cambial meristematic activity had a radial formation ratio of wood-to-phloem of 4.1:0.4.
It is striking to see that there was high activity of the phellogen during these first 6 years of the tree life, which was comparable to the cambial activity in terms of cellular volume production: cork represented on average 47.3 % of the cross-sectional area, where wood and phloem represented respectively 43.3% and 9.7% (Table 2). This large amount of cork confers an increased protection to the tree, namely regarding insulation against high temperatures and the lowering of the probability of a tree being killed by fire and the increase of crown regeneration (Catry et al., 2012).
3.1. Chemical composition of cork, phloem and wood
The summative composition of cork, phloem and wood in the Q. suber samples is presented in Table 3, by provenance and in average, including the monomeric composition of polysaccharides regarding neutral and acid monosaccharides, and acetyl groups. The data obtained show that cork, phloem and wood differ chemically, with cork clearly differentiating itself regarding summative composition as well as component ratios and monomeric composition.
Table 3.
Chemical summative composition (% of o.d. mass) and monomeric composition of polysaccharides (% of total monomeric units) of Quercus suber cork, phloem and wood from three provenances (P14, P15, P19) as mean and standard deviation of three trees and two aliquots, and their mean standard deviation (three trees, three provenances, two aliquots).
| Cork |
Phloem |
Wood |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P14 | P15 | P19 | Mean | P14 | P15 | P19 | Mean | P14 | P15 | P19 | Mean | |
| Ash | 0.7 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.2) | 3.1 (0.7) | 2.7 (1.0) | 2.8 (0.2) | 2.7 (1.0) | 1.1 (0.2) | 1.2 (0.1) | 1.1 (0.1) | 1.1 (0.1) |
| Total extractives | 10.4 (0.4) | 12.6 (0.7) | 12.1 (1.8) | 11.7 (1.4) | 3.9 (0.5) | 4.8 (1.4) | 5.0 (0.8) | 4.5 (1.0) | 5.0 (0.7) | 5.8 (0.3) | 5.9 (0.7) | 5.6 (0.7) |
| CH2Cl2 | 4.8 (0.3) | 5.3 (0.5) | 5.1 (0.6) | 5.1 (0.5) | 0.1 (0.03) | 0.2 (0.05) | 0.2 (0.04) | 0.2 (0.04) | 0.3 (0.05) | 0.3 (0.03) | 0.4 (0.07) | 0.3 (0.06) |
| EtOH | 2.1 (0.6) | 4.1 (0.8) | 3.1 (0.7) | 3.1 (1.0) | 1.5 (0.4) | 1.7 (0.4) | 1.4 (0.3) | 1.5 (0.4) | 1.2 (0.5) | 1.9 (0.2) | 1.5 (0.5) | 1.6 (0.5) |
| H2O | 3.4 (0.4) | 3.3 (0.6) | 3.8 (0.7) | 3.5 (0.6) | 2.3 (0.2) | 2.9 (1.6) | 3.4 (1.0) | 2.8 (1.1) | 3.4 (1.1) | 3.5 (0.4) | 4.0 (0.7) | 3.7 (0.8) |
| Suberin | 42.7 (2.8) | 43.3 (6.3) | 41.0 (4.7) | 42.3 (4.7) | - | - | - | - | - | - | - | - |
| Total lignin | 24.9 (1.3) | 23.4 (2.8) | 23.8 (2.1) | 23.3 (2.1) | 38.4 (1.2) | 37.8 (3.0) | 37.9 (1.0) | 38.0 (1.9) | 22.6 (0.6) | 24.5 (1.3) | 23.1 (0.9) | 23.4 (1.2) |
| Klason | 24.2 (1.3) | 22.7 (2.7) | 23.0 (2.1) | 24.1 (2.1) | 35.9 (1.3) | 35.3 (3.2) | 35.5 (0.8) | 35.6 (1.9) | 19.7 (0.8) | 21.6 (1.4) | 20.4 (0.7) | 20.6 (1.3) |
| Soluble | 0.7 (0.09) | 0.7 (0.1) | 0.9 (0.08) | 0.7 (0.1) | 2.5 (0.2) | 2.4 (0.3) | 2.4 (0.3) | 2.5 (0.3) | 2.9 (0.3) | 2.9 (0.2) | 2.6 (0.4) | 2.8 (0.3) |
| Polysaccharides | 16.8 (1.6) | 15.2 (4.4) | 16.6 (4.1) | 16.2 (3.2) | 48.5 (5.3) | 51.6 (2.1) | 47.3 (1.1) | 49.1 (2.3) | 66.9 (1.7) | 64.8 (5.1) | 62.1 (2.3) | 64.6 (3.6) |
| Monosaccharides (% total monosaccharides) | ||||||||||||
| Arabinose | 17.1 (2.1) | 19.0 (6.9) | 17.0 (3.0) | 17.7 (4.0) | 2.3 (0.2) | 2.6 (0.6) | 2.3 (0.5) | 2.4 (0.4) | 1.5 (0.2) | 1.4 (0.3) | 1.7 (0.2) | 1.5 (0.2) |
| Xylose | 29.3 (1.8) | 27.1 (7.1) | 30.5 (3.5) | 29.0 (4.3) | 38.5 (1.5) | 37.5 (3.1) | 41.0 (2.8) | 39.0 (2.7) | 25.6 (1.5) | 27.8 (1.5) | 29.7 (1.3) | 27.7 (2.2) |
| Galactose | 6.4 (1.0) | 7.0 (1.8) | 6.5 (1.3) | 6.7 (1.3) | 1.8 (0.01) | 2.3 (0.5) | 1.7 (0.2) | 2.0 (0.4) | 2.4 (0.6) | 2.4 (0.7) | 2.2 (0.3) | 2.3 (0.5) |
