Abstract
Sentinel lymph node biopsy alone, without complete axillary lymph node dissection, is the standard treatment of the axilla nodal chain in early-stage breast cancer patients presenting a negative sentinel lymph node. The updated results of the IBCSG 23-01 randomized trial recently provided evidence that this approach could be extended to early-stage breast cancer patients presenting only micrometastasis in the sentinel lymph node.
On the other hand, patients with large operable or locally advanced breast cancer and clinically positive lymph nodes currently receive neoadjuvant chemotherapy and sentinel lymph node biopsy, which is then followed by complete axillary node dissection if the sentinel lymph node till contains tumor residue, regardless of the extent of nodal disease. Assuming that patients presenting only a micrometastatic sentinel lymph node after neoadjuvant chemotherapy are clinically equivalent to the IBCSG 23-01 early-breast cancer patients with only micrometastatic sentinel node, then complete axillary dissection would be unneeded also in these subset of patients in the neoadjuvant setting. The multicenter uncontrolled non-inferiority trial NEONOD 2 we here present was designed to assess this hypothesis, i.e. whether or not omission of complete axillary nodal clearance worsens prognosis in patients with sentinel node resulting only micrometastatic after neoadjuvant chemotherapy.
Keywords: Infiltrating breast cancer, Clinically positive axilla, Neoadjuvant chemotherapy, Sentinel lymph node biopsy, Axillary lymph node dissection, Outcome
Abbreviations
- BC
breast cancer
- SLN
sentinel lymph node
- SLNB
sentinel lymph node biopsy
- ALND
axillary lymph node dissection
- NAC
neoadjuvant chemotherapy
- RCT
randomized clinical trial
- LABC
locally advanced breast cancer
- cN
clinically negative node
- cN+
clinically positive node
- OS
overall survival
- DFS
disease-free survival
- LRDFS
locoregional disease-free survival
- DDFS
distant disease-free survival
- pCR
pathological complete response
- SIR
successful identification rate
- FNR
false negative rate
- BCS
breast conserving surgery
- RT
radiotherapy
- ER
estrogen receptor
- PgR
progesterone receptor
- HER2
human epidermal growth factor receptor 2
1. Introduction
In the last twenty years, the management of breast cancer (BC) patients has been characterized by a constant trend towards less invasive axillary surgery. Two main strategies have contributed to this intent: the development of the sentinel lymph node biopsy (SLNB) procedure [1] and the introduction of neoadjuvant chemotherapy (NAC) [2].
1.1. Sentinel lymph node biopsy
The main purpose of SLNB, initially developed in early-breast BC (cT1-cT2/cN0) patients, is staging the axilla through the pathological evaluation of the sentinel lymph node (SLN), thus allowing to appropriately choose the subsequent axillary management. The high negative predictive value of SLNB implies that patients with negative SLN(pN0) most probably have no additional axillary nodes involved. Consequently, these patients can be spared the standard complete axillary lymph node dissection (ALND), with the ensuing advantage of decreased morbidity and improved quality of life [3,4]. Randomized clinical trials (RCT) demonstrated that SLNB is indeed equivalent to ALND in terms of locoregional disease control and survival in SLN-negative early-breast BC patients [3,[5], [6], [7], [8], [9]].
Moreover, the IBCSG 23-01 RCT reported that performing ALND represents overtreatment in early-stage BC patients whose SLN presents only micrometastasis (foci >0.2 mm - ≤2 mm, pN1mi)) and has no benefit on patient outcome in terms of either disease-free survival (DFS) or overall survival (OS) [10,11].
1.2. Neoadjuvant chemotherapy
Neoadjuvant chemotherapy is currently included in the management of selected BC patients as those with cT3-cT4 tumors or those with locally advanced breast cancer (LABC) to either convert inoperable tumors into resectable ones or reduce operable tumors to a dimension compatible with breast-conserving surgery [12,13]. Patients presenting clinically positive nodes (cN+) most benefit from NAC considering the 40–60% pathological complete response (pCR) observed at the axilla level after treatment [14,15]. In addition, the achievement of an axillary pCR, together with residual breast tumor or not, strongly correlates with a more favorable prognosis [16,17].
