Abstract
Recent years have witnessed the expansion of tissue failures and diseases. The uprising of regenerative medicine converges the sight onto stem cell-biomaterial based therapy. Tissue engineering and regenerative medicine proposes the strategy of constructing spatially, mechanically, chemically and biologically designed biomaterials for stem cells to grow and differentiate. Therefore, this paper summarized the basic properties of embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and adult stem cells. The properties of frequently used biomaterials were also described in terms of natural and synthetic origins. Particularly, the combination of stem cells and biomaterials for tissue repair applications was reviewed in terms of nervous, cardiovascular, pancreatic, hematopoietic and musculoskeletal system. Finally, stem-cell-related biomanufacturing was envisioned and the novel biofabrication technologies were discussed, enlightening a promising route for the future advancement of large-scale stem cell-biomaterial based therapeutic manufacturing.
Keywords: Stem cells, Biomaterial, Regenerative medicine, Biofabrication, Biomanufacturing
Graphical abstract
1. Introduction
Stem cell, a particular cell type with self-renewing ability, has the potential to differentiate into different cell lineages and has gradually become the most versatile and valuable cell source for organ transplantation, disease treatment and cosmetology. Technically, the term stem cell can be divided into three specific categories: embryonic stem cell, induced pluripotent stem cell and adult stem cell, depending on the cell's developmental potential. All of them play a crucial role in tissue regeneration. However, some therapeutic defects such as low efficiency of differentiation protocols, high risk of teratoma formation and poor immune compatiblility [1] remain unsolved when the stem cells are used alone. Therefore, a new reliable approach has been applied - the introduction of advanced biomaterials.
Biomaterials are usually designed to possess appropriate biochemical and biophysical properties - including benign molecular compatibility, high porosity and suitable mechanical strength - mimicking the microenvironment of natural extracellular matrix (ECM) [2]. Such an artificial environment may either serve as a bio-adhesive surface for 2D cell culture, for example hydrogel microarrays used for supporting stem cell attachment and proliferation [3], or as 3D scaffold for a specific cell type to interact with and to control their function, thereby inducing the multi-spatial and temporal cellular processes of tissue formation and regeneration. Compared to the conventional cell type-specific biomaterial [4], the novel stem cell-interacting biomaterial is designed to be capable of meeting the need of diverse cell types due to the presence of bioactive cues in the biomaterials [5].
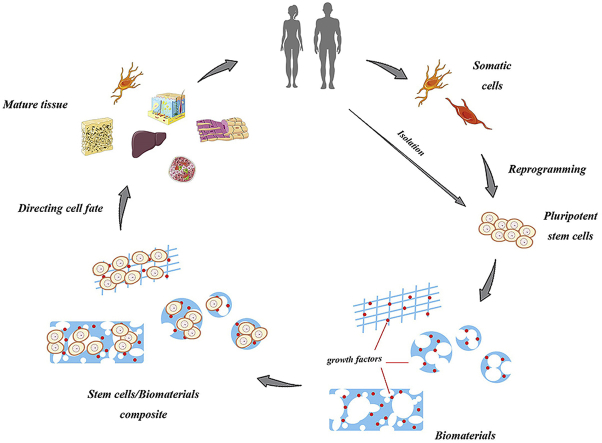
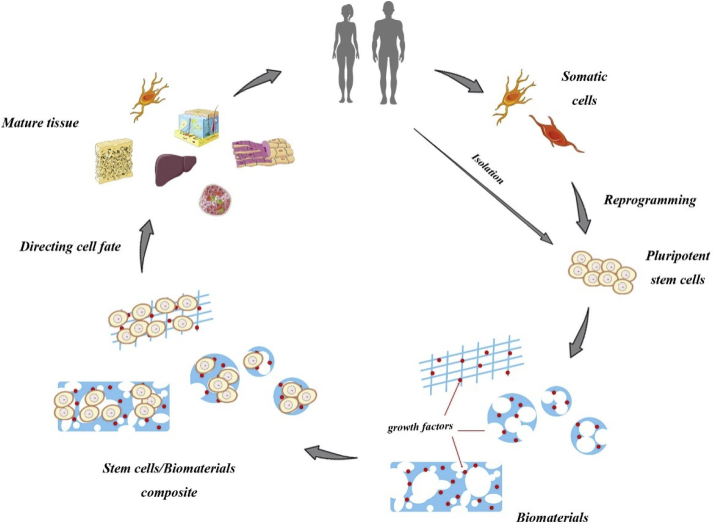
Almost 140 different combinations between stem cells and biomaterials are reported in recent studies, and the efficiency and properties of the cell-material interactions included morphology, vitality, cytotoxicity, apoptosis and proliferation [6]. As depicted in Fig. 1, the ultimate goal of the study is to identify materials that can regulate cell function as well as to find the appropriate biomaterial-stem cell combination for human body.
Fig. 1.
Schematic of stem cells and biomaterials application.
In this review we firstly outlined the various properties of stem cells and biomaterials, respectively. We then summarized the innovative application of biomaterial-stem cell interactions into clinical therapy for tissue and organ regeneration.
2. Stem cell
The primary merit of stem cells is their infinite proliferation competence. Due to their distinct proliferative properties, stem cell has also been referred as an “undifferentiated” cell type. Whether the stem cells remain self-renewal or turn into differentiated functional cells is mainly determined by the intrinsic state or the microenvironment in which stem cells reside—niche [7]. Naturally, stem cell keeps the balance between quiescence and activation. Considering the process of division, it can be categorized into asymmetric division (a retained stem cell and a daughter cell) and symmetric division (two daughter cells). Stem cells can be specified as totipotent stem cells (capable of producing all cell types of body), pluripotent stem cells (capable of producing all cells of the embryo), multipotent stem cells (capable of producing cells impacted by the microenvironment) and unipotent stem cells (capable of producing only one or two types of cells).
In mammals, only zygotes and spores are totipotent, and they will develop into embryonic lineages and extraembryonic lineages. Extraembryonic cells form the placenta, whereas the inner cell mass (ICM) becomes pluripotent, possessing the capacity to give rise to all cell types of the body. When the ICM is isolated and cultured in vitro, we can formulate embryonic stem cells (ESCs) that are able to generate every cell type within the human body [8].
While pluripotent stem cells expand and differentiate, part of them are maintained locally and have the ability to either self-renew or generate specialized cell types for a confined tissue, making them multipotent stem cells. Examples include neural stem cells, hematopoietic stem cell and mammary stem cell etc. [[9], [10], [11]], all of which play important roles in both organogenesis and tissue regeneration. In general, most adult stem cells (ASCs) are multipotent and have limited potency and finite periods of regeneration. ASCs are derived from patient or their parent without ethical issues and are widely used for therapy such as leukemia and radiotherapy [12,13]. Unlike the pluripotent and multipotent stem cells, unipotent stem cells have the lowest differentiation potential along only one lineage, however, the fact that adult unipotent germline stem cells can give rise to reproducible germline-derived pluripotent stem cells [14], addresses more potential to the unipotent stem cells.
At the beginning of human developmental studies, researchers used cells from teratocarcinomas, a cancer line derived from germ cells [15]. The problems, including out-of-control differentiation into multiple cell types, called for a more feasible way to find tractable model for studying human cells and disease in vitro. In following years, scientists demonstrated that by using different transcription factors (e.g. Oct 3/4, Sox 2, Klf4 and c-Myc), differentiated somatic cells could be reprogrammed to become induced pluripotent stem cell (iPSC) [16]. These iPSCs are reset to the pluripotent state primed for cloning and medical treatment similar to that of the ESCs. Therefore, the complete pluripotency of those reprogrammed cells broadened the source of stem cells and promised the future of cell therapy.
A brief summary of ESC, ASC, and iPSC are described in Table 1.
Table 1.
The main types of stem cells.
| Stem cell type | Source | Examples | Differentiation capacity | Ref |
|---|---|---|---|---|
| Embryonic stem cell | Derived from ICM of the embryo | H1, H9 | Capable to differentiate into any cell type of the body and infinite regeneration | [17,18] |
| Adult stem cell | Isolated from restricted organ | Neural/lung/hematopoietic stem cell | Limited potency and finite periods of regeneration | [19,20] |
| Induced pluripotent stem cell | Reprogrammed from somatic cells | Reprogrammed fibroblasts | Similar to that of the embryonic stem cell | [[21], [22], [23]] |
3. Biomaterial for stem cell culture
Biomaterials serve as non-viable materials in medicine to repair malfunctional tissues and organs. The past five decades have witnessed tremendous growth in biomaterial science and engineering as a result of vast investment in developing new products. Biomaterials comprise of two species: natural and synthetic materials. As discussed earlier, a specific environment is required for stem cell survival. Therefore, to mimic the in vivo microenvironment, biomaterials open up a new avenue for regulating stem cell fate via cell-matrix interactions. Biomaterial scaffolds can provide cell adhesion sites and maintain the merits of stem cells. In contrast to traditional 2D culture, the novel 3D biomaterial scaffolds construct a more satisfactory microenvironment for stem cells by including both chemical and physical signals across the ECM. Upon well-designed configuration, scaffolds can directly regulate cell signaling and trigger lineage-specific differentiation of stem cells by chemical cues or cell-matrix interactions [24].
With the growing interest in utilizing biomaterial-based approaches, the properties of the biomaterials were found to affect stem cell lineage specification. Hence, surface, mechanical, electrical, electrostrictional, morphological and chemical properties must be precisely considered when designing a new scaffold [25]. After elaborate selections, the cell adhesion, cell transportation, cell differentiation and matrix organization can be modulated to direct stem cell differentiation. Table 2 summarized typical biomaterials for stem cell culture and the detailed properties of each category will be unfolded in the following part.
Table 2.
Biomaterials for stem cell culture.
| Types | Examples | Properties | Application | |
|---|---|---|---|---|
| Natural biomaterial | Collagen, hyaluronic acid, gelatin, laminin, fibrin | Good biocompatibility | Cartilage/bone repair | |
| Less immune responsive | Osteochondral repair | |||
| Self-existing biosignal | Cornea repair | |||
| Short degradation period | Nerve regeneration | |||
| Poor mechanical strength | Coating matrix | |||
| Synthetic biomaterial | Polymer | PLA, PLGA, PCL, PEG, PVA, PHEMA, PMMA | Easy modification Properties can be designed |
All kinds of stem cell culture and tissue repair |
| Ceramic | HA, TCP, bioactive glass | Good mechanical strength Poor degradability Poor tensile property |
Additives in bone tissue engineering | |
| Metal | Titanium, titanium alloy, stainless steel, cobalt alloy | Good compressive strength Good fatigue resistance Non-degradable Non-bioadhesive |
Orthopedic and dental treatment | |
3.1. Natural biomaterials
With the goal of mimicking the 3D ECM to regulate stem cell behavior, certain natural biomaterials have been adopted to support stem cell proliferation and differentiation, including collagen, gelatin, hyaluronic acid hydrogels, fibrin, glycosaminoglycans (GAGs), alginate, matrigel, silk and hydroxyapatite (HA), etc. These materials exhibit specific advantages, including similar mechanical and adhesive properties as the natural ECM, while batch variability, short degradation period, difficulty in purification and quality control make up the main disadvantages of these materials.
Collagen, present in all connective tissue, acts as main component of ECM with superior biocompatibility, due to the fact that collagen-derived acellular ECM would not cause serious adverse immune responses [26]. Of note, the biodegradability of collagen makes it a better choice in skin tissue restoration because of its high rate of degradation [27]. While it is the most abundant protein in animals, collagen is difficult to obtain for research and clinical treatment, leading to the production of recombinant collagen for unlimited supply [28]. Moreover, collagen-based biomaterials are now used for cartilage regeneration treating osteochondral defects [29] and cornea defects [[30], [31], [32]].
