Abstract
Introduction
There is a significant need for disease-modifying therapies to treat and prevent dementia, including Alzheimer's disease. Availability of real-world observational information and new analytic techniques to analyze large volumes of data can provide a path to aid drug discovery.
Methods
Using a self-controlled study design, we examined the association between 2181 medications and incidence of dementia across four US insurance claims databases. Medications associated with ≥50% reduction in risk of dementia in ≥2 databases were examined.
Results
A total of 117,015,066 individuals were included in the analysis. Seventeen medications met our threshold criteria for a potential protective effect on dementia and fell into five classes: catecholamine modulators, anticonvulsants, antibiotics/antivirals, anticoagulants, and a miscellaneous group.
Discussion
The biological pathways of the medications identified in this analysis may be targets for further research and may aid in discovering novel therapeutic approaches to treat dementia. These data show association not causality.
Keywords: Dementia, Alzheimer's disease, Real-world evidence, Self-control cohort design, Disease-modifying therapy, Treatment options, Drug discovery
1. Background
The current landscape of available treatment options for dementia, including Alzheimer's disease (AD), is limited, and researchers have outlined the need for new initiatives to arrive at much-needed breakthrough medications [1]. Nearly all forms of dementia are irreversible [2], and instead of offering a cure, available medications only address symptoms of the disease. Currently, the most popular treatment options are acetylcholinesterase inhibitors (AChEIs) which have been the first-line pharmacotherapy for AD for more than 20 years [3], and memantine, which can be used alone or in combination with an AChEI [4], [5]. The benefits of these treatments are modest in many cases, and they do not reverse the progression of the disease or offer a potential cure. There is a significant need for disease-modifying therapies, and recent progress on the development of such treatments is especially limited when compared with major breakthroughs in other domains such as cancer and HIV [6]. Development of therapeutics for disease modification in AD has been both unsuccessful and limited to a small number of targets [7]. In recent years, there has been an increase in the development of non–amyloid-based therapies, and there is a wide consensus in the field that new approaches are needed to diversify discovery and development to identify truly novel and effective treatments [8], [9].
Pharmacoepidemiology and observational research has played a role historically in the identification of potential therapeutic targets for disease-modifying treatments to treat dementia. In t' Veld et al. conducted a large population-based cohort study and examined an association between nonsteroidal anti-inflammatory drug use and the reduced risk of dementia and AD [10], and others have used similar study designs to detect potential protective effects and/or risks of antihypertensive medications [11], [12], antioxidants [13], and hormone therapies [14]. These large prospective observational studies are useful for studying specific treatments of interest, but they are expensive and cannot be easily scaled to study many exposures.
Recently, administrative health insurance claims data have become widely available to researchers. This type of data captures a vast amount of health care information, and when paired with appropriate analytic techniques, it can play a key role in informing therapeutic development [6], [15]. The use of claims data is already seen as a vital resource for studying the safety and effectiveness of medications [16], [17], [18], but real-world data including administrative health data, electronic medical records, and other population level data might provide insights into disease etiology and mechanisms to contribute to the development of novel interventions. In one of an increasing number of examples of the use of insurance claims data, Zissimopoulos et al. examined potential protective effects of statins on incident dementia within a Medicare population [19]. Here, we aim to expand upon this approach to identify new potential treatment pathways for dementia that can be targets for further future research. Recent improvements in computational efficiency and statistical methodologies allow us to implement approaches not feasible a few years ago, specifically the ability to study the association between thousands of medications with many outcomes in millions of patients. This study leverages the availability of these health care data sources and analytic methods to identify potential new pathways for the treatment of dementia.
2. Methods
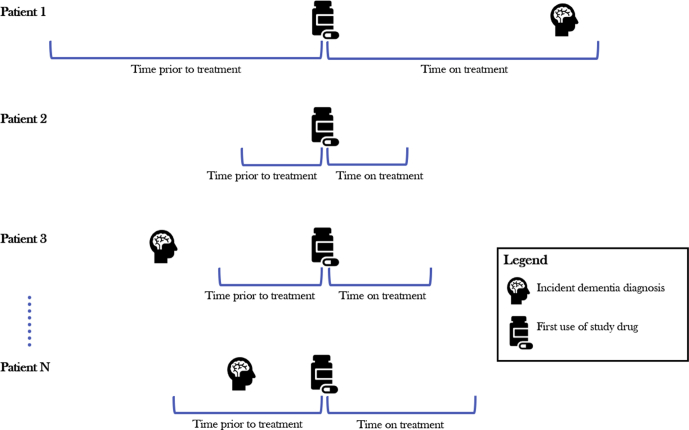
This study used a self-controlled cohort design in which individuals served as their own controls. This design tends to produce less biased estimates with higher predictive accuracy than other more commonly used designs, such as case-control studies [20]. Fig. 1 illustrates the study design for a single medication. A separate analysis was run for every medication in the database. This framework was applied to a previous study examining treatments associated with the risk of parkinsonism [21].
