Abstract
Atopic dermatitis is a chronic, relapsing, non-contiguous, exudative eczema/dermatitis, which represents a complex, multi-factorial disorder, due to an impairment of the stratum corneum barrier. Currently available drugs have a low skin bioavailability and may give rise to severe adverse events. Nanotechnologies, including nano-particles, liposomes, nano-gels, nano-mixtures, nano-emulsions and other nano-carriers, offer unprecedented solutions to these issues, enabling: i) the management of different clinical forms of atopic dermatitis, especially the recalcitrant ones, i) a better bio-availability and trans-dermal drug targeted delivery at the inflammation site, ii) dose control, iii) significant improvements both in clinical symptoms and immune responses, iv) with less adverse events being reported and a better safety profile. However, some nano-sized structures could amplify and even worsen symptoms in particularly susceptible individuals. Furthermore, most studies included in the present systematic review have been conducted in-vitro or in-vivo, with few randomized controlled clinical trials (RCTs). Future investigations should adopt this design in order to enable scholars achieving robust findings and evidence. Therefore, given the above-mentioned shortcomings, further research in the field is urgently warranted.
Keywords: Nanodermatology, Nanobiotechnologies, Atopic dermatitis
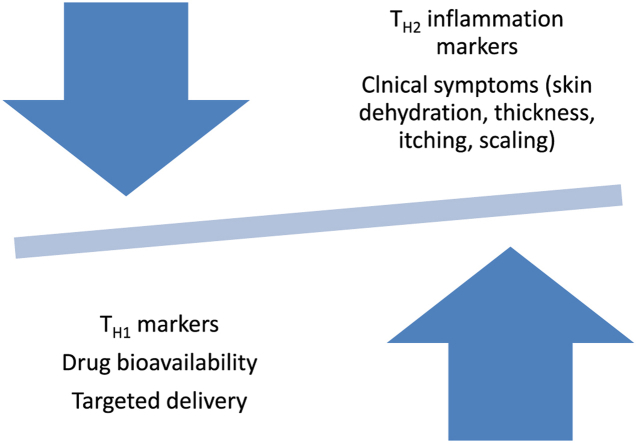
Graphical abstract
The effects of nanobiotechnologies-based pharmaceutics in atopic dermatitis.
Highlights
-
•
Atopic dermatitis is a chronic, relapsing eczema/dermatitis, due to an impairment of the stratum corneum barrier.
-
•
Currently available drugs have a low skin bioavailability and may give rise to severe adverse events.
-
•
Nanotechnologies offer unprecedented solutions, enabling the management of different clinical forms of atopic dermatitis.
1. Atopic dermatitis: physiopathology and burden
Atopic dermatitis is a chronic, relapsing, non-contiguous, exudative eczema/dermatitis, which represents a complex, multi-factorial disorder, due to an impairment of the stratum corneum barrier [[1], [2], [3]]. This results in a disturbed skin function and an increase in trans-epidermal water loss (TEWL), which leads to dehydration, and in a series of inflammatory processes characterized by the production and release of several cytokines, chemokines and interleukins (including interleukin type 1 beta or IL-1β, IL-4, IL-5, IL-6, IL-8, IL10, IL-12p70, IL-13, IL-17 and IL-17A, IL-19, IL-33 and thymic stromal lymphopoietin, or TSLP) [4,5].
These molecules and others, such as tumor necrosis factor-alpha or TNF-α and interferon-γ or IFN-γ, promote lipogenesis, activating lipids that play a major role in the skin barrier but also in delivering biochemical signals, which, in its turn, increases inflammation levels and the risk of infections.
Atopic dermatitis is also characterized by alterations in the apoptotic cascades [6]. Furthermore, an excessive amount of elastase in peripheral blood neutrophils creates an imbalance between the concentrations of the proteolytic enzyme and its endogenous inhibitors and leads to disrupted elastic fiber organization [[7], [8], [9]].
Summarizing, despite being not still fully understood, atopic dermatitis is traditionally regarded as a T helper type 2 lymphocytes-(TH2)-mediated disease, whilst, recently, advancements in the field of basic science have revealed that some other immune actors may play a crucial role, like T helper type 17 lymphocytes (TH17) and T helper type 22 lymphocytes (TH22), as well as eosinophils and mast cells that degranulate contributing to the inflammatory microenvironment [10].
From a clinical standpoint, patients suffering from atopic dermatitis complain of dryness, erythema, scaling and fissuring [1,2]. Atopic dermatitis may occur anytime during life but its onset and development should be regarded in the contest of “atopic march”; the series of events that start from the skin and may affect mainly the respiratory system (with disorders such as rhinitis, asthma, sinusitis) and eye (conjunctivitis) [11].
Current management of atopic dermatitis comprises cyclosporine A, topical calcineurin inhibitors (tacrolimus and pimecrolimus), and corticosteroids (that can be administered in a systemic or topical way). Only recently atopic dermatitis has started to benefit from targeted therapy, due to the advent of dupilumab, an anti-IL4/13 biologic, that is capable to effectively solve also the patients resistant to systemic treatments [12,13].
Synergically with the revolution in the treatment and management of atopic dermatitis, clinicians have started to distinguish and better study the late-onset atopic dermatitis that typically is more pleomorphic and less recognized, deserving a more accurate differential diagnosis [14,15]. Each patient is different, in terms of prognosis, burden of co-morbidities [16], and contraindications to the conventional systemic treatments (hepatic or renal) or even to targeted therapy (ocular disorders). Nanotechnologies, taking into account these aspects, can pave the way to an individualized treatment and management of atopic dermatitis patients, in that drug delivery, controlled release, dosage and skin permeation/retention play a key role [17].
2. Nanotechnology meets atopic dermatitis: current solutions
Nanotechnology-based therapeutics [18,19], including nanoparticles, nanogels, nanomixtures, nanoemulsions and other nanocarriers, have been explored as potential treatments for atopic dermatitis.
In this review, we have systematically searched PubMed/MEDLINE mining its entire content since inception, without any time or language constraint, utilizing the following string of key-words: (nanocarrier OR nanocarriers OR nanotube OR nanotubes OR nanogel OR nanogels OR nanoemulsion OR nanoemulsions OR nanostructure OR nanostructures OR nanostructured OR nanosize OR nanosized OR nanovector OR nanovectors OR nanocapsule OR nanocapsules OR nanoencapsulation OR nanoencapsulated OR liposome OR liposomes OR cubosome OR cubosomes OR nanovesicle OR nanovesicles OR transfersome OR transfersomes OR nanotechnology OR nanotechnologies OR nanobiotechnology OR nanobiotechnologies OR nanotherapeutics OR nanoformulation OR nanoformulations OR nanovehicle OR nanovehicles OR nanodrug OR nanodrugs OR nanodelivery OR nanocosmetic OR nanocosmetics OR nanocosmeceuticals OR nanoshell OR nanoshells) AND (“atopic dermatitis” OR “atopic eczema”). Medical subject headings (MeSH) terms and wild-card (i.e., truncated words) options, were used where appropriate.