Extracted from cartilage, hyaluronic acid hydrogels (also known as hyaluronan) serve as a native component and play an essential role in cartilage homeostasis and biomechanical integrity, including morphogenesis, proliferation, cellular signaling and wound repair [[33], [34], [35]]. Hyaluronic acid hydrogels has been used in tissue repair and regeneration as well as adriamycin-induced cytotoxicity prevention by forming a bioartificial stem cell niche [35]. Hyaluronic acid hydrogels were also photo-crosslinked for the application in chondrogenesis where almost all of the encapsulated mesenchymal stem cells (MSCs) survived [36].
Gelatin, derived from collagen, is now widely employed as a scaffold material for cartilage tissue engineering due to its biocompatibility, biodegradability and ability to form hydrogels [37]. Gelatin can be functionalized with unsaturated methacrylamide to create covalently bound hydrogels for encapsulating stem cells. Of note, gelatin presents a better performance in biomechanical and biochemical properties when compared with other frequently-used hydrogels like alginate and agarose [38].
Matrigel is a soluble basement membrane extract from Engelbreth-Holm-Swarm (EHS) murine sarcoma. It is composed of laminin, type IV collagen, nestin, heparin sulfate glycoprotein, as well as growth factors and matrix metalloproteinases. At room temperature, matrigel polymerizes to form a biologically active 3D matrix, which mimics the structure, composition, physical properties and functions of the cell basement membrane in vivo, benefitting the culture and differentiation of cells in vitro [[39], [40], [41]].
Another classic tissue-derived biomaterial scaffold is made of fibrin, which presents superior properties for providing a microenvironment for stem cells. For instance, nerve growth factor β-NGF was covalently incorporated with fibrin scaffold to produce neurons and oligodendrocytes [42,43]. However, plasmin inhibitor had to be co-operated to avoid unexpected degradation of the 3D scaffold caused by the ESCs [44].
3.2. Synthetic biomaterials
Although natural biomaterials have favored biocompatibility and self-existing biosignals, the frail mechanical strength and difficulty in modification limit their broader applications. To overcome these obstacles, synthetic scaffolds have become a solution. As a designed component, the structure and relative mass of a synthetic biomaterial can be controlled at will. Nevertheless, synthetic biomaterials are not consummate for this application since they lack cell adhesion properties and biological signals and thus cannot direct cell fate on their own. Notably, biocompatibility and bioresorbability of the synthetic composite frequently acts as the most essential hurdle in stem cell culture, and many studies are being conducted to solve these issues.
3.2.1. Synthetic polymers
Polymers serve as the most prevalent type of biomaterials. Commonly used polymers for stem cell culture include polylactic acid (PLA), poly (lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), polyethylene glycol (PEG), polyhydroxyl ethyl methacrylate (PHEMA) and polyvinyl alcohol (PVA). Lactic acid polymers have a long application history since their invention in the 1700s and are now widely used in various fields [45]. PLA and PLGA exhibit superiority including biocompatibility, biodegradability, bioresorbability, low immunogenicity and low toxicity over other synthetic polymers, making them favorable materials as 3D scaffolds for applications in dentistry, plastic surgery and so on [46]. With surface coating of polydopamine, PLA has been proven to promote and regulate the human adipose-derived stem cell adhesion, proliferation and differentiation [47]. PCL was mixed with PLA to improve the thermal resistance and mechanical properties of engineered tissues [48]. PEG is well accepted for human MSC osteogenic differentiation, as PEG gels provide abundant interspace for nutrient and waste diffusion between stem cells and the extracellular matrix [49,50]. Moreover, glucosamine-modified PEG hydrogel for cartilage restoration has shown an enhanced biocompatibility, while preventing fibrosis and hypertrophic cartilage markers [51].
3.2.2. Synthetic ceramics
Calcium phosphate, bioactive glasses and calcium phosphate cements are common ceramic additives for directing stem cell differentiation in the field of orthopedics, dentistry and bone tissue engineering. Though ceramics have poor degradability and tensile properties, the superior osteo-inductive and osteo-conductive characteristics and mechanical properties make them popular in bone tissue engineering. In order to enhance the initial mechanical strength and provide sufficient stiffness for the bone recovery device, hydroxyapatite-based calcium phosphate and bioactive glasses are usually added to form a composite for optimized osteo-fate directing [52,53]. The addition of bioceramics can be also used to enhance the porosity of a polymer implant for improved nutrition and waste transport throughout the scaffolds by introducing micro-/nanoscale voids characteristic of the ceramics [54]. However, the use of a ceramic scaffold can be sensitive. For example, while calcium phosphate cements possessed a unique injectable property and brilliant biocompatibility in vivo, the release of phosphate from an unmodified scaffold lowered medium pH and hampered cell proliferation in vitro [55]. By sintering the scaffolds at a very high temperature, Link et al. [55] altered the calcium phosphate content to a more stable form and the resulting scaffolds improved the specification of osteoblast-like cells in vitro. These results indicated that the physiochemical state of bioceramics played a crucial part in cytotoxicity and both in vitro and in vivo performance needed to be taken into account to comprehensively understand the function of bioceramics for clinical application.
3.2.3. Synthetic metals
Titanium, titanium alloys, stainless steels and cobalt alloys are the commonly used metals that contribute to bone regeneration, especially for orthopedic and dental treatments. Stainless steel implants serve as the most accepted materials because of easy procurement; however, it is hard to control the cell-metal interactions on the surface of the materials due to non-specific protein adsorption and cell adhesion, which leads to sub-optimal integration with the host tissue. A recent study aimed to overcome these deficiencies by covalently tethering adhesive peptides to functionalized stainless steel to control stem cell attachment [56]. Another study for improving the biocompatibility of stainless steels by utilizing ZrO2 and SiO2/ZrO2 coating showed that the proliferation of stem cells was dependent on stainless steel scaffold surface properties [57]. Titanium and tantalum demonstrated satisfactory biocompatibility, anticorrosion and excellent mechanical properties which could improve MSC multilineage differentiation in vitro by providing adequate plots for cell adhesion in 3D porous scaffolds [58]. In addition to increasing cell viability, titanium and tantalum could also conserve the immunophenotypic features of MSCs [59]. Moreover, a tantalum film may be deposited onto titanium alloy (Ti6Al4V) by filtered cathodic vacuum arc deposition (FCVAD) to enhance the mechanical and anticorrosion properties as well as cytocompatibility for mammalian bone MSCs [60]. Another innovative study exploited a two-layer coating comprised of a tantalum layer and a polymer-titanium hybrid layer on medical devices to enhance the biocompatibility for MSCs [61]. These results support that the titanium, tantalum, and their alloys are suitable for the biomaterial scaffolds.
While metallic biomaterials stand out in compressive strength and fatigue resistance, potential risks exist for current applications include toxic ion release and non-degradable fixtures. Promisingly, magnesium (Mg) and its alloys could serve as a novel substrate to settle these issues. The elastic modulus and compressive yield strength of Mg is similar to that of natural bone, so it can provide an imitative physical microenvironment for cell culture. Furthermore, the magnesium cation is one of the natural trace elements in human body and thus it is relatively harmless upon degradation [62,63]. However, several parameters for 3D scaffold design in MSC differentiation need to be investigated in detail, and more efforts are required to unveil the complex mechanism [64] and the functional pathways of Mg and its alloys.
3.2.4. Synthetic graphene
Recently, one of the 2D complanate structure biomaterials, graphene, has come into sight. Graphene has plenty of marvelous characteristics such as adequate reaction area, active surface chemistry and functional electrical/thermal conductivity [65,66]. Graphene and its derivatives could serve as a biocompatible and biodegradable preconcentration platform for MSC growth and osteogenesis, through firm non-covalent binding. The differentiation could be modulated by interactions of growth factors and π–π stacking as well as electrostatic and hydrogen bonding [67]. Interestingly, graphene and its derivatives could also act as ultrasensitive bio-detection materials for immunosensors of the Nanog protein to quantify the pluripotency of stem cells [68]. There have been studies demonstrating the merits of graphene in stem cell culture; nevertheless, we have to admit that the mechanism of stem cell-graphene interactions are barely understood and there is still a demand for further studies to shift the graphene-based stem cell niche from 2D planes to 3D structures.
4. Biomaterials & stem cell applications
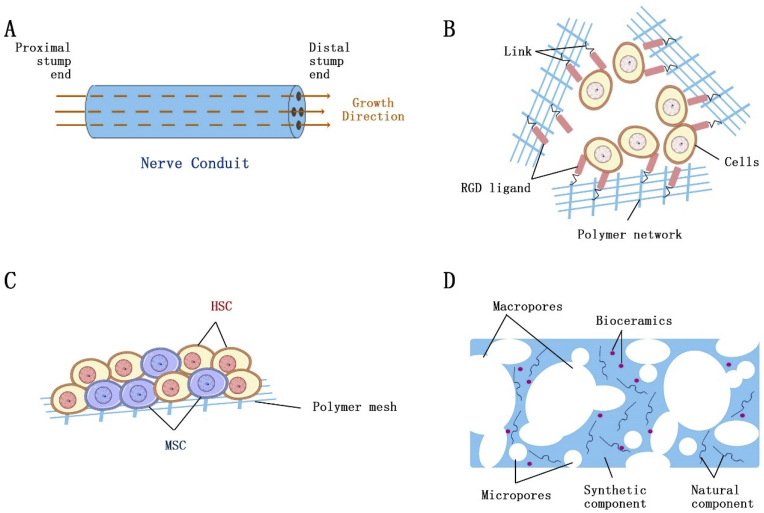
Biomaterials play an essential role in the field of tissue engineering and regenerative medicine. The diversity and versatility of these biomaterials have promoted a bloom of development in the field. With the increasing demand for tissue repair, a plethora of biomaterials are modified and exploited in various applications with regard to specific tissue type, as illustrated in Fig. 2 and listed in Table 3. In this section, detailed applications of novel biomaterials to direct stem cells into different lineages for tissue repair will be discussed accordingly.
Fig. 2.
Different models of biomaterials for various tissue types. A. Conduit-based scaffold for peripheral nerve regeneration; B. Encapsulation for cardiac tissue and pancreatic islet regeneration; C. Co-culture of MSC and HSC for hematopoietic system repair; D. Bi-modal porous scaffold for bone/cartilage tissue regeneration.
Table 3.
Biomaterials used in different tissue types for specific functions.
| Tissue type | Biomaterials | Stem cell types | Applications | Ref. |
|---|---|---|---|---|
| Nerve tissue | Collagen | Nerve progenitor cell | Central nerve regeneration | [69] |
| Poly (l-lactic acid)-co-poly-(3 caprolactone) | MSC | To enhance differentiation into neurons | [70] | |
| PLGA | KT 98 | To increase proliferation rate | [71] | |
| PEDOT-HA | PC12 | To enhance synapse growth | [72] | |
| Collagen-fibrin | Schwann cell | Peripheral nerve regeneration | [73] | |
| Cardiovascular tissue | Alginate | MSC | To encapsulate cells | [74] |
| RGD peptide | MSC | To promote cell attachment | [74] | |
| GFOGER hydrogel | Cardiac progenitor cell | To induce cardiomyocyte differentiation | [75] | |
| Pancreatic islet | Alginate, agarose, PEG, PLGA, PLLA | Any iPSC, ESC | To encapsulate cells | [[76], [77], [78], [79]] |
| Hematopoietic system | Tropoelastin | HSC | To enhance proliferation | [80] |
| Collagen | HSC | To modulate matrix elasticity | [81] | |
| Collagen, PEG, PCL | HSC, MSC | To support co-culture of HSC and MSC | [[82], [83], [84]] |
4.1. Nerve tissue
Neurological diseases are a severe threat to human health: about 46.8 million people suffer from dementia and Alzheimer's Disease (AD) and over 10 million patients were diagnosed with Parkinson's Disease (PD) [95,96]. However, lacking the ability of spontaneous regeneration, the repair/reconstruction of nervous system needs to be associated with therapeutic intervention, in which nerve tissue engineering scaffolds are designed to direct the formation of new neurons from stem cells in vitro, for the replacement of damaged or malfunctional nerve tissue.
