Abstract
Citrus is one of the largest output fruits in the word. In China, the major orange variety is the Citrus reticulate Blanco (Ponkan). The peels are discarded as waste material, its comprehensive utilization is urgently needed. In this work, hydrodistillation method was developed to extract citrus essential oil (EO) from Blanco peel. With the optimal extraction conditions, the EO yield was more than 3%. By GC-MS analysis, 53 compounds were identified from the citrus EO. Terpenes compounds accounted for 71.2%, especially d-limonene (major composition) accounted for 58.9%. The obtained citrus EO showed remarkable antibacterial activity against Cutibacterium acnes (C. acnes, Formerly P. acnes) and common microorganisms such as S. aureus, B. subtilis, and E. coli. Even compared with the common antibiotics (such as erythromycin, clindamycin, and tetracycline) for acne therapy, its antibacterial activity against C. acnes is more excellent. This work provides a potential therapy material for the treatment of acne.
Keywords: Biochemical engineering, Antimicrobial agent, Natural product chemistry, Food technology, Antimicrobial, Essential oil, Citrus reticulate Blanco, Cutibacterium acnes, Acne, Antibacterial activity, Citrus peel
Biochemical engineering; Antimicrobial agent; Natural product chemistry; Food technology; Antimicrobial; Essential oil; Citrus reticulate blanco; Cutibacterium acnes; Acne; Antibacterial activity; Citrus peel.
1. Introduction
Citrus is one of the four largest harvested fruits in the world, and its yield and consumption rank first. With increasing demands, citrus production record is still creating. According to FAO report, global citrus output increased from 115.18 million tons to 178.48 million tons from 2010 to 2015. Citrus peel accounts for 25%–40% of the total fruit weight, and its annual output is as high as 10 million tons in China. As such, it's an important biological resource to be comprehensively utilized (Negro et al., 2016). Compendium of Materia Medica (a Chinese Medical Classics by Li Shizhen) records that orange peel (i.e. dried orange peel) is an important traditional Chinese medicine and can relieve vomiting, diarrhea, phlegm and cough. Citrus essential oil (EO) is an important biologically active substance from citrus peel. It is intensively collected in oil glands of the citrus peel (Boussaada et al., 2007). On the average, it accounts for about 1–3% fresh weight of citrus peel (Njoroge et al., 2005). The citrus EO is composed of tens to hundreds of various compounds, which depend on the citrus variety and growth environment (Sharma et al., 2017). Also, its ingredient varies markedly according to ripeness of the fruit and extraction method (Dosoky and Setzer, 2018; González-Mas et al., 2019; Guo et al., 2018). Citrus EO is widely used in food, chemical industry, medical treatment, and other fields because of its pleasant aroma, antioxidant properties, and antimicrobial activity. Especially its natural product characteristics is attractive. The previous research indicated that citrus EO had broad-spectrum antibacterial activity to bacteria and yeasts, and its antimicrobial activity mainly depends on the components of EO (Dosoky and Setzer, 2018; Guo et al., 2018; Reyes-Jurado et al., 2019).
Acne is a chronic inflammatory dermatosis involving hair follicles and sebaceous glands which often occurs in the face, chest, and back. The incidence of acne is as high as 85%. Although it is not a life-threatening disease, it has a great psychological impact on patient's life (Dreno et al., 2019). Cutibacterium acnes (C. acnes, formerly Propionibacterium acnes) is the dominant pathogen bacterial to acne, which is an anaerobic Gram-positive Corynebacterium (Scharschmidt, 2019). Consequently, C. acnes has been recognized as one of the main targets for acne treatment (Webster, 1996). The bacteriostasis or killing of C. acnes are one of the key routes to prevent and treat acne. Currently, erythromycin and clindamycin are the main antibiotics for treating C. acne (Shaw and Kennedy, 2007; Ruga et al., 2018). The traditional antibiotic therapy to acne have various side effects. For instance, it can lead to dryness, redness, irritation of the skin and hyperpigmentation, and in an extreme instance, malpractice can lead to antibiotic resistance of the bacteria (Coates et al., 2002; Dessinioti and Katsambas, 2017). Traditional medical plants and their extracts provide good choices for the treatment of C. acne and show excellent results in bacteriostasis research (Hamdy et al., 2017; Jeong and Kim, 2017; Poomanee et al., 2018). EOs from other plant extracts have good antimicrobial properties and they are valid against C. acne (Millerm A et al., 2015; Murbach Teles Andrade et al., 2018). Citrus EO contains a lot of bioactive substances (Dosoky and Setzer, 2018). Previous studies showed that it had obvious antimicrobial activities such as against Escherichia coli, Salmonella enteritidis, Staphylococcus epidermidis, Listeria monocytogenes, Acinetobacter baumannii, Mycobacterium smegmatis (Mitropoulou et al., 2017; Reyes-Jurado et al., 2016). However, the antimicrobial activity of citrus EO against C. acne has not been reported. If citrus EO possesses the antibacterial activity against C. acne, it can therefore be as a potential natural product for acne therapy to replace antibiotics. Based on its product characteristics, it must be very attractive and well received by acne patients.