1.3. Sentinel lymph node biopsy in the setting of neoadjuvant chemotherapy
As NAC may induce axilla fibrosis and alter the lymphatic drainage, major problems associated with SLNB are the successful SLN identification rate (SIR) and the SLN false-negative rate (FNR). In a previous study, we provided evidence that SLNB performed after NAC is feasible and accurately assesses the axillary response to NAC, thus properly predicting the axilla status, in cN+ LABC patients [18]. Three recent multicenter prospective studies further substantiated these findings in larger cohorts of NAC-treated cT0-T4/cN1-N2 BC patients [[19], [20], [21]]. The satisfactorily high SLN SIR (87.6%–97.2%) and low SLN FNR (5.1%–14.2%) reported in our and in these studies have significant clinical indications, as encouraging an increased use of only SLNB in patients fully responding to preoperative chemotherapy instead of performing ALND by default in all NAC-treated cN+ patients.
2. Study purpose
2.1. Rationale
Patients with residual axillary disease after NAC have a worse prognosis as compared to patients presenting a pCR [16,17]. However, whether the tumor burden (isolated tumor cells (ITC), micrometastasis, macrometastasis) influences this worsening in the same way is not quite clear. In particular, the clinical significance of post-NAC axillary micrometastasis remains to be ascertained.
Analyzing the SLN pathological status in a pilot cohort of patients initially diagnosed cN+ but downstaged to ycN- upon NAC, we obtained preliminary evidences that patients with micrometastatic SLN (SLNypN1mi)have DFS and OS similar to those of patients with disease-free SLN (SLNypN0). By contrast, these outcomes were significantly worse in patients with macrometastatic SLN (SLNypN1-3) [unpublished personal communication]. These findings are in line with a recent study that assessed prognosis according to the extent of axillary tumor residue in cN+/NAC-treated patients and showed that ypN0 and ypN1mi patients have similar DFS and OS at long term while ypN1-3 patients have a significantly less favorable prognosis [22]. Considering early-stage BC patients who do not receive NAC, the recent 10-year follow-up of the above-mentioned IBCSG 23-01 RCT corroborated the 5-year initial findings, i.e. that omitting ALND in SLNpN1mi patients does not jeopardize patient outcome [11].
2.2. Hypothesis
Assuming that NAC-treated cN+ patients downstaged SLNypN1mi at definitive evaluation are clinically equivalent to SLNpN1mi early-BC patients, then ALND would be unneeded for this patient subsetin the NAC setting. On the other hand, axillary micrometastases may have a different prognostic value after NAC compared to the adjuvant setting. Only large-scale RCTs would properly assess these two hypotheses.
However, the low occurrence rate of micrometastatic SLN (4–8%) [21, 22 and unpublished personal communication], the limited rate of axillary relapse (4% at 8y of FU) [unpublished personal communication] and the relatively low incidence of distant metastases (20–25% at 8y of FU) [unpublished personal communication] observed after NAC would preclude any RCT to achieve in a reasonable time powered conclusions on the risk of recurrence in cN+ patients presenting SLNypN1mi and not undergoing ALND.
To circumvent this difficulty, the Clinical Institute Humanitas of Milan (Italy) is promoting the NEONOD 2 trial that we here describe.