Natural biomaterials, such as collagen, alginate, gelatin, chitin, elastin and hyaluronic acid, are widely used scaffold matrices for nerve tissue repair due to their excellent biocompatibility and cell-adhesive properties. For example, aligned and oriented 3D collagen hydrogels, prepared by mechanical strain stimulation after gelation, were proven to enhance neuronal growth compared to 2D culture and proven to direct an elongated morphology of both single-cell neuron and nerve tissue explant [97]. Scaffolds compatible with stem cells are favored as they hold the potential of directing differentiation of stem cells into any neural and glial cell type that are required in the treatment. Winter et al. [69] devised transplantable tubular hydrogel-collagen micro-columns, mimicking glial tubes, to guide nerve progenitor cells for central nervous system regeneration. Moreover, collagen-based scaffolds have been shown to preserve in a hypothermic condition for up to 4 days without affecting cell viability and metabolic activity, indicating a promising feasibility of clinical and commercial translation of this system [98]. Nonetheless, natural scaffolds suffer from relatively-weak mechanical properties, and thus synthetic biomaterials were introduced into natural scaffold. For example, poly-(l-lactic acid)-co-poly-(3 caprolactone) was mixed with collagen to form a nerve tissue-targeted nanofibrous scaffold that enhanced the differentiation of MSCs into neurons [70]. The incorporation of hyaluronic acid doped-poly (3,4-ethylenedioxythiophene) (PEDOT-HA) nanoparticles into chitosan/gelatin (CS/Gel) scaffold led to better mechanical properties and improved substrate conductivity, enhancing the direction of synapse growth [72].
The expanding demand for nerve injury treatment poses an increase in the study of synthetic scaffolds, as they can overcome the problems encountered by traditional allografts and autografts, including limited availability and immune rejection. Synthetic scaffolds may be rationally designed and engineered with suitable biodegradability, biocompatibility, porosity, hydrophilicity and mechanical strength for the injured site. The availability of different synthetic biomaterials with different functions [99], including poly l-lactic acid (PLLA), PLA, PCL, polyglycerol sebacate, PLGA, poly-3-hydroxybutyrate (PHB), polyamide, polydioxanone, poly-ε-caprolactone-co-ethyl ethylene phosphate (PCLEEP), poly-D. l-lactide-co-caprolactone (PDLLCL), PVA, poly acrylonitrile-co-methylacrylate (PAN-MA) and a copolymer of methyl methacrylate and acrylic acid (PMMAAA), has enabled the incorporation of various neurotropic factors for neuronal repair. Of note, a conduit-based technology, a specific scaffold that could provide an appropriate microenvironment for peripheral nerve regeneration over long nerve gaps or in the case of large-sized nerves, has been an active research area in both fundamental studies and clinical trials. In this nerve conduit, the proximal and distal nerve stumps are plugged into the two ends of the tube respectively, and axons are formed from the proximal nerve and eventually germinated maturely at the end of the distal nerve. Due to the restricted growth by encapsulated conduit, the probability of immune rejection and neuromas formation will decrease. For example, a gelatin cryogel 3D conduit was shown to recover the transected peripheral nerve and reduce the risk of scarring other tissues [100]. A hybrid structure guidance conduit by wrapping PLGA microfiber bundles in a flat micro/nanostructured PLGA membrane was also shown to increase cell proliferation and promote neurite outgrowth [71]. In another in vivo test [73], collagen-fibrin conduit rods containing Schwann cells increased axonal differentiation in the midsection and distal part of the injured nerve after 4 weeks, providing strong proof of peripheral nerve regeneration using conduit biomaterials.
4.2. Cardiovascular tissue
Cardiovascular disease is a leading cause of death in the world. Patients suffering from ischemic heart disease including myocardial infarction (MI), cardiac arrests and stable/unstable angina, increase significantly along with the progress of tobacco, unhealthy living habits, overdrinking and other detrimental factors. Tissue engineering serves as a reliable option to cure these disorders by exploiting bone marrow-derived stem cells, ESCs, parthenogenetic stem cells and iPSCs. While direct injection of cardiac stem cells have achieved some progress in remuscularization of the heart, difficulties in increasing cell survival and efficient tissue integration [101] call for functional biomaterials to facilitate heart regeneration. The ideal scaffolds for cardiac tissue should follow the nature of ECM and porous architecture for muscularization and vascularization. The porous 3D architecture provides attachment sites for stem cells, promoting the interactions between implanted cells and the host tissue for vascularization [102].
The earliest attempt to retrieve normal heart tissue in vitro was attained in 1994 by culturing chicken embryonic heart cells in collagen I [103]. Afterwards, multiple natural (matrigel, collagen, fibrin, alginate, hyaluronan, chitosan etc.) and synthetic (peptide-amphiphile nanofibers, PEG hydrogels etc.) biomaterials were developed for treating injured heart [104]. Even without cells, alginate [105], fibrin [106], methylcellulose [107] and hyaluronic acid [108] themselves were reported to restore heart function. Incorporation of follistatin-like 1 into a collagen scaffold stimulated de novo cardiomyogenesis after MI in vivo [109], further indicating that these acellular scaffolds could direct cardiac fate and become a potential therapy for cardiac repair.
Considering the complexity of heart tissue, stem cell-based scaffold is more efficient and attractive, and the field has become flooded by the combination of hPSC-derived cardiomyocytes (CMs) and biomaterials. Hydrogels were often applied for cardiac repair. Particularly, alginate hydrogels were used to encapsulate stem cells for CM differentiation with enhanced cell attachment and survival. Yu et al. [74] produced Arg-Gly-Asp (RGD, major recognition receptor from adhesive ECM, blood and cell surface proteins) modified alginate microspheres to encapsulate MSCs for cardiac regeneration. While both the encapsulated MSCs and MSCs alone could induce similar angiogenesis, the former showed long-term survival in the infarcted area. A study of ECM-derived GFOGER hydrogels mimicking collagen adhesive site revealed that induced cardiomyogenesis of cardiac progenitor cells was accompanied by the reduction of reparative growth factor release in vitro [75]. However, these hydrogels did not rescue cardiac function or reduce reparative growth factor level in rats undergoing ischemia-reperfusion, suggesting different mechanisms between in vivo and in vitro tissue regeneration. Interestingly, 3D PEG hydrogels containing thiosulfate cyanide sulfur transferase (TST) were fabricated to catalyze H2S production for a minimal ischemic effect and reperfusion damage in the implant site while stimulating angiogenesis [110]. The increased proliferation of human cardiac progenitor cells suggested the possibility of endogenous gasotransmitter on cardiac regeneration. Likewise, a plenty of studies aim to identify reliable stem cell-materials grafts for cardiac repair, but a lack of mechanistic understanding of cardiac regeneration slows down the pace in clinic study.
4.3. Pancreatic islet
Diabetes mellitus is one of the major non-communicable diseases characterized by hyperglycemia. It is a serious and chronic disease that could further lead to the dysfunction and damage of many other organs, including eyes, ears, blood vessels, kidneys and nerves. There are three forms of diabetes - Type 1, Type 2 and Gestational diabetes. Type 1 diabetes is mainly caused by the failure of pancreas to produce insulin due to the loss of β cells from an autoimmunity, and therefore, the regeneration of insulin-producing β cells holds the key in Type 1 diabetes treatment. Conventional methods including allo- and xeno-pancreatic islet transplantation often encounter with insufficient donors or severe immune rejection. Alternatively, stem cell-derived pancreatic β cells are considered as an unlimited source, and autologous patient-specific iPSC-derived β cells are supposed to generate minor immunoreaction. Furthermore, semi-permeable porous scaffolds provide better seeding and a protective microenvironment for pancreatic cells/tissues. In conclusion, incorporation of pancreatic progenitor cells into biomaterials for grafting without immunosuppression becomes an ideal method in future diabetes treatment.
Encapsulation of islet cells in biomaterials, in which the encapsulated cells were isolated from the immune system, was widely investigated to avoid auto-immune attack. Both Bratlie et al. [103] and Scharp et al. [102] reviewed structural approaches for islet encapsulation, and summarized the prevalent use of alginate, agarose, tissue-engineered chondrocytes, polyacrylates and PEG to produce macro-/micro-/nano-devices, conformal coating and layer-by-layer coating for encapsulation. Upon linked with directing factors, these scaffolds could specify pancreatic fate. A recent study invented an activin A-grafted gelatin-PLGA nanoparticle (PLGA NP) scaffold to induce endoderm formation from iPSCs in the precence of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) prior to pancreatic differentiation [78]. This pioneering work proposed a guidance for the production of pancreatic cells from iPSCs in polymer scaffolds. For example, stem cell-derived β cells were encapsulated in TMTD (triazole-thiomorpholine dioxide) modified alginates for in vivo glycemic control for 174 days without exploiting imunosuppression [79].
Numerous pre-clinical trials using encapsulated stem cell-derived human β cells were performed in non-immunosuppressed diabetic animal models, yet few of them were successfully confirmed in clinical human patients for the past three decades [111]. Scharp and Marchetti [76] owed the difficulties in encapsulated islet clinical therapy to the following cues: a) poor translation of treatment results obtained from rodents to human, b) acute loss of encapsulated cells due to hypoxia, c) transplantation antigen sensitization to the implant recipient, d) not enough cell quantities implanted. While the first reason could only be solved upon plenty of clinical human trials, the other three could be overcome by proper design of the scaffold/cell composite. Most of the unsuccessful islet implantations were caused by the low survival rate of the cells due to poor vascularization around the implanted site [112]. Proper vascularization between encapsulated cells and host tissue can be achieved by the directing function of engineered porous tunnels, or by co-encapsulation of vascular cells [113,114]. Kaufman-Francis et al. [115] co-cultured pancreatic islets, human umbilical vein endothelial cells (HUVECs) and human fibroblast cells on highly porous PLLA/PLGA scaffolds, leading to an increased insulin secretion by 50%, an enhanced islet survival and the formation of functional tube-like endothelial vessels.
4.4. Hematopoietic system
The hematopoietic system, comprised of bone marrow, spleen, tonsils and lymph nodes, serves as a blood producer. Hematopoietic stem cells (HSCs), residing in the bone marrow (main source) and cord-blood, hold the proliferation and immune potential to generate all cellular components to maintain the blood circulatory function. This renders HSCs as an essential core for the transplantation treatment of hematopoietic disorders and cancers [116]. However, the fate of a long-term HSC includes four stages: self-renewal, differentiation, emigration from bone marrow to blood and apoptosis [116]. Therefore the organization of hematopoiesis signaling pathways is rather complicated. Cell to cell, cell to cytokine and cell to ECM interactions, as well as the microenvironment parameters, such as O2 concentration, permeability, transcription factors, cytokines, stability, etc., all devoted to the ex vivo expansion of HSCs [117], shaping great challenges for the design of biomaterials for hematopoietic system therapy.
HSCs were considered strongly related to bone component and Calvi et al. [118] confirmed that osteoblastic cells were the regulatory component in the HSC niche in vivo. In this sense, the ECM properties of HSC were quite similar to that of osteoblasts. Nonetheless, while stiffness was widely studied for bone scaffolds, recent research revealed an emphasized impact of elasticity for the HSC expansion in biomaterials. Hoist et al. [80] found that elimination of elasticity sensing ability of HSCs could neutralize the proliferation enhancement on elastic biomaterials. More recently, Choi et al. [81] fabricated collagen-coated polyacrylamide scaffolds and discovered the spreading and morphology of hematopoietic stem and progenitor cell (HSPC) were responsive to the substrate elasticity, validating the important role of matrix biophysical properties in HSC survival. In addition, a 3D structure was proved to be more beneficial for HSC expansion as compared to 2D culture, since higher ratio of surface area to volume provided more efficient cellular interactions [119].
