Abstract
Background
Infections with multidrug resistant Acinetobacter baumannii in immunocompromised patients are life-threatening. Therapeutic options are rare in this context, but patients are dependent on an effective antibiotic therapy. Thus, new antibiotic strategies are deemed necessary.
Case presentation
This case report recounts the therapeutic drug monitoring-guided meropenem therapy of a 32 years old patient admitted with acute exacerbation of cystic fibrosis. Veno-venous extracorporeal membrane oxygenation was initiated on the first day of admission to the intensive care unit. The patient showed insufficient serum trough levels of meropenem despite the maximum approved dose (2g every 8h) was administered which was due to augmented renal clearance. Through continuous infusion of the same cumulative dose, target levels were reached. On day 17 of admission, the patient underwent successful double-lung-transplant surgery and extracorporeal membrane oxygenation was ended. Unfortunately, the donor's lung was colonized with a multidrug resistant Acinetobacter baumannii that was positive for OXA-23 carbapenemase. Hence a combination therapy of intravenous sulbactam, tigecycline, meropenem and inhalative colistin was established, with a known minimal inhibitory concentration for meropenem of 32 mg/l. Under continuous infusion of 8 g meropenem/day, serum levels exceeded 32 mg/l over 12 days. The patient was transferred from the intensive care unit to a general ward without any signs of infection.
Conclusions
Therapeutic drug monitoring-guided meropenem may be a sound new therapeutic option in eradicating multidrug resistant Acinetobacter and offer a novel therapeutic option in the field of personalized medicine.
Keywords: Meropenem, Acinetobacter baumannii, Bacterial resistance, Therapeutic drug monitoring, Augmented renal clearance, Lung transplantation
1. Background
Infections with multi-drug resistant (MDR) Acinetobacter baumannii (A. baumannii) are associated with high mortality rates in lung transplant patients [1]. A risk factor for these infections is the colonization of the donor's lung, which is the most likely source of infection for patients undergoing transplantation [2]. The recovery of the immunocompromised organ recipient is then dependent on an effective antibiotic therapy.
Standard dosing of antibiotics in intensive-care-unit (ICU) patients runs the risk of low serum concentrations due to altered physiological conditions such as augmented renal clearance (ARC) and increased volume of distribution [3]. Low serum-concentrations in combination with multiresistant bacteria at a higher than normal minimal inhibitory concentrations (MIC) lead to subtherapeutic antibiotic exposure, with the consequence of treatment failure and the selection of more resistant pathogens. As such, standard dosing would be an inadequate strategy in this setting [4]. There are limited treatment options for MDR A. baumannii infections and inappropriate initial therapy is associated with increased mortality. Novel antibiotics and combination therapy of existing drugs are deemed necessary in this context [5].
In this case report we describe a modern therapeutic drug monitoring (TDM)-guided meropenem therapy to achieve effective target levels for MDR A. baumannii in a patient undergoing lung transplantation.
2. Case presentation
A 32 years old patient was admitted to our ICU with the diagnosis of pulmonary exacerbation and known cystic fibrosis. The patient was colonized with a multidrug resistant Pseudomonas aeruginosa and he received anti-infective therapy with intravenous cefepime, which was switched to meropenem at day 4.
First lab results showed normal values except an elevated creatinine clearance (ClCr) of 156 ml/min and CRP of 17.7 mg/dl (normal range < 0.5 mg/dl).
The patient decompensated under non-invasive CPAP/ASB-ventilation within the first 24 hours. Consequently, he was cannulated with a 31F single-needle veno-venous ECMO under general anesthesia as part of a “bridge-to-transplant” concept. On day 17, the patient was allocated a lung and the ECMO therapy was finished on the first postoperative day.
The serum level of meropenem was monitored daily. Initially, an intermittent i.v. dosing regimen of meropenem (2g every 8 hours) was applied resulting in low serum trough concentrations of 1.22–2.13 mg/l. The dosage regimen was then switched to continuous infusion of 6 g meropenem daily resulting in appropriate serum levels of 17.3–23.2 mg/l and stable ClCr values.