3. Patients and methods
3.1. Patients, study group allocation and treatments
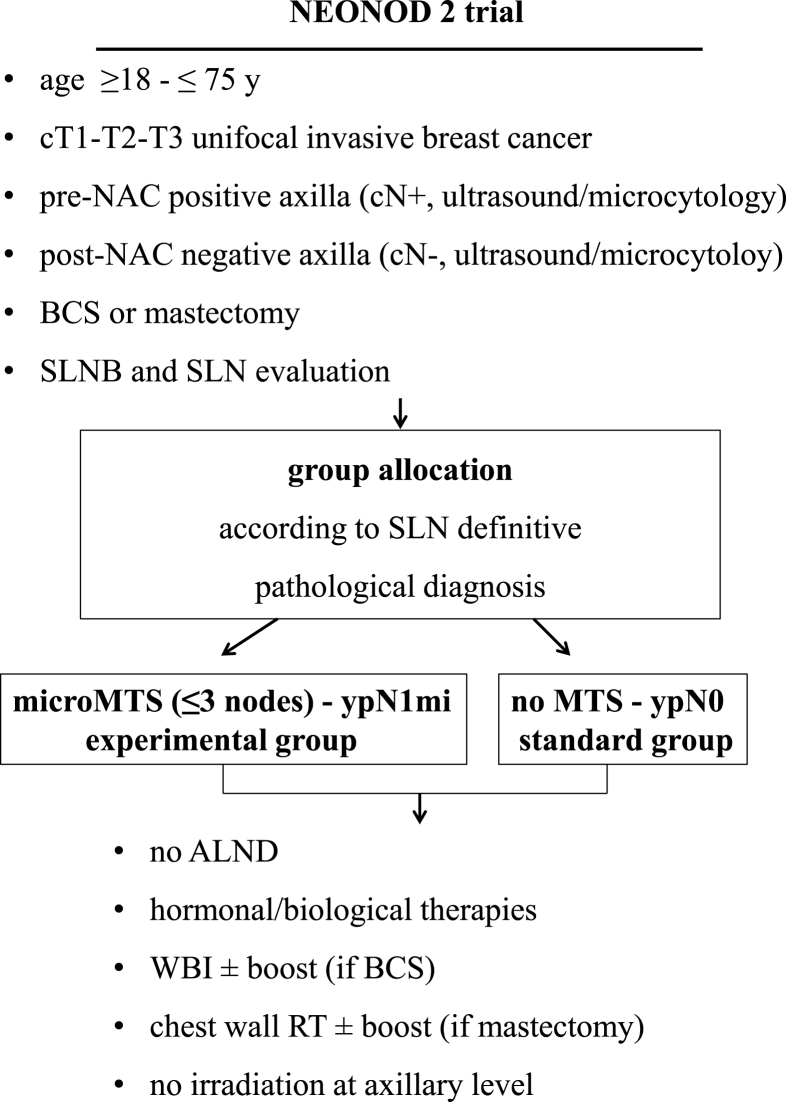
NEONOD 2 is a prospective uncontrolled non-inferiority trial to assess whether the preservation of axillary lymph nodes in cN+ patients candidate to NAC, downstaged cN- after NAC and diagnosed ypN1mi at definitive evaluation, does not worsen the prognosis in terms of recurrence and survival rates as compared to cN+ patients downstaged ypN0 for whom the ALND omission is current standard practice (Fig. 1).
Fig. 1.
NEONOD 2 trial: study design.
NAC, neoadjuvant chemotherapy; BCS, breast conserving surgery; SLNB, sentinel lymph node biopsy; SLN, sentinel lymph node; MTS, metastasis; ALND, axilaary
lymph node dissection; WBI, whole breast irradiation; RT, radiotherapy.
Table 1 summarizes patient eligibility criteria. The study population includes patients aged ≥18-≤75 years with infiltrating breast carcinoma (cT1-cT3) and clinically positive axillary nodes (cN+), candidate to NAC and subsequent SLNB. Among them, only those presenting post-NAC clinically negative nodes (cN-) and pathologically negative SLNs or micrometastatic SLNs (up to 3 nodes with micrometastasis) are retained and allocated to the experimental group (ypN1mi) or the standard group (ypN0). To insure homogeneity of SLN evaluation, the histopathological assessment of the SLNs retrieved is standardized among the participating centers. Each SLN is bisected along its major axis and serial sectioning at 200 μm intervals is performed in each half of the node. Sections are stained first with hematoxylin–eosin. If this histological evaluation results equivocal, additional serial sections are tested by immunohistochemistry for the presence of cytokeratins.