| Glucose | 43.7 (1.6) | 43.4 (2.6) | 42.9 (0.8) | 43.3 (1.6) | 43.7 (3.3) | 43.8 (4.4) | 42.8 (3.3) | 43.4 (3.2) | 60.4 (1.1) | 57.9 (0.5) | 58.5 (2.1) | 58.9 (1.6) |
| Galacturonic acid | 3.1 (0.3) | 3.1 (0.7) | 2.7 (0.2) | 3.0 (0.46) | 2.1 (0.6) | 2.5 (0.7) | 1.8 (0.4) | 2.1 (0.6) | 1.7 (0.2) | 1.8 (0.3) | 1.4 (0.1) | 1.7 (0.3) |
| Glucuronic acid | 0.3 (0.0) | 0.3 (0.1) | 0.4 (0.1) | 0.34 (0.0) | 0.3 (0.3) | 0.5 (0.3) | 0.1 (0.0) | 0.3 (0.3) | 0.4 (0.2) | 0.3 (0.2) | 0.1 (0.0) | 0.3 (0.2) |
| Acetic acid | - | - | - | - | 11.2 (1.3) | 10.8 (1.8) | 10.2 (1.4) | 10.7 (1.4) | 8.0 (0.7) | 8.2 (1.7) | 6.4 (1.1) | 7.5 (1.4) |
It is well known that suberin is the major structural component of cork and that it is not present in phloem and wood (Pereira, 2007). The chemical composition of cork (Table 3) with 44.8 % suberin is in accordance with published values (Pereira, 2013). The suberin-to-lignin ratio was 1.8 similar to the reported value of 2.0, and the relation cellulose-to-hemicelluloses, determined by the ratio glucose-to-other-sugars was 1:1.3, similar to the reported 1:1.2 (Pereira, 2013, 2015). The cellulose and hemicelluloses totalled 16.2% of the cell wall structural components (Table 3). Hemicelluloses were mainly composed by arabinoxylans with a significant proportion of galactose (6.7% of the total monomeric units) and including uronic acids (3.3% of the total) but without acetyl groups. Similar predominance of arabinoxylans in virgin cork hemicelluloses is reported in the literature e.g. xylose and arabinose representing 40.4% of total sugars (Pereira, 2007).
Cork contains a high content of extractives corresponding to 11.7%, of which a significant proportion of 44% are lipophilic compounds. This is in accordance with the reported range of values found in cork (e.g. Pereira, 2013; Bento et al., 2001; Sen et al., 2016). The chemical composition of cork from the cork oak is in general similar to that of cork from other species (Leite and Pereira, 2017) although chemical differences between species arise e.g. in extractives content and composition (for example in Quercus cerris, Sen et al., 2010; or in Pseudotsuga menziesii, Cardoso et al., 2018).
Phloem and wood are lignocellulosic tissues produced by the cambium that have transport and mechanical support functions and which are specialized into different cell types e.g. respectively sieve elements and vessels for transport, and sclereids and fibers for support (Lourenço et al., 2016; Sousa et al., 2009). Phloem and wood have a moderate content of extractives (4.5% vs. 5.6%), mostly polar compounds corresponding to respectively 4.3% and 5.3% (Table 3). Phloem is more lignified than wood (38.0% vs. 23.4%) and has less polysaccharides (49.1% vs. 64.6%) with some differences in composition: the relation glucose-to-other sugars was 1:1.3 in phloem and 1:0.7 in wood. The cork oak wood chemical composition is in line with reported values (Leal et al., 2008a).
The chemical difference between the tissues is also noticeable by near-infrared spectroscopy. NIR spectra of cork present distinctive bands (1730, 1762, 2310 and 2349 nm) allowing a clear discrimination between the cork and the wood and phloem tissues (Figure 3 and Figure 4). The 1730 nm band is probably due to the first overtone of C–H stretching from CH2 groups and was assigned by Schwanninger et al. (2011) to cellulose because it was only found in spruce cellulose, although they also refer that, combination bands of C–H stretching vibration and the 1st overtone of C–H deformation vibration of methylene and methyl groups can also appear in this range. Workman and Weyer (2008) assigned the 1762 nm band to the 1st overtone of C–H stretching due to the symmetric C–H methylene (CH2).
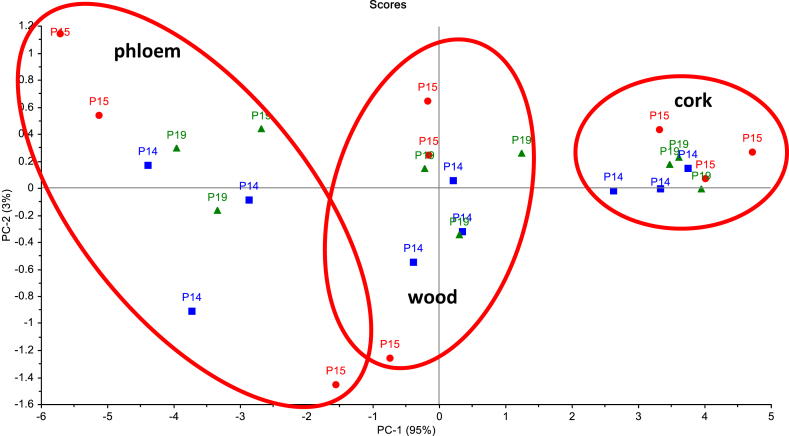
Figure 4.
Principal component analysis of NIR raw spectra for cork, phloem and wood from 6-year-old Quercus suber trees from three provenances (P14, P15, P19).