Due to the potential application value of citrus EO in acne therapy and the effect of citrus variety on the EO component, a comprehensive study was conducted here for the first time to extract EO from Citrus reticulate Blanco (Ponkan) peel, its chemical component was analyzed and the antimicrobial activity against C. acne was rightly evaluated. Ponkan is the most widely planted citrus variety in China with the largest output. The comprehensive utilization of its peel is essential to the Ponkan industry.
2. Materials and methods
2.1. Reagents and raw materials
Fresh Citrus reticulate Blanco (Ponkan), originated in Luxi county of Hunan Province, China, was purchased from the local market. The fruit was harvested at the end of October in it natural maturation stage. The peel was peeled from fresh fruits and rinsed with clean water. The peel was softly dried at 45 °C in a drying oven by a flowing air for 24 h. The moisture content was about 40% by weight on a dry basis. It was crushed in a plants grinder, stored and sealed at a temperature of 4 °C. Wilkins-Chalgren Anaerobe Broth, Anaerobe Basal Agar, Oxoid™ Tryptone, Soya peptone, and Oxoid™ yeast extract were purchased from Thermo Scientific™ (Shanghai, China). The beef extract was purchased from Beijing Shuangxuan Microbe Medium Products Plant (Beijing, China). Cholesterol, Petroleum ether, sodium chloride, and other general reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). d-limonene, clindamycin, erythromycin, and tetracycline were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Staphylococcus aureus ATCC25923, Bacillus subtilis CMCC63501, Escherichia coli ATCC25922 were obtained from China General Microbiological Culture Collection Center (CGMCC) and preserve at the Industrial Biotechnology Laboratory in Wuhan University of Science & Technology (WUST). C. acnes was isolated from the acne pus of a patient and preserve at the Industrial Biotechnology Laboratory in WUST.
Microorganism culture medium: Tryptone soy agar (TSA) was used for S. aureus culture, composed of tryptone 15.0 g, soya peptone 5.0 g, sodium chloride 5.0 g, agar 13.0 g, distilled water 1.0 L, pH 7.3. Nutrient broth agar was used for B. subtilis culture, composed of peptone 5.0 g, beef extract 3.0 g, NaCl 5.0 g, agar 15.0g, distilled water 1.0 L, pH 7.0. Luria-Bertani agar was used for E. coli culture, composed of Tryptone 10.0 g, Yeast extract 5.0 g, NaCl 10.0 g, distilled water 1.0 L, and pH7.4. Wilkins-Chalgren Anaerobe Broth and Anaerobe Basal Agar was used for C. acnes culture, also composed of Wilkins-Chalgren Anaerobe Broth 33.0g with distilled water 1.0 L or Anaerobe Basal Agar 45.8 g with distilled water 1.0 L.
2.2. Extraction of EO from citrus peel
Citrus EO was extracted from citrus peel by hydrodistillation. Hydrodistillation is a variant of traditional steam distillation method for the extraction of EO from plant in the laboratory. A micro Dean-Stark apparatus was used for hydrodistillation. 50 g of crushed citrus peel was placed into the flask along with 250 mL distilled water (in a ratio of 1g solid: 5ml water), the samples materials was immersed directly into the distilled water. The solid-liquid mixture was heated until it boiled under atmospheric pressure. The volatile aroma compounds and water formed an azeotropic (azeotropes) mixture, condensed and divided due to their density difference and immiscibility. After subjecting the citrus material to hydrodistillation for 2 h, the essential oil was collected and isolated. Subsequently, the essential oil was dried with Na2SO4, collected into sealed vials and stored in a refrigerator (4 °C) for further use. Thus, gas chromatography coupled with mass spectrometry (GC-MS) analysis and antibacterial activity evaluation.
To obtain the optimal extraction conditions, the effect of hydrodistillation time, citrus peel grinding degree, and additional salts on citrus EO yield was investigated. To study the reciprocal influence of independent variables (hydrodistillation time, citrus peel grinding degree and additional reagent ie. an amount of NaCl) on the citrus EO yield, a 3 factors 3 levels orthogonal experiment design L9(3ˆ3) optimization experiments were performed, shown in Table 1. Its data were analyzed by IBM SPSS Statistics 24.0.
Table 1.
Factors and levels of orthogonal experiment design.
| Leve | Factors |
||
|---|---|---|---|
| A (Grinding degree/Mesh) | B (Hydrodistillation time/h) | C (NaCl/%) | |
| 1 | 10 | 0.5 | 1 |
| 2 | 20 | 1 | 2 |
| 3 | 30 | 2 | 3 |
The yield of essential oil was expressed as gram per 100 g of the fruit on a wet weight basis.