Fig. 1.
The self-controlled cohort study design. An example studying the association between one medication and incident dementia by including all patients who were exposed to the medication. It shows incident dementia occurring during the time a patient was on treatment (patient 1), not occurring in patient's history (patient 2), occurring outside of the observation windows and therefore not counted in either period (patient 3) and occurring during the unexposed control period (patient N). This approach was repeated for all 2181 medications identified in the database.
2.1. Exposure and control definition
Medications were identified according to the RxNorm ingredient [22]. Individuals were identified at the time they first filled the medication of interest. An exposure period was defined as the period starting with initiation of the medication until discontinuation or end of observation, allowing for a maximum of the medication supply plus a 30-day gap between consecutive fills. The time directly preceding the exposure and similar in length to the exposure period served as the unexposed (i.e., control) period.
Medications primarily given as acute treatments during hospitalizations (e.g., heparin) and single-use rescue medications were excluded. In addition, medications already being used for treating dementia were excluded from the analysis due to the confounded relationship to the outcome introduced by the study design. Because these medications are used to treat the condition, exposure to these medications will typically come after the first diagnosis of dementia and rarely before. This leads to a much higher incidence of the condition in the unexposed period compared with the exposed period and thus artificially reduces the relative risk when using this study design.
2.2. Outcome definition
Subjects with an incident dementia diagnosis were identified as those having at least two claims containing a diagnosis for dementia (ICD-9-CM and ICD-10-CM codes in Supplementary Table 1) on distinct service dates and occurring within 12 months of each other. Our dementia definition included AD, primary and secondary vascular dementia, among others. The date of the first diagnosis for dementia was defined as the date of incident dementia diagnosis. The codes used to identify dementia were based on a validation study that found good agreement between these diagnosis codes and a confirmed diagnosis of dementia found in a medical chart review [23].
2.3. Data sources
The analysis was executed in four US-based administrative claims databases. Each database contains data from adjudicated health insurance claims (e.g., inpatient, outpatient/emergency department, and outpatient pharmacy) and health plan enrollment information. Briefly, the four databases included in this study were as follows:
-
1.
IBM MarketScan® Commercial Database (CCAE): Includes data from 142 million individuals enrolled in employer-sponsored insurance health plans.
-
2.
IBM MarketScan® Multi-State Medicaid Database (MDCD): A claims database for 26 million Medicaid enrollees from multiple states.
-
3.
IBM MarketScan® Medicare Supplemental Database (MDCR): Includes data for more than 9 million retirees with primary or Medicare supplemental coverage through privately insured fee-for-service, point-of-service, or capitated health plans.
-
4.
Optum© De-Identified Clinformatics® Data Mart Database. Includes 84 million members with private health insurance, who are fully insured in commercial plans or in administrative services only and Medicare Advantage (Medicare Advantage Prescription Drug coverage). The population is representative of US commercial claims patients (0–65 years old) with some Medicare (65 + years old).
Data elements included were outpatient pharmacy dispensing claims (coded with National Drug Codes), inpatient and outpatient medical claims which provide diagnosis codes (coded in ICD-9-CM or ICD-10-CM) associated with a visit. The use of the IBM MarketScan and Optum claims databases was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subjects research.
2.4. Statistical analysis
For each treatment, incidence rates (IRs) of dementia were calculated for the exposed and unexposed periods. For the exposed period, IRexposed is defined as the number of all individuals with incident dementia diagnosed during the exposed period divided by the sum of exposed time across all patients. Similarly, IRunexposed was calculated as the number of all individuals with incident dementia diagnosed during the unexposed period divided by the sum of unexposed time across all exposed patients. An incident rate ratio (IRR) was then calculated as the IRexposed divided by the IRunexposed. If there is no association between the medication and dementia, the expectation is that cases of incident dementia will be equally distributed before and after initiation of the medication, leading to an IRR of 1.0. An IRR >1.0 indicates more cases identified after initiation of the medication, whereas an IRR <1.0 indicates fewer cases identified after initiation.
We applied three strict criteria to identify medications with potential protective benefits in dementia:
-
1.
The medication must have been associated with at least a 50% reduction in the incidence of dementia (i.e., IRR ≤0.5), and
-
2.
the association must have been statistically significant (P < .05) in at least two of the four databases, and
-
3.
there must have been no evidence of the contrary association of significant risk between the medication and dementia in any of the databases, defined as having an IRR >2.0 with P < .05.