The first microbiological results of the donor's lungs were positive for MDR A. baumannii carrying an OXA-23 carbapenemase with MIC levels of 156 mg/l for sulbactam, 1 mg/l for tigecycline, 32 mg/l for meropenem and 3 mg/l for rifampicin, respectively. All other antibiotics tested, including polymyxins and cotrimoxazole, showed even higher resistance levels.
Lacking alternative options and facing the high risk of serious infection of the transplanted lung with MDR A. baumannii in an immunocompromised patient, high dose sulbactam, high dose tigecycline and TDM-guided meropenem were initiated based on test results. Meropenem was infused continuously with 8g/d leading to an average serum concentration of 39.6 mg/l (see Fig. 1). Colistin p.i. was administered as prophylaxis for further infections according to our standard protocol, if the donor organ hails from a country with high risk of MDR-pathogen colonization.
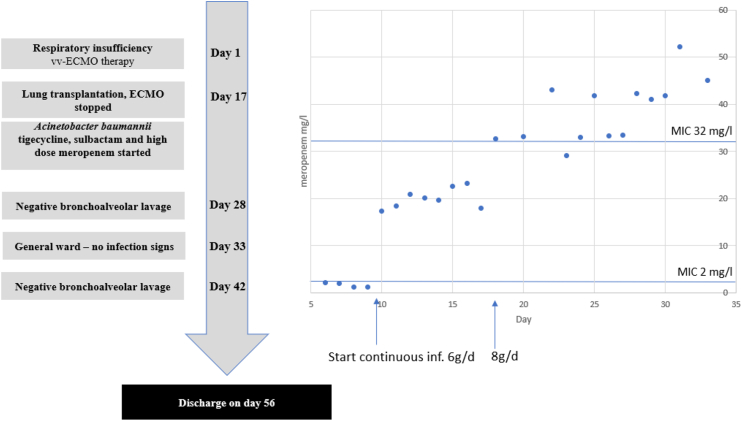
Fig. 1.
Timeline of the case. On the right, meropenem serum concentrations are shown from day 5 to day 33. The potential MIC (EUCAST) of the relevant pathogen Pseudomonas aeruginosa before transplantation is marked with the horizontal line at 2 mg/l. Through intermittent infusion, none of values exceeded the reference line. Only continuous infusion achieved sufficient Css serum levels. On day 17, the regimen was switched to 8g/day due to the new relevant pathogen Acinetobacter baumannii and higher target serum levels.
Antibiotic therapy was stopped on day 33 and the patient was transferred from the ICU to a general ward without any signs of infection.
3. Discussion
In this case report pharmacokinetic/pharmacodynamics (PK/PD) optimization of high-dose TDM-guided continuous-infusion of meropenem revealed a rational and effective approach in the treatment of MDR A. baumannii.
There is no clear evidence for the best therapeutic regimen for patients colonized with MDR A. baumannii. The German S2k guidelines recommend a combination therapy with tigecycline and colistin in carbapenem-resistant A. baumannii and due to lack of data provides no recommendation regarding a continuous carbapenem infusion in carbapenem-resistant bacteria [6]. The antibiotic regimen chosen in our case was a combination of tigecycline, sulbactam and TDM-guided high dose meropenem as continuous infusion.
3.1. Pharmacokinetic alterations by ECMO
The extent of pharmacokinetic changes caused by ECMO therapy is not conclusively known and specific dosage guidelines for ECMO patients are lacking. ECMO-related physiological changes such as systemic inflammatory response syndrome (SIRS) seem to be responsible for an increased volume of distribution especially in hydrophilic substances such as meropenem [7]. In a study by Shekar et al. on eleven ECMO patients, the increased volume of distribution was compensated by reduced clearance [8]. Exactly the opposite occurred in this case, since an ARC, which is common in young critically ill patients, was present [9]. This constellation led to very low serum concentrations with an intermittent dosing regimen even when the maximum approved dose was administered. Avoidance of insufficient concentrations could only be achieved by TDM guided continuous infusion. We therefore recommend in accordance with a recently published study on 44 patients with ECMO therapy a regular TDM in ECMO patients with good renal function to avoid insufficient serum levels and to use alternative dosing regimens such as continuous infusion [10].