Apart from substrate mechanical properties, mesenchymal stromal cells also played a crucial role in HSC function [120], and therefore attempts to co-culture HSC with stromal cells were broadly studied. Collagen [82], PEG [83] and PCL [84] scaffolds were fabricated for the co-culturing of HSC with stromal cells, regardless of their source, all of which led to an improved proliferation of HSCs. Stromal cells were often conceived as support biomaterial, mimicking the biological component of HSC niche in bone marrow. Biological cues were further incorporated in the scaffolds to direct HSC activity. Mahadik et al. [121] used a methacrylamide-functionalized gelatin hydrogel to covalently immobilize stem cell factors (SCF), which could selectively maintain HSC multipotency. The incorporation of SDF-1α into PLGA scaffolds was shown to improve HSC recruitment and reduce subsequent inflammatory responses [122]. In conclusion, specific biomaterials can be tuned mechanically, chemically or biologically to meet the criteria for the hematopoietic therapy.
4.5. Bone/cartilage tissue
Nowadays, aging and bone injuries have led to a prominent problem of bone tissue loss. To address the clinical demand, a plethora of efforts have employed tissue engineering approaches, comprised of biomaterials and stem cells, to promote bone and cartilage regeneration [[123], [124], [125]].
Different cell types have been investigated for bone repair. Straightforwardly, ESCs were cultivated on 3D osteoconductive scaffolds to form large and compact bone constructs primed for transplantation [126]. Osteogenic potential of bone marrow-derived mesenchymal stem cell MSC was also validated to become any bone/cartilage component, including osteoblasts, chondrocytes, myocytes and adipocytes [128]. While whether or not the age of the MSC donor affects the cell viability and functionality still under debate [129,130], the ‘aging’ of MSC itself in terms of reduced proliferation and differentiation capacity [129], and its limited quantity in a body restricts its large-scale applications. On this account, iPSCs [131] or adipose-derived stem cell (ADSC) [132] were identified as a more reliable cell source for bone regeneration. iPSCs surpasses the limitation of cell quantities and age and may be applied to three strategies for bone regeneration [131]: a) generating MSCs through an embryoid body (EB); b) generating MSCs without an EB; c) generating bone tissue bypassing MSC or EB. iPSCs were demonstrated to have similar or even superior osteogenic capacity to MSC. On the other hand, ADSC is a specific type of MSC but with distinct advantages over those derived from bone marrow: better angiogenic and osteogenic properties, higher proliferation and differentiation capacity, larger percentage of stem cell progenitors in tissue [[133], [134], [135]] and most importantly, less affected by the donor age [132,136]. The elevated angiogenesis and osteogenesis of ADSCs rendered better performance in a pre-clinical bone allografting trial [137].
The basic design of biomaterials for bone/cartilage tissue engineering emphasizes pore morphology and structure to direct osteo-differentiation, which happen to be the most crucial characteristics of a scaffold. Cells tend to attach, proliferate and migrate on pores larger than 100 μm, and pores smaller than 50 μm are beneficial for nutrient and waste transport [138,139]. Salerno et al. [140] found macro-porosity was favored by colonization of osteoblast MG63 in bi-modal porous PCL scaffolds. Particularly, diameters in the range of 380–450 μm could promote chondrocyte and osteoblast growth, while sizes ranging from 290 to 310 μm could accelerate bone differentiation [141]. The introduction of osteo-inductive and osteo-conductive structures into biomaterials has recently become a promising method to produce heterogeneity in the structures, mimicking that of natural bones [142]. For example, Rodrigues et al. [143] constructed a starch/PCL scaffold containing an osteogenic layer and a chondrogenic layer for improved osteoarthritic treatment.
Likewise, natural biomaterials, including collagen, gelatin [85], hyaluronic acid [87], fibrin [88], alginate [144] et al., are widely reported for bone tissue engineering. However, natural biomaterials are often taken as a composite component merely to enhance biocompatibility due to a strict demand of mechanical properties for bone tissue engineering. Bioceramics such as HA, tricalcium phosphate and bioactive glasses [89] are often used as additives to enhance their mechanical properties, osteo-inductivity and osteo-conductivity. In addition, GAG [90] was introduced to promote scaffold, cellular and biological cue interactions as it has strong affinity to growth factors for directing cell fate. PLA [91], PLGA [92], PCL [93] and polyurethane (PU) [94] are popular synthetic biomaterials for bone/cartilage tissue engineering. Among them, PCL stood out for its superior short-term and long-term biocompatibility due to non-inflammatory and nontoxic degradation products [[145], [146], [147]], unlike acid-releasing PLA and PLGA. Moreover, the degradation of PCL was rather slow (>1 year), matching the regrowth of natural bones and its medical application was approved by FDA [141].
5. Stem cell biomanufacturing
Biomanufacturing uses biological systems including enzymes, microorganisms, cells, tissues, plants and animals to manufacture commercial products for agricultural, food, energy, material and pharmaceutical industries [148]. Biomanufacturing can be traced back to 1860s, when fermentation products appeared on the market [148]. Today, biomanufacturing can cover a wide range of chemical products [149], regenerative medicine [150] and even metal biorefinery [151]. However, most of stem cell-associated biomanufacturing is limited to the manufacturing or fabrication of tissue engineering devices. An exception was conducted by Google who invested €250,000 for a program to make hamburgers from cow stem-cell-derived muscles [152]. While the majority of literature relevant to biomanufacturing and stem cells concentrates on the design of bioreactors [[153], [154], [155]] or product quality control [156], those focusing on the large-scale production of stem-cell-associated therapeutic products brought in the term biofabrication.
Biofabrication is a vigorous and young field that is capturing attention in tissue engineering and regenerative medicine. Biofabrication utilizes automated processes such as additive manufacturing technologies to produce 3D biomaterial-based cell culture systems [157]. Biofabrication is characterized by cell-based building blocks, bio-inspired fabrication methods and biological products [158]. Accordingly, the bio-products of biofabrication are mostly hybrids of stem cells and biomaterials, including scaffolds, microcarriers, microgels, etc. Because the ultimate goal of biofabrication in tissue engineering and regenerative medicine is to achieve a favored spatial organization of constructs for stem cells to differentiate and mature on after implantation, all of the cell types and biomaterials mentioned above can be employed, and the focus of biofabrication becomes more of the methodologies of ‘fabrication’ than on the selection of building blocks. Bajaj et al. [159] reviewed common biofabrication techniques, including solvent casting, particle leaching, freeze drying, gas foaming, bioprinting and photolithography. Among these, novel techniques like bioprinting and photolithography are legitimate approaches for biofabrication as they allowed for the involvement of live cells. Likewise, Groll et al. [157] categorized the biofabrication strategies (additive manufacturing methodologies) into bioprinting and bioassembly based on the organization mechanism of building blocks.
5.1. Bioprinting
The evolution of biofabrication was parallel with the development of bioprinting [157]. The intimate relationship between the two dimmed the inconsistent concepts and often misguided the public to consider them as interchangeable. Actually, bioprinting is a frequently-used strategy of biofabrication, defined as the process for patterning and assembling cells, tissues, molecular cues and biomaterials [160]. The minimum fabrication unit of bioprinting is down to molecular level and when cells, cell aggregates, biomaterials or bioactive molecules are small enough, they are considered ‘bioink’ [161,162] and can be bioprinted. There are two types of bioprinting featured by the printing equipment: drop-based and extrusion. Drop-based bioprinting uses an inkjet and deposits bioink dropwise to form a gel or solid scaffold [161]. Extrusion bioprinting employs a mechanical extruder to continuously deposit bioink [163].
Bauwens et al. [164] bioprinted different sizes of hESC/poly (dimethylsiloxane) (PDMS) composite and found that colonies containing different germ-layer-biased hESCs had distinct differentiation capacity, indicating a size-motivated effect of the bioprinting product. The size control idea was further progressed by Dias et al. [165] using laser direct-writing to print ESCs onto gelatin. Other than ESCs, iPSCs could also be bioprinted into gelatin methacrylic (GelMA) microfibrous scaffolds for formation of an endothelial-integrated myocardium by an extruder [166] or into alginate hydrogels for hepatocyte differentiation by a drop-based printing platform [167]. For the broad application in a variety of tissue repairs, MSCs and tissue-specific progenitor cells were bioprinted with regard to the target tissue. Gaebel et al. [168] co-seeded human umbilical vein endothelial cells (HUVEC) with hMSCs onto a urethane urea (PEUU) cardiac patch in a programmed pattern by a laser-induced-forward-transfer (LIFT) cell printing technique and found restored vessel function to the infarct area in mice. Koch et al. [169] used the same technique to deliver fibroblasts with hMSCs onto a gold sheet for skin formation. Gao et al. [170] bioprinted hMSCs together with bioceramics to form poly (ethylene glycol) dimethylacrylate (PEGDMA) scaffolds for bone/cartilage tissue repair. Ma et al. [171] bioprinted hepatic progenitors, HUVECs and hMSCs onto GelMA and glycidol methacrylate-hyaluronic acid (GMHA) chips to generate a liver model for drug screening and pathophysiology studies. Huang et al. [172] bioprinted epithelial progenitors with EGF onto gelatin alginate substrate for sweat gland regeneration.
Bioprinting techniques are versatile for pluripotent, multipotent and unipotent stem cell, and have a broad application. Gel-forming biomaterials (alginate, PEG), together with solid-forming materials (PLGA, PCL), bioactive ceramics and glasses, as well as growth factors can all be used as bioink for a programmed printing process.
5.2. Bioassembly
Another biofabrication strategy is bioassembly. Unlike bioprinting, bioassembly starts with large pre-formed cellular constructs such as large cell aggregates, cell fibers, cell sheets and even microtissues. Bioassembly refers to the automated assembly of pre-generated cellular devices produced by cell driven self-organization or cell-material hybridization technologies [157]. The minimum building block unit of bioassembly is a pre-formed cellular device large enough for automated assembly.
The assembling unit can come in the form of microcarriers or microgels. Many researchers [[173], [174], [175]] demonstrated the long-term maintenance, self-renewal capacity and pluripotency of hESCs cultured on microcarriers. These microcarriers may be further assembled by different methods for tissue engineering applications. For instance, Xie et al. [176] employed a pressurized-CO2 assisted technique to assemble PLGA microscaffolds containing ESCs layer by layer for tissue engineering. The cells in microcarriers were not restricted to ESCs. The same group also bioassembled hMSC microscaffolds and demonstrated preserved protein and DNA activity [177]. Furthermore, Guduric et al. used a PLA membrane with human bone marrow stromal cells and endothelial progenitor cells for a layer-by-layer bioassembly [178]. After 7 days of culture, cell migration and osteoblastic differentiation were observed, suggesting a potential application in bone tissue engineering. In addition to cells, microtissues were also applied as building blocks for bioassembly. Mekhileri et al. [179] pre-differentiated chondrogenic microtissues, and sequentially plotted the microtissues and PEGT/PBT polymer scaffold layer-by-layer using a fluidic-based bioassembly system. Comparable cell viability was obtained between the bioassembled cell-biomaterial hybrids and microtissues.
In summary, the evolution of bioassembly extends the working unit of bioprinting to larger blocks, broadens the methodologies to organize complex structures with a scaffold, and paves the way for scale-up in biofabrication, thus making up to the stem-cell-associated biomanufacturing.