The assignment in the spectral region of the combination bands, that include the 2310 and 2350 nm bands, is difficult due to the high number of possibilities for the coupling of vibrations (Schwanninger et al., 2011). However, Prades et al. (2012, 2014) assigned the 2300–2360 nm region to CH combination bands of antisymmetric and symmetric stretching plus one deformation mode in the CH and CH2 structures. Workman and Weyer (2008) assigned the 2310 nm band to the 2nd overtone of C–H fundamental bending due to lipids, and the 2349 nm band to the functional group of C–H methylene C–H, associated with linear aliphatic R(CH2)NR, due to aliphatic hydrocarbons as representative of cork suberin chemical structure (Pereira, 2007).
The differences between phloem and xylem are observed in NIR spectra with bands around 1428 nm in the 2nd derivative (1450 nm in raw spectra) and 1722 nm. In asymmetric bands of the NIR spectrum (Figure 3a), the position of the 2nd derivative can deviate from the position of the band in the spectrum. The band around 1428 nm (1450 nm) is due to bond vibrations from the 1st overtone of O–H stretching, and it was assigned to amorphous polysaccharides of wood, including free and weakly H-bonded OH of cellulose, free and weakly H-bonded OH: O(6)–H(6) of cellulose, glucomannan, and O(2)–H(2) of cellulose and xylan (Fackler and Schwanninger, 2010). Recently, Liang et al. (2020) highlighted the existence of a strong absorption signal at 1454 nm corresponding to the 1st overtone of O–H stretching from phenolic groups present in lignin. The band around 1722 nm that helps to differentiate phloem and xylem tissues, was assign by Fackler and Schwanninger (2010) to 1st overtone of C–H stretching in furanoses and pyranoses of hemicelluloses (xylan and glucomannan).
The band at 2267 nm is present in the NIR spectra of all tissues and can contribute to differentiate between the tissues; however, it is difficult to assign this band as several bands can be seen in 2nd derivatives of the spectra of milled wood lignin and in celluloses and hemicelluloses (Schwanninger et al., 2011). Sandak et al. (2013) assigned the 2270 nm band to semi-crystalline and/or crystalline regions in cellulose, particularly to CH2 stretching and deformation in cellulose. Liang et al. (2020) assigned bands at 2267 nm and 2383 nm to combination bands of O–H stretching and C–O stretching, as well as C–H stretching and C=C stretching. In this work we tentatively assigned this band to lignin because it appears in the lignin Klason spectra from the same samples (not shown). Toscano et al. (2017) already highlighted this peak as one of the most relevant wavelengths for the discrimination between bark and wood samples.
All spectra were analysed by principal component analysis and Figure 4 presents their projection on the plane defined by the first two principal components, cumulatively representing 98% of the total original data variance. Three separated clusters are observed in the score plot, showing high clustering tendency of the NIR data from the three tissues. The dispersion of the observations within each group highlights the significant variability associated with the tree and that provenances were a non-significant factor of chemical variation.
The results obtained here for the chemical differences between cork, phloem and wood confirm the values obtained in the previous study that analysed the three tissues from one cork oak tree (Lourenço et al., 2016). The present work with a more ambitious sampling of three provenances and three trees per provenance, thereby allows the consolidation of the findings and an insight into the factors of chemical variation (provenance and tree).
There are very few studies comparing the chemical composition of phloem and cork in the bark of other species that are in accordance with the results obtained in this study e.g. in Pseudotsuga menziesii (Ferreira et al., 2016; Cardoso et al., 2019) and in Quercus cerris (Sen et al., 2010).
Regarding the chemical variation related to the provenances, no statistically significant differences were found for almost all the chemical features. The results show that only ethanol extractives in cork have provenances as a factor for chemical variation (p = 0.026). The studied provenances belong to the same broad cork production region, corresponding to a coastal region south of the river Tagus (e.g. the maximal distance between the provenances locations is around 125 km). For cork, the lack of chemical differences between regions was already reported in a large-scale study with sampling of 29 sites in six regions of Portugal (Pereira, 2013) as well as in a study with seven provenances in Spain (Conde et al., 1998). However, some chemical variation was found between the trees within a provenance (Table 3). This between-tree variation was also noticed for cork chemical composition, including suberin monomeric composition (Bento et al., 2001).
3.2. Lignin composition
Analytical pyrolysis is a powerful tool to evaluate lignin composition of plant tissues allowing lignin chemical classification based on its precursors proportion (Lourenço et al., 2019). The results obtained in the present study show that cork, phloem and wood have different types of lignin (Table 4): cork lignin is mainly constituted by G units with also an important proportion of H units, but with few S units (H:G:S 1:2.5:0.3, S/G 0.12); while phloem and wood lignins have mainly G and S units, but contain also H units; wood has more syringyl units (H:G:S of 1:2.0:4.5 and S/G of 2.3).
Table 4.