The IRRs and 95% confidence intervals were reported for analyses in each of the four databases. A meta-analysis with random effects was then performed to pool results across the four databases into a single effect estimate and 95% confidence interval. The I2 measure was used to measure heterogeneity of the associations across the data sources.
3. Results
A total of 117,015,066 individuals were included in the analysis across the four databases. There were 2181 individual medications studied, 17 of which met our threshold criteria described previously. Average exposure time varied across the medications; while patients received most of the treatments for an average of 3 to 12 months, the average time on antibiotics was much shorter (Table 1).
Table 1.
Results from the pooled meta-analyses across four US claims databases
| Medication | N exposed | Mean ± SD treatment time (days)∗ | Cases in exposed period | Cases in unexposed period | IRR | Lower 95% | Upper 95% | P value | I2 |
|---|---|---|---|---|---|---|---|---|---|
| Catecholamine modulators | |||||||||
| Atomoxetine | 407,883 | 292 ± 308 | 80 | 112 | 0.69 | 0.39 | 1.20 | .189 | 0.68 |
| Mirtazapine | 864,577 | 329 ± 330 | 9586 | 20,665 | 0.49 | 0.43 | 0.55 | <.001 | 0.94 |
| Linezolid† | 131,921 | 28 ± 53 | 85 | 178 | 0.48 | 0.37 | 0.62 | <.001 | 0.00 |
| Anticoagulants | |||||||||
| Fondaparinux | 81,391 | 99 ± 246 | 45 | 116 | 0.38 | 0.27 | 0.54 | <.001 | 0.00 |
| Enoxaparin | 1,772,960 | 32 ± 90 | 1516 | 4569 | 0.42 | 0.31 | 0.57 | <.001 | 0.95 |
| Antibiotics/antivirals | |||||||||
| Ampicillin | 710,193 | 32 ± 87 | 170 | 339 | 0.50 | 0.41 | 0.61 | <.001 | 0.04 |
| Cefpodoxime | 245,868 | 14 ± 60 | 140 | 514 | 0.28 | 0.21 | 0.39 | <.001 | 0.46 |
| Cefuroxime | 3,452,799 | 17 ± 56 | 685 | 1732 | 0.44 | 0.35 | 0.54 | <.001 | 0.77 |
| Cefdinir | 7,408,135 | 15 ± 35 | 481 | 988 | 0.59 | 0.43 | 0.82 | .002 | 0.85 |
| Emtricitabine | 61,916 | 358 ± 354 | 66 | 97 | 0.62 | 0.39 | 1.01 | .054 | 0.48 |
| Anticonvulsants | |||||||||
| Valproate | 553,341 | 303 ± 297 | 3786 | 12,356 | 0.36 | 0.26 | 0.49 | <.001 | 0.99 |
| Oxcarbazepine | 252,034 | 313 ± 332 | 584 | 1103 | 0.49 | 0.44 | 0.54 | <.001 | 0.00 |
| Levetiracetam | 351,986 | 351 ± 371 | 3506 | 6927 | 0.53 | 0.42 | 0.66 | <.001 | 0.96 |
| Miscellaneous | |||||||||
| Acamprosate | 62,145 | 158 ± 173 | 40 | 90 | 0.45 | 0.30 | 0.68 | <.001 | 0.16 |
| Quinidine | 17,789 | 332 ± 291 | 231 | 617 | 0.39 | 0.29 | 0.53 | <.001 | 0.72 |
| Palonosetron | 385,832 | 99 ± 82 | 349 | 687 | 0.51 | 0.45 | 0.58 | <.001 | 0.00 |
| Pegfilgrastim | 305,935 | 86 ± 57 | 177 | 349 | 0.53 | 0.41 | 0.70 | <.001 | 0.44 |
Abbreviations: SD, standard deviation; IRR, incidence rate ratio.
Treatment duration calculated within patients who had an outcome of dementia in either the exposed or unexposed periods.
Linezolid is an antibiotic whose mechanism of action is acting as a monoamine oxidase inhibitor and therefore is included in the category of catecholamine modulators.
The 17 candidate medications fell into five main categories: catecholamine modulators, anticoagulants, anticonvulsants, antibiotics/antivirals, and a miscellaneous group.