Table 1.
Enrollment criteria.
| Inclusion |
|---|
| A. Before surgery (clinical evaluation) |
|
|
|
|
|
|
|
|
|
| B. Intra-operative or post-surgery (definitive pathological diagnosis) |
| B1. Inclusion in the experimental group |
|
|
|
| B.2 Inclusion in the standard group |
|
|
|
| Exclusion |
|
|
|
|
|
|
| SLNB, sentinel lymph node biopsy; NAC, neoadjuvany chemotherapy; SLN, sentinel lymph node; ITC, isolated tumor cell |
Patients receive breast conserving surgery (BCS) or mastectomy but no axillary intervention. Based on the biological and pathological features of the tumor, a multidisciplinary committee establish post-surgery treatments including hormonal/biological therapies and radiotherapy (RT). Regarding RT, irradiation procedures are evaluated together with the AIRO (Italian Association of Radiotherapy and clinical Oncology) and standardized to avoid discrepancies between centers. More precisely, patients receive either whole-breast irradiation ± boost (BCS) or chest wall/reconstruction (if cT3) irradiation ± boost (mastectomy). Irradiation procedures include 3D conformational RT, intensity modulated RT or volumetric modulated Arc/tomotherapy but no high tangent fields. In any case, even in the absence of axillary dissection in the experimental group (ypN1mi), axillary level I/II nodes as well as station III/IV nodes and mammary internal chain are never irradiated, as of what is routine procedure in the standard group (ypN0). The avoidance of axillary irradiation in both groups may prevent differences in the regional recurrence rate.
Overall, the post-operative treatment is guided essentially by the bio-pathological features of the tumor and by the response (complete or not) to the neoadjuvant therapy, and is carried out according to the guidelines of each participating center which are all in line with the AIOM guidelines (Associazione Italiana di Oncologia Medica). Anyhow, would post-operative treatments result different between the two groups, survival analyses are adjusted also for this parameter.
All procedures used in this trial are conducted in accordance with the Declaration of Helsinki.
All patients are informed of the purpose, advantages and risks of the study and give a signed consent before enrollment.
In that respect, we would bring to attention that in many countries SLNB alone is not an accepted standard of care for patients who present initially with positive lymph nodes (cN1). Many guidelines still recommend ALND or Targeted Axillary Dissection (TAD). However, in all centers participating to the NEONOD 2 trial, SLNB is nowadays current practice when cN+ patients are downstaged cN0 (as estimated through instrumental assessment and possibly cyto-microhistology) after neoadjuvant chemotherapy.
It should also be noted that micrometastatic spread and isolated tumour cells are prognostically equivalent to N0 disease, with local as well as systemic treatment options selected according to other tumor and patient parameters. Based on the results of the IBCSG 23–01 trial [10], further axillary treatment is not required when a SLN presents micrometastasis (0.2–2 mm). The purpose of the NEONOD 2 trial is precisely to assess whether this approach applies also to NAC- treated ypN1mi patients.
3.2. Trial monitoring and quality control
Along the whole experimental process and follow-up assessment, all procedures are supervised through Monitor site visits including pre-trial monitoring visit, trial initiation visit, routine monitoring visit and close-out visit. In particular, the routine visits are aimed at assessing whether the study is accurately conducted according to the protocol and to the GCP (Good Clinical Practice) and at giving support to the investigational team in solving eventual problems. Regarding the filling in of the Case Report Forms (CRFs), quality control are performed at two levels: electronic queries are released extemporarily at the time of filling every single form if potential errors are detected; queries are periodically released on the whole database.
3.3. Statistical considerations
Primary endpoint is DFS. Secondary endpoints are OS, locoregional disease-free survival (LRDFS) and distant disease-free survival (DDFS).