Py-GC/MS results obtained for Quercus suber cork, phloem and wood from three provenances (P14, P15, P19) classified by chemical families (% of total area), as well as S/G and H:G:S lignin ratios.
| Pyrolysis |
Cork |
Phloem |
Wood |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compounds | P14 | P15 | P19 | Mean (STDEV) | P14 | P15 | P19 | Mean (STDEV) | P14 | P15 | P19 | Mean (STDEV) |
| Lignin | 13.2 | 12.4 | 12.1 | 12.6 (1.0) | 15.0 | 14.6 | 13.5 | 14.4 (1.6) | 10.4 | 12.0 | 9.9 | 10.7 (1.9) |
| S | 1.0 | 0.8 | 0.5 | 0.8 (0.2) | 6.3 | 5.6 | 4.6 | 5.5 (1.2) | 6.3 | 7.2 | 5.4 | 6.3 (1.7) |
| G | 7.0 | 6.5 | 5.4 | 6.3 (1.0) | 5.5 | 5.2 | 4.9 | 5.2 (0.7) | 2.5 | 3.0 | 2.6 | 2.7 (1.7) |
| H | 2.5 | 2.5 | 2.6 | 2.5 (0.5) | 1.8 | 2.1 | 2.3 | 2.0 (0.3) | 1.2 | 1.3 | 1.6 | 1.4 (0.4) |
| Others | 2.7 | 2.7 | 3.6 | 3.0 (0.5) | 1.4 | 1.6 | 1.7 | 1.6 (0.3) | 0.3 | 0.4 | 0.3 | 0.4 (0.1) |
| S/G | 0.14 | 0.12 | 0.09 | 0.12 | 1.2 | 1.1 | 0.92 | 1.1 | 2.5 | 2.4 | 2.1 | 2.3 |
| H:G:S | 1:2.9:0.4 | 1:2.6:0.3 | 1:2.0:0.2 | 1:2.5:0.3 | 1:3.1:3.5 | 1:2.5:2.7 | 1:2.2:2.0 | 1:2.6:2.7 | 1:2.1:5.1 | 1:2.3:5.4 | 1:1.6:3.3 | 1:2.0:4.5 |
| Carbohydrates | 26.2 | 24.3 | 25.6 | 25.4 (4.2) | 57.7 | 57.4 | 60.8 | 58.6 (2.1) | 64.1 | 62.7 | 62.4 | 63.1 (1.6) |
| Pyran | 7.7 | 7.0 | 6.8 | 7.2 (1.6) | 17.3 | 15.7 | 17.7 | 16.9 (2.2) | 24.1 | 20.9 | 22.2 | 22.4 (2.1) |
| Furan | 4.5 | 4.2 | 4.5 | 4.4 (0.7) | 5.6 | 5.7 | 5.9 | 5.7 (0.3) | 6.0 | 6.2 | 6.0 | 6.1 (0.2) |
| Low molecular | 11.0 | 10.3 | 11.0 | 10.8 (1.7) | 27.1 | 28.5 | 28.8 | 28.1 (2.0) | 25.9 | 27.2 | 26.0 | 26.3 (1.5) |
| Others | 3.1 | 2.8 | 3.2 | 3.0 (0.7) | 7.8 | 7.5 | 8.4 | 7.9 (0.6) | 8.1 | 8.3 | 8.3 | 8.2 (0.7) |
| Suberin | 33.2 | 33.0 | 32.9 | 33.0 (2.4) | - | - | - | - | - | - | - | - |
| Fatty acid | 7.4 | 7.4 | 7.9 | 7.6 (0.7) | - | - | - | - | - | - | - | - |
| Alkane | 1.9 | 2.1 | 1.6 | 1.9 (0.3) | - | - | - | - | - | - | - | - |
| Alkene | 18.1 | 17.5 | 17.5 | 17.7 (1.3) | - | - | - | - | - | - | - | - |
| Alkadiene | 4.2 | 4.4 | 4.4 | 4.3 (0.5) | ||||||||
| Not identified | 1.7 | 1.6 | 1.5 | 1.6 (0.3) | - | - | - | - | - | - | - | - |
As far as we know, only Lourenço et al. (2016) studied in detail the lignin composition in these tissues after lignin isolation and reported a lignin compositional profile similar to the one reported here, e.g. the S/G ratio was 0.10 in cork, 0.62 in phloem and 1.66 in wood. These results show that lignification is a heterogeneous process and the lignin monomeric composition depends on tissue and cell type (Barros et al., 2015; Ruel et al., 1999). Lignification has implications upon the protection and strength of the individual cells and therefore on the tissues. For instance, tracheary elements present cell walls that are able to support negative pressure of sap ascent which can be provided by the presence of G-units; whereas fibers and sclereids offer mechanical strength and are typically constituted by S-units (Terashima and Fukushima, 1989; Higuchi, 1990). This might explain the different monomeric composition of cork, phloem and wood found in Q. suber: cork constitutes a homogeneous tissue of phellem cells that possess more G-lignin, while phloem has a large proportion of sclereids and wood more fibers, both mainly constituted by S-units (Barceló, 1997).
Another justification for the differences could be the time of lignification i.e., the deposition of the lignin units in the cell wall is believed to be successive, first the H-units, followed by the G-units and at last the S-units (Terashima et al., 1986; Chesson et al., 1997). The incorporation of G-units is in the early to late stages of cell formation, whereas the S-units are deposited mainly during the middle and late stages (Terashima et al., 1986; Fukushima and Terashima, 1991; Rencoret et al., 2011). Chesson et al. (1997) also refer for the later stage a variation in the deposition of G and S units with cell type.
It is known that plants are able to incorporate substantial amounts of different components to respond to external conditions as an adaptive survival strategy. For instance, in poplar after wounding the xylem cells became thicker with more guaiacyl lignin, a mechanism associated with a plant response to increase resistance (Schmitt et al., 2006). However, it is difficult to establish the relation between lignin content, composition and localization with the specific cell type and function (Neutelings, 2011).
3.3. Analytical pyrolysis
Table 4 presents a summary of the main derived group of compounds obtained by Py-CG/MS analysis of cork, phloem and wood. The Supplementary data includes more detailed information (e.g. identification of pyrolysis compounds, their origin and mean value in percentage of the total chromatographic area by provenance).