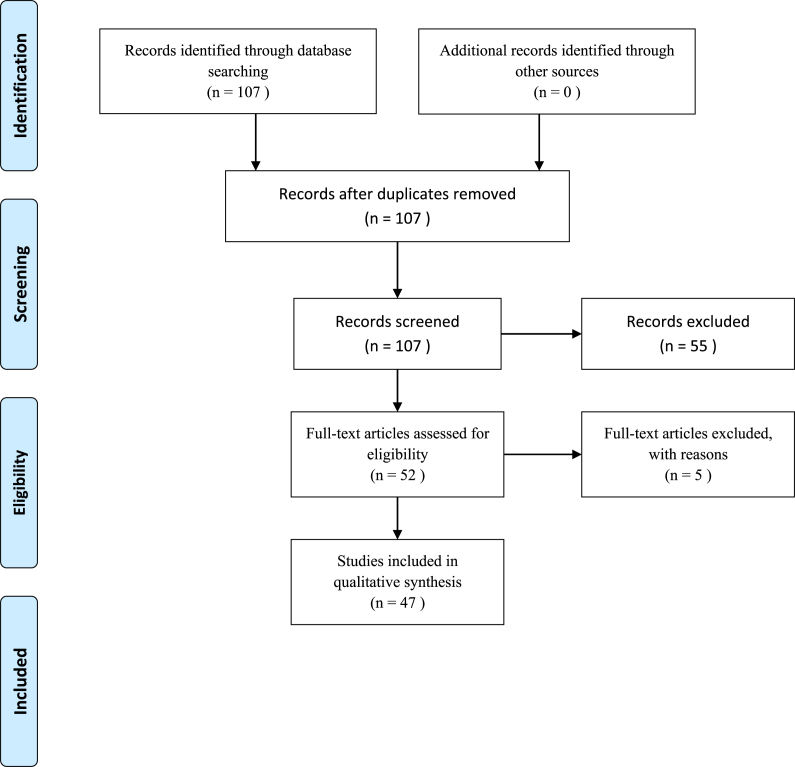
The working team was multi-disciplinary, comprising of two experts in dermatology (G.D. and P.D.M.P.), an expert in research methodology (N.L.B.) and an expert in biophysics and nanotechnology (R.E.). We followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines [20]. Studies conducted in animals for veterinary purposes, being out of scope for the present review, were excluded [[21], [22], [23], [24]]. Review articles, if existing, were scanned for increasing the chance of getting potentially relevant articles but were excluded from the present review. Extensive, iterative cross-referencing was performed, checking the list of references of each eligible study until no new study could be found. Further details are pictorially shown in Fig. 1.
Fig. 1.
Literature search strategy adopted in the present systematic review.
Nano-sized formulations found are described in the following paragraphs.
3. Atopic dermatitis, nanoparticles and nanocarriers
Nanoparticles and nanocarriers seem to have optimal rheological properties, antimicrobial effects and capability to restore skin conditions. These nanostructures, such as silver (Ag-NPs) and silver-lipid or poly(lactic acid) (PLA-NPs) nanoparticles [[25], [26], [27], [28], [29]] appear promising in the treatment and management of patients with atopic dermatitis.
Pandey and coworkers [30] fabricated hyaluronic acid-decorated betamethasone valerate-loaded chitosan nanoparticles (BMV-HA-CNPs, nano-size of 300 ± 28 nm, positively charged zeta potential of 58 ± 8 mV, entrapment efficiency of 86 ± 5.6% and loading capacity of 34 ± 7.2%. Drug diffusion and permeation efficiency profiles appeared to be optimal and promising for atopic dermatitis management.
Rosado and colleagues [31] fabricated hydrocortisone-loaded poly(ε-caprolactone) nanoparticles (PCL-NPs) by modified solvent displacement method, reporting good physical-chemical properties.
Kim and collaborators [32] evaluated the efficacy of cyclosporin A-loaded solid lipid nanoparticles (CsA-SLN) prepared by hot homogenization method (size approximately of 73 nm, negative surface charge of −16 mV). The product was tested in murine models, displaying a 2-fold higher skin penetration and reducing the production and release of IL-4 and IL-5 by T helper type 2 (TH2) lymphocytes.
Kang and coauthors [33] evaluated thermosensitive, solid lipid nanoparticles loaded with tacrolimus (TCR-SLNs), comparing its efficacy versus 0.1% Protopic® in a murine model. TCR-SLNs proved effective in drug delivery, allowing tacrolimus to reach the deepest skin layers.
Singh and Pople [[34], [35], [36]] fabricated tacrolimus-loaded lipid nanoparticles by high pressure homogenization technique. Statistically significant better drug controlled release and skin permeating profiles could be found with respect to the reference ointment, Protopic®. Immune responses were inhibited by 3.5 times, with fewer adverse events reported.
Zhuo and colleagues [37] developed hyaluronic acid-decorated tacrolimus-loaded nanoparticles (HA-TCS-CS-NPs) and tested them in-vitro. Drug release kinetics, permeation and efficacy were proved to be satisfactory.
Yu and coworkers [38] assessed the feasibility of exploiting chitosan-based nanoparticles loaded with tacrolimus and combined with nicotinamide (FK506-NIC-CS-NPs), in order to mitigate or counteract the insurgence of adverse effects associated with high doses of FK506, potentially occurring during long-term treatment. The formulation was tested in animal models (BALB/c mice with 1-chloro-2, 4-dinitrobenzene (DNCB)-induced atopic dermatitis). FK506-NIC-CS-NPs based formulation was compared with a commercially available ointment (Protopic®). Delivery and permeation of FK506 was increased up to 92.2%, enabling to spare dose of FK506.
Siddique and coauthors [[39], [40], [41]] exploited nanoparticles for a targeted delivery of systemic corticosteroids at the inflammation site. In a murine model, topically applied cationic polymeric chitosan-based nanoparticles (CSNPs) loaded with anti-inflammatory hydrocortisone and antimicrobial hydroxytyrosol (HC-HT-CSNPs, size of 228.5 ± 7 nm and positive surface charge of 39±5 mV) displayed optimal epidermal and dermal permeation, reaching the deepest skin layers with an efficiency 2.46-fold higher with respect to the commercial reference formulation. No evidence of toxicity could be found.
Similarly, Hussain et al. [[42], [43], [44]] explored the effects of hydrocortisone-loaded chitosan-based nanoparticles (HC-CNPs) in a model of 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. HC-NPs resulted in down-regulated inflammatory cascades (reduced production of IgE, IL-4, IL-5, IL-6, IL-13, IL-12p70, IFN-γ, TNF- α. release of histamine, expression of prostaglandin-E2 and vascular endothelial growth factor-α, VEGF- α), in the sera and skin. At a cellular level, events such as fibroblast infiltration and fragmentation of elastic fibers were inhibited or drastically reduced.
Huang and collaborators [45] fabricated 7,3′,4′-trihydroxyisoflavone based nanoparticles by planetary ball mill preparation under a solvent-free process (734THIN) using polyvinylpyrrolidone K30 as excipient. This formulation inhibited the expression of COX-2 and MMP-9 by down-regulating the “Mitogen-Activated Protein Kinase” (MAPK)-related signaling cascade.
Dessy and coworkers [46] prepared polymeric nano-particles based formulations loaded with anti-inflammatory Dead Sea Water (DSW) minerals (calcium, magnesium, sodium, potassium, zinc and strontium). Nanoparticles consisted of Poly (maleic anhydride-alt-butyl vinyl ether) 5% grafted with monomethoxy poly(ethyleneglycol) 2000 MW (PEG) and 95% grafted with 2-methoxyethanol (VAM41-PEG), prepared using a combined mini-emulsion/solvent evaporation process, and had a nano-size of 300 nm. Drug delivery and release properties were found to be satisfactory.
Nagaich and Gulati [47] fabricated nanocarriers loaded with betamethasone valerate, which demonstrated a permeation profile 2.59 folds higher than commercially available betamethasone valerate gel and an extended anti-inflammatory effect (up to 16.5%).
Finally, Tessema and collaborators [48] fabricated nano-carriers loaded with oat-derived phytoceramides, including lecithin-based microemulsions and starch-based nanoparticles, using Carbopol®980 as a gel. The delivery system demonstrated appropriate physical-chemical properties.