According to the only published report [22], the 5-y cumulative rates of recurrence and death among patients with cN+/T2-4 BC, undergone NAC and ALND, are similar in the ypN0 group and the ypN1mi group and are approximately 30% and 20%, respectively. Consequently, the non-inferiority thresholds have been set for the present trial at the same values as reported in that study [22]. This implies that the null hypothesis to be rejected is that the 5y-DFS in the experimental group is 70%, and that the alternative hypothesis (i.e. non-inferiority) assumes that the experimental strategy (no ALND) is associated with a 5y-DFS of 80%, comparable to what is expected today in similar patients undergoing ALND after NAC.
3.4. Study power and sample size
To reject with alpha = 0.05 (1-sided) and power = 80% (under H1: 5y-DFS = 80%) the null hypothesis that ALND avoidance in the experimental group is associated with a 5-y DFS of 70%, 850 consecutive patients (ypN0 and ypN1mi) need to be enrolled, among whom ≈130 ypN1mi patients, and followed for at least 5 years. To stop the study if evidence arises that the experimental treatment is indeed inferior to the standard procedure, yearly futility analyses assessing DFS and OS are scheduled and the study is expected to cease should the probability (conditional power) of rejecting the null hypothesis of inferiority fall below 20% assuming that the alternative hypothesis (5y DFS = 80%) is true.
4. Statistical analyses
DFS, OS, LRDFS and DDFS are assessed using the Product Limit Estimator of Kaplan-Meier and the log-rank test. To avoid introducing bias in the survival analyses due heterogeneity between groups for some prognostic factor(s), multivariate Cox regressions are performed assessing survival after adjustment for the main histopathological factors, i.e. number of SLN retrieved, SLN status (negative vs micrometastatic), age, menopausal status, type of breast surgery, tumor size, tumor stage, grading, lymphovascular invasion, hormonal receptor status (ER/PgR), Ki67 level, HER2 status. If post-operative treatments between groups result different, survival analyses are adjusted also for this parameter.
The analyses are conducted both considering all patients included in the study (Intention-to-Treat principle) and excluding patients who did not undergo the treatment assigned (per protocol).
5. Concluding considerations
The study protocol has been approved by the ethical committee of the Clinical Institute Humanitas and its registration within the clinicaltrials.gov database is currently in progress. Patients enrollment will start at this center within the end of 2019.
Other 25–30 centers, including those participating to our currently ongoing RCT SINODAR ONE [23] are now in the process of submitting the protocol to their respective ethical committees. Patient recruitment is expected to last ≈3 years. All patients will be followed for at least 5 years after surgery. Follow-up assessments include clinical evaluation every 6 months for the first 5 years and thereafter annual breast mammography, breast ecography, and axillary ecography.
Fundings
This work is supported by grants from both Walgreens Boots Alliance and SIAS Autostrade.
Ethical approval
The study protocol was approved by the ethical committee of the Clinical Institute Humanitas.
All surgical procedures and clinical treatments used in this trial are conducted in accordance with the International Good Clinical Practice Guidelines and the Declaration of Helsinki.
All patients are fully apprised of the purpose, advantages and risks of the study and sign an informed consent prior to enrollment.
Declaration of competing interest
None.