5.3. Scale-up production of stem cells
Stem cells outstand in future therapeutic applications. Nonetheless, the realization of their full potential requires sufficient amount of stem cells, and thus their large-scale production becomes an important issue in stem cell engineering. As mentioned above, the manufacturing of stem cell products often associates with bioreactors, while the complex nature of stem cell culture has limited the direct transfer of 2D culture conditions to 3D bioreactor production. Stem cell fate to either maintain pluripotency or differentiate is specified by the combinatorial influence of biochemical factors, cell-cell interactions, and cell-matrix interactions in the culture environment. Stem cell fate can also be impacted by the mechanical stimuli from its surrounding fluid when the cells are introduced to bioreactors for scaling up. Kinney et al.180. reviewed and summarized the effect of hydrodynamic environments on stem cell aggregation, metabolism and phenotype in suspension culture. Importantly, they envisioned cell aggregates as a promising method for large-scale cell production [181], while it remains challenging to maintain the differentiation homogeneity of the stem cell aggregates due to the altered cue profile caused by size effect. Biomaterials are then introduced for more homogenous cell assembly [182] as they could regulate stem cell expansion and differentiation by mechanically interacting with cytoskeleton [183], or by harnessing the binding and release of both exogenous and endogenous growth factors [184]. Of note, the controlled spatial delivery of biochemical cues through biomaterials could overcome the diffusion limitation and eliminate heterogeneity of stem cell aggregates [185]. In particular, natural or synthetic microcarriers, with or without functional modification via bioactive groups (DEAE, collagen) for better cell attachment [186], were often applied in bioreactors to engineer stem cell proliferation and differentiation [187,188]. Microencapsulation of stem cells using alginate, agarose, or hyaluronic acid hydrogel was another biomaterial application to avoid agglomeration while preserving cell-cell and cell-matrix contact [187]. For instance, a series of scalable thermoreversible PEG-based or hyaluronic acid-based hydrogel systems [[189], [190], [191]] have been developed for cell spheroid encapsulation to protect cells from hydrodynamic stresses and prevent excessive agglomeration, leading to a 20-fold higher cell yield as compared to traditional suspension culture. While the introduction of microcarriers or microencapsulation could solve one or several problems, they were not perfect solutions as the biomaterials used may exert new problems, such as microcarrier clumping, difficult monitoring and observing of culture condition, and additional separation step of materials and cells [192].
Apart from cell expansion, large-scale generation and manufacturing of iPSCs from patient's somatic cells is another challenge for personalized medicine [193,194]. The current manipulation process to produce clinical-grade iPSCs is time-consuming, labor-intensive, and expensive [195]. For example, the total cost reached up to $50,000 to produce iPSCs for one patient to meet the clinical needs [196]. On this account, Lin and his colleagues developed an alginate hydrogel tube system (AlgTubes) to efficiently reprogram human fibroblasts into iPSCs [195], which resulted in high purity of iPSCs that express pluripotent markers OCT3/4, NANOG, ALP and SSEA4 on day 30 and maintained a long-term (20 passages) pluripotency. This AlgTube system was also applicable to other bio-manufacturing processes, such as biofabrication [197]. The simplicity, good reproducibility, cost-effective and feasible integration of induction, expansion and differentiation of AlgTubes enabled it a promising tool for future manufacturing of iPSCs to meet the various demand for personalized medicine at larger-scale.
The preservation and transportation of large quantities of stem cells represents another challenge in stem cell manufacturing. The afore-mentioned AlgTubes proposed a simple transportation pattern in hydrogels but its preservation and storage capacity was not evaluated. Li and co-workers discussed various cryopreservation bioprocesses [198] and formulations [199] for large-scale banking of iPSCs, but similar work was not conducted in other studies. Additionally, the online monitoring of cell aggregates in bioreactors and the fact that different cells require distinct culture patterns and quality control measurements [180] make the design of a universal large-scale bioreactors an even more challenging task, which requires a comprehensive master of the stem cell production process, the follow-up transportation and preservation process, and the rational bioreactor design. Even with these challenges, the promise biomaterials hold for the stem cell manufacturing offers tremendous potential for expanding the scope of stem cell-based therapies.
6. Conclusion
Stem cells are gaining attention due to their self-renewal ability and capacity of differentiating into any specific cell type. Pluripotent, multipotent and unipotent stem cells draw different interests in a variety of applications, including drug screening, disease modeling and regenerative medicine. However, immune sensitivity limits the application of stem cells in clinical trials. With the aid of biomaterials, these barriers can be overcome. Natural biomaterials are highly biocompatible, while synthetic biomaterials can be rationally designed for a particular purpose. The incorporation of stem cells into structured and modified biomaterials increases the competence of restoring and repairing dysfunction tissues. The well-organized spatial properties of a biomaterial or scaffold in turn can provide a protective and sometimes inducible microenvironment for the stem cells, mimicking the natural ECM. The stem cell-biomaterial system has been applied to various tissue treatments, including heart, nerve, pancreatic islet, hematopoietic system and bone. The appearance of biofabrication technologies enables the precise design and scalable biomanufacturing of cell-based scaffolds using program-controlled bioprinting or bioassembly.
Declaration of competing interest
None.
Acknowledgement
The authors are grateful for the startup funding from the Davidson School of Chemical Engineering and the College of Engineering at Purdue University, United States.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2019.11.002.
Contributor Information
Yibo Xu, Email: xu1242@purdue.edu.
Chuanxin Chen, Email: 11328028@zju.edu.cn.
Peter B. Hellwarth, Email: phellwa@purdue.edu.
Xiaoping Bao, Email: bao61@purdue.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Drukker M. Recent advancements towards the derivation of immune-compatible patient-specific human embryonic stem cell lines. Semin. Immunol. 2008;20(2):123–129. doi: 10.1016/j.smim.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Burdick J.A., Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A. 2009;15(2):205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson D.G., Levenberg S., Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 2004;22(7):863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 4.Boontheekul T., Hill E.E., Kong H.-J., Mooney D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13(7):1431–1442. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- 5.Bratt-Leal A.M., Carpenedo R.L., Ungrin M.D., Zandstra P.W., McDevitt T.C. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32(1):48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuss S., Apel C., Buttler P., Denecke B., Dhanasingh A., Ding X., Grafahrend D., Groger A., Hemmrich K., Herr A., Jahnen-Dechent W., Mastitskaya S., Perez-Bouza A., Rosewick S., Salber J., Wӧltje M., Zenke M. Assessment of stem cell/biomaterial combinations for stem cell-based tissue engineering. Biomaterials. 2008;29(3):302–313. doi: 10.1016/j.biomaterials.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Carlesso N., Cardoso A.A. Stem cell regulatory niches and their role in normal and malignant hematopoiesis. Curr. Opin. Hematol. 2010;17(4):281–286. doi: 10.1097/MOH.0b013e32833a25d8. [DOI] [PubMed] [Google Scholar]
- 8.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Liu S., Dontu G., Wicha M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7(3):86. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2008;71(1):241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 11.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-Current views. Stem Cells. 2007;25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 12.Rigotti G., Marchi A., Galiè M., Baroni G., Benati D., Krampera M., Pasini A., Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007;119(5):1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 13.Kami M., Hamaki T., Miyakoshi S., Murashige N., Kanda Y., Tanosaki R., Takaue Y., Taniguchi S., Hirai H., Ozawa K., Kasai M. Allogeneic haematopoietic stem cell transplantation for the treatment of adult T-cell leukaemia/lymphoma. Br. J. Haematol. 2003;120(2):304–309. doi: 10.1046/j.1365-2141.2003.04054.x. [DOI] [PubMed] [Google Scholar]
- 14.Ko K., Tapia N., Wu G., Kim J.B., Bravo M.J.A., Sasse P., Glaser T., Ruau Dm, Han D.W., Greber B., Hausdӧrfer K., Sebastiano V., Stehling M., Fleischmann B.K., Brüstle O., Zenke M., Schӧler H.R. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5(1):87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Martin G.R. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Young R.A. Control of the embryonic stem cell state. Cell. 2011;144(6):940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilic D., Ogeilvie C. Concise review : human embryonic stem cells — what have we done? What are we doing? Where are we going? Stem Cells. 2017;35(1):17–25. doi: 10.1002/stem.2450. [DOI] [PubMed] [Google Scholar]
- 19.Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Aguilera A., Leukemia Á. The hematopoietic stem-cell niche in health and leukemia. Cell. Mol. Life Sci. 2017;74:579–590. doi: 10.1007/s00018-016-2306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K., Tanabe K., Ohnuki M., Narita M., Icchisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 23.Meissner A., Wernig M., Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 2007;25(10):1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 24.Lutolf M.P., Gilbert P.M., Blau H.M. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martino S., D'Angelo F., Armentano I., Kenny J.M., Orlacchio A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012;30(1):338–351. doi: 10.1016/j.biotechadv.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Charriere G., Bejot M., Schnitzler L., Ville G., Hartmann D.J. Reactions to a bovine collagen implant. Clinical and immunologic study in 705 patients. J. Am. Acad. Dermatol. 1989;21(6):1203–1208. doi: 10.1016/s0190-9622(89)70330-3. [DOI] [PubMed] [Google Scholar]
- 27.Yannas I.V., Burke J.F., Orgill D.P., Skrabut E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215(4529):174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- 28.Yang C., Hillas P.J., Báez J.A., Nokelainen M., Balan J., Tang J., Spiro R., Polarek Jw. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18(2):103–119. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 29.Freyria A.-M., Ronzière M.-C., Cortial D., Galois L., Hartmann D., Herbage D., Mallein-Gerin F. Comparative phenotypic analysis of articular chondrocytes cultured within type I or type II collagen scaffolds. Tissue Eng. A. 2009;15(6):1233–1245. doi: 10.1089/ten.tea.2008.0114. [DOI] [PubMed] [Google Scholar]
- 30.Trottier V., Marceau-Fortier G., Germain L., Vincent C., Fradette J. Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells. 2008;26(10):2713–2723. doi: 10.1634/stemcells.2008-0031. [DOI] [PubMed] [Google Scholar]
- 31.Grueterich M., Espana E.M., Tseng S.C.G. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv. Ophthalmol. 2003;48(6):631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Dravida S., Gaddipati S., Griffith M., Merrett K., Lakshmi Madhira S., Sangwan V.S., Vemuganti G.K. A biomimetic scaffold for culturing limbal stem cells: a promising alternative for clinical transplantation. J. Tissue Eng. Regenerat. Med. 2008;2(5):263–271. doi: 10.1002/term.91. [DOI] [PubMed] [Google Scholar]