4. Nano-capsules
Marto and coworkers [49] explored the feasibility of topically delivering a novel synthetic human neutrophil elastase inhibitor (ER143) by means of a starch-based nanoparticulate system (StNC), demonstrating good permeation and retention profiles.
MD and colleagues [50] developed an ad hoc nano-encapsulated delivery system for atopic dermatitis: chitosan nanoparticles loaded with betamethsone valerate (BMV-CS-NPs, nano-size of 250 ± 28 nm, positively charged zeta potential of 58 ± 8 mV). The formulation showed satisfactory entrapment efficiency and loading capacity.
5. Lipid nano-mixtures and nano-emulsions
Berardesca and coworkers [51] assessed the effectiveness of a topical skin lipid mixture containing ceramide-3 and nanoparticles alone or combined with topical corticosteroids administered until clearance or for a period of 8 weeks. Authors found improvements both in terms of skin physiology and barrier properties and clinical symptoms.
Yilmaz and Borchert [52] investigated the efficacy of positively charged oil/water nano-emulsions (PN) containing ceramide-3B and ceramide-3, cholesterol, and palmitic acid (PNSC). Creams of PNSC were compared to PN, negatively charged oil/water nano-emulsion and SC lipids (NNSC) prepared by high-pressure homogenization and stabilized with a thickener (Carbopol-940) and a commercially available cream (Physiogel®). Each formulation was tested in a sample of 14 healthy females aged 25–50 years. All products were found to increase skin properties in terms of hydration and elasticity, with PNSC being significantly more effective than PN and NNSC, suggesting a role of phytosphingosine, lipids and ceramides.
Similarly, Neubert and coworkers [53] compared different nano-formulations: a colloidal microemulsion based of novel dimeric ceramides, a ceramides-based cream with ethoxydiglycol as penetration enhancer and a nano-formulation, finding that the former had the lowest penetration and permeation profiles, whilst the use of a penetration enhancer significantly improved the physical-chemical properties of the drug.
Bernardi et al. [54] assessed the effectiveness of rice bran oil nano-emulsions (10% rice bran oil, 10% surfactants sorbitan oleate/PEG-30 castor oil, 0.05% anti-oxidant and 0.50% preservatives formulated in distilled water) utilizing low energy emulsification methods, such as the phase diagram method. The product was found to be stable, displaying a low irritation potential, improving skin moisture and maintaining pH values within its normal range.
Baspinar and coauthors [55,56] prepared positively charged prednicarbate nanoemulsions using high pressure homogenization method. The formulation was found to be stable and potentially adequate for the treatment and management of atopic dermatitis patients.
Verma and Fahr [57] utilized a lipid mixture, NAT-8539, to enhance the topical delivery of cyclosporin A (NAT-8539-CyA), with vesicles in the range 56.6–100.6 nm of diameter and ethanol between 10% and 20%. Depending on the concentration of ethanol and the nano-size of vesicles, the nano-mixture proved to be effective in reaching the deepest skin layers.
Finally, Espinoza and colleagues [58] improved the delivery of pioglitazone, a peroxisome proliferator-activated receptor agonist, by fabricating a nano-emulsion (PGZ-NE). This formulation was proved to decrease several inflammatory cytokines, such as IL-6, IL-1β and TNF-α.
6. Liposomes, nanoliposomes and nanovesicles
Due to skin barrier defects and impairment, the stratum spinosum and stratum granulosum of atopic dermatitis patients appear to be rich in lamellar, ovoid, membrane-coating granules (MCGs), comprising of extruded, parallel disks that give rise to the formation of continuous lamellae [[59], [60], [61], [62], [63]]. Being structurally similar, liposomes can be utilized for the management of atopic dermatitis, with a hydrating effect on the stratum corneum [63], as well as being able to act as carriers of bioactive compounds [62,63].
The first pioneering study to investigate the effects of a liposomal preparation dates back to the nineties. Korting et al. [64] used a beta-methasone dipropionate (0.039%)-loaded liposome (BDP-liposome). For this purpose, they recruited 10 patients in a double-blind, randomized, paired trial and administered BDP-liposome for two weeks versus a commercial propylene glycol-gel containing 0.064% beta-methasone dipropionate. The liposomal preparation proved effective, significantly reducing inflammation and erythema and scaling.
Based on these findings, Eroğlu and coworkers [65] prepared betamethasone valerate/diflucortolone valerate-loaded chitosan-based liposomes (nano-size of 220–350 nm), with the formulation proving to be safe and effective in ameliorating atopic dermatitis symptoms in an animal model.
Ibaraki and colleagues [66,67] explored the potential effects of a topically delivered small interfering RNA (siRNA)-based treatment using AT1002 combined with a flexible, highly permeable anti-nuclear factor-kappa B (NF-κB) (RelA)-encapsulated 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine/cholesteryl hemisuccinate (siRelA-DOPE/CHEMS) liposome. AT1002 was chosen in that is a peptide able to open and finely modulate tight junctions [68,69]. The formulation was tested in in-vitro and in-vivo (animal and human) models.
Another peptide which may be of interest for the treatment of atopic dermatitis is Pep-1. Kang and coauthors [70] explored in a murine model (NC/Nga mice) the effects of a peptide-conjugated elastic liposomal formulation (comprising of phosphatidylcholine, Tween 80, N-[4-(p-maleimidophenyl)butyryl]-phosphatidylethanolamine or MPB-PE) of taxifolin glycoside (TXG-Pep1-EL, nano-size of 130 nm, zeta potential of 25 mV and deformability index value of 60). Authors found optimal skin delivery, permeation and retention profiles, as well as improvements in skin properties in terms of hydration, elasticity and immune responses.
Kang et al. [71] developed oregonin-loaded elastic, highly flexible liposomes consisting of soybean phosphatidylcholine and Tween 80 (85:15 water/water %) (ORG-EL, nanosize of 130 nm and 4-fold greater deformability index than conventional liposomes). Permeation properties of the nanocarriers were excellent and were further increased by the addition of a peptide, Trans-activating transcriptional activator (Tat), which can modulate and open tight junctions (ORG-EL-Tat). Concentrations of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), IL-4, IgE, and eosinophils in skin or blood were found to be significantly reduced.
Tat peptide (0.16 water/water %) was utilized also to facilitate skin delivery and permeation of a natural immune-modulator, hirsutenone, by means of ad hoc devised liposomes consisting of phosphatidylcholine and Tween 80 (85:15 water/water %) (HST-EL-Tat), prepared by thin-film hydration method, in an investigation carried out by Kang and coworkers [72]. Levels of iNOS, COX-2, IL-4, IL-13, IgE, and eosinophil were decreased in a murine model.
Augustin and colleagues [73] tested the effectiveness and the anti-septic, anti-inflammatory properties of liposomal polyvinyl-pyrrolidone (PVP)-iodine (3%) hydrogel administered for up to 4 weeks in a prospective, single-arm, uncontrolled, open-label phase II, pilot clinical trial recruiting 20 patients with atopic dermatitis. The “Global Clinical Severity” (GCS), pain, quality of life and “Eczema Area and Severity Index” (EASI) scores significantly improved. Formulation was well tolerated, with generally mild adverse events being reported.
Liposomes loaded with 18β-glycyrrhetinic acid, the major metabolite of glycyrrhizin, extracted from licorice root, and characterized by anti-microbial, anti-oxidative properties, appear to be another promising therapeutic solution for the management of atopic dermatitis [74].
Jung and coauthors [75] improved the topical skin delivery of cobalamin/vitamin B12 by preparing a liposomal hydrogel of adenosylcobalamin (Lipo-AdCbl, nano-size of 106.4 ± 2.2 nm) by thin film hydration method. Skin permeability was increased up to 17-times in a murine model (NC/Nga mice) with ameliorated symptoms and immune responses.