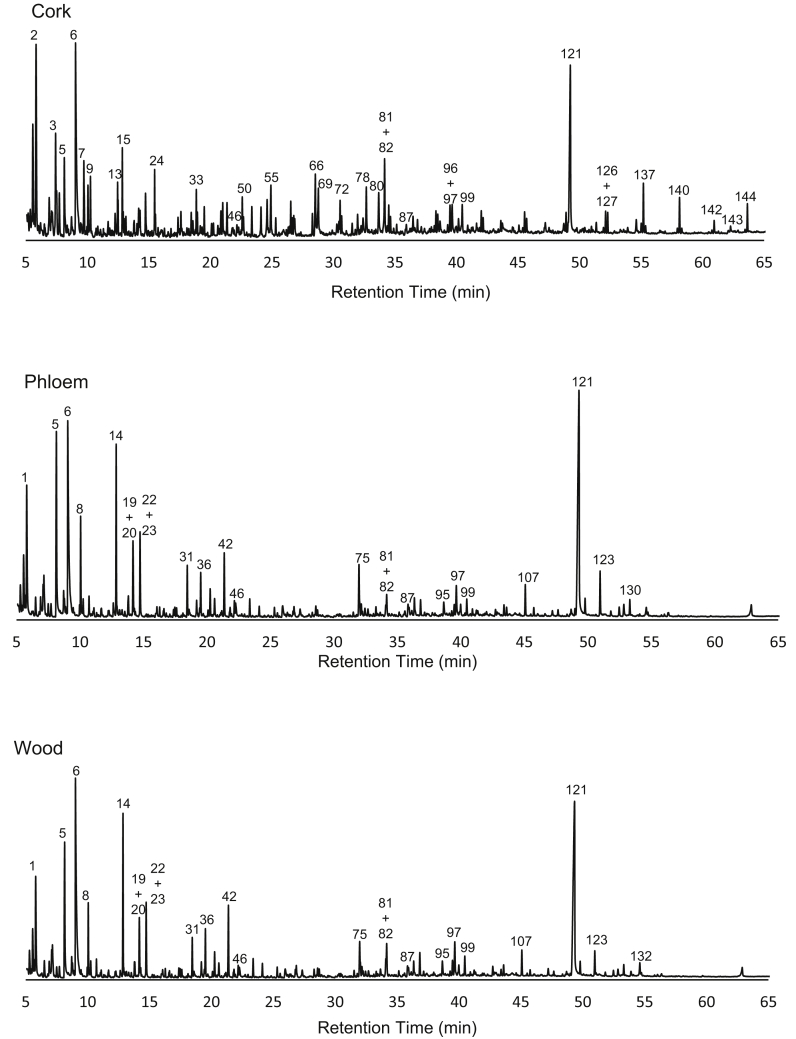
In addition to lignin composition (as mentioned above), analytical pyrolysis also allows insight into the composition of the other structural components (Figure 2). The pyrolytic degradation of suberin is dominated by the presence in the pyrolysis products of different families belonging to aliphatic products, which include fatty acids (7.4%), alkanes (1.9%), alkenes (18.1%), alkadienes (4.2%) and other unidentified aliphatic products (1.7%), as summarised in Table 4. These aliphatic compounds included homologous series with different chain lengths from 6 to 22 carbons. The alkenes and alkadiene carbon chains ranged from 9 to 22 carbons, the main compounds being 1-hexene (C6:1), 1-heptene (C7:1), 1-octene (C8:1), 1,8-nonadiene (C8:2) and 1,15-hexadiene (C6:2). The identification of long chain fatty acids was difficult due to the impossibility to identify the molecular ion, and some peaks (peaks 142–164, Supplementary data) were not identified accurately. Thus, only a small amount of fatty acids with short carbon chains was identified such as C8:0 (1.5%, peak 66), C7:1 (1.3%, peak 55) and C8:1 (1.0%, peak 69). Marques and Pereira (2014) did not identify fatty acids from the pyrolysis of different corks, due to decarboxylation and decarbonylation reactions, and instead found alkenes, alkadienes and alkanes. The pyrolysis conditions, namely temperature and flow rates certainly influence the thermochemical degradation reactions and therefore the pyrograms (Marques and Pereira, 2014).
Figure 2.
Py-GC/MC pyrograms of cork, phloem and wood from 6-year-old Quercus suber trees. 1: 2-oxo-propanal; 2: 1-hexene (C6:1); 3: 1-heptene (C7:1); 5: hydroxyacetaldehyde; 6: acetic acid; 7: 1-octene (C8:1); 8: acetol; 9: toluene; 14: 3-hydroxypropanal; 19: CH3–CO–CHOH–CHO; 20: CHO–CH2–CH2–CHO; 22: furfural; 23: 2-cyclopenten-1-one; 31: 2-hydroxy-2-cyclopenten-1-one; 33: 1-undecene (C11:1); 36: Not identified sugar; 42: 4-hydroxy-5,6-dihydro-(2H)-pyran-2-one; 46: methyl-dihydro-(2H)-pyran-2-one; 50: 1-dodecene (C12:1); 55: 6-heptenoic acid (C7:1); 66:octanoic acid (C8:0); 69: 7-octanoic acid (C8:1); 72: 1-tetradecene (C14:1); 75: Not identified sugar; 78: 8-nonenoic acid (C9:1); 80: 1,5-anhydro-arabinofuranose; 81: 2,3-dihydrobenzofuran; 82: 4-vinylguaiacol; 87: 5-hydroxymethylfurfural; 95: 2-hydroxymethyl-5-hydroxy-2,3-dihydro-(4H)-pyran-4-one; 96: trans-isoeugenol; 97: similar to 1,5-anhydro-arabinofuranose; 99: vanillin; 107: 4-vinylsyringol; 121: levoglucosan; 123: syringaldehyde; 126: 1-eicosene (C20:1); 127: 1,19-eicosadiene (C20:2); 130: acetosyringone; 132: trans-coniferaldehyde; 137: 1-heneicosene (C21:2); 140: 1-docosene (C22:1); 142–144: Not identified suberin derivatives.