- 33.Toole B.P. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 34.Toole B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 35.Burdick J.A., Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23(12):H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung C., Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. A. 2009;15(2):243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuurman W., Levett P.A., Pot M.W., van Weeren P.R., Dhert W.J.A., Hutmacher D.W., Melchels F.P.W., Klein T.J., Malda J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013;13(5):551–561. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 38.Bulcke AI Van Den, Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Macromolecules. 2000;1(1):31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 39.Rowland T.J., Miller L.M., Blaschke A.J., Doss E.L., Bonham A.J., Hikita S.T., Johnson L.V., Clegg D. Roles of integrins in human induced pluripotent stem cell growth on matrigel and vitronectin. Stem Cells Dev. 2010;19(8):1231–1240. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 40.Uemura M., Refaat M.M., Shinoyama M., Hayashi H., Hashimoto N., Takahashi J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J. Neurosci. Res. 2009;88(3) doi: 10.1002/jnr.22223. NA-NA. [DOI] [PubMed] [Google Scholar]
- 41.Kleinman H.K., Martin G.R. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Sakiyama-Elbert S.E., Panitch A., Hubbell J.A. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 2001;15(7):1300–1302. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- 43.Willerth S.M., Faxel T.E., Gottlieb D.I., Sakiyama-Elbert S.E. The effects of soluble growth factors on embryonic stem cell differentiation inside of fibrin scaffolds. Stem Cells. 2007;25(9):2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willerth S.M., Arendas K.J., Gottlieb D.I., Sakiyama-Elbert S.E. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27(36):5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan L., Yu X., Wan P., Yang K. Biodegradable materials for bone repairs: a review. J. Mater. Sci. Technol. 2013;29(6):503–513. [Google Scholar]
- 46.Tyler B., Gullotti D., Mangraviti A., Utsuki T., Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016;107:163–175. doi: 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Kao C.-T., Lin C.-C., Chen Y.-W., Yeh C.-H., Fang H.-Y., Shie M.-Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C. 2015;56:165–173. doi: 10.1016/j.msec.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 48.Jeong H., Rho J., Shin J.-Y., Lee D.Y., Hwang T., Kim K.J. Mechanical properties and cytotoxicity of PLA/PCL films. Biomed Eng Lett. 2018;8(3):267–272. doi: 10.1007/s13534-018-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuttelman C.R., Tripodi M.C., Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J. Biomed. Mater. Res. 2004;68A(4):773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 50.Almany L., Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26(15):2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 51.Yao H., Xue J., Wang Q. Glucosamine-modified polyethylene glycol hydrogel-mediated chondrogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C. 2017;79:661–670. doi: 10.1016/j.msec.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 52.Kenny S.M., Buggy M. Bone cements and fillers: a review. J. Mater. Sci. Mater. Med. 2003;14(11):923–938. doi: 10.1023/a:1026394530192. [DOI] [PubMed] [Google Scholar]
- 53.Hutmacher D.W., Schantz J.T., Lam C.X.F., Tan K.C., Lim T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regenerat. Med. 2007;1(4):245–260. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 54.Habraken W.J.E.M., Wolke J.G.C., Jansen J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007;59(4–5):234–248. doi: 10.1016/j.addr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Link D.P., van den Dolder J., Wolke J.G.C., Jansen J.A. The cytocompatibility and early osteogenic characteristics of an injectable calcium phosphate cement. Tissue Eng. 2007;13(3):493–500. doi: 10.1089/ten.2006.0015. [DOI] [PubMed] [Google Scholar]
- 56.Alas G.R., Agarwal R., Collard D.M., García A.J. Peptide-functionalized poly[oligo(ethylene glycol) methacrylate] brushes on dopamine-coated stainless steel for controlled cell adhesion. Acta Biomater. 2017;59:108–116. doi: 10.1016/j.actbio.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Śmieszek A., Donesz-Sikorska A., Grzesiak J., Krzak J., Marycz K. Biological effects of sol–gel derived ZrO2 and SiO2/ZrO2 coatings on stainless steel surface—in vitro model using mesenchymal stem cells. J. Biomater. Appl. 2014;29(5):699–714. doi: 10.1177/0885328214545095. [DOI] [PubMed] [Google Scholar]
- 58.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21(7):667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 59.Blanco J.F., Sánchez-Guijo F.M., Carrancio S., Muntion S., García-Briñon J., del Cañizo M.-C. Titanium and tantalum as mesenchymal stem cell scaffolds for spinal fusion: an in vitro comparative study. Eur. Spine J. 2011;20(S3):353–360. doi: 10.1007/s00586-011-1901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hee A.C., Cao H., Zhao Y., Jamali S.S., Bendavid A., Martin P.J. Cytocompatible tantalum films on Ti6Al4V substrate by filtered cathodic vacuum arc deposition. Bioelectrochemistry. 2018;122:32–39. doi: 10.1016/j.bioelechem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Cortecchia E., Pacilli A., Pasquinelli G., Scandola M. Biocompatible two-layer tantalum/titania-polymer hybrid coating. Biomacromolecules. 2010;11:2446–2453. doi: 10.1021/bm100619t. [DOI] [PubMed] [Google Scholar]
- 62.Yazdimamaghani M., Razavi M., Vashaee D., Moharamzadeh K., Boccaccini A.R., Tayebi L. 2017. Porous Magnesium-Based Scaffolds for Tissue Engineering. [DOI] [PubMed] [Google Scholar]
- 63.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Luthringer B.J.C., Willumeit-Römer R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene. 2016;575(1):9–20. doi: 10.1016/j.gene.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 65.Balandin A.A., Ghosh S., Bao W., Calizo I., Teweldebrhan D., Miao F., Lau C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8(3):902–907. doi: 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- 66.Dreyer D.R., Park S., Bielawski C.W., Ruoff R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39(1):228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 67.Lee W.C., Lim C.H.Y.X., Shi H. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–7341. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 68.Chikkaveeraiah B.V., Soldà A., Choudhary D., Maran F., Rusling J.F. Ultrasensitive nanostructured immunosensor for stem and carcinoma cell pluripotency gatekeeper protein NANOG. Nanomedicine. 2012;7(7):957–965. doi: 10.2217/nnm.11.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winter C.C., Katiyar K.S., Hernandez N.S., Song Y.J., Struzyna L.A., Harris J.P., Cullen D.K. Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration. Acta Biomater. 2016;38:44–58. doi: 10.1016/j.actbio.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prabhakaran M.P., Venugopal J.R., Ramakrishna S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials. 2009;30(28):4996–5003. doi: 10.1016/j.biomaterials.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 71.Peng S.-W., Li C.-W., Chiu I.-M., Wang G.-J. Nerve guidance conduit with a hybrid structure of a PLGA microfibrous bundle wrapped in a micro/nanostructured membrane. Int. J. Nanomed. 2017;12:421–432. doi: 10.2147/IJN.S122017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S., Guan S., Zhu Z., Li W., Liu T., Ma X. Hyaluronic acid doped-poly(3,4-ethylenedioxythiophene)/chitosan/gelatin (PEDOT-HA/Cs/Gel) porous conductive scaffold for nerve regeneration. Mater. Sci. Eng. C. 2017;71:308–316. doi: 10.1016/j.msec.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 73.Schuh C.M.A.P., Day A.G.E., Redl H., Phillips J. An optimized collagen-fibrin blend engineered neural tissue promotes peripheral nerve repair. Tissue Eng. A. 2018;24(17–18):1332–1340. doi: 10.1089/ten.tea.2017.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu J., Du K.T., Fang Q., Gu Y., Mihardja S.S., Sievers R.E., Wu J.C., Lee R.J. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials. 2010;31(27):7012–7020. doi: 10.1016/j.biomaterials.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 75.Bhutani S., Nachlas A.L.Y., Brown M.E., Pete T., Johnson C.T., Garcia A.J., Davis M.E. Evaluation of hydrogels presenting extracellular matrix-derived adhesion peptides and encapsulating cardiac progenitor cells for cardiac repair. ACS Biomater. Sci. Eng. 2018;4(1):200–210. doi: 10.1021/acsbiomaterials.7b00502. (Tables) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scharp D.W., Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 77.Bratlie K.M., York R.L., Invernale M.A., Langer R., Anderson D.G. Materials for diabetes therapeutics. Adv. Healthc. Mater. 2012;1(3):267–284. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuo Y.-C., Liu Y.-C., Rajesh R. Pancreatic differentiation of induced pluripotent stem cells in activin A-grafted gelatin-poly(lactide-co-glycolide) nanoparticle scaffolds with induction of LY294002 and retinoic acid. Mater. Sci. Eng. C. 2017;77:384–393. doi: 10.1016/j.msec.2017.03.265. [DOI] [PubMed] [Google Scholar]
- 79.Vegas A.J., Veiseh O., Gürtler M., Millman J.R., Pagliuca F.W., Bader A.R., Doloff J.C., Li J., Chen M., Olejnik K., Tam H.H., Jhunjhunwala S., Langan E., Aresta-Dasilva S., Gandham S., McGarrigle J.J., Bochenek M.A., Hollister-Lock J., Oberholzer J., Greiner D.L., Weir G.C., Melton D.A., Langer R., Anderson D.G. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22(3):306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holst J., Watson S., Lord M.S., Eamegdool S.S., Bax D.V., Nivison-Smith L.B., Kondyurin A., Ma L., Oberhauser A.F., Weiss A.S., Rasko J.E.J. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 2010;28(10):1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 81.Choi J.S., Harley B.A.C. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials. 2012;33(18):4460–4468. doi: 10.1016/j.biomaterials.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Leisten I., Kramann R., Ventura Ferreira M.S. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials. 2012;33(6):1736–1747. doi: 10.1016/j.biomaterials.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 83.Raic A., Rödling L., Kalbacher H., Lee-Thedieck C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 2014;35(3):929–940. doi: 10.1016/j.biomaterials.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 84.Ventura Ferreira M.S., Jahnen-Dechent W., Labude N. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials. 2012;33(29):6987–6997. doi: 10.1016/j.biomaterials.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 85.Celikkin N., Mastrogiacomo S., Jaroszewicz J., Walboomers X.F., Swieszkowski W. Gelatin methacrylate scaffold for bone tissue engineering: the influence of polymer concentration. J. Biomed. Mater. Res. A. 2018;106(1):201–209. doi: 10.1002/jbm.a.36226. [DOI] [PubMed] [Google Scholar]
- 87.Cui N., Qian J., Liu T., Zhao N., Wang H. Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering. Carbohydr. Polym. 2015;126:192–198. doi: 10.1016/j.carbpol.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 88.Noori A., Ashrafi S.J., Vaez-Ghaemi R., Hatamian-Zaremi A., Webster T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017;12:4937–4961. doi: 10.2147/IJN.S124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarker B., Hum J., Nazhat S.N., Boccaccini A.R. Combining collagen and bioactive glasses for bone tissue engineering: a review. Adv. Healthc. Mater. 2015;4(2):176–194. doi: 10.1002/adhm.201400302. [DOI] [PubMed] [Google Scholar]
- 90.Salbach J., Rachner T.D., Rauner M. Regenerative potential of glycosaminoglycans for skin and bone. J. Mol. Med. 2012;90(6):625–635. doi: 10.1007/s00109-011-0843-2. [DOI] [PubMed] [Google Scholar]
- 91.Ramakrishna H., Li T., He T., Temple J., King M.W., Spagnoli A. Tissue engineering a tendon-bone junction with biodegradable braided scaffolds. Biomater. Res. 2019;23(1):1–12. doi: 10.1186/s40824-019-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Y., Wang Z., Zhou H., Li L., Zhu Q., Zhang P. An injectable hydroxyapatite/poly(lactide-co-glycolide) composite reinforced by micro/nano-hybrid poly(glycolide) fibers for bone repair. Mater. Sci. Eng. C. 2017;80:326–334. doi: 10.1016/j.msec.2017.04.121. [DOI] [PubMed] [Google Scholar]