3.2. Effectiveness of tigecycline, sulbactam and colistin
The off-label use of tigecycline to treat pulmonary infections is regarded with skepticism in the literature since the concentrations found in epithelial lining fluids are low [11]. Nevertheless, when administered to treat pulmonary infections, a high dose regimen is recommended by the guidelines [6]. In this case, the patient received a likely effective high-dose tigecycline therapy with a MIC of 1 mg/l.
High dose sulbactam is described to be effective in the therapy of MDR-A. baumannii infections [12]. A case series of 22 patients showed that there is no correlation between the MIC and the clinical cure rate under sulbactam-therapy in a MIC range of 2.0–32 mg/l [13]. But a mechanism that might cause sulbactam-resistance in A. baumannii isolates is the OXA-23 carbapenemase [14]. As a MIC of 156 mg/l is extremely high and the isolate was equipped with a potentially powerful resistance mechanism, the effectiveness of sulbactam therapy in this patient remains elusive.
For colistin there is described synergistic effect with meropenem [15]. Given a MIC of > 8mg/l of the tested A. baumannii strain against colistin a strong effect is unlikely.
3.3. Effectiveness of high dose meropenem
There is, to the best of our knowledge, no published data examining a TDM-guided high dose meropenem therapy in MDR-A. baumannii infections, but there are indications hinting at its effectiveness. Lenhard et al. showed in time kill curves and a hollow fiber infection model a meropenem-dose dependent killing rate of carbapenem resistant A. baumannii strains. These strains were partly equipped with an OXA-23-carbapenemase. An intensified meropenem dose alone did not kill off carbapenem resistant A. baumannii strains, but there was a dose dependent effect when meropenem was administered in combination with colistin. The doses needed to optimize the killing rates were above the maximum approved dose of 2g every 8h. The author concluded that a dose increase in meropenem could boost the likelihood of bacterial eradication in carbapenem resistant A. baumannii infections [16]. Pea et al. described a high-dose TDM-guided continuous meropenem infusion in 13 cases of KPC-Klebsiella pneumonia isolates with a MIC of 16–64 mg/l for meropenem. Reaching the target PK/PD-Indices for meropenem was the most relevant index of clinical success, although this was with combination therapy. Neither the actual MIC nor the number of active antimicrobial agents had a significant impact on the clinical outcome. The authors suggested the implementation of high-dose meropenem as combination therapy in treating KPC-Klebsiella pneumonia whenever it is possible to achieve relevant PK/PD-indices [17]. We are describing, for the first time here, the use of TDM-guided continuous meropenem infusion for A. baumannii with very high MICs.
4. Conclusions
-
1)
TDM is essential for patients with ARC undergoing ECMO-therapy. Alternative dosage regimens such as continuous infusion can avoid subtherapeutic levels.
-
2)
TDM-guided high-dose meropenem offers a novel therapeutic option in the field of personalized medicine for the treatment of MDR-A. baumannii that is not reported to date.
-
3)
In an urgent race to promote the use of rational anti-infective therapy, TDM-guided meropenem may be a sound new therapeutic option in eradicating MDR bacteria.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for anonymous publication of this case report. A copy of the written consent is available upon request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, ornot-for-profit sectors.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Authors’ contributions
UL, MZ an CS conceived the idea for the article. UL and CS drafted the manuscript, all authors revised it critically. MP and JJ performed analytical work, IS, MZ, LF and UL treated the patient in the hospital. All authors approved the submitted manuscript.
Declaration of competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgements
English language editing assistance was provided by Anne Guo.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.100966.