Goindi and coworkers [76,77] enhanced skin delivery of cetirizine/levocetirizine dihydrochloride, a piperazine-derived second-generation anti-histaminic drug, by combining Phospholipon®90G and edge activators. The formulation was found to have good penetration and permeation profiles in a murine model, significantly reducing dermal eosinophil count, erythema and itching scores with respect to conventional ointments and creams.
Liposomes can be utilized also for gene delivery. For instance, Kim and collaborators [78] developed IL-13 antisense oligonucleotide complexed with cationic elastic liposome (IL-13-ASO-cEL). The formulation was able to suppress IL-13 production and release. by up to 70%, as well as IL-4 and IL-5.
Finally, liposomal formulations can as well act as carriers of stem cells or purified extracts from stem cells, as in the investigation by Jahn and colleagues [79]. Authors fabricated proliposomes consisting of soy phosphatidyl choline, Poloxamer-407, ethanol and sorbitol, loaded with advanced adipose stem cell derived protein extract (AAPE, nano-size of 589 ± 3.6 nm, negatively charged zeta potential of 51.33 ± 0.36 mV). Proliposomes were stable with good physical-chemical properties.
7. Atopic dermatitis, ethosomes, nanoethosomes, cubosomes, nanofibrils and other nanocomposites
Akhtar and colleagues [80] developed β-cylcoethosomes based on β-cycloamylose and carbomer 934P gel (nano-size of 228.33 ± 1.23 nm), demonstrating good skin permeability, whereas Li and coworkers [81,82] prepared ethosomes loaded with tacrolimus, obtaining satisfactory pharmacological effects.
Izumi et al. [83] assessed the effect of chitin nanofibrils (CNFs) in a murine model. CNFs proved as effective as the topical application of corticosteroids. From a molecular standpoint, CNFs seem to reduce NF-κB, COX-2, iNOS, and IgE levels.
Kwon and Kim [84] fabricated lipid cubosomes, loaded with water-soluble extracts of Houttuynia cordata and tested them in hairless mice. The formulation was found to decrease IgE and IL-4 expression, while stimulating IFN-γ expression.
Shershakova and coworkers [85] exploited water-soluble forms of fullerene C60 prepared by exhaustive dialysis of water-organic C60 solution against water (nano-size of 100 nm and negatively charged zeta-potential of 30 mV). In a murine model female BALB/c mice, improvements in immune responses (reduced concentrations of IgE and TH2 cytokines, with increased levels of TH1 cytokines) and skin barrier function (increased levels of Foxp3+ and filaggrin expression) were detected.
8. Nanotechnology and atopic dermatitis: current challenges
Despite their hope and promises, nanotechnology-based solution can have also triggering effect on the induction of allergic reactions and the clinical amplification of such symptoms. Utilizing a mouse model, Kang and collaborators [86] found that 5- but not 100-nm nano-sized Ag-NPs induce release of granule from tryptase-positive mast cells, as well as lead to increased reactive oxygen species (ROS, hydrogen peroxide and mitochondrial superoxide) levels and intracellular calcium concentrations.
Similar adverse advents have been reported for titanium dioxide, silica and zinc oxide-based nanoparticles [[87], [88], [89], [90]].
However, a final consensus on the safety of nanotechnological devices is yet to be established, needing more convincing investigations that expert scientists should consider and appraise carefully. Further research in this field is, therefore, warranted.
9. Future prospects
Nanotechnology-based therapeutics appear promising also in preventing atopic dermatitis. Oral oligodeoxynucleotide nanocapsules (iODN-CAPs/iSG3-CAPs) specifically targeting the gut Peyer's patches, after being administered and taken up by macrophages, are able to induce an effective immune response. If continuously administered, iODN-CAPs/iSG3-CAPs in the long-term seem to display inhibitory/suppressive effects, preventing the insurgence of skin lesions and reduced epidermal thickness and elasticity typical of atopic dermatitis. From a molecular standpoint, this formulation binds to the “Signal Transducer and Activator of Transcription 6” (STAT-6), inhibiting its phosphorylation. STAT-6 gene is involved in the etiopathogenesis of atopic dermatitis, increasing its propensity and risk, leading to disseminated viral infections [91]. Signaling pathways and IL-4 related cascades, that are usually mediated by binding of allergens to TH2 cells or environmental stimuli, are, therefore, inhibited.
Finally, most studies included in the present systematic review have been conducted in-vitro or in-vivo, with few randomized controlled clinical trials (RCTs). Future investigations should adopt this design in order to enable scholars achieving robust findings and evidence.
10. Conclusions
Nanotechnology-based solutions appear promising in the management of atopic dermatitis, especially the recalcitrant forms, enabling: i) a better bio-availability and trans-dermal drug targeted delivery at the inflammation site, displaying better physical-chemical properties, permeation, retention and diffusion profiles, ii) dose control, iii) improvements both in clinical symptoms and immune responses, inhibiting inflammatory cascades and pathways up to 3–5 times and positively impacting on patient's quality of life, iv) with less adverse events being reported [[92], [93], [94], [95], [96], [97], [98], [99]].
However, given the above-mentioned shortcomings, further research in the field is urgently warranted.
Funding
None.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.David Boothe W., Tarbox J.A., Tarbox M.B. Atopic dermatitis: pathophysiology. Adv. Exp. Med. Biol. 2017;1027:21–37. doi: 10.1007/978-3-319-64804-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Torres T., Ferreira E.O., Gonçalo M., Mendes-Bastos P., Selores M., Filipe P. Update on atopic dermatitis. Acta Med. Port. 2019 Sep 2;32(9):606–613. doi: 10.20344/amp.11963. [DOI] [PubMed] [Google Scholar]
- 3.Aburai K., Yoshino S., Sakai K., Sakai H., Abe M., Loiseau N., Holleran W., Uchida Y., Sakamoto K. Physicochemical analysis of liposome membranes consisting of model lipids in the stratum corneum. J. Oleo Sci. 2011;60(4):197–202. doi: 10.5650/jos.60.197. [DOI] [PubMed] [Google Scholar]
- 4.Klonowska J., Gleń J., Nowicki R.J., Trzeciak M. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets. Int. J. Mol. Sci. 2018 Oct 9;19(10) doi: 10.3390/ijms19103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahina R., Maeda S. A review of the roles of keratinocyte-derived cytokines and chemokines in the pathogenesis of atopic dermatitis in humans and dogs. Vet. Dermatol. 2017 Feb;28(1) doi: 10.1111/vde.12351. 16-e5. [DOI] [PubMed] [Google Scholar]