2.3. Analysis of citrus EO chemical components by GC-MS
The components of the citrus EO were identified by GC–MS analyses (González-Mas et al., 2019). The samples were diluted in 1000 fold with dichloromethane. The Gas chromatography-mass spectrometry (Agilent GC-MSD GC6890-MS5973) was incorporated with a capillary column (DB-Wax, 30 m × 0.25 mm id, film thickness 0.25 μm). The inlet temperature was 250 °C. Helium was used as the carrier gas with 1 mL/min. The split ratio was 10:1. The oven temperature was programmed: initial temperature was kept at 40 °C for 8 min, and increased from 40 °C to 140 °C with 3 °C/min, further increased to 250 °C with 10 °C/min, and held at 250 °C for 10 min. FID and MSD transfer line temperatures were set at 300 and 250 °C, respectively. EI mass spectra (70 eV) were acquired over the m/z range of 35–550. Most of the compounds were identified according to Kovats Indexes in reference to n-alkanes and mass spectra (the NIST/NBS, Wiley libraries collection). For the determination of compound contents, the relative area percentages obtained by FID were used.
2.4. Antimicrobial activity evaluation
Generally, there are two routes to assess EO antimicrobial activity. One is disk diffusion assay, and the other is minimum inhibitory concentration (MIC) (Millerm A et al., 2015; Mitropoulou et al., 2015; Mitropoulou et al., 2017). The two evaluation methods were conducted in this work.
Disk diffusion assay was performed initially to evaluate the antimicrobial activity of citrus EO against C. acnes. Comparing with common microorganism such as S. aureus, B. subtilis, E. coli were also applied. Sterile water and d-limonene were used as the negative and positivecontrols respectively. The bacterial suspensions were 10–103 fold when diluted in Ringer's solution. 100 μL of appropriate diluted bacterial suspensions was spread on the corresponding agar 100 mm diameter Petri dishes, in order to provide initial inoculums of 105 or 107 CFU/mL. Subsequently, 6 mm diameter sterile dry paper disks were placed onto the inoculated agar surface containing 5 μL of citrus EO or of its positive and negative control (d-limonene and water). The S. aureus, B. subtilis, E. coli, Petri dishes were incubated at 37 °C for 24 h. And to C. acnes, Petri dishes were incubated at 37 °C for 48 h under anaerobic condition. After incubation, the inhibition zones were measured in mm. All experiments were carried out at least in triplicates and the mean values were are presented.
To further evaluate the antimicrobial efficiency of citrus EO against C. acnes, we compared its antimicrobial activity with the traditional antibiotics (such as clindamycin 0.1 mg/mL, erythromycin 0.1 mg/mL, and tetracycline 50 μg/mL) against C. acnes using disk diffusion assay. The experimental conditions were the same as former method. The inhibition zones were measured as the antimicrobial activity index.
The minimal inhibition concentration (MIC) values were obtained using the serial dilution bioassay. Bacterial strain inoculums were cultured for 24 h with Wilkins-Chalgren Anaerobe broth. Citrus EO was in series of two-fold and diluted with medium broth resulting in final oil concentrations of 20.00, 10.00, 5.00, 2.50, and 1.25 μL/mL. The 96-well microplate was prepared with 95 μL of medium broth and 5 μL of the C. acnes seed broth into each well. Then, 100 μL of serially diluted citrus EO dilutions were added into the 10 consecutive rows wells with duplicate rows for one dilution. The last two rows wells were used as the negative control, containing 195 μL of medium broth and 5 μL of seed broth without citrus EO. The final volume was 200 μL in each well. After incubating for 48 h at a temperature of 37 °C and under anaerobic condition, bacterial growth was recorded by a microplate reader (Multiskan FC, Thermo Fisher Scientific, Shanghai, China). The MIC was defined as the lowest concentration of citrus EO to inhibit the growth of the microorganisms. The experimental groups were replicated three times.