- 93.Daly A.C., Kelly D.J. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials. 2019;197(December 2018):194–206. doi: 10.1016/j.biomaterials.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 94.Niu H., Ma Y., Wu G., Duan B., Wang Y., Yuan Y., Liu C. Multicellularity-interweaved bone regeneration of BMP-2-loaded scaffold with orchestrated kinetics of resorption and osteogenesis. Biomaterials. 2019;216:119216. doi: 10.1016/j.biomaterials.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 95.Venkatesh K., Sen D. Mesenchymal stem cells as a source of dopaminergic neurons: a potential cell based therapy for Parkinson's disease. Curr. Stem Cell Res. Ther. 2017;12(4):326–347. doi: 10.2174/1574888X12666161114122059. [DOI] [PubMed] [Google Scholar]
- 96.Arber C., Lovejoy C., Wray S. Stem cell models of Alzheimer's disease: progress and challenges. Alzheimer's Res. Ther. 2017;9(1):42. doi: 10.1186/s13195-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Antman-Passig M., Levy S., Gartenberg C., Schori H., Shefi O. Mechanically oriented 3D collagen hydrogel for directing neurite growth. Tissue Eng. A. 2017;23(9–10):403–414. doi: 10.1089/ten.TEA.2016.0185. [DOI] [PubMed] [Google Scholar]
- 98.Day A.G.E., Bhangra K.S., Murray-Dunning C., Stevanato L., Phillips J.B. The effect of hypothermic and cryogenic preservation on engineered neural tissue. Tissue Eng. C Methods. 2017;23(10):575–582. doi: 10.1089/ten.tec.2017.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sensharma P., Madhumathi G., Jayant R.D., Jaiswal A.K. Biomaterials and cells for neural tissue engineering: current choices. Mater. Sci. Eng. C. 2017;77:1302–1315. doi: 10.1016/j.msec.2017.03.264. [DOI] [PubMed] [Google Scholar]
- 100.Tao J., Hu Y., Wang S., Zhang J., Liu X., Gou Z., Cheng H., Liu Q., Zhang Q., You S., Gou M. A 3D-engineered porous conduit for peripheral nerve repair. Sci. Rep. 2017;7:46038. doi: 10.1038/srep46038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weinberger F., Mannhardt I., Eschenhagen T. Engineering cardiac muscle tissue: a maturating field of research. Circ. Res. 2017;120(9):1487–1500. doi: 10.1161/CIRCRESAHA.117.310738. [DOI] [PubMed] [Google Scholar]
- 102.Ruvinov E., Cohen S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2016;96:54–76. doi: 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 103.Eschenhagen T., Fink C., Remmers U., Scholz H., Wattchow J., Weil J., Zimmermann W., Dohmen H.H., Schӓfer H., Bishopric N., Wakatsuki T., Elson E.L. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11(8):683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 104.Hirt M.N., Hansen A., Eschenhagen T. Cardiac tissue engineering. Circ. Res. 2014;114(2):354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- 105.Ruvinov E., Leor J., Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32(2):565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 106.Tang J., Vandergriff A., Wang Z., Hensley M.T., Cores J., Allen T.A., Dinh P.-U., Zhang J., Caranasos T.G., Cheng K. A regenerative cardiac patch formed by spray painting of biomaterials onto the heart. Tissue Eng. C Methods. 2017;23(3):146–155. doi: 10.1089/ten.tec.2016.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mihardja S.S., Gonzales J.A., Gao D., Sievers R.E., Fang Q., Stillson C.A., Yu J., Peng M., Lee R.J. The effect of a peptide-modified thermo-reversible methylcellulose on wound healing and LV function in a chronic myocardial infarction rodent model. Biomaterials. 2013;34(35):8869–8877. doi: 10.1016/j.biomaterials.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 108.Jha A.K., Tharp K.M., Browne S., Ye J., Stahl A., Yeghiazarians Y., Healy K.E. Matrix metalloproteinase-13 mediated degradation of hyaluronic acid-based matrices orchestrates stem cell engraftment through vascular integration. Biomaterials. 2016;89:136–147. doi: 10.1016/j.biomaterials.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei K., Serpooshan V., Hurtado C., Diez-Cunado M., Zhao M., Maruyama S., Zhu W., Fajardo G., Noseda M., Nakamura K., Tian X., Liu Q., Wang A., Matsuura Y., Bushway P., Cai W., Savchenko A., Mahmoudi M., Schneider M.D., van Den Hoff M.J.B., Butte M.J., Yang P.C., Walsh K., Zhou B., Bernstein D., Mercola M., Ruiz-Lozano P. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mauretti A., Neri A., Kossover O., Seliktar D., Nardo P Di, Melino S. Design of a novel composite H2S-Releasing hydrogel for cardiac tissue repair. Macromol. Biosci. 2016;16(6):847–858. doi: 10.1002/mabi.201500430. [DOI] [PubMed] [Google Scholar]
- 111.Calafiore R., Basta G. Clinical application of microencapsulated islets: Actual prospectives on progress and challenges. Adv. Drug Deliv. Rev. 2014;67–68:84–92. doi: 10.1016/j.addr.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 112.Mattsson G., Jansson L., Carlsson P.-O. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51(5):1362–1366. doi: 10.2337/diabetes.51.5.1362. http://www.ncbi.nlm.nih.gov/pubmed/11978631 [DOI] [PubMed] [Google Scholar]
- 113.Borg D.J., Welzel P.B., Grimmer M. Macroporous biohybrid cryogels for co-housing pancreatic islets with mesenchymal stromal cells. Acta Biomater. 2016;44:178–187. doi: 10.1016/j.actbio.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 114.Kasoju N., Kubies D., Fábryová E., Kříž J., Kumorek M.M., Sticová E., Rypáček F. In vivo vascularization of anisotropic channeled porous polylactide-based capsules for islet transplantation: the effects of scaffold architecture and implantation site. Physiol. Res. 2015;64(1):S75–S84. doi: 10.33549/physiolres.933138. http://www.ncbi.nlm.nih.gov/pubmed/26447597 [DOI] [PubMed] [Google Scholar]
- 115.Kaufman-Francis K., Koffler J., Weinberg N., Dor Y., Levenberg S. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS One. 2012;7(7):1–10. doi: 10.1371/journal.pone.0040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shizuru J.A., Negrin R.S., Weissman I.L. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu. Rev. Med. 2002;56(1):509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 117.Zhang C.C., Kaba M., Ge G., Xie K., Tong W., Hug C., Lodish H.F. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 2006;12(2):240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., Milner L.A., Kronenberg H.M., Scadden D.T. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 119.Eskandari F., Allahverdi A., Nasiri H., Azad M., Kalantari N., Soleimani M., Zare-Zardini H. Nanofiber expansion of umbilical cord blood hematopoietic stem cells. Iran J Pediatr Hematol Oncol. 2015;5(4):170–178. [PMC free article] [PubMed] [Google Scholar]
- 120.Wagner W., Roderburg C., Wein F., Dichlmann A., Frankhauser M., Schubert R., Eckstein V., Ho A.D. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25(10):2638–2647. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 121.Mahadik B.P., Pedron Haba S., Skertich L.J., Harley B.A.C. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials. 2015;67:297–307. doi: 10.1016/j.biomaterials.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thevenot P.T., Nair A.M., Shen J., Lotfi P., Ko C.-Y., Tang L. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31(14):3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Howdle S.M., Yang X.B., Oreffo R.O.C., Shakesheff K.M., Whitaker M.J., Sebald W., Clarke N. Human osteoprogenitor bone formation using encapsulated bone morphogenetic protein 2 in porous polymer scaffolds. Tissue Eng. 2004;10(7–8):1037–1045. doi: 10.1089/ten.2004.10.1037. [DOI] [PubMed] [Google Scholar]
- 124.Steele J.A.M., Stevens M.M., McCullen S., Williams C.K., Bismarck A., Purcell M., Howdle S.M., Shakesheff K.M., Tang M., Lee K.-Y. Porous copolymers of ε-caprolactone as scaffolds for tissue engineering. Macromolecules. 2013;46(20):8136–8143. [Google Scholar]
- 125.Mehta M., Schmidt-Bleek K., Duda G.N., Mooney D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Deliv. Rev. 2012;64(12):1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marolt D., Campos I.M., Bhumiratana S., Koren A., Petridis P., Zhang G., Spitalnik P.F., Grayso W.L., Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109(22):8705–8709. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saltz A., Kandalam U. Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: a review. J. Biomed. Mater. Res. A. 2016;104(5):1276–1284. doi: 10.1002/jbm.a.35647. [DOI] [PubMed] [Google Scholar]
- 129.Fickert S., Schroter-Bobsin U., Groß A.F., Hempel U., Wojciechowski C., Rentsch C., Corbeil D., Gunther K.P. Human mesenchymal stem cell proliferation and osteogenic differentiation during long-term ex vivo cultivation is not age dependent. J. Bone Miner. Metab. 2011;29(2):224–235. doi: 10.1007/s00774-010-0215-y. [DOI] [PubMed] [Google Scholar]
- 130.Kretlow J.D., Jin Y.Q., Liu W., Zhang W.J., Hong T.H., Zhou G., Baggett L.S., Mikos A.G., Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:1–13. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bastami F., Nazeman P., Moslemi H., Rezai Rad M., Sharifi K., Khojasteh A. Induced pluripotent stem cells as a new getaway for bone tissue engineering: a systematic review. Cell Prolif. 2017;50(2) doi: 10.1111/cpr.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dufrane D. Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant. 2017;26(9):1496–1504. doi: 10.1177/0963689717721203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 134.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., March K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 135.Liao H.-T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells. 2014;6(3):288. doi: 10.4252/wjsc.v6.i3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen H.T., Lee M.J., Chen C.H. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell Mol. Med. 2012;16(3):582–593. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schubert T., Xhema D., Vériter S., Schubert M., Behets C., Delloye C., Gianello P., Dufrane D. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials. 2011;32(34):8880–8891. doi: 10.1016/j.biomaterials.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 138.Salerno A., Oliviero M., Di Maio E., Iannace S., Netti P.A. Design and preparation of μ-bimodal porous scaffold for tissue engineering. J. Appl. Polym. Sci. 2007;106:3335–3342. [Google Scholar]
- 139.Chen C.X., Peng H.H., Guan Y.X., Yao S.J. Morphological study on the pore growth profile of poly(ε-caprolactone) bi-modal porous foams using a modified supercritical CO2 foaming process. J. Supercrit. Fluids. 2019;143:72–81. [Google Scholar]
- 140.Salerno A., Di Maio E., Iannace S., Netti P.A., Zeppetelli S. Processing/structure/property relationship of multi-scaled PCL and PCL-HA composite scaffolds prepared via gas foaming and NaCl reverse templating. Biotechnol. Bioeng. 2010;108(4):963–976. doi: 10.1002/bit.23018. [DOI] [PubMed] [Google Scholar]
- 141.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer - polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35(10):1217–1256. [Google Scholar]
- 142.Motamedian S.R., Hosseinpour S., Ahsaie M.G., Khojasteh A. Smart scaffolds in bone tissue engineering: a systematic review of literature. World J. Stem Cells. 2015;7(3):657. doi: 10.4252/wjsc.v7.i3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodrigues M.T., Lee S.J., Gomes M.E., Reis R.L., Atala A., Yoo J.J. Bilayered constructs aimed at osteochondral strategies: the influence of medium supplements in the osteogenic and chondrogenic differentiation of amniotic fluid-derived stem cells. Acta Biomater. 2012;8(7):2795–2806. doi: 10.1016/j.actbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 144.Venkatesan J., Bhatnagar I., Manivasagan P., Kang K.-H., Kim S.-K. Alginate composites for bone tissue engineering: a review. Int. J. Biol. Macromol. 2015;72:269–281. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 145.Sawyer A.A., Song S.J., Susanto E. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials. 2009;30(13):2479–2488. doi: 10.1016/j.biomaterials.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 146.Schantz J.T., Hutmacher D.W., Lam C.X.F., Lim T.C., Chou N., Guldberg R.E., Teoh S.H. Repair of calvarial defects with customized tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in Vivo. Tissue Eng. 2003;9:S127–S139. doi: 10.1089/10763270360697030. [DOI] [PubMed] [Google Scholar]
- 147.Fukushima K., Feijoo J.L., Yang M.C. Comparison of abiotic and biotic degradation of PDLLA, PCL and partially miscible PDLLA/PCL blend. Eur. Polym. J. 2013;49(3):706–717. [Google Scholar]
- 148.Zhang Y.H.P., Sun J., Ma Y. Biomanufacturing: history and perspective. J. Ind. Microbiol. Biotechnol. 2017;44(4–5):773–784. doi: 10.1007/s10295-016-1863-2. [DOI] [PubMed] [Google Scholar]