- 6.Szymanski L., Cios A., Lewicki S., Szymanski P., Stankiewicz W. Fas/FasL pathway and cytokines in keratinocytes in atopic dermatitis - manipulation by the electromagnetic field. PLoS One. 2018 Oct 4;13(10) doi: 10.1371/journal.pone.0205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A., Schalkwijk J., Happle R., van de Kerkhof P.C. Elastase-inhibiting activity in scaling skin disorders. Acta Derm. Venereol. 1990;70(2):147–151. [PubMed] [Google Scholar]
- 8.Peng W., Novak N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy. 2015 Mar;45(3):566–574. doi: 10.1111/cea.12495. [DOI] [PubMed] [Google Scholar]
- 9.Neshkova E., Puzhko S., Dotsenko V., Nenasheva N., Yarovaya G., Gorjachkina L. Activity of leukocyte elastase in patients' plasma is a significant indicator of atopic diseases. Immunopharmacology. 1996 Jun;33(1–3):383–386. doi: 10.1016/0162-3109(96)00092-6. [DOI] [PubMed] [Google Scholar]
- 10.Wiedow O., Wiese F., Streit V., Kalm C., Christophers E. Lesional elastase activity in psoriasis, contact dermatitis, and atopic dermatitis. J. Investig. Dermatol. 1992 Sep;99(3):306–309. doi: 10.1111/1523-1747.ep12616644. [DOI] [PubMed] [Google Scholar]
- 11.Han H., Roan F., Ziegler S.F. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017 Jul;278(1):116–130. doi: 10.1111/imr.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fargnoli M.C., Esposito M., Ferrucci S., Girolomoni G., Offidani A., Patrizi A., Peris K., Costanzo A., Malara G., Pellacani G., Romanelli M., Amerio P., Cristaudo A., Flori M.L., Motolese A., Betto P., Patruno C., Pigatto P., Sirna R., Stinco G., Zalaudek I., Bianchi L., Boccaletti V., Cannavò S.P., Cusano F., Lembo S., Mozzillo R., Gallo R., Potenza C., Rongioletti F., Tiberio R., Grieco T., Micali G., Persechino S., Pettinato M., Pucci S., Savi E., Stingeni L., Romano A., Argenziano G. Dupilumab Italian National Access Program (Dup-INAP group). Real-life experience on effectiveness and safety of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J. Dermatol. Treat. 2019 Oct 24:1–20. doi: 10.1080/09546634.2019.1682503. [DOI] [PubMed] [Google Scholar]
- 13.Damiani G., Calzavara-Pinton P., Stingeni L., Hansel K., Cusano F. “Skin Allergy” group of SIDeMaST, ADOI (Associazione Dermatologi Ospedalieri Italiani), and “SIDAPA” (Società Italiana di Dermatologia Allergologica, Professionale e Ambientale), Pigatto PD. Italian Guidelines for therapy of Atopic Dermatitis- adapted from Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) Dermatol. Ther. 2019 Oct 17 doi: 10.1111/dth.13121. [DOI] [PubMed] [Google Scholar]
- 14.Barrett M., Luu M. Differential diagnosis of atopic dermatitis. Immunol. Allergy Clin. N. Am. 2017 Feb;37(1):11–34. doi: 10.1016/j.iac.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Stingeni L., Bianchi L., Hansel K., Corazza M., Gallo R., Guarneri F., Patruno C., Rigano L., Romita P., Pigatto P.D., Calzavara-Pinton P. Skin Allergy" group of SIDeMaST and "SIDAPA" (Società Italiana di Dermatologia Allergologica, Professionale e Ambientale). Italian Guidelines in Patch Testing - adapted from the European Society of Contact Dermatitis (ESCD) G. Ital. Dermatol. Venereol. 2019 Jun;154(3):227–253. doi: 10.23736/S0392-0488.19.06301-6. [DOI] [PubMed] [Google Scholar]
- 16.Paller A., Jaworski J.C., Simpson E.L., Boguniewicz M., Russell J.J., Block J.K., Tofte S., Dunn J.D., Feldman S.R., Clark A.R., Schwartz G., Eichenfield L.F. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am. J. Clin. Dermatol. 2018 Dec;19(6):821–838. doi: 10.1007/s40257-018-0383-4. [DOI] [PubMed] [Google Scholar]
- 17.van der Schaft J., Thijs J.L., Garritsen F.M., Balak D., de Bruin-Weller M.S. Towards personalized treatment in atopic dermatitis. Expert Opin. Biol. Ther. 2019 May;19(5):469–476. doi: 10.1080/14712598.2019.1583204. [DOI] [PubMed] [Google Scholar]
- 18.Wiesenthal A., Hunter L., Wang S., Wickliffe J., Wilkerson M. Nanoparticles: small and mighty. Int. J. Dermatol. 2011 Mar;50(3):247–254. doi: 10.1111/j.1365-4632.2010.04815.x. [DOI] [PubMed] [Google Scholar]
- 19.Puglia C., Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin. Drug Deliv. 2012 Apr;9(4):429–441. doi: 10.1517/17425247.2012.666967. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner I., Geh K.J., Hubert M., Winter G., Weber K., Classen J., Klinger C., Mueller R.S. Preliminary evaluation of cytosine-phosphate-guanine oligodeoxynucleotides bound to gelatine nanoparticles as immunotherapy for canine atopic dermatitis. Vet. Rec. 2017 Jul 29;181(5):118. doi: 10.1136/vr.104230. [DOI] [PubMed] [Google Scholar]
- 22.Prélaud A.R., Fuchs S., Weber K., Winter G., Coester C., Mueller R.S. In vitro effects of CpG oligodeoxynucleotides delivered by gelatin nanoparticles on canine peripheral blood mononuclear cells of atopic and healthy dogs - a pilot study. Vet. Dermatol. 2013 Oct;24(5) doi: 10.1111/vde.12056. 494-e117. [DOI] [PubMed] [Google Scholar]
- 23.Puigdemont A., Brazís P., Ordeix L., Dalmau A., Fuertes E., Olivar A., Pérez C., Ravera I. Efficacy of a new topical cyclosporine A formulation in the treatment of atopic dermatitis in dogs. Vet. J. 2013 Aug;197(2):280–285. doi: 10.1016/j.tvjl.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Mueller R.S., Veir J., Fieseler K.V., Dow S.W. Use of immunostimulatory liposome-nucleic acid complexes in allergen-specific immunotherapy of dogs with refractory atopic dermatitis - a pilot study. Vet. Dermatol. 2005 Feb;16(1):61–68. doi: 10.1111/j.1365-3164.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- 25.Gloor M. How do dermatological vehicles influence the horny layer? Skin Pharmacol. Physiol. 2004 Nov-Dec;17(6):267–273. doi: 10.1159/000081111. [DOI] [PubMed] [Google Scholar]
- 26.Keck C.M., Schwabe K. Silver-nanolipid complex for application to atopic dermatitis skin: rheological characterization, in vivo efficiency and theory of action. J. Biomed. Nanotechnol. 2009 Aug;5(4):428–436. doi: 10.1166/jbn.2009.1053. [DOI] [PubMed] [Google Scholar]
- 27.Rujido-Santos I., Naveiro-Seijo L., Herbello-Hermelo P., Barciela-Alonso M.D.C., Bermejo-Barrera P., Moreda-Piñeiro A. Silver nanoparticles assessment in moisturizing creams by ultrasound assisted extraction followed by sp-ICP-MS. Talanta. 2019 May 15;197:530–538. doi: 10.1016/j.talanta.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 28.Bianco C., Visser M.J., Pluut O.A., Svetličić V., Pletikapić G., Jakasa I., Riethmuller C., Adami G., Larese Filon F., Schwegler-Berry D., Stefaniak A.B., Kezic S. Characterization of silver particles in the stratum corneum of healthy subjects and atopic dermatitis patients dermally exposed to a silver-containing garment. Nanotoxicology. 2016 Dec;10(10):1480–1491. doi: 10.1080/17435390.2016.1235739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keck C.M., Anantaworasakul P., Patel M., Okonogi S., Singh K.K., Roessner D., Scherrers R., Schwabe K., Rimpler C., Müller R.H. A new concept for the treatment of atopic dermatitis: silver-nanolipid complex (sNLC) Int. J. Pharm. 2014 Feb 28;462(1–2):44–51. doi: 10.1016/j.ijpharm.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Pandey M., Choudhury H., Gunasegaran T.A.P., Nathan S.S., Md S., Gorain B., Tripathy M., Hussain Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 2019 Apr;9(2):520–533. doi: 10.1007/s13346-018-0480-1. [DOI] [PubMed] [Google Scholar]