2.5. Statistical analysis
All experiments were performed in triplicate. The data represents the mean of triplicate values. The corresponding standard deviation was calculated. IBM SPSS Statistics 24.0 was applied for the data analysis.
3. Results and discussion
3.1. Extraction of EO from citrus peel
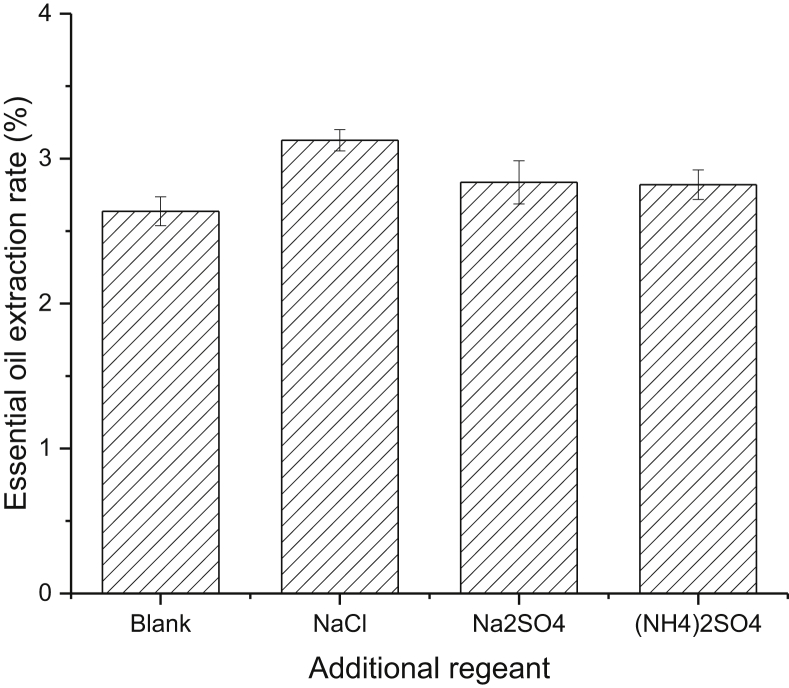
Citrus EO was extracted from ponkan peel by hydrodistillation. From the pre-experiment, there was an indications that the hydrodistillation time, citrus peel grinding degree and additives were important factors which has the tendency to affect the citrus EO yield. Hence, the effect of citrus peel grinding degree, hydrodistillation time and additives on citrus EO yield were investigated. The results were shown in Figure 1 to Figure 3. From the results, citrus peel grinding degree, hydrodistillation time and additional NaCl obviously affected the citrus EO yield. Extending the extraction time to 2 h, the citrus EO yield would reach to its highest about 3.1%. The grinding degree was another key factor to citrus EO yield, the highest citrus EO yield could be obtained as the citrus peel scrap through 20–30 mesh (i.e. with about 0.83 to 0.55 mm size as shown in Figure 2). Smaller particle size favored EO extraction since it had a better mass transfer. However, instance where the particle size was too small, the particles were easily aggregated, thereby reducing the EO extraction. Addition of some salt will promote the EO extraction yield (Weng et al., 2019). We investigated the commonly used salt, NaCl, Na2SO4 and (NH4)2SO4, which promote citrus EO extraction. The results were shown in Figure 3. It confirmed that common salts could promote citrus EO extraction to some degree. Especially, adding NaCl has a sigificant promoting efficiency of EO extraction, the yield was enhanced about 20% compared with the control. Addition of salt promote the EO extraction due to the fact that it help the oil sacs of plant cells to be released from the citrus peel.
Figure 1.
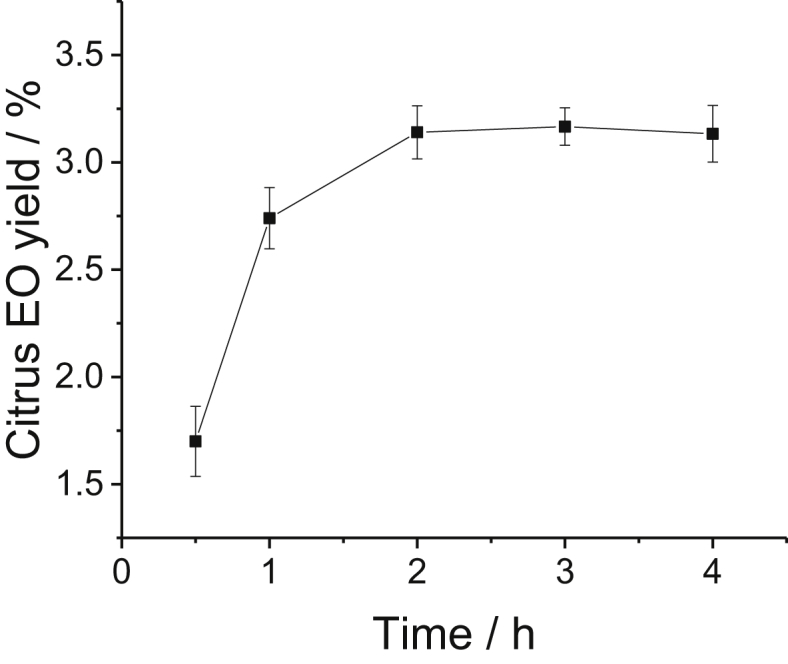
Effect of hydrodistillation time on citrus EO extraction yield.
Figure 3.
Effect of additional salts of on citrus EO extraction yield.
Figure 2.

Effect of grinding degree on citrus EO extraction yield.
To study the reciprocal influence of independent variables (hydrodistillation time, citrus peel grinding degree and additional reagent (the amount of NaCl) on citrus EO extraction efficiency, an orthogonal experiment design optimization experiments were performed as shown in Table 1. The experiment result was shown in Table 2, and the variance analysis was given in Table 3. From the variance analysis results, the significance of the three factors to citrus EO extraction is A (Grinding degree)>B (Hydrodistillation time)>C (NaCl). According to Table 2, the optimal condition was A3B3C2. With this condition, i.e. 30 Mesh citrus peel and 2 h hydrodistillation time with adding 2% NaCl, citrus EO yield reached 3.26%. These optimal conditions were further verified by an additional experiment and the yield of citrus EO was 3.23%.
Table 2.
Orthogonal experiment result.
| Entry | Factors |
|||
|---|---|---|---|---|
| A | B | C | Citrus EO yield/% | |
| 1 | 1 | 1 | 1 | 1.36 |
| 2 | 1 | 2 | 2 | 1.49 |
| 3 | 1 | 3 | 3 | 1.78 |
| 4 | 2 | 1 | 2 | 2.08 |
| 5 | 2 | 2 | 3 | 2.21 |
| 6 | 2 | 3 | 1 | 2.32 |
| 7 | 3 | 1 | 3 | 2.78 |
| 8 | 3 | 2 | 1 | 2.86 |
| 9 | 3 | 3 | 2 | 3.26 |
| K1 | 2.46 | 2.17 | 2.38 | |
| K2 | 2.36 | 2.54 | 2.12 | |
| K3 | 2.57 | 2.67 | 2.12 | |
| R | 1.42 | 0.38 | 0.097 | |
Table 3.