Abbreviations
- MDR
multidrug resistant
- ICU
intensive care unit
- ARC
augmented renal clearance
- MIC
minimal inhibitory concentration
- TDM
therapeutic drug monitoring
- ECMO
extracorporeal membrane oxygenation
- ClCr
Creatinine clearance
- PK/PD
pharmacokinetic/pharmacodynamics
- SIRS
systemic inflammatory response syndrome
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Trottier V., Namias N., Pust D.G., Nuwayhid Z., Manning R., Marttos Antonio C., Jr. Outcomes of acinetobacter baumannii infection in critically ill surgical patients. Surg. Infect. 2007 Aug 1;8(4):437–444. doi: 10.1089/sur.2006.029. [DOI] [PubMed] [Google Scholar]
- 2.Len O., Gavaldà J., Blanes M., Montejo M., San Juan R., Moreno A. Donor infection and transmission to the recipient of a solid allograft. Am. J. Transplant. 2008 Nov;8(11):2420–2425. doi: 10.1111/j.1600-6143.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 3.Ehmann L., Zoller M., Minichmayr I.K., Scharf C., Maier B., Schmitt M.V. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit. Care. 2017 Dec;21(1):263. doi: 10.1186/s13054-017-1829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udy A.A., Roberts J.A., Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013 Dec;39(12):2070–2082. doi: 10.1007/s00134-013-3088-4. [DOI] [PubMed] [Google Scholar]
- 5.Iii J.P.L., Zhanel G.G., Clark N.M. Infections due to acinetobacter baumannii in the ICU: treatment options. Semin. Respir. Crit. Care Med. 2017 Jun;38(03):311–325. doi: 10.1055/s-0037-1599225. [DOI] [PubMed] [Google Scholar]
- 6.S82-006l_S2k_Parenterale_Antibiotika_2018-1.pdf. https://www.awmf.org/uploads/tx_szleitlinien/S82-006l_S2k_Parenterale_Antibiotika_2018-1.pdf [Internet]. [cited 2018 Nov 27]. Available from.
- 7.Cheng V., Abdul-Aziz M.-H., Roberts J.A., Shekar K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2017 Oct 19;10(5) doi: 10.21037/jtd.2017.09.154. S629-S641–S641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekar K., Fraser J.F., Taccone F.S., Welch S., Wallis S.C., Mullany D.V. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit. Care. 2014 Dec 12;18(6):565. doi: 10.1186/s13054-014-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huttner A., Von Dach E., Renzoni A., Huttner B.D., Affaticati M., Pagani L. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int. J. Antimicrob. Agents. 2015 Apr 1;45(4):385–392. doi: 10.1016/j.ijantimicag.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Bouglé A., Dujardin O., Lepère V., Ait Hamou N., Vidal C., Lebreton G. Pharmecmo: therapeutic drug monitoring and adequacy of current dosing regimens of antibiotics in patients on Extracorporeal Life Support. Anaesth. Crit. Care Pain Med. 2019 Oct;38(5):493–497. doi: 10.1016/j.accpm.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Conte J.E., Golden J.A., Kelly M.G., Zurlinden E. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents. 2005 Jun;25(6):523–529. doi: 10.1016/j.ijantimicag.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Liu Q., Chen Z., Li C. Efficacy of sulbactam for the treatment of Acinetobacter baumannii complex infection: a systematic review and meta-analysis. J. Infect. Chemother. 2017 May;23(5):278–285. doi: 10.1016/j.jiac.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira M., Costa S., de Pedri E., van der Heijden I., Levin A. The minimal inhibitory concentration for sulbactam was not associated with the outcome of infections caused by carbapenem-resistant Acinetobacter sp. treated with ampicillin/sulbactam. Clinics. 2013 Apr 25;68(4):569–573. doi: 10.6061/clinics/2013(04)21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y., Xu Q., Li T., Fu Y., Shi Y., Lan P. OXA-23 is a prevalent mechanism contributing to sulbactam resistance in diverse acinetobacter baumannii clinical strains. Antimicrob. Agents Chemother. 2019 Jan 1;63(1) doi: 10.1128/AAC.01676-18. e01676-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marie M.A.M., Krishnappa L.G., Alzahrani A.J., Mubaraki M.A., Alyousef A.A. A prospective evaluation of synergistic effect of sulbactam and tazobactam combination with meropenem or colistin against multidrug resistant Acinetobacter baumannii. Bosn. J. Basic Med. Sci. 2015 Oct 14;15(4):24–29. doi: 10.17305/bjbms.2015.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenhard J.R., Bulitta J.B., Connell T.D., King-Lyons N., Landersdorfer C.B., Cheah S.-E. High-intensity meropenem combinations with polymyxin B: new strategies to overcome carbapenem resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2017 Jan 1;72(1):153–165. doi: 10.1093/jac/dkw355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pea F., Della Siega P., Cojutti P., Sartor A., Crapis M., Scarparo C. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int. J. Antimicrob. Agents. 2017 Feb 1;49(2):255–258. doi: 10.1016/j.ijantimicag.2016.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.