The pyrolysis products derived from carbohydrates also showed differences between cork, phloem and wood: the ratio of pyran:furan structures was 1.6, 3.0 and 3.7 respectively, while the low molecular compounds represented a substantial proportion of the total carbohydrate-derived compounds (42.5%, 48.0% and 41.7% respectively, Table 4). The quantification of hexose and pentose type of compounds cannot be made by pyrolysis data, since their degradation produces the same compounds, except for levoglucosan (peak 121) that is derived exclusively from cellulose, and for 4-hydroxy-5,6-dihydro-2H-pyran-2-one (peak 42) that is a pentosan marker (Faix et al., 1991). Other hexose markers were defined (peaks 5, 8, 46, 79, 87, 95, 121) and for pentoses, peaks 42 and 80 (Marques et al., 1994). Under these assumptions, the ratio hexoses/pentoses was 3.7, 7.8, 11.5 respectively for cork, phloem and wood; Marques et al., (1994) reported for cork an even lower ratio of 1.3. This shows the importance of pentoses in the polysaccharides of cork corresponding to the main proportion of arabinoxylans (Pereira, 1988).
Analytical pyrolysis has been proposed for chemical quantification, e.g. lignin content (Lourenço et al., 2019; Meier and Faix, 1992). However, in the conditions used in this work, namely a pyrolysis temperature of 650 °C, an important amount of low mass pyrolytic compounds was generated, that could not be assigned as originating from a specific component (near 30% of the chromatogram area). Therefore, the specific components are under or overestimated in accordance with the intensity of their thermal degradation. For instance, cork thermal behaviour showed that suberin is more thermally resistant (Sen et al., 2012, 2014; Pereira, 2015). The effect of pyrolysis temperature was also discussed by Marques and Pereira (2014) who proposed 650 °C for the pyrolysis of cork-containing materials. In consequence, lignin content determined by pyrolysis is by far lower than the results attained by chemical analysis (12.6% vs. 26.4% of extractive-free cork, Table 4 vs. Table 3) while the under-estimation of suberin content was of smaller magnitude (33.1% vs. 37.4% of extractive-free cork). Therefore, the quantification by pyrolysis of structural components of plant materials, namely those of complex nature, should be made with caution.
The comparison of the analytical pyrolysis data regarding provenances showed no significant differences e.g. the compositional pyrolytic profile regarding lignin and suberin was similar (Table 4).
4. Conclusions
The focus of this study was to evaluate chemical differences in young Quercus suber trees between the three stem tissues (cork, phloem and wood) corresponding to physiological derived differences and between provenances and trees growing under the same edaphoclimatic conditions, therefore corresponding to genetic variation.
The chemical composition of cork was remarkably different from both phloem and wood. Cork has suberin as the major structural component followed by a lignin with a great proportion of G units but with few S units, while phloem and wood are mainly constituted by polysaccharides and lignin which was characterized by increasing amounts of S units from phloem to wood.
No chemical differences were found between provenances but among tree variation was present, showing genetic distinction at the individual tree level. NIR spectroscopy and principal component analysis allowed to differentiate cork, phloem and wood, with high clustering tendency of the NIR data from the three tissues while the dispersion within each group highlighted the significant variability associated with the tree and that provenances were a non-significant factor of chemical variation.
Declarations
Author contribution statement
Ana Lourenco: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ricardo Costa: Performed the experiments; compile the data.
Vanda Oliveira: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Helena Pereira: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by FCT (Fundação para a Ciência e Tecnologia, Portugal) by financing the Forest Research Center (UID/AGR/00239/2019). Vanda Oliveira was supported by FCT through a postdoctoral grant (SFRH/BPD/118037/2016). Ana Lourenço was supported by FCT through a research contract (DL 57/2016/CP1382/CT0007).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
A word of appreciation to Duarte M. Neiva and Jorge Gominho for their help in the chemical analysis and discussion of the results.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Almeida M.H., Lourenço M.J., Sampaio T., Nunes A.M., Varela M.C., Faria C., Chambel M.R., Pereira J.S. Congresso Internacional “Sobrais, fábricas e comerciantes. Passado, Presente e Futuro da Actividade Corticeira”. February; Palafrugell (Girona): 2005. Five years results of provenance trials of Quercus suber in Portugal; pp. 16–18. [Google Scholar]
- Barceló A.R. Lignification in plant cell walls. Int. Rev. Cytol. 1997;176:87–132. [PubMed] [Google Scholar]