References
- 1.Krag D., Weaver D., Ashikaga T., Moffat F., Klimberg V.S., Shriver C. The sentinel node in breast cancer – a multicenter validation study. N. Engl. J. Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B., Brown A., Mamounas E., Wieand S., Robidoux A., Margolese R.G. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 3.Mansel R.E., Fallowfield L., Kissin M., Goyal A., Newcombe R.G., Dixon J.M. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANACH trial. J. Natl. Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 4.Ashikaga T., Krag D.N., Land S.R., Julian T.B., Anderson S.J., Brown A.M. Morbidity results of the NASBP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J. Surg. Oncol. 2010;102:111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veronesi U., Paganelli G., Viale G., Luini A., Zurrida S., Galimberti V. A randomized comparison of sentinel node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 6.Zavagno G., De Salvo G.L., Scalco G., Bozza F., Barutta L., Del Bianco P. A randomized clinical trial of sentinel node biopsy versus standard axillary treatment in breast cancer : results of the Sentinella/GIVOM trial. Ann. Surg. 2008;247:207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 7.Canavese G., Catturich A., Vecchio C., Tomei D., Gipponi M., Villa G. Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: results of a randomized trial. Ann. Oncol. 2009;10:1001–1007. doi: 10.1093/annonc/mdn746. [DOI] [PubMed] [Google Scholar]
- 8.Krag D.N., Anderson S.J., Julian T.B., Brown A.M., Harlow S.P., Costantino G.P. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canavese G., Bruzzi P., Catturich A., Tomei D., Carli F., Garrone E. Sentinel lymph node biopsy versus axillary dissection in node-negative early-stage breast cancer: 15-year follow-up update of a randomized clinical trial. Ann. Surg. Oncol. 2016;23:2494–2500doi. doi: 10.1245/s10434-016-5177-4. [DOI] [PubMed] [Google Scholar]
- 10.Galimberti V., Cole B.F., Zurrida S., Viale G., Luini A., Veronesi P. Axillary dissection versus no axillary dissection in patients with sentinel node micrometastasis (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galimberti V., Cole B.F., Viale G., Veronesi P., Vicini E., Intra M., Mazzarol G., Massaru International Breast Cancer Study Group Trial 23-01. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018 Oct;19(10):1385–1393. doi: 10.1016/S1470-2045(18)30380-2. [DOI] [PubMed] [Google Scholar]
- 12.Bonadonna G., Veronesi U., Brambilla C., Ferrari L., Luini A., Greco M. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J. Natl. Cancer Inst. 1990;82:1539–1545. doi: 10.1093/jnci/82.19.1539. [DOI] [PubMed] [Google Scholar]
- 13.King T.A., Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015;12:335–343. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- 14.Budzar A.U., Valero V., Ibrahim N.K., Francis D., Broglio K.R., Theriault R.L. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin. Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 15.Mamtani A., Barrio A.V., King T.A., Van Zee K.J., Plitas G., Pilewskie M. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastasis ? Results of a prospective study. Ann. Surg. Oncol. 2016;23:3467–3474. doi: 10.1245/s10434-016-5246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennessy B.T., Hortobagyi G.N., Rouzier R., Kuerer H., Sneige N., Budzar A.U. Outcome after pathological complete eradication of cytologically proven breast cancer node metastases following primary chemotherapy. J. Clin. Oncol. 2005;23:9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 17.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 18.Canavese G., Dozin B., Vecchio C., Tomei D., Villa G., Carli F. Accuracy of sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with locally advanced breast cancer and clinically positive axillary nodes. Eur. J. Surg. Oncol. 2011;37:688–694doi. doi: 10.1016/j.ejso.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Boughey J.C., Suman V.J., Mittendorf E.A., Ahrendt G.M., Wilke L.G., Taback B. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. J. Am. Med. Assoc. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuehn T., Bauerfeind I., Fehm T., Fleige B., Hausschild M., Helms G. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 21.Boileau J.F., Poirier B., Basik M. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J. Clin. Oncol. 2015;3:258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 22.Van Nijnatten T.J.A., Simons J.M., Moossdorff M., de Munck L., Lobbes M.B.I., van der Pol C.C. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients : isolated tumor cells and micrometastasis carry a better prognosis than macrometastases. Breast Canc. Res. Treat. 2017;163:159–166. doi: 10.1007/s10549-017-4157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinterri C., Canavese G., Bruzzi P., Dozin B. SINODAR ONE, an ongoing randomized clinical trial to assess the role of axillary surgery in breast cancer patients with one or two macrometastatic sentinel nodes. Breast. 2016;30:197–200. doi: 10.1016/j.breast.2016.06.016. [DOI] [PubMed] [Google Scholar]