Orthogonal experiment variance analysis.
| Factor | Sum of square | Df | mean-square | F value | P value | Sig. |
|---|---|---|---|---|---|---|
| A | 3.04 | 2 | 1.52 | 216.37 | 0.003 | ** |
| B | 0.23 | 2 | 0.11 | 23.73 | 0.04 | * |
| C | 0.016 | 2 | 0.008 | 1.62 | 0.381 | |
| Error | 0.010 | 2 | 0.005 |
* is represented as significant.
** is represented as the most significant.
According to these results, the commonly used hydrodistillation is an efficient technology for citrus EO extraction. The highest EO yield reached about 3%, which meet the 1–3% fresh weight of citrus peel on average (Lemes et al., 2018), also it is similar to the CP-EO from Citrus aurantifolia peel reported by Lemes et al. (2018). But the citrus EO yield was significantly higher than what Zhang et al. reported, which is only about 0.5% (Zhang et al., 2019). In that case, the citrus variety used (Citrus aurantifolia) maybe the reason, as well as the different extraction techniques employed influenced the EO yield. Compared to the previous reported yield, citrus EO yield in this was very outstanding. This indicates that Citrus reticulate Blanco (Ponkan) peels is an attractive raw material for EO with adequate development value.
3.2. Chemical composition of citrus EO
The chemical composition of citrus EO was analyzed by GC-MS, results shown in Table 4. In total, 53 compounds were identified from the citrus EO, representing 96.87%. Among them the main class of compounds in citrus EO were terpenes, representing 71.2%. The maximum constituent was d-limonene, representing 58.9%. The outcome of chemical composition of EO from Blanco (Ponkan) peels was consistent with previous report that limonene was the main constituent of citrus EO (Dosoky and Setzer, 2018; González-Mas et al., 2019; Guo et al., 2018; Lemes et al., 2018; Zhang et al., 2019). Other compounds, such as lauric acid (4.89%), 1-methyl-1,4-cyclohexadiene (3.46%), methyl linoleate (3.12%), myristic acid (3.0%), (E,E,E)-2,6,10-trimethyl-2,6,9,11-dodecanetetraen-1-al (2.43%), palmitic acid (2.32%), β-myrcene (1.51%), were secondary constituent, each representing more than 1.5%.
Table 4.
Chemical constitutes and relatively content of citrus EO.
| No. | Compound | Relatively content/% | No. | Compound | Relatively content/% |
|---|---|---|---|---|---|
| 1 | α-Phellandrene | 0.05 | 28 | Caryophyllene | 0.10 |
| 2 | α-Pinene | 0.51 | 29 | γ-Elemene | 0.91 |
| 3 | β-Pinene | 0.2 | 30 | (E)-Geranylacetone | 0.3 |
| 4 | β-Myrcene | 1.51 | 31 | (6E)-7,11-Dimethyl-3-methylene-1,6,10-dodecatriene | 0.43 |
| 5 | p-Cymene | 0.69 | 32 | (−)−α-Cubebene | 0.53 |
| 6 | d-Limonene | 58.9 | 33 | β-Selinene | 0.12 |
| 7 | β-Ocimene | 0.11 | 34 | δ-Selinene | 0.06 |
| 8 | 1-Methyl-1,4-Cyclohexadiene | 3.46 | 35 | α-Caryophyllene | 0.06 |
| 9 | Terpinolene | 0.22 | 36 | α-Muurolene | 0.07 |
| 10 | 1,3-Cyclohexadiene | 0.41 | 37 | 2,6-Di-tert-butyl-4-methylphenol | 0.16 |
| 11 | 2-Cyclopenten-1-one | 0.05 | 38 | Cadinene | 0.71 |
| 12 | p-Mentha-1,3,8-Triene | 0.45 | 39 | Germacrene D | 0.45 |
| 13 | 1-(1,4-dimethyl-3-cyclohexen-1-yl)-Ethanone | 0.10 | 40 | anti- (+) - Nerolidol | 1.1 |
| 14 | Terpene ketones | 0.22 | 41 | Lauric acid | 4.89 |
| 15 | α-Terpineol | 0.07 | 42 | Spathulenol | 1.23 |
| 16 | Decaldehyde | 0.41 | 43 | δ- Cadinol | 0.34 |
| 17 | Thymol methyl ether | 0.12 | 44 | β- Orange aldehyde | 3.1 |