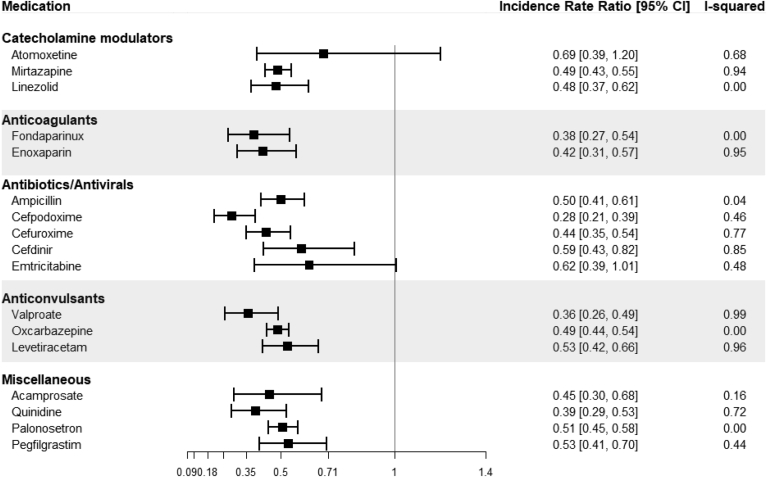
Some of the most consistent findings were within catecholamine modulators whose pooled effects ranged from a 52% decrease in incidence to a 31% decrease (pooled IR and 95% confidence interval): mirtazapine, an antidepressant (0.49 [0.43–0.55]), linezolid, an antibiotic (0.48 [0.37–0.62]), and atomoxetine, an attention-deficit/hyperactivity disorder treatment (0.69 [0.39–1.2]) (Fig. 2). A pair of anticoagulants were also found to have a strong negative association with incident dementia—fondaparinux and enoxaparin. There were a handful of antibiotics and an antiviral medication (ampicillin, cefpodoxime, cefuroxime, cefdinir, and emtricitabine) identified in the analysis. In addition, a group of anticonvulsants (valproate, oxcarbazepine, levetiracetam) had consistently strong protective associations. There were several other miscellaneous medications found to have protective associations, including acamprosate (an N-methyl-D-aspartate [NMDA] receptor antagonist and modulator of GABA receptors, used to treat alcohol dependence), quinidine (a class Ia antiarrhythmic agent associated with sodium channel interference), palonosetron (an antiemetic, 5-ht3 receptor antagonist), and pegfilgrastim (a granulocyte colony-stimulating factor). A full list of results within each of the databases and the pooled effects estimates from the meta-analysis can be found in Supplementary Table 2.
Fig. 2.
Forest plots of meta-analyses results for the medications found to have protective associations with dementia. Each bar represents the result of a meta-analysis for the pooled effect across the four claims databases. Abbreviation: CI, confidence interval.
In several cases, the I2 statistic indicated significant variability (>0.75) across the four data sources. However, this was mostly due to narrow confidence intervals that did not overlap with each other while the magnitudes of the point estimates were qualitatively consistent. For example, the effect estimates for mirtazapine ranged from 0.42 to 0.59, all of which indicate strong protective effects, yet the I2 is 0.94. Because of this, we examined each effect in the individual databases to infer heterogeneity, rather than relying solely on the I2 statistic.
4. Discussion
This study examined the association between more than 2000 medications and their association with incident dementia across four US administrative claims databases. By using strict criteria, we were able to identify 17 medications that showed a strong, consistent, protective association with the dementia outcome. Identifying potential treatment pathways is an important first step to discovering new, effective medications for dementia. The treatments we identified fell into five main categories: catecholamine modulators, anticoagulants, anticonvulsants, antibiotics/antivirals, and a miscellaneous group.
The most interesting of these groups may be the catecholamine modulators as it contains multiple medications working on a specific biologic pathway that could be explored further in a clinical setting. Atomoxetine is a serotonin and norepinephrine reuptake inhibitor used for the treatment of attention-deficit/hyperactivity disorder and is currently being studied for the treatment of patients with mild cognitive impairment [24]. Mirtazapine, a noradrenergic and specific serotonergic antidepressant, has been studied as a potential treatment for agitated patients with AD; however, research never moved beyond a small pilot study [25]. Linezolid, an antibiotic typically used for highly resistant bacteria, is a monoamine oxidase inhibitor and has antidepressant-like properties [26] though it is not used specifically as an antidepressant. The interesting aspect of this group is that each of the three medications are used to treat distinct conditions. Therefore, it is less likely that results are confounded by the presence of a single, common comorbid condition. For example, the negative association between antidepressant use and incident dementia may be partly confounded by the study design: because depression is common among patients diagnosed with AD, it follows that treatment for depression could more often come after a diagnosis of dementia than before, leading to a protective effect estimate. However, the association with atomoxetine and linezolid suggests the findings are due to more than this confounding relationship, as these medications would not be given as antidepressive treatments. This supports a hypothesis that the modulation of catecholamines may affect the development of dementia and deserves further inspection.