- 31.Rosado C., Silva C., Reis C.P. Hydrocortisone-loaded poly(ε-caprolactone) nanoparticles for atopic dermatitis treatment. Pharm. Dev. Technol. 2013 May-Jun;18(3):710–718. doi: 10.3109/10837450.2012.712537. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.T., Jang D.J., Kim J.H., Park J.Y., Lim J.S., Lee S.Y., Lee K.M., Lim S.J., Kim C.K. Topical administration of cyclosporin A in a solid lipid nanoparticle formulation. Die Pharmazie. 2009 Aug;64(8):510–514. [PubMed] [Google Scholar]
- 33.Kang J.H., Chon J., Kim Y.I., Lee H.J., Oh D.W., Lee H.G., Han C.S., Kim D.W., Park C.W. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int. J. Nanomed. 2019 Jul 18;14:5381–5396. doi: 10.2147/IJN.S215153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh K.K., Pople P. Safer than safe: lipid nanoparticulate encapsulation of tacrolimus with enhanced targeting and improved safety for atopic dermatitis. J. Biomed. Nanotechnol. 2011 Feb;7(1):40–41. doi: 10.1166/jbn.2011.1191. [DOI] [PubMed] [Google Scholar]
- 35.Pople P.V., Singh K.K. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis-Part II: in vivo assessment of dermatopharmacokinetics, biodistribution and efficacy. Int. J. Pharm. 2012 Sep 15;434(1–2):70–79. doi: 10.1016/j.ijpharm.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 36.Pople P.V., Singh K.K. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus, Part II--in vivo assessment, drug targeting, efficacy, and safety in treatment for atopic dermatitis. Eur. J. Pharm. Biopharm. 2013 May;84(1):72–83. doi: 10.1016/j.ejpb.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Zhuo F., Abourehab M.A.S., Hussain Z. Hyaluronic acid decorated tacrolimus-loaded nanoparticles: efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr. Polym. 2018 Oct 1;197:478–489. doi: 10.1016/j.carbpol.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Yu K., Wang Y., Wan T., Zhai Y., Cao S., Ruan W., Wu C., Xu Y. Tacrolimus nanoparticles based on chitosan combined with nicotinamide: enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose. Int. J. Nanomed. 2017 Dec 22;13:129–142. doi: 10.2147/IJN.S150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddique M.I., Katas H., Amin M.C., Ng S.F., Zulfakar M.H., Jamil A. In-vivo dermal pharmacokinetics, efficacy, and safety of skin targeting nanoparticles for corticosteroid treatment of atopic dermatitis. Int. J. Pharm. 2016 Jun 30;507(1–2):72–82. doi: 10.1016/j.ijpharm.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Siddique M.I., Katas H., Amin M.C.I.M., Ng S.F., Zulfakar M.H., Buang F., Jamil A. Minimization of local and systemic adverse effects of topical glucocorticoids by nanoencapsulation: in vivo safety of hydrocortisone-hydroxytyrosol loaded chitosan nanoparticles. J. Pharm. Sci. 2015 Dec;104(12):4276–4286. doi: 10.1002/jps.24666. [DOI] [PubMed] [Google Scholar]
- 41.Siddique M.I., Katas H., Jamil A., Mohd Amin M.C.I., Ng S.F., Zulfakar M.H., Nadeem S.M. Potential treatment of atopic dermatitis: tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Drug Deliv. Transl. Res. 2019 Apr;9(2):469–481. doi: 10.1007/s13346-017-0439-7. [DOI] [PubMed] [Google Scholar]
- 42.Hussain Z., Katas H., Mohd Amin M.C., Kumolosasi E., Buang F., Sahudin S. Self-assembled polymeric nanoparticles for percutaneous co-delivery of hydrocortisone/hydroxytyrosol: an ex vivo and in vivo study using an NC/Nga mouse model. Int. J. Pharm. 2013 Feb 28;444(1–2):109–119. doi: 10.1016/j.ijpharm.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Hussain Z., Katas H., Mohd Amin M.C., Kumolosasi E. Efficient immuno-modulation of TH1/TH2 biomarkers in 2,4-dinitrofluorobenzene-induced atopic dermatitis: nanocarrier-mediated transcutaneous co-delivery of anti-inflammatory and antioxidant drugs. PLoS One. 2014 Nov 14;9(11):e113143. doi: 10.1371/journal.pone.0113143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain Z., Katas H., Mohd Amin M.C., Kumolosasi E., Sahudin S. Downregulation of immunological mediators in 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions by hydrocortisone-loaded chitosan nanoparticles. Int. J. Nanomed. 2014 Nov 5;9:5143–5156. doi: 10.2147/IJN.S71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang P.H., Tseng C.H., Lin C.Y., Lee C.W., Yen F.L. Preparation, characterizations and anti-pollutant activity of 7,3',4'-trihydroxyisoflavone nanoparticles in particulate matter-induced HaCaT keratinocytes. Int. J. Nanomed. 2018 Jun 1;13:3279–3293. doi: 10.2147/IJN.S153323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dessy A., Kubowicz S., Alderighi M., Bartoli C., Piras A.M., Schmid R., Chiellini F. Dead Sea Minerals loaded polymeric nanoparticles. Colloids Surfaces B Biointerfaces. 2011 Oct 15;87(2):236–242. doi: 10.1016/j.colsurfb.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 47.Nagaich U., Gulati N. Preclinical assessment of steroidal nanostructured lipid carriers based gels for atopic dermatitis: optimization and product development. Curr. Drug Deliv. 2018;15(5):641–651. doi: 10.2174/1567201814666170918163615. [DOI] [PubMed] [Google Scholar]
- 48.Tessema E.N., Gebre-Mariam T., Paulos G., Wohlrab J., Neubert R.H.H. Delivery of oat-derived phytoceramides into the stratum corneum of the skin using nanocarriers: formulation, characterization and in vitro and ex-vivo penetration studies. Eur. J. Pharm. Biopharm. 2018 Jun;127:260–269. doi: 10.1016/j.ejpb.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 49.Marto J., Ruivo E., Lucas S.D., Gonçalves L.M., Simões S., Gouveia L.F., Felix R., Moreira R., Ribeiro H.M., Almeida A.J. Starch nanocapsules containing a novel neutrophil elastase inhibitor with improved pharmaceutical performance. Eur. J. Pharm. Biopharm. 2018 Jun;127:1–11. doi: 10.1016/j.ejpb.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Md S., Kuldeep Singh J.K.A., Waqas M., Pandey M., Choudhury H., Habib H., Hussain F., Hussain Z. Nanoencapsulation of betamethasone valerate using high pressure homogenization-solvent evaporation technique: optimization of formulation and process parameters for efficient dermal targeting. Drug Dev. Ind. Pharm. 2019 Feb;45(2):323–332. doi: 10.1080/03639045.2018.1542704. [DOI] [PubMed] [Google Scholar]