| 18 | Terpineol | 0.04 | 45 | (E,E,E)-2,6,10-trimethyl-2,6,9,11-dodecanetetraen-1-al | 2.43 |
| 19 | cis-1-Methyl-4-isopropyl-2-cyclohexen-1-ol | 0.13 | 46 | Myristic acid | 3.0 |
| 20 | Perillaldehyde | 0.07 | 47 | Diisobutyl phthalate | 0.42 |
| 21 | Carvacrol | 0.07 | 48 | Methyl palmitate | 1.14 |
| 22 | Undecanal | 0.07 | 49 | Palmitic acid | 2.32 |
| 23 | Citronellone acetate | 0.54 | 50 | Ethyl palmitate | 0.01 |
| 24 | Nerol acetate | 0.14 | 51 | Methyl linoleate | 3.12 |
| 25 | Decanoic acid | 0.17 | 52 | Trihexadecane | 0.73 |
| 26 | Geranyl butyrate | 0.09 | 53 | Eicosane | 0.11 |
| 27 | β-Elemene | 0.23 | Total | 96.87 |
3.3. Antimicrobial activity of the citrus EO against C. acnes
The antimicrobial activity of the citrus EO against C. acnes was firstly assayed by disk diffusion assay. Comparation of its antimicrobial activity against E. coli, S. aureus and B. subtilis were also conducted. The major constituent, d-limonene, was simultaneously assessed. The inhibition zones results were given in Table 5. These results evidenced that C. acnes was sensitive to citrus EO and its major constituent, d-limonene. As expected, the other chosen microbes were also sensitive to citrus EO and d-limonene. This is consistent with the reported results by Mitropoulou et al. (2017) and Guo et al. (2018). Of note, the large inhibition zones (21 mm) were observed in E. coli. While the inhibition zones for C. acnes (13.5 mm) is larger than that of B. subtilis (9.3 mm). To our knowledge, this is the first report on antimicrobial activity of citrus EO against C. acnes, also it is the first report that anaerobic bacterium is sensitive to citrus EO.
Table 5.
Antibacterial activity of the citrus EO to bacterial taxa by the disk diffusion assay as diameter of inhibition zones.
| Sample | Diameter of inhibition zones of various bacterial/mm |
|||
|---|---|---|---|---|
| E. coli | S. aureus | B. subtilis | C. acnes | |
| blank | NDa | ND | ND | ND |
| Citrus EO | 21.0 ± 1.52 | 15.5 ± 1.33 | 9.3 ± 0.67 | 13.5 ± 1.25 |
| d-Limonene | 16.0 ± 1.14 | 9.8 ± 0.81 | 5.6 ± 0.57 | 7.7 ± 0.63 |
ND indicates no inhibition zone.
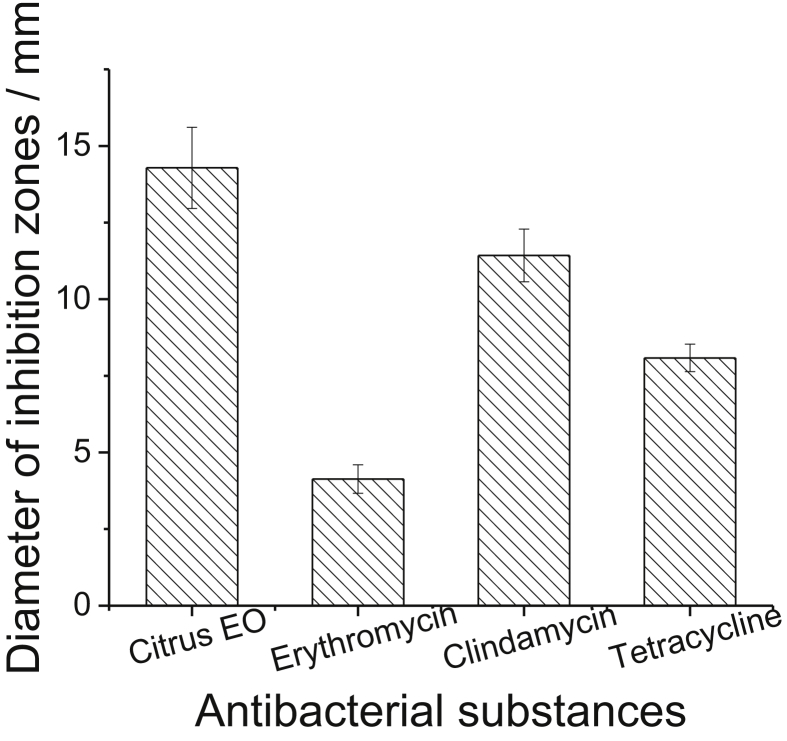
To assess the feasibility of citrus EO as a potential therapy against C. acnes as an alternative for traditional antibiotics, we further evaluated the antimicrobial activity of the citrus EO and common acne antibiotics (i.e. erythromycin, clindamycin, and tetracycline) against C. acnes by disk diffusion assay. Results were given in Figure 4. It can be seen that the inhibition zones diameter of C. acnes was largest for citrus EO, and it followed by clindamycin, tetracycline and erythromycin. Compared with the traditional antibiotics by disk diffusion, the antimicrobial activity of citrus EO against C. acnes was about 1.2-fold to clindamycin, 1.8-fold to tetracycline, and almost 3.5-fold to erythromycin. Mitropoulou reported that citrus medica essential oil perform almost similar antibacterial activity against E. coli and S. aureus with gentamycin (Mitropoulou et al., 2017). Simultaneously, the previous reported work shows that citrus EO is enough and safe to human (Dosoky and Setzer, 2018). This indicates that citrus EO could be applied as an antibacterial active substance against C. acnes pathogenic bacterium to replace traditional antibiotics.