A pair of anticoagulants were also associated with a lower incidence of dementia. We examined another common anticoagulant, warfarin, and saw a protective effect, though the association was not strong enough to meet our a priori threshold criteria and therefore was not included in our results (data not shown). This finding is consistent with recent studies that have found similar, large protective effects of anticoagulants on the onset of dementia [27], [28], which could be through maintaining cerebral blood flow and decreasing the risk of vascular damage to the brain. Most evidence supporting this association is based on observational research within patients having atrial fibrillation; further evidence from a variety of data sources and a broader population is warranted.
Anticonvulsants also showed consistent protective associations across multiple medications. Current evidence is mixed on the use of these medications for treatment of behavioral symptoms within patients who have dementia [29], [30] and on the association between anticonvulsant use and the incidence of dementia and AD [31], [32]. However, there is evidence that anticonvulsants may have neuroprotective effects [33], [34], and neuroprotective strategies may be vital in delaying the onset of AD or slowing its progression [35], [36]. In fact, the anticonvulsant levetiracetam is currently being investigated as an AD treatment [37].
The association with multiple antibiotics and an antiviral medication (emtricitabine used for the treatment of HIV) is interesting. Persistent subclinical bacterial and viral central nervous system infections have been postulated as pathogenesis of dementia [38]; therefore, it can be hypothesized that antibiotic or antiviral exposure could lead to the successful treatment of subclinical infections avoiding a chronic brain immune response that could lead to accumulation of beta-amyloid protein that is the hallmark characteristic of dementia [39].
There were four other medications that did not fit neatly into the other classes highlighted here. Pegfilgrastim is a granulocyte colony-stimulating factor, a class of medications that has been studied recently for its potential effectiveness as a treatment for AD with promising early results regarding cognition, memory, and behavioral function [40], [41]. Their potential effectiveness stems from their ability to mobilize hematopoietic stem/progenitor cells that promote brain repair by releasing growth factors and immunomodulatory cytokines [41].
Early research in mice has found that the NMDA antagonist acamprosate, in combination with baclofen and standards of care, may be effective in alleviating cognitive deficits, consistent with the protective association shown in our study [42]. The AD treatment memantine works by blocking NMDA receptor and there has been keen interest in further exploration of NMDA receptor pharmacology for the treatment of dementia [43].
Quinidine may be effective for treating agitation in Alzheimer's patients [44], and it is possible that existing use of that medication in already diagnosed patients may be contributing to the protective effects found in this study. We did not find any evidence that the antiemetic palonosetron is being targeted specifically for use in patients with dementia. Therefore, the temporal relationship between use of this medication and disease onset is less confounded by the design of the self-controlled study and suggest further exploration is warranted.
There are several strengths of this study. The self-controlled study design allowed individuals to serve as their own control and thus control for any time-invariant covariates (e.g., genetics). The analysis described in this study includes 8724 statistical models, each with a unique combination of exposure, outcome, and database. We applied multiple filter criteria to allow us to review such a vast number of results and avoid erroneous findings due to multiple comparisons and overly narrow confidence intervals associated with the study design. We required that the medication must have been associated with at least a 50% reduction in the incidence of dementia, a very large effect in observational research, and the association must have been replicated in at least one of the other databases. Regardless, potential type 1 error was not a major concern of this study as the goal was to generate new targets for future research rather than to conclude definitive causal effects. Because of the strict filtering criteria, we found that when prior evidence of an association between dementia and a medication was mixed, it typically did not meet our inclusion threshold; for example, simvastatin, for which a large observational study found a strong protective association (HR = 0.46) [45], but no association was found in a phase 3 clinical trial [46] or in another observational study [47]. In our study, there was only a slight protective association (meta-analysis HR = 0.81); consequently, it did not meet our threshold criteria and was not included in our results. Similarly, prior research has found protective associations with use of nonsteroidal anti-inflammatory drugs [10] and potassium sparing diuretics [11], but in our study, these associations were not strong enough to be included in the results (e.g., meta-analysis HR for aspirin = 0.60, coxibs = 0.88, aldosterone antagonists = 0.87).
Another major benefit of the study design was that it does not require a large amount of computational resources, allowing us to perform the thousands of models relatively quickly, a framework that can then be expanded to any outcome of interest.
Although the self-controlled cohort design accounts for many potential confounding factors, it does not control for time-variant influences. A major risk factor of dementia is age, which varies over the course of follow-up. However, age—and thus risk of dementia—is greater in the post-treatment period versus the control period and more likely to bias the results toward showing a risk of the outcome rather than a protective effect.