- Barros J., Serk H., Granlund I., Pesquet E. The cell biology of lignification in higher plants. Ann. Bot. 2015;115:1053–1074. doi: 10.1093/aob/mcv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento M.F.S., Pereira H., Cunha M.A., Moutinho A.M.C., van der Berg K.J., Boon J.J. A study of variability of suberin composition in cork from Quercus suber L. using thermally assisted transmethylation GC-MS. J. Anal. Appl. Pyrolysis. 2001;57:45–55. [Google Scholar]
- Blanco M., Villarroya I. NIR spectroscopy: a rapid-response analytical tool. TrAC Trends Anal. Chem. (Reference Ed.) 2002;21:240–250. [Google Scholar]
- Cardoso S., Ferreira J., Miranda I., Pereira H. Age variation of Douglas-Fir bark chemical composition. J. Wood Chem. Technol. 2018;38:385–396. [Google Scholar]
- Cardoso S., Quilhó T., Pereira H. Influence of cambial age on the bark structure of Douglas-fir. Wood Sci. Technol. 2019;53:191–210. [Google Scholar]
- Catry F.X., Branco M., Sousa E., Caetano J., Naves P., Nóbrega F. Presence and dynamics of ambrosia beetles and other xylophagous insects in a Mediterranean cork oak forest following fire. Ecol. Manag. 2017;404:45–54. [Google Scholar]
- Catry F.X., Moreira F., Pausas J.G., Fernandes P.M., Rego F., Cardillo E., Curt T. Cork oak vulnerability to fire: the role of bark harvesting, tree characteristics and abiotic factors. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson A., Provan G.J., Russell W., Scobbie L., Chabbert B., Monties B. Characterisation of lignin from parenchyma and sclerenchyma cell walls of the maize internode. J. Sci. Food Agric. 1997;73:10–16. 199701)73:1<10::AID-JSFA697>3.0.CO;2-E. [Google Scholar]
- Conde E., Cadahia E., Garcia-Vallejo M.C., Adrados J.R.C. Chemical characterization of reproduction cork from Spanish Quercus suber. J. Wood Chem. Technol. 1998;18:447–469. [Google Scholar]
- Fackler K., Schwanninger M. Polysaccharide degradation and lignin modification during Brown rot of spruce wood: a polarised fourier transform near infrared study. J. Near Infrared Spectrosc. 2010;18(6):403–416. [Google Scholar]
- Faix O., Fortman I., Bremer J., Meier D. Thermal degradation products of wood. Gas chromatographic separation and mass spectrometric characterization of polysaccharide derived products. Holz als Roh-und Werkstoff. 1991;49:213–219. [Google Scholar]
- Ferreira J.P.A., Miranda I., Gominho J., Pereira H. Chemical characterization of cork and phloem from Douglas fir outer bark. Holzforschung. 2016;70(5):475–583. [Google Scholar]
- Fortes M.A., Rosa M.E., Pereira H. IST Press; Lisboa: 2004. A Cortiça. [Google Scholar]
- Fukushima K., Terashima N. Heterogeneity information of lignin. XIV. Formation and structure of lignin in differentiating xylem of Ginkgo biloba. Holzforschung. 1991;45:87–94. [Google Scholar]
- Graça J., Pereira H. Cork suberin: a glyceryl based polyester. Holsforschung. 1997;51:225–234. [Google Scholar]
- Graça J., Pereira H. The periderm development in Quercus suber. IAWA J. 2004;25:325–335. [Google Scholar]
- Higuchi T. Lignin biochemistry: biosynthesis and biodegradation. Wood Sci. Technol. 1990;24:23–63. [Google Scholar]
- Knapic S., Machado J.S., Pereira H. Properties of cork oak wood related to solid wood flooring performance. Constr. Build. Mater. 2012;30:569–573. [Google Scholar]
- Leal S., Sousa V.B., Knapic S., Gominho J., Callot H., Machado J.S., Louzada J.L., Pereira H. Cork oak wood properties. In: Vázquez-Piqué J., Pereira H., González-Pérez A., editors. Suberwood – New Challenges for the Integration of Cork Oak Forests and Products. University of Huelva; Spain: 2008. pp. 393–402. [Google Scholar]
- Leal S., Nunes E., Pereira H. Cork oak (Quercus suber L.) wood growth and vessel characteristics variations in relation to climate and cork harvesting. Eur. J. For. Res. 2008;127:33–41. [Google Scholar]
- Leite C., Pereira H. Cork-containing barks – a review. Frontiers in Materials. 2017;3:63. [Google Scholar]
- Liang L., Wei L., Fang G., Xu F., Deng Y., Shen K., Tian Q., Wu T., Zhu B. Prediction of holocellulose and lignin content of pulp wood feedstock using near infrared spectroscopy and variable selection. Spectrochim. Acta: Mol Biomol Spectrosc. 2020;225:117515. doi: 10.1016/j.saa.2019.117515. [DOI] [PubMed] [Google Scholar]
- Lourenço A., Gominho J., Curt M.D., Revilla E., Villar J.C., Pereira H. Steam explosion as a pretreatment of Cynara cardunculus prior to delignification. Ind. Eng. Chem. Res. 2017;56:424–433. [Google Scholar]
- Lourenço A., Gominho J., Pereira H. Chemical characterization of lignocellulosic materials by analytical pyrolysis. In: Kusch Peter., editor. Chapter of Book “Analytical Pyrolysis”. Publisher; 2019. 88 pages. [Google Scholar]
- Lourenço A., Rencoret J., Chematova C., Gominho J., Gutiérrez A., del Río J.C., Pereira H. Lignin composition and structure differs between xylem, phloem and phellem in Quercus suber L. Front. Plant Sci. 2016;7:1612. doi: 10.3389/fpls.2016.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A.V., Pereira H., Meier D., Faix O. Quantitative analysis of cork (Quercus suber L.) and milled cork lignin by FTIR spectroscopy, analytical pyrolysis and total hydrolysis. Holzforschung. 1994;48(Suppl):43–50. [Google Scholar]
- Marques A.V., Pereira H. Aliphatic bio-oils from corks: a Py-GC/MS study. J. Anal. Appl. Pyrolysis. 2014;109:29–40. [Google Scholar]