- 51.Berardesca E., Barbareschi M., Veraldi S., Pimpinelli N. Evaluation of efficacy of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: a multicenter study. Contact Dermatitis. 2001 Nov;45(5):280–285. doi: 10.1034/j.1600-0536.2001.450505.x. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz E., Borchert H.H. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema--an in vivo study. Int. J. Pharm. 2006 Jan 13;307(2):232–238. doi: 10.1016/j.ijpharm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Neubert R.H., Sonnenberger S., Dobner B., Gray C.W., Jr., Barger K.N., Sevi-Maxwell K., Sommer E., Wohlrab J. Controlled penetration of a novel dimeric ceramide into and across the stratum corneum using microemulsions and various types of semisolid formulations. Skin Pharmacol. Physiol. 2016;29(3):130–134. doi: 10.1159/000445776. [DOI] [PubMed] [Google Scholar]
- 54.Bernardi D.S., Pereira T.A., Maciel N.R., Bortoloto J., Viera G.S., Oliveira G.C., Rocha-Filho P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J. Nanobiotechnol. 2011 Sep 28;9:44. doi: 10.1186/1477-3155-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baspinar Y., Keck C.M., Borchert H.H. Development of a positively charged prednicarbate nanoemulsion. Int. J. Pharm. 2010 Jan 4;383(1–2):201–208. doi: 10.1016/j.ijpharm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Baspinar Y., Borchert H.H. Penetration and release studies of positively and negatively charged nanoemulsions--is there a benefit of the positive charge? Int. J. Pharm. 2012 Jul 1;430(1–2):247–252. doi: 10.1016/j.ijpharm.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 57.Verma D.D., Fahr A. Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin A. J. Control. Release. 2004 May 31;97(1):55–66. doi: 10.1016/j.jconrel.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 58.Espinoza L.C., Silva-Abreu M., Calpena A.C., Rodríguez-Lagunas M.J., Fábrega M.J., Garduño-Ramírez M.L., Clares B. Nanoemulsion strategy of pioglitazone for the treatment of skin inflammatory diseases. Nanomedicine. 2019 Jul;19:115–125. doi: 10.1016/j.nano.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Yamamoto Y., Shigemori S., Watanabe T., Oshiro K., Wang X., Wang P., Sato T., Yonekura S., Tanaka S., Kitazawa H., Shimosato T. Inhibitory/suppressive oligodeoxynucleotide nanocapsules as simple oral delivery devices for preventing atopic dermatitis in mice. Mol. Ther. 2015 Feb;23(2):297–309. doi: 10.1038/mt.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howell M.D., Gao P., Kim B.E., Lesley L.J., Streib J.E., Taylor P.A., Zaccaro D.J., Boguniewicz M., Beck L.A., Hanifin J.M., Schneider L.C., Hata T.R., Gallo R.L., Kaplan M.H., Barnes K.C., Leung D.Y. The signal transducer and activator of transcription 6 gene (STAT6) increases the propensity of patients with atopic dermatitis toward disseminated viral skin infections. J. Allergy Clin. Immunol. 2011 Nov;128(5):1006–1014. doi: 10.1016/j.jaci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fartasch M., Diepgen T.L. The barrier function in atopic dry skin. Disturbance of membrane-coating granule exocytosis and formation of epidermal lipids? Acta Derm. Venereol. Suppl. 1992;176:26–31. [PubMed] [Google Scholar]
- 62.Werner Y., Lindberg M., Forslind B. Membrane-coating granules in "dry" non-eczematous skin of patients with atopic dermatitis. A quantitative electron microscopic study. Acta Derm. Venereol. 1987;67(5):385–390. [PubMed] [Google Scholar]
- 63.Schmid M.H., Korting H.C. Liposomes for atopic dry skin: the rationale for a promising approach. Clin. Investig. 1993 Aug;71(8):649–653. doi: 10.1007/BF00184495. [DOI] [PubMed] [Google Scholar]
- 64.Korting H.C., Zienicke H., Schäfer-Korting M., Braun-Falco O. Liposome encapsulation improves efficacy of betamethasone dipropionate in atopic eczema but not in psoriasis vulgaris. Eur. J. Clin. Pharmacol. 1990;39(4):349–351. doi: 10.1007/BF00315408. [DOI] [PubMed] [Google Scholar]
- 65.Eroğlu İ., Azizoğlu E., Özyazıcı M., Nenni M., Gürer Orhan H., Özbal S., Tekmen I., Ertam İ., Ünal İ., Özer Ö. Effective topical delivery systems for corticosteroids: dermatological and histological evaluations. Drug Deliv. 2016 Jun;23(5):1502–1513. doi: 10.3109/10717544.2014.960981. [DOI] [PubMed] [Google Scholar]
- 66.Ibaraki H., Kanazawa T., Kurano T., Oogi C., Takashima Y., Seta Y. Anti-RelA siRNA-encapsulated flexible liposome with tight junction-opening peptide as a non-invasive topical therapeutic for atopic dermatitis. Biol. Pharm. Bull. 2019;42(7):1216–1225. doi: 10.1248/bpb.b19-00259. [DOI] [PubMed] [Google Scholar]
- 67.Kanazawa T., Hamasaki T., Endo T., Tamano K., Sogabe K., Seta Y., Ohgi T., Okada H. Functional peptide nanocarriers for delivery of novel anti-RelA RNA interference agents as a topical treatment of atopic dermatitis. Int. J. Pharm. 2015 Jul 15;489(1–2):261–267. doi: 10.1016/j.ijpharm.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Uchida T., Kanazawa T., Kawai M., Takashima Y., Okada H. Therapeutic effects on atopic dermatitis by anti-RelA short interfering RNA combined with functional peptides Tat and AT1002. J. Pharmacol. Exp. Ther. 2011 Aug;338(2):443–450. doi: 10.1124/jpet.111.180042. [DOI] [PubMed] [Google Scholar]
- 69.Uchida T., Kanazawa T., Takashima Y., Okada H. Development of an efficient transdermal delivery system of small interfering RNA using functional peptides, Tat and AT-1002. Chem. Pharm. Bull. (Tokyo) 2011;59(2):196–201. doi: 10.1248/cpb.59.196. [DOI] [PubMed] [Google Scholar]
- 70.Kang M.J., Eum J.Y., Park S.H., Kang M.H., Park K.H., Choi S.E., Lee M.W., Kang K.H., Oh C.H., Choi Y.W. Pep-1 peptide-conjugated elastic liposomal formulation of taxifolin glycoside for the treatment of atopic dermatitis in NC/Nga mice. Int. J. Pharm. 2010 Dec 15;402(1–2):198–204. doi: 10.1016/j.ijpharm.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 71.Kang M.J., Eum J.Y., Jeong M.S., Choi S.E., Park S.H., Cho H.I., Cho C.S., Seo S.J., Lee M.W., Choi Y.W. Facilitated skin permeation of oregonin by elastic liposomal formulations and suppression of atopic dermatitis in NC/Nga mice. Biol. Pharm. Bull. 2010;33(1):100–106. doi: 10.1248/bpb.33.100. [DOI] [PubMed] [Google Scholar]
- 72.Kang M.J., Eum J.Y., Jeong M.S., Park S.H., Moon K.Y., Kang M.H., Kim M.S., Choi S.E., Lee M.W., Lee D.I., Bang H., Lee C.S., Joo S.S., Li K., Lee M.K., Seo S.J., Choi Y.W. Tat peptide-admixed elastic liposomal formulation of hirsutenone for the treatment of atopic dermatitis in NC/Nga mice. Int. J. Nanomed. 2011;6:2459–2467. doi: 10.2147/IJN.S24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Augustin M., Goepel L., Jacobi A., Bosse B., Mueller S., Hopp M. Efficacy and tolerability of liposomal polyvinylpyrrolidone-iodine hydrogel for the localized treatment of chronic infective, inflammatory, dermatoses: an uncontrolled pilot study. Clin. Cosmet. Investig. Dermatol. 2017 Sep 22;10:373–384. doi: 10.2147/CCID.S141887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kowalska A., Kalinowska-Lis U. 18β-Glycyrrhetinic acid: its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019 Aug;41(4):325–331. doi: 10.1111/ics.12548. [DOI] [PubMed] [Google Scholar]