There are other limitations to this study. The evidence gathered from this research is based on administrative claims data. Although not perfect, the diagnosis codes used to identify dementia in this study have good validity when compared with diagnoses found in medical charts [23] and comprised a broad definition of dementia with the inclusion of AD, vascular dementia, and other conditions. However, this program of research was designed to identify a manageable number of hypotheses of biological pathways not to confirm a causal association. Results may be biased in the protective direction if the treatment is already being used to treat dementia or conditions that are comorbid with dementia, such as for managing symptoms of agitation and depression. The self-controlled cohort analysis may not be ideal for chronic outcomes due to the limited amount of time patients remain on a medication after initiating therapy. However, if this were true, we would expect no difference in the rate of dementia before or after exposure, which was not the case for the several medications highlighted in our study. This is because individuals with very short exposure periods will add very little information to the analysis due to the exposure and control periods being too short to capture outcomes. Thus, patients with long exposure periods are the ones contributing the most information, and long exposures are most likely to be meaningful for outcomes of chronic conditions such as dementia.
Prior research examining the association between medications and incident dementia has implemented a lag-time into the study design to remove one or two years of observation time which directly preceded the outcome [10], [48]. However, applying this design element into our infrastructure was not feasible. Using a lag in a self-controlled cohort design, in which the index date is the date an individual initiated a drug, is not straightforward. Implementing a lag in our study would require that individuals are exposed to the medication for a minimum amount of time, after which outcomes may be captured. A similar lag would also have to be applied to the preexposure control period to minimize any bias due to differing times at risk. This would lead to a large amount of patient follow-up time that is excluded from the analysis, resulting in a much shorter observation time and ultimately a major loss in sample. For example, if a lag of two years is preferred, it would require four years of observation to be ignored (two years before and after initiation of the medication), leaving very few patients with a relevant amount of preexposure and postexposure time during which cases could be captured. Further work may build upon the findings in this paper to create a more tailored approach for a specific exposure of interest in which a study design could be implemented with an appropriate lag time.
This study identified associations between treatments and dementia, and results should not be used to infer causal relationships. Instead, the findings can be used to generate hypotheses for new therapeutic candidates for treating or preventing dementia.
5. Conclusion
There is a large unmet need for an effective disease-modifying therapy to treat dementia. The primary treatments for the disease have been around for more than 20 years, and new strategies are needed to help aid the drug discovery process. Our approach used the enormous amount of available, real-world observational claims data to perform large-scale analytics. This allowed us to start by studying the relationship between thousands of existing medications and incident dementia and end up with fewer than 20 medications in a handful of drug classes that may deserve further attention. This approach provides tangible targets for more rigorous research that can aid in the discovery of new and effective dementia treatments. Subsequent work will extend this approach to identify other potential treatment pathways to help accelerate treatments for patients with highly debilitating disorders.
Research in context.
-
1.
Systematic Review: The authors reviewed the literature using traditional sources (e.g., PubMed, Google Scholar) and online publications (e.g., policy briefs). Throughout the literature, there is an urgency to find effective, disease modifying treatments for dementia, including Alzheimer's disease, by using new research strategies.
-
2.
Interpretation: Using a novel research framework, we studied more than 2000 medications and found 17 were associated with a reduced risk of dementia. The information gained from this study can be used to generate hypotheses for potential new disease modifying therapeutics.
-
3.
Future Direction: New hypotheses regarding effective treatment pathways for preventing, delaying, or reversing the effects of dementia that may be produced from these results include studying the role of: (1). modulation of catecholamines, (2). maintaining cerebral blood flow via anticoagulants, (3). the neuroprotective effect of anticonvulsants, (4). granulocyte colony–stimulating factors, and (5). early detection and treatment of subclinical central nervous system infections.
Footnotes
All authors are employees of Janssen Research & Development, LLC and stockholders of Johnson & Johnson.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.07.012.