Figure 4.
Comparison of the antibacterial activity against C. acnes between citrus EO and common acne antibiotics.
The minimum inhibitory concentration (MIC) usually used to detect the antimicrobial efficacy of antimicrobial agents is a basic indicator of drug detection. This experiment was carried out to determine the antimicrobial activity of citrus EO against C. acnes, it provided basic application data for citrus EO application as an antimicrobial agents for C. acnes. The MIC of citrus EO to C. acnes and common microbe E. coli, S. aureus and B. subtilis was detected. Also, the d-limonene was used as a positive control. The results were given in Table 6. The MIC of citrus EO to C. acnes was 2.5 μL/mL, and it was 1.25 μL/mL to E. coli and 2.5 μL/mL to S. aureus. Guo et al. reported that the MIC of Cold Pressed Gannan orange EO was 1.56 μL/mL to E. coli and 3.13 μL/mL to S. aureus (Guo et al., 2018). Their results were almost consistent with our results. The subtle difference maybe due to varied citrus, Gannan orange in their case and ponkan in our case. The minimum concentration was about 2.5 μL/mL when citrus EO was used to treat acne. Acording to the disk diffusion assay and MIC results, E. coli, S. aureus and B. subtilis were also sensitive to citrus EO. This shows that citrus EO can be a potential antibacterial agent against these bacterials.
Table 6.
MIC of citrus EO and limonene to various bacterium.
| Sample | MIC/μL/mL |
|||
|---|---|---|---|---|
| E. coli | S. aureus | B. subtilis | C. acnes | |
| Citrus EO | 1.25 | 2.50 | 10.00 | 2.50 |
| d-Limonene | 2.5 | 5.00 | 20.00 | 5.00 |
4. Conclusion
In order to make full use of wastes from citrus processing industry, a thorough study was carried out on Citrus reticulate Blanco (Ponkan) peels. This citrus is one of the most maximum output orange variety around China. It is significant to note that peels of this citrus variety provides outstanding EO yield, more than 3%. GC-MS analysis of C. reticulate essential oil led to identification of 53 chemical components representing 96.87% (Table 4). Terpenes form the main class compounds, representing 71.2%, and d-limonene representing 58.9%. It is appealing that the obtained citrus peel EO showed remarkable antibacterial activity against C. acnes, which provides a potential therapy for the treatment of acne. However, further research is needed to investigate their biological activities mechanism and mode of action with C. acnes in order to use this EO at the commercial level.
Declarations
Author contribution statement
He-Shuai Hou, Emmanuel Mintah Bonku: Performed the experiments; Wrote the paper.
Rong Zhai: Performed the experiments.
Rong Zeng: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ya-Li Hou: Analyzed and interpreted the data.
Zhong-Hua Yang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Can Quan: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the National Natural Science Foundation of China (grant no. 21376184), Foundation from the Educational Commission of Hubei Province of China (grant no. D20121108), the National Key Research and Development Project (2017YFF0205803, Ministry of Science and Technology of China), and the National Institute of Metrology of China (21-AKY1615).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Zhong-Hua Yang, Email: yangzh@wust.edu.cn.
Can Quan, Email: quancan@nim.ac.cn.
References
- Boussaada O., Skoula M., Kokkalou E., Chemli R. Chemical variability of flowers, leaves, and peels oils of four sour orange provenances. J. Essent. Oil Bear. Plants. 2007;10:453–464. [Google Scholar]
- Coates P., Vyakrnam S., Eady E.A., Jones C.E., Cove J.H., Cunliffe W.J. Prevalence of antibioticresistant proprionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br. J. Dermatol. 2002;146:840–848. doi: 10.1046/j.1365-2133.2002.04690.x. [DOI] [PubMed] [Google Scholar]
- Dessinioti C., Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin. Dermatol. 2017;35:163–167. doi: 10.1016/j.clindermatol.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Dosoky N.S., Setzer W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19071966. pii: E1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreno B., Bordet C., Seite S., Taieb C. Acne relapses: impact on quality of life and productivity. J. Eur. Acad. Dermatol. Venereol. 2019;33:937–943. doi: 10.1111/jdv.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mas M.C., Rambla J.L., López-Gresa M.P., Blázquez M.A., Granell A. Volatile compounds in citrus essential oils: a comprehensive review. Front. Plant Sci. 2019;10:12. doi: 10.3389/fpls.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Liu K., Deng W., Zhong B., Yang W., Chun J. Chemical composition and antimicrobial activity of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall) peel essential oils. Food Sci. Nutr. 2018;6:1431–1437. doi: 10.1002/fsn3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy A.A., Kassem H.A., Awad G.E.A., El-Kady S.M., Benito M.T., Doyagüez E.G., Jimeno M.L., Lall N., Hussein A.A. In-vitro evaluation of certain Egyptian traditional medicinal plants against Propionibacterium acnes. South Afr. J. Bot. 2017;109:90–95. [Google Scholar]