- 75.Jung S.H., Cho Y.S., Jun S.S., Koo J.S., Cheon H.G., Shin B.C. Topical application of liposomal cobalamin hydrogel for atopic dermatitis therapy. Die Pharmazie. 2011 Jun;66(6):430–435. [PubMed] [Google Scholar]
- 76.Goindi S., Kumar G., Kaur A. Novel flexible vesicles based topical formulation of levocetirizine: in vivo evaluation using oxazolone-induced atopic dermatitis in murine model. J. Liposome Res. 2014 Sep;24(3):249–257. doi: 10.3109/08982104.2014.899365. [DOI] [PubMed] [Google Scholar]
- 77.Goindi S., Kumar G., Kumar N., Kaur A. Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech. 2013 Dec;14(4):1284–1293. doi: 10.1208/s12249-013-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S.T., Lee K.M., Park H.J., Jin S.E., Ahn W.S., Kim C.K. Topical delivery of interleukin-13 antisense oligonucleotides with cationic elastic liposome for the treatment of atopic dermatitis. J. Gene Med. 2009 Jan;11(1):26–37. doi: 10.1002/jgm.1268. [DOI] [PubMed] [Google Scholar]
- 79.Jahn A., Song C.K., Balakrishnan P., Hong S.S., Lee J.H., Chung S.J., Kim D.D. AAPE proliposomes for topical atopic dermatitis treatment. J. Microencapsul. 2014;31(8):768–773. doi: 10.3109/02652048.2014.932027. [DOI] [PubMed] [Google Scholar]
- 80.Akhtar N., Verma A., Pathak K. Investigating the penetrating potential of nanocomposite β-cycloethosomes: development using central composite design, in vitro and ex vivo characterization. J. Liposome Res. 2018 Mar;28(1):35–48. doi: 10.1080/08982104.2016.1254241. [DOI] [PubMed] [Google Scholar]
- 81.Li G., Fan Y., Fan C., Li X., Wang X., Li M., Liu Y. Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012 Sep;82(1):49–57. doi: 10.1016/j.ejpb.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Li G., Fan C., Li X., Fan Y., Wang X., Li M., Liu Y. Preparation and in vitro evaluation of tacrolimus-loaded ethosomes. Sci. World J. 2012;2012:874053. doi: 10.1100/2012/874053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Izumi R., Azuma K., Izawa H., Morimoto M., Nagashima M., Osaki T., Tsuka T., Imagawa T., Ito N., Okamoto Y., Saimoto H., Ifuku S. Chitin nanofibrils suppress skin inflammation in atopic dermatitis-like skin lesions in NC/Nga mice. Carbohydr. Polym. 2016 Aug 1;146:320–327. doi: 10.1016/j.carbpol.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 84.Kwon T.K., Kim J.C. In vitro skin permeation and anti-atopic efficacy of lipid nanocarriers containing water soluble extracts of Houttuynia cordata. Drug Dev. Ind. Pharm. 2014 Oct;40(10):1350–1357. doi: 10.3109/03639045.2013.819883. [DOI] [PubMed] [Google Scholar]
- 85.Shershakova N., Baraboshkina E., Andreev S., Purgina D., Struchkova I., Kamyshnikov O., Nikonova A., Khaitov M. Anti-inflammatory effect of fullerene C60 in a mice model of atopic dermatitis. J. Nanobiotechnol. 2016 Jan 25;14:8. doi: 10.1186/s12951-016-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang H., Kim S., Lee K.H., Jin S., Kim S.H., Lee K., Jeon H., Song Y.G., Lee S.W., Seo J., Park S., Choi I.H. 5 nm silver nanoparticles amplify clinical features of atopic dermatitis in mice by activating mast cells. Small. 2017 Mar;13(9) doi: 10.1002/smll.201602363. [DOI] [PubMed] [Google Scholar]
- 87.Yanagisawa R., Takano H., Inoue K., Koike E., Kamachi T., Sadakane K., Ichinose T. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp. Biol. Med. 2009 Mar;234(3):314–322. doi: 10.3181/0810-RM-304. [DOI] [PubMed] [Google Scholar]
- 88.Hirai T., Yoshioka Y., Takahashi H., Ichihashi K., Udaka A., Mori T., Nishijima N., Yoshida T., Nagano K., Kamada H., Tsunoda S., Takagi T., Ishii K.J., Nabeshi H., Yoshikawa T., Higashisaka K., Tsutsumi Y. Cutaneous exposure to agglomerates of silica nanoparticles and allergen results in IgE-biased immune response and increased sensitivity to anaphylaxis in mice. Part. Fibre Toxicol. 2015 Jun 26;12:16. doi: 10.1186/s12989-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ilves M., Palomäki J., Vippola M., Lehto M., Savolainen K., Savinko T., Alenius H. Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part. Fibre Toxicol. 2014 Aug 14;11:38. doi: 10.1186/s12989-014-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rancan F., Gao Q., Graf C., Troppens S., Hadam S., Hackbarth S., Kembuan C., Blume-Peytavi U., Rühl E., Lademann J., Vogt Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. A.ACS Nano. 2012 Aug 28;6(8):6829–6842. doi: 10.1021/nn301622h. [DOI] [PubMed] [Google Scholar]
- 91.Shao M., Hussain Z., Thu H.E., Khan S., Katas H., Ahmed T.A., Tripathy M., Leng J., Qin H.L., Bukhari S.N.A. Drug nanocarrier, the future of atopic diseases: advanced drug delivery systems and smart management of disease. Colloids Surfaces B Biointerfaces. 2016 Nov 1;147:475–491. doi: 10.1016/j.colsurfb.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 92.Palmer B.C., DeLouise L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules. 2016 Dec 15;21(12) doi: 10.3390/molecules21121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hussain Z., Sahudin S., Thu H.E., Shuid A.N., Bukhari S.N., Kumolosasi E. Recent advances in pharmacotherapeutic paradigm of mild to recalcitrant atopic dermatitis. Crit. Rev. Ther. Drug Carrier Syst. 2016;33(3):213–263. doi: 10.1615/CritRevTherDrugCarrierSyst.2016015219. [DOI] [PubMed] [Google Scholar]
- 94.Okada H. Drug discovery by formulation design and innovative drug delivery systems (DDS) Yakugaku Zasshi. 2011;131(9):1271–1287. doi: 10.1248/yakushi.131.1271. [DOI] [PubMed] [Google Scholar]
- 95.Zoschke C., Schilrreff P., Romero E.L., Brandner J.M., Schafer-Korting M. Dendritic nanoparticles for cutaneous drug delivery--testing in human skin and reconstructed human skin. Curr. Pharmaceut. Des. 2015;21(20):2784–2800. doi: 10.2174/1381612821666150428142515. [DOI] [PubMed] [Google Scholar]
- 96.Patel P., Patel H., Panchal S., Mehta T. Formulation strategies for drug delivery of tacrolimus: an overview. Int. J. Pharm. Investig. 2012 Oct;2(4):169–175. doi: 10.4103/2230-973X.106981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun L., Liu Z., Cun D., Tong H.H., Zheng Y. Application of nano- and micro-particles on the topical therapy of skin-related immune disorders. Curr. Pharmaceut. Des. 2015;21(19):2643–2667. doi: 10.2174/1381612821666150416100516. [DOI] [PubMed] [Google Scholar]
- 98.Palmer B.C., DeLouise L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules. 2016 Dec 15;21(12) doi: 10.3390/molecules21121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shao M., Hussain Z., Thu H.E., Khan S., Katas H., Ahmed T.A., Tripathy M., Leng J., Qin H.L., Bukhari S.N.A. Drug nanocarrier, the future of atopic diseases: advanced drug delivery systems and smart management of disease. Colloids Surfaces B Biointerfaces. 2016 Nov 1;147:475–491. doi: 10.1016/j.colsurfb.2016.08.027. [DOI] [PubMed] [Google Scholar]