Supplementary Data
References
- 1.Taylor J., Marjanovic S., Nolte E., Pollitt A., Rubin J. Treatment for dementia: learning from breakthroughs for other conditions. Rand Health Q. 2016;6:5. [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathi M., Vibha D. Reversible dementias. Indian J Psychiatry. 2009;51:S52–S55. [PMC free article] [PubMed] [Google Scholar]
- 3.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer L., Bennett K., McGreevy C., Williams D. A population-based study of dosing and persistence with anti-dementia medications. Eur J Clin Pharmacol. 2013;69:1467–1475. doi: 10.1007/s00228-013-1483-y. [DOI] [PubMed] [Google Scholar]
- 5.Peskind E.R., Potkin S.G., Pomara N., Ott B.R., Graham S.M., Olin J.T. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14:704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer's Disease International World Alzheimer report 2018. The state of the art of dementia research: new frontiers. 2018. https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf
- 7.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimer's Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khachaturian A.S., Hayden K.M., Mielke M.M., Tang Y., Lutz M.W., Gustafson D.R. Future prospects and challenges for Alzheimer's disease drug development in the era of the NIA-AA Research Framework. Alzheimer's Dement. 2018;14:532–534. doi: 10.1016/j.jalz.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 10.in t' Veld B.A., Ruitenberg A., Hofman A., Launer L.J., van Duijn C.M., Stijnen T. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. New Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 11.Chuang Y.-F., Breitner J.C.S., Chiu Y.-L., Khachaturian A., Hayden K., Corcoran C. Use of diuretics is associated with reduced risk of Alzheimer's disease: the Cache County Study. Neurobiol Aging. 2014;35:2429–2435. doi: 10.1016/j.neurobiolaging.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z., Fratiglioni L., Zhu L., Fastbom J., Winblad B., Viitanen M. Occurrence and progression of dementia in a community population aged 75 years and older: relationship of antihypertensive medication use. Arch Neurol. 1999;56:991–996. doi: 10.1001/archneur.56.8.991. [DOI] [PubMed] [Google Scholar]
- 13.Kryscio R.J., Abner E.L., Caban-Holt A., Lovell M., Goodman P., Darke A.K. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer's Disease by Vitamin E and Selenium Trial (PREADViSE) JAMA Neurol. 2017;74:567–573. doi: 10.1001/jamaneurol.2016.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca W.A., Grossardt B.R., Shuster L.T. Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol. 2014;389:7–12. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman R.E., Anderson S.A., Dal Pan G.J., Gray G.W., Gross T., Hunter N.L. Real-world evidence - what is it and what can it tell us? New Engl J Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 16.Berger M.L., Sox H., Willke R.J., Brixner D.L., Eichler H.G., Goettsch W. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Value Health. 2017;20:1003–1008. doi: 10.1016/j.jval.2017.08.3019. [DOI] [PubMed] [Google Scholar]
- 17.Gagne J.J., Han X., Hennessy S., Leonard C.E., Chrischilles E.A., Carnahan R.M. Successful comparison of US Food and Drug Administration Sentinel Analysis Tools to traditional approaches in quantifying a known drug-adverse event association. Clin Pharmacol Ther. 2016;100:558–564. doi: 10.1002/cpt.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stang P.E., Ryan P.B., Racoosin J.A., Overhage J.M., Hartzema A.G., Reich C. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med. 2010;153:600–606. doi: 10.7326/0003-4819-153-9-201011020-00010. [DOI] [PubMed] [Google Scholar]
- 19.Zissimopoulos J.M., Barthold D., Brinton R.D., Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer Disease Association between statin exposure and Alzheimer Disease by Sex/Race Association between statin exposure and Alzheimer disease by sex/race. JAMA Neurol. 2017;74:225–232. doi: 10.1001/jamaneurol.2016.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan P.B., Stang P.E., Overhage J.M., Suchard M.A., Hartzema A.G., DuMouchel W. A comparison of the empirical performance of methods for a risk identification system. Drug Saf. 2013;36:S143–S158. doi: 10.1007/s40264-013-0108-9. [DOI] [PubMed] [Google Scholar]
- 21.Cepeda M.S., Kern D.M., Seabrook G.R., Lovestone S. Comprehensive real-world assessment of marketed medications to guide Parkinson’s drug discovery. Clin Drug Investig. 2019 doi: 10.1007/s40261-019-00830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. National Library of Medicine Unified medical language system® (UMLS®): RxNorm. 2019. https://www.nlm.nih.gov/research/umls/rxnorm/
- 23.Taylor D.H., Jr., Østbye T., Langa K.M., Weir D., Plassman B.L. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimer's Dis. 2009;17:807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ClinicalTrials.gov Identifier NCT01522404: Effects of Atomoxetine in Mild Cognitive Impairment (ATX-001) 2018. https://clinicaltrials.gov/ct2/show/study/NCT01522404
- 25.Cakir S., Kulaksizoglu I.B. The efficacy of mirtazapine in agitated patients with Alzheimer's disease: A 12-week open-label pilot study. Neuropsychiatr Dis Treat. 2008;4:963–966. doi: 10.2147/ndt.s3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel W.W. Antidepressant activity of linezolid. J Neuropsychiatry Clin Neurosci. 2013;25:E59. doi: 10.1176/appi.neuropsych.12030047. [DOI] [PubMed] [Google Scholar]
- 27.Ding M., Fratiglioni L., Johnell K., Santoni G., Fastbom J., Ljungman P. Atrial fibrillation, antithrombotic treatment, and cognitive aging. A population-based study. 2018;91:e1732–e1740. doi: 10.1212/WNL.0000000000006456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mongkhon P., Naser A.Y., Fanning L., Tse G., Lau W.C.Y., Wong I.C.K. Oral anticoagulants and risk of dementia: a systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehavioral Rev. 2019;96:1–9. doi: 10.1016/j.neubiorev.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher D., Herrmann N. Antiepileptic drugs for the treatment of agitation and aggression in dementia: do they have a place in therapy? Drugs. 2014;74:1747–1755. doi: 10.1007/s40265-014-0293-6. [DOI] [PubMed] [Google Scholar]
- 30.Konovalov S., Muralee S., Tampi R.R. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatrics. 2008;20:293–308. doi: 10.1017/S1041610207006540. [DOI] [PubMed] [Google Scholar]
- 31.Taipale H., Gomm W., Broich K., Maier W., Tolppanen A.M., Tanskanen A. Use of antiepileptic drugs and dementia risk-an analysis of Finnish health register and German health insurance data. J Am Geriatr Soc. 2018;66:1123–1129. doi: 10.1111/jgs.15358. [DOI] [PubMed] [Google Scholar]
- 32.Helmstaedter C., Beghi E., Elger C.E., Kalviainen R., Malmgren K., May T.W. No proof of a causal relationship between antiepileptic drug treatment and incidence of dementia. Comment on: Use of antiepileptic drugs and dementia risk-an analysis of Finnish health register and German health insurance data. Epilepsia. 2018;59:1303–1306. doi: 10.1111/epi.14432. [DOI] [PubMed] [Google Scholar]
- 33.Pitkanen A., Kubova H. Antiepileptic drugs in neuroprotection. Expert Opin Pharmacother. 2004;5:777–798. doi: 10.1517/14656566.5.4.777. [DOI] [PubMed] [Google Scholar]
- 34.Willmore L.J. Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav. 2005;7:S25–S28. doi: 10.1016/j.yebeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Longo F.M., Massa S.M. Neuroprotective strategies in Alzheimer's disease. NeuroRx. 2004;1:117–127. doi: 10.1602/neurorx.1.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer A.M. Neuroprotective therapeutics for Alzheimer's disease: progress and prospects. Trends Pharmacological Sciences. 2011;32:141–147. doi: 10.1016/j.tips.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov Identifier NCT03489044: An Investigation of Levetiracetam in Alzheimer's Disease (ILiAD) 2018. https://clinicaltrials.gov/ct2/show/NCT03489044
- 38.Maheshwari P., Eslick G.D. Bacterial infection and Alzheimer's disease: a meta-analysis. J Alzheimer's Dis. 2015;43:957–966. doi: 10.3233/JAD-140621. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D.K., Choi S.H., Washicosky K.J., Eimer W.A., Tucker S., Ghofrani J. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash A., Medhi B., Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-beta induced experimental model of Alzheimer's disease. Pharmacol Biochem Behav. 2013;110:46–57. doi: 10.1016/j.pbb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Ramos J., Cimino C., Avila R., Rowe A., Chen R., Whelan G. Pilot study of granulocyte-colony stimulating factor for treatment of Alzheimer's disease. J Alzheimer's Dis. 2012;31:843–855. doi: 10.3233/JAD-2012-120196. [DOI] [PubMed] [Google Scholar]
- 42.Cholet N., Foucquier J., Murphy N., Guedj M., Nabirotchkin S., Hajj R. A combination of acamprosate and baclofen (Pxt864) synergizes with standards of care for the treatment of Alzheimer's disease. Alzheimer's Dement. 2017;13:P948. [Google Scholar]
- 43.Ogden K.K., Traynelis S.F. New advances in NMDA receptor pharmacology. Trends Pharmacol Sci. 2011;32:726–733. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummings J.L., Lyketsos C.G., Peskind E.R., Porsteinsson A.P., Mintzer J.E., Scharre D.W. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314:1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- 45.Wolozin B., Wang S.W., Li N.-C., Lee A., Lee T.A., Kazis L.E. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano M., Bell K.L., Galasko D., Galvin J.E., Thomas R.G., van Dyck C.H. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zandi P.P., Sparks D.L., Khachaturian A.S., Tschanz J., Norton M., Steinberg M. Do statins reduce risk of incident dementia and Alzheimer disease?: the Cache County Study. JAMA Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 48.Power M.C., Weuve J., Sharrett A.R., Blacker D., Gottesman R.F. Statins, cognition, and dementia—systematic review and methodological commentary. Nat Rev Neurol. 2015;11:220–229. doi: 10.1038/nrneurol.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.