- 149.Clomburg J.M., Crumbley A.M., Gonzalez R. Industrial biomanufacturing: the future of chemical production. Science (80- ) 2017;(6320):355. doi: 10.1126/science.aag0804. [DOI] [PubMed] [Google Scholar]
- 150.Roh K.-H., Nerem R.M., Roy K. Biomanufacturing of therapeutic cells: state of the art, current challenges, and future perspectives. Annu Rev Chem Biomol Eng. 2016;7(1):455–478. doi: 10.1146/annurev-chembioeng-080615-033559. [DOI] [PubMed] [Google Scholar]
- 151.Murray A.J., Zhu J., Wood J., Macaskie L.E. A novel biorefinery: Biorecovery of precious metals from spent automotive catalyst leachates into new catalysts effective in metal reduction and in the hydrogenation of 2-pentyne. Miner. Eng. 2017;113(March):102–108. [Google Scholar]
- 152.Khademhosseini A., Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11(10):1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 153.Srinivasan G., Morgan D., Varun D., Brookhouser N., Brafman D.A. An integrated biomanufacturing platform for the large-scale expansion and neuronal differentiation of human pluripotent stem cell-derived neural progenitor cells. Acta Biomater. 2018;74:168–179. doi: 10.1016/j.actbio.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Misener R., Allenby M.C., Fuentes-Garí M., Gupta K., Wiggins T., Panoskaltsis N., Pistikopoulos E.N., Mantalaris A. Stem cell biomanufacturing under uncertainty: a case study in optimizing red blood cell production. AIChE J. 2018;64(8):3011–3022. doi: 10.1002/aic.16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Patel D.B., Santoro M., Born L.J., Fisher J.P., Jay S.M. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol. Adv. 2018;36(8):2051–2059. doi: 10.1016/j.biotechadv.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jin J., Tarrant R.D., Bolam E.J., Angell-Manning P., Soegaard M., Pattinson D.J., Dulal P., Silk S.E., Marshall J.M., Dabbs R.A., Nugent F.L., Barrett J.R., Hjerrild K.A., Poulsen L., Jorgensen T., Brenner T., Baleanu I.N., Parracho H.M., Tahiri-Alaoui A., Whale G., Moyle S., Payne R.O., Minassian A.M., Higgins M.K., Detmers F.J., Lawrie A.M., Douglas A.D., Smith R., de Jongh W.A., Berrie E., Ashfield R., Draper S.J. Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells. npj Vaccines. 2018;3(1) doi: 10.1038/s41541-018-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Groll J., Boland T., Blunk T., Burdick J.A., Cho D.-W., Dalton P.D., Derby B., Forgacs G., Li Q., Mironov V.A., Moroni L., Nakamura M., Shu W., Takeuchi S., Vozzi G., Woodfield T.B.F., Xu T., Yoo J.J., Malda J. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016;8(1) doi: 10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 158.Luo X. Biofabrication in microfluidics: a converging fabrication paradigm to exploit biology in microsystems. J. Bioeng. Biomed. Sci. 2013;02(03):2–4. [Google Scholar]
- 159.Bajaj P., Schweller R.M., Khademhosseini A., West J.L., Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16(1):247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mironov V., Reis N., Phil D., Derby B. Bioprinting : a beginning. Tissue Eng. 2006;12(4):631–634. doi: 10.1089/ten.2006.12.631. [DOI] [PubMed] [Google Scholar]
- 161.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 162.Yu Y., Ozbolat I.T. Tissue strands as “bioink” for scale-up organ printing. 2014 36th Annu Int Conf IEEE Eng Med Biol Soc EMBC. 2014;2014:1428–1431. doi: 10.1109/EMBC.2014.6943868. [DOI] [PubMed] [Google Scholar]
- 163.Wang W., Huang B., Byun J.J., Bártolo P. Assessment of PCL/carbon material scaffolds for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019;93(October 2018):52–60. doi: 10.1016/j.jmbbm.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 164.Bauwens C.L., Peerani R., Niebruegge S., Woodhouse K.A., Kumacheva E., Husain M., Zandstra P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26(9):2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 165.Dias A.D., Unser A.M., Xie Y., Chrisey D.B., Corr D.T. Generating size-controlled embryoid bodies using laser direct-write. Biofabrication. 2014;6(2) doi: 10.1088/1758-5082/6/2/025007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Y.S., Arneri A., Bersini S., Shin S.R., Zhu K., Goli-Malekabadi Z., Aleman J., Colosi C., Busignani F., Dell'Erba V., Bishop C., Shupe T., Demarchi D., Moretti M., Rasponi M., Dokmeci M.R., Atala A., Khademhosseini A. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Faulkner-jones A., Greenhough S., King J.A., Reid J.A., Mollica P.A., Johnson G.D., Faulkner-Jones A., Fyfe C., Cornelissen D., Gardner J., King J. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini- livers in 3D. Biofabrication. 2015;7(4):44102. doi: 10.1088/1758-5090/7/4/044102. [DOI] [PubMed] [Google Scholar]
- 168.Gaebel R., Ma N., Liu J., Guan J., Koch L., Klopsch C., Gruene M., Toelk A., Wang W., Mark P., Wang F., Chichkov B., Li W., Steinhoff G. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32(35):9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 169.Koch L., Kuhn S., Sorg H., Gruene M., Schlie S., Gaebel R., Polchow B., Reimers K., Stoelting S., Ma N., Vogt P.M., Steinhoff G., Chichkov B. Laser printing of skin cells and human stem cells. Tissue Eng. C Methods. 2010;16(5):847–854. doi: 10.1089/ten.TEC.2009.0397. [DOI] [PubMed] [Google Scholar]
- 170.Gao G., Schilling A.F., Yonezawa T., Wang J., Dai G., Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014;9(10):1304–1311. doi: 10.1002/biot.201400305. [DOI] [PubMed] [Google Scholar]
- 171.Ma X., Qu X., Zhu W., Li Y.-S., Yuan S., Zhang H., Liu J., Wang P., Lai C.S.E., Zanella F., Feng G.-S., Sheikh F., Chien S., Chen S. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. 2016;113(8):2206–2211. doi: 10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang S., Yao B., Xie J., Fu X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016;32:170–177. doi: 10.1016/j.actbio.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 173.Phillips B.W., Horne R., Lay T.S., Rust W.L., Teck T.T., Crook J.M. Attachment and growth of human embryonic stem cells on microcarriers. J. Biotechnol. 2008;138(1–2):24–32. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 174.Oh S.K.W., Chen A.K., Mok Y., Chen X., Lim U.M., Chin A., Choo A.B.H., Reuveny S. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2(3):219–230. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 175.Lock L.T., Tzanakakis E.S. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng. A. 2009;15(8):2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie Y., Yang Y., Kang X., Li R., Volakis L.I., Zhang X., Lee L.J., Kniss D.A. Bioassembly of three-dimensional embryonic stem cell-scaffold complexes using compressed gases. Biotechnol. Prog. 2009;25(2):535–542. doi: 10.1002/btpr.151. [DOI] [PubMed] [Google Scholar]
- 177.Yang Y., Xie Y., Kang X., Lee L.J., Kniss D.A. Assembly of three-dimensional polymeric constructs containing cells/biomolecules using carbon dioxide. J. Am. Chem. Soc. 2006;128(43):14040–14041. doi: 10.1021/ja066157u. [DOI] [PubMed] [Google Scholar]
- 178.Guduric V., Metz C., Siadous R., Bareille R., Levato R., Engel E., Fricain J.C., Devillard R., Luzanin O., Catros S. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J. Mater. Sci. Mater. Med. 2017;28(5) doi: 10.1007/s10856-017-5887-6. [DOI] [PubMed] [Google Scholar]
- 179.Hooper G.J., Brown G.C.J., Lim K.S., Mutreja I., Mekhiileri N.V., Schon B.S., Woodfield T.B.F. Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication. 2017;10(2) doi: 10.1088/1758-5090/aa9ef1. [DOI] [PubMed] [Google Scholar]
- 180.Kinney M.A., Sargent C.Y., Mcdevitt T.C. The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng. B Rev. 2011;17(4):249–262. doi: 10.1089/ten.teb.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fridley K.M., Kinney M.A., Mcdevitt T.C. Hydrodynamic modulation of pluripotent stem cells. Stem Cell Res. Ther. 2012;3(6):45–53. doi: 10.1186/scrt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mattys O.B., Hookway T.A., McDevitt T. Design principles for engineering of tissues from human pluripotent stem cells. Curr Stem Cell Reports. 2016;2(1):43–51. doi: 10.1007/s40778-016-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Murphy W.L., McDevitt T.C., Engler A.J. Materials as stem cell regulators. Nat. Mater. 2014;13(6):547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kinney M.A., Hookway T.A., Wang Y., Mcdevitt T.C. Engineering three-dimensional stem cell morphogenesis for the development of tissue models and scalable regenerative therapeutics. Ann. Biomed. Eng. 2014;42(2):352–367. doi: 10.1007/s10439-013-0953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sart S., Schneider Y., Li Y., Agathos S.N. Stem cell bioprocess engineering towards cGMP production and clinical applications. Cytotechnology. 2014;66:709–722. doi: 10.1007/s10616-013-9687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Agathos S.N., Sart S., Li Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol. Prog. 2013;29(6):1354–1367. doi: 10.1002/btpr.1825. [DOI] [PubMed] [Google Scholar]
- 187.Li Y., Liu M., Yang S. Dendritic cells derived from pluripotent stem cells: potential of large scale production. World J. Stem Cells. 2014;6(1):1–10. doi: 10.4252/wjsc.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bardy J., Chen A.K., Lim Y.M., Wu S., Wei S. Microcarrier Suspension Cultures for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue Eng. C Methods. 2013;19(2):166–180. doi: 10.1089/ten.TEC.2012.0146. [DOI] [PubMed] [Google Scholar]
- 189.Li Q., Wang Q., Wang O., Shao K., Lin H., Lei Y. A simple and scalable hydrogel-based system for culturing protein-producing cells. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lei Y., Schaffer D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. 2013;110(52):E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ekerdt B.L., Fuentes C.M., Lei Y., Adil M.M., Ramasubramanian A., Segalman R.A., Schaffer D.V. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv. Healthc. Mater. 2018;7(12):1–22. doi: 10.1002/adhm.201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Alves P.M., Serra M., Brito C. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30(6):350–359. doi: 10.1016/j.tibtech.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 193.Paull D., Sevilla A., Zhou H., Hahn A.K., Kim H., Napolitano C., Tsankov A., Shang L., Krumholz K., Jagadeesan P., Woodard C.M., Sun B., Vilboux T., Zimmer M., Forero E., Moroziewicz D.N., Martinez H., Malicdan M.C.V., Weiss K.A., Vensand L.B., Dusenberry C.R., Polus H., Sy K.T.L., Kahler D.J., Gahl W.A., Solomon S.L., Chang S., Meissner A., Eggan K., Noggle S.A. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods. 2015;12:885. doi: 10.1038/nmeth.3507. [DOI] [PubMed] [Google Scholar]
- 194.Beers J., Linask K.L., Chen J.A., Siniscalchi L.I., Lin Y. A cost-effective and efficient reprogramming platform for large- scale production of integration- free human induced pluripotent stem cells in chemically defined culture. Sci. Rep. 2015;5:11319. doi: 10.1038/srep11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin H., Li Q., Du Q., Wang O., Wang Z., Akert L., Carlson M.A., Zhang C., Subramanian A., Zhang C., Lunning M., Li M., Lei Y. Integrated generation of induced pluripotent stem cells in a low-cost device. Biomaterials. 2019;189:23–36. doi: 10.1016/j.biomaterials.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 196.Neofytou E., O'Brien C.G., Couture L.A., Wu J.C. Hurdles to clinical translation of human induced pluripotent stem cells. J. Clin. Investig. 2015;125(7):2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li Q., Lin H., Du Q., Liu K., Wang O., Evans C., Christian H., Zhang C., Lei Y. Scalable and physiologically relevant microenvironments for human pluripotent stem cell expansion and differentiation Scalable and physiologically relevant microenvironments for human pluripotent stem cell expansion and differentiation. Biofabrication. 2018;10 doi: 10.1088/1758-5090/aaa6b5. [DOI] [PubMed] [Google Scholar]
- 198.Li Y., Ma T. Bioprocessing of cryopreservation for large-scale banking of human pluripotent stem cells. Biores Open Access. 2012;1(5):205–214. doi: 10.1089/biores.2012.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ma T., Li Y. Cryopreservation of pluripotent stem cell aggregates in defined protein-free formulation. Biotechnol. Prog. 2013;29(1):143–153. doi: 10.1002/btpr.1653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.