- Jeong W.Y., Kim K. Anti-Propionibacterium acnes and the anti-inflammatory effect of Aloe ferox miller components. J. Herb. Med. 2017;9:53–59. [Google Scholar]
- Lemes R.S., Alves C.C.F., Estevam E.B.B., Santiago M.B., Martins C.H.G., Santos T.C.L.D., Crotti A.E.M., Miranda M.L.D. Chemical composition and antibacterial activity of essential oils from Citrus aurantifolia leaves and fruit peel against oral pathogenic bacteria. An. Acad. Bras. Cienc. 2018;90:1285–1292. doi: 10.1590/0001-3765201820170847. [DOI] [PubMed] [Google Scholar]
- Millerm A.B., Cates R.G., Lawrence M., Soria J.A., Espinoza L.V., Martinez J.V., Arbizú D.A. The antibacterial and antifungal activity of essential oils extracted from Guatemalan medicinal plants. Pharm. Biol. 2015;53:548–554. doi: 10.3109/13880209.2014.932391. [DOI] [PubMed] [Google Scholar]
- Mitropoulou G., Fitsiou E., Stavropoulou E., Papavassilopoulou E., Vamvakias M., Pappa A., Oreopoulou A., Kourkoutas Y. Composition, antimicrobial, antioxidant, and antiproliferative activity of Origanum dictamnus (dittany) essential oil. Microb. Ecol. Health Dis. 2015;26:26543. doi: 10.3402/mehd.v26.26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulou G., Fitsiou E., Spyridopoulou K., Tiptiri-Kourpeti A., Bardouki H., Vamvakias M., Panas P., Chlichlia K., Pappa A., Kourkoutas Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT – Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;84 344-35216. [Google Scholar]
- Murbach Teles Andrade B.F., Nunes Barbosa L., Bérgamo Alves F.C., Pereira Marques A.F., Albano M., Mores Rall V.L., Brüggemann H., Fernandes Júnior A. The impact of Cymbopogon martinii essential oil on Cutibacterium (formerly Propionibacterium) acnes strains and its interaction with keratinocytes. J. Pharm. Pharmacol. 2018;70:1688–1699. doi: 10.1111/jphp.13011. [DOI] [PubMed] [Google Scholar]
- Negro V., Mancini G., Ruggeri B., Fino D. Citrus waste as feedstock for bio-based products recovery: review on limonene case study and energy valorization. Bioresour. Technol. 2016;214:806–815. doi: 10.1016/j.biortech.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Njoroge S.M., Koaze H., Karanja P.N., Sawamura M. Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis) Flavour Fragrance J. 2005;20:80–85. [Google Scholar]
- Poomanee W., Chaiyana W., Mueller M., Viernstein H., Khunkitti W., Leelapornpisid P. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe. 2018;52:64–74. doi: 10.1016/j.anaerobe.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Reyes-Jurado F., Navarro-Cruz A.R., Ochoa-Velasco C.E., Palou E., López-Malo A., Ávila-Sosa R. Essential oils in vapor phase as alternative antimicrobials: a review. Crit. Rev. Food Sci. Nutr. 2019;18:1–10. doi: 10.1080/10408398.2019.1586641. [DOI] [PubMed] [Google Scholar]
- Reyes-Jurado F., López-Malo A., Palou E. Antimicrobial activity of individual and combined essential oils against foodborne pathogenic bacteria. J. Food Prot. 2016;79:309–315. doi: 10.4315/0362-028X.JFP-15-392. [DOI] [PubMed] [Google Scholar]
- Ruga R., Kingkaew K., Tamsampaoloet K., Chavasiri W. Enhancing antibacterial activity against Propionibacterium acnes and Staphylococcus aureus by combination of tetracycline with selected compounds. Chem. Lett. 2018;47:1538–1541. [Google Scholar]
- Scharschmidt T.C. Antibiotics for acne-a pilot study of collateral damage to the skin microbiome. JAMA Dermatol. 2019;155:419–421. doi: 10.1001/jamadermatol.2018.5146. [DOI] [PubMed] [Google Scholar]
- Sharma K., Mahato N., Cho M.H., Lee Y.R. Converting citrus wastes into value-added products: economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Shaw L., Kennedy C. The treatment of acne. J. Paediatr. Child Health. 2007;17:385–389. [Google Scholar]
- Webster G.F. Acne. In: Callen J.P., editor. Current Problems in Dermatology. first ed. Mosby A time mirror company; Philadelphia, Pennsylvania: 1996. pp. 238–268. [Google Scholar]
- Weng J., Wei M.M., Wu S.J., Liu Y.Q., Li S.R., Ye Y.Y., Wang M., Wang D. High-value utilization of Citrus peel: efficient extraction of essential oil and preparation of activated carbon. BioResources. 2019;14:3899–3913. [Google Scholar]
- Zhang H., Lou Z., Chen X., Cui Y., Wang H., Kou X., Ma C. Effect of simultaneous ultrasonic and microwave assisted hydrodistillation on the yield, composition, antibacterial and antibiofilm activity of essential oils from Citrus medica L. var. sarcodactylis. J. Food Eng. 2019;244:126–135. [Google Scholar]