- Meier D., Faix O. Pyrolysis-gas-chromatography-mass spectroscopy. In: Lin S.Y., Dence C.W., editors. Methods in Lignin Chemistry. 1992. (Springer Series in Wood Science). New York. [Google Scholar]
- Neutelings G. Lignin variability in plant cell walls: contribution of new models. Plant Sci. 2011;181:379–386. doi: 10.1016/j.plantsci.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Oliveira V., Rosa M.E., Pereira H. Variability of the compression properties of cork. Wood Sci. Technol. 2014;48(5):937–948. [Google Scholar]
- Oliveira V., Van den Bulcke J., Van Acker J., de Schryver T., Pereira H. Cork structural discontinuities studied with X-ray microtomography. Holzforschung. 2016;70(1):87–94. [Google Scholar]
- Pereira H., Graça J., Baptista C. The effect of growth rate on the structure and compressive properties of cork. IAWA J. 1992;13:389–396. [Google Scholar]
- Pereira H., Rosa M.E., Fortes M.A. The cellular structure of cork from Quercus suber L. IAWA (Int. Assoc. Wood Anat.) Bull. 1987;8(3):213–218. [Google Scholar]
- Pereira H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1988;22:211–218. [Google Scholar]
- Pereira H. Elsevier Publications; Amsterdam: 2007. Cork: Biology, Production and Uses; p. 336. [Google Scholar]
- Pereira H. Variability of the chemical composition of cork. Bioresources. 2013;8(2):2246–2256. [Google Scholar]
- Pereira H. The rationale behind cork properties: a review of structure and chemistry. Bioresources. 2015;10(3):6207–6229. [Google Scholar]
- Pinto-Correia T., Ribeiro N., Sá-Sousa P. Introducing the montado, the cork and holm oak agroforestry system of Southern Portugal. Agrofor. Syst. 2011;82:99–104. [Google Scholar]
- Prades C., Gómez-Sánchez I., García-Olmo J., González-Adrados J.R. Discriminant analysis of geographical origin of cork planks and stoppers by near infrared spectroscopy. J. Wood Chem. Technol. 2012;32(1):66–85. [Google Scholar]
- Prades C., Gómez-Sánchez I., García-Olmo J., González-Adrados J.R. Application of VIS/NIR spectroscopy for estimating chemical, physical and mechanical properties of cork stoppers. Wood Sci Tecchnol. 2014;48(4):811–830. [Google Scholar]
- Rencoret J., Gutiérrez A., Nieto L., Jiménez-Barbero J., Faulds C., Kim H., Ralph J., Martínez A.T., del Río J.C. Lignin composition and structure in young versus adult Eucalyptus globulus plants. Plant Physiol. 2011;155:667–682. doi: 10.1104/pp.110.167254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnan Å., van den Berg F.W., Engelsen S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. (Reference Ed.) 2009;28(10):1201–1222. [Google Scholar]
- Ruel K., Burlat V., Joseleau J.P. Relationship between ultrastructural topochemistry of lignin and wood properties. IAWA J. 1999;20(2):203–211. [Google Scholar]
- Sampaio T., Branco M., Guichoux E., Petit R.J., Pereira J.S., Varela M.C., Almeida M.H. Does the geography of cork oak origin influence budburst and leaf pest damage? For. Ecol. Manag. 2017;373:33–43. [Google Scholar]
- Sandak A., Ferrari S., Sandak J., Allegretti O., Terziev N., Riggio M. Monitoring of wood decay by near infrared spectrocopy. Adv. Mater. Res. 2013;778:802–809. [Google Scholar]
- Schmitt U., Singh A., Frankenstein C., Moller R. Cell wall modifications in woody stems induced by mechanical stress. New Zeland J For Sci. 2006;36(1):72–86. [Google Scholar]
- Schwanninger M., Rodrigues J.C., Fackler K. A review of band assignments in near infrared spectra of wood and wood components. J. Near Infrared Spectrosc. 2011;19:287–308. [Google Scholar]
- Sen A., Bulcke JV den, Defoirdt N., Acker J.V., Pereira H. Thermal behavior of cork and cork components. Thermochim. Acta. 2014;582:94–100. [Google Scholar]
- Sen A., Miranda I., Pereira H. Temperature-induced structural and chemical changes in cork from Quercus cerris. Ind. Crops Prod. 2012;37:508–513. [Google Scholar]
- Sen A., Miranda I., Santos S., Graça J., Pereira H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crops Prod. 2010;31:417–422. [Google Scholar]
- Sen A., Zhianski M., Glushkova M., Petkova K., Ferreira J., Pereira H. Chemical composition and cellular structure of corks from Quercus suber trees planted in Bulgaria and Turkey. Wood Sci. Technol. 2016;50:1261–1276. [Google Scholar]
- Sousa V.B., Leal S., Quilhó T., Pereira H. Characterization of cork oak (Quercus suber) wood anatomy. IAWA J. 2009;30:149–161. [Google Scholar]
- Terashima N., Fukushima K., Takabe K. Heterogeneity in formation of lignin. VIII: an autoradiographic study on the formation of guaiacyl and syringyl lignin in Magnolia kobus DC. Holzforschung. 1986;40:101–105. [Google Scholar]
- Terashima N., Fukushima K. Biogenesis and structure of macromolecular lignin in the cell wall of tree xylem as studied by microautoradiography. Plant Cell Wall Polymers. ACS (Am. Chem. Soc.) Symp. Ser. 1989;399 Chapter 11: 160–168. [Google Scholar]
- Toscano G., Rinnan A., Pizzi A., Mancini M. The use of near-infrared (NIR) spectroscopy and principal component analysis (PCA) to discriminate bark and wood of the most common species of the pellet sector. Energy & Fuels. 2017;31:2814–2821. [Google Scholar]
- Workman J., Weyer L. CRC Press, Taylor & Francis Group; NW: 2008. Practical Guide to Interpretive Near-Infrared Spectrocopy; p